The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders
Abstract
:1. Introduction
2. Ferroptosis and Central Neurological Disorders
2.1. Mechanism and Pathway of Ferroptosis
2.2. Ferroptosis in Central Neurological Disorders
2.3. Cognitive Dysfunction Caused by Ferroptosis
3. Neuroinflammation in Central Neurological Disorders
4. The Link between Neuroinflammation and Ferroptosis
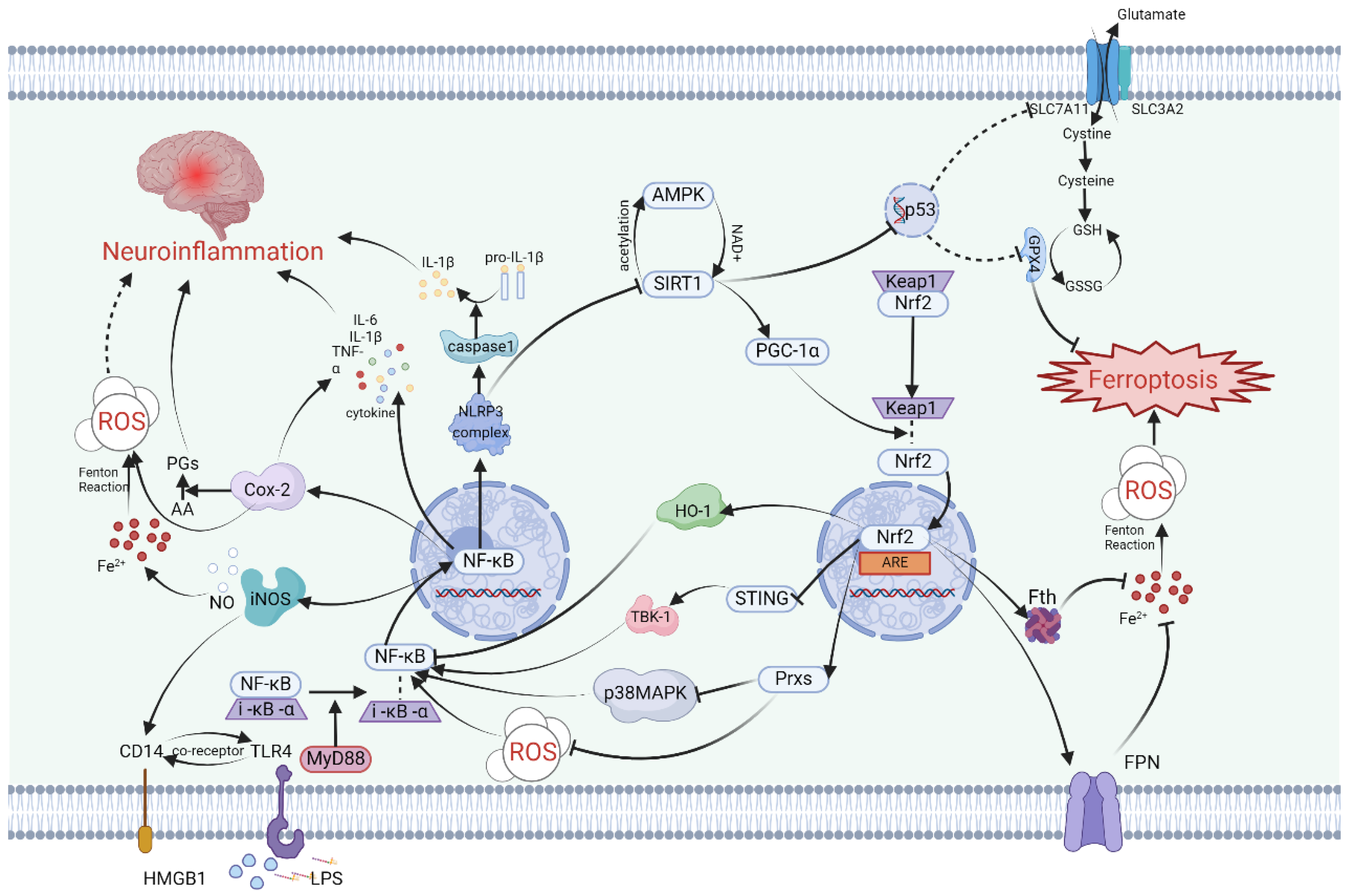
4.1. Molecular Mechanisms and Signaling Pathways
4.1.1. SIRT1
4.1.2. Nrf2
4.1.3. NF-κB
4.1.4. PTGS2/Cox-2
4.1.5. iNOS/NO•
4.2. Cell Phenotype and Specific Structure
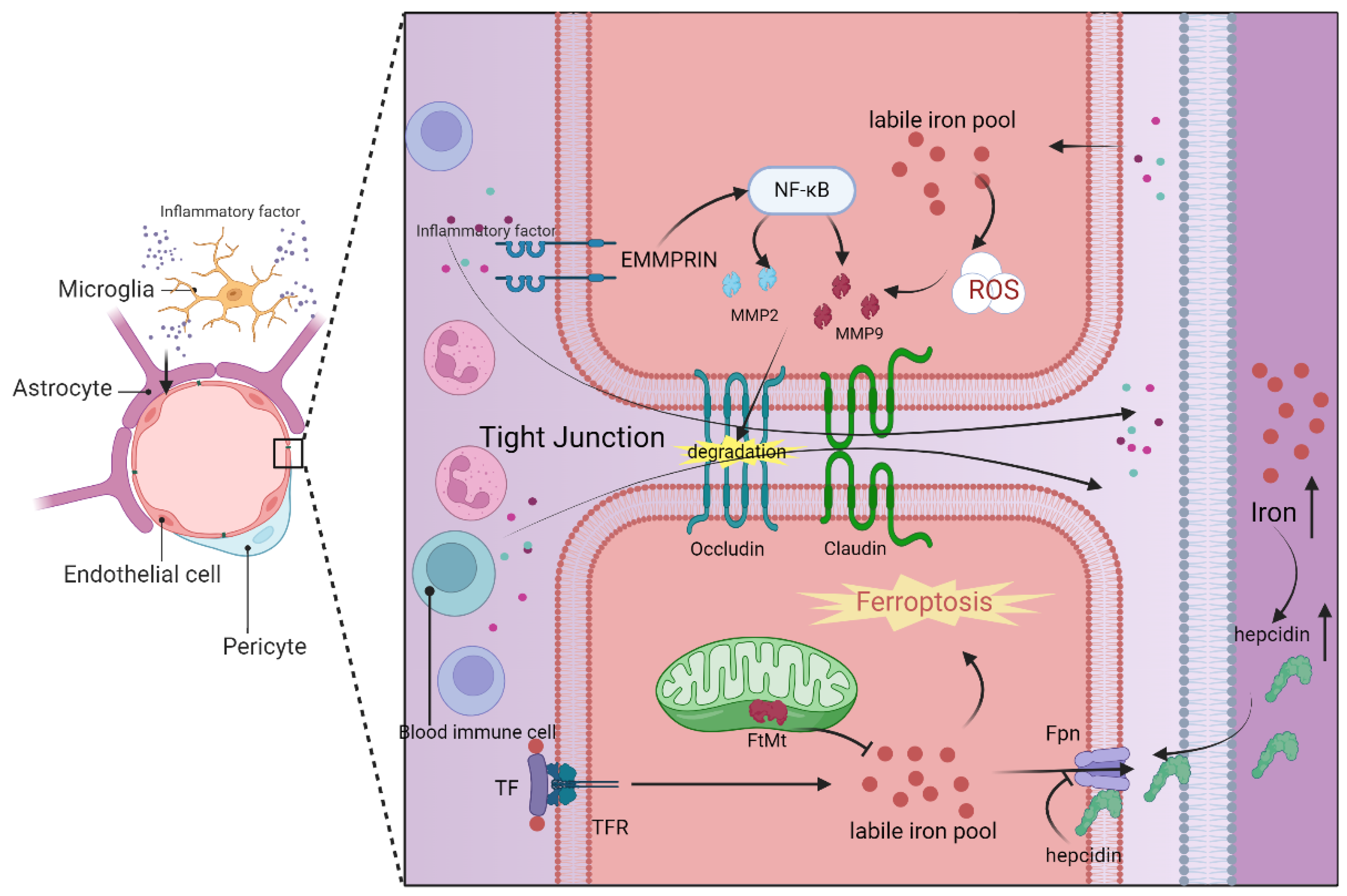
4.2.1. Blood–Brain Barrier
4.2.2. Microglia
4.2.3. Astrocytes
4.2.4. Neutrophils and NETs
4.2.5. T Cells
5. Conclusions
6. Futures Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sauve, F.; Nampoothiri, S.; Clarke, S.A.; Fernandois, D.; Ferreira Coelho, C.F.; Dewisme, J.; Mills, E.G.; Ternier, G.; Cotellessa, L.; Iglesias-Garcia, C.; et al. Long-COVID cognitive impairments and reproductive hormone deficits in men may stem from GnRH neuronal death. EBioMedicine 2023, 96, 104784. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Hong, D.K.; Kang, B.S.; Lee, S.H.; Choi, S.; Kim, H.J.; Lee, S.M.; Suh, S.W. Engineered Mesenchymal Stem Cells Over-Expressing BDNF Protect the Brain from Traumatic Brain Injury-Induced Neuronal Death, Neurological Deficits, and Cognitive Impairments. Pharmaceuticals 2023, 16, 436. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Jiang, R.; Zhang, W.; Yang, Z.; Li, L.L.; Shimozawa, M.; Tambaro, S.; Mayer, J.; Zhang, B.; Li, M.; et al. An App knock-in rat model for Alzheimer’s disease exhibiting Abeta and tau pathologies, neuronal death and cognitive impairments. Cell Res. 2022, 32, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fan, Z.; Rauh, M.; Buchfelder, M.; Eyupoglu, I.Y.; Savaskan, N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene 2017, 36, 5593–5608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shaabani, S.; Vowinkel, K.; Trombetta-Lima, M.; Sabogal-Guaqueta, A.M.; Chen, T.; Hoekstra, J.; Lembeck, J.; Schmidt, M.; Decher, N.; et al. Novel SK channel positive modulators prevent ferroptosis and excitotoxicity in neuronal cells. Biomed. Pharmacother. 2024, 171, 116163. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, A.; Gerhard, A.; Banati, R.B. In vivo imaging of neuroinflammation. Eur. Neuropsychopharmacol. 2002, 12, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Adrian, M.; Weber, M.; Tsai, M.C.; Glock, C.; Kahn, O.I.; Phu, L.; Cheung, T.K.; Meilandt, W.J.; Rose, C.M.; Hoogenraad, C.C. Polarized microtubule remodeling transforms the morphology of reactive microglia and drives cytokine release. Nat. Commun. 2023, 14, 6322. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Sun, W.; Zhang, M. Morin Suppresses Astrocyte Activation and Regulates Cytokine Release in Bone Cancer Pain Rat Models. Phytother. Res. 2017, 31, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Guo, S.; Wei, B.; Liu, W.; Li, G.; Li, X.; Liu, J.; Jin, L.; Duan, C. Nrf-2 modulates excitability of hippocampal neurons by regulating ferroptosis and neuroinflammation after subarachnoid hemorrhage in rats. Brain Res. Bull. 2024, 207, 110877. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Jiang, Y.; Shi, B.; Zhang, Z.; Zhang, Z. Selenomethionine alleviates decabromodiphenyl ether-induced oxidative stress and ferroptosis via the NRF2/GPX4 pathway in the chicken brain. J. Hazard. Mater. 2024, 465, 133307. [Google Scholar] [CrossRef] [PubMed]
- Mumbauer, S.; Pascual, J.; Kolotuev, I.; Hamaratoglu, F. Ferritin heavy chain protects the developing wing from reactive oxygen species and ferroptosis. PLoS Genet. 2019, 15, e1008396. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xu, W.; Tang, X.; Liu, X.; Cheng, Y.; Wu, Y.; Xie, Z.; Wu, X.; He, X.; Wang, Q.; et al. Canonical Wnt signaling works downstream of iron overload to prevent ferroptosis from damaging osteoblast differentiation. Free Radic. Biol. Med. 2022, 188, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhou, C.; Jing, Q.; Li, Y.; Yang, J.; Yang, C.; Wang, L.; Hu, J.; Li, H.; Wang, H.; et al. FTH promotes the proliferation and renders the HCC cells specifically resist to ferroptosis by maintaining iron homeostasis. Cancer Cell Int. 2021, 21, 709. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Luo, X.; Bai, X.; Lv, Y.; Weng, X.; Zhang, S.; Leng, Y.; Huang, J.; Dai, X.; Wang, Y.; et al. Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic. Biol. Med. 2023, 201, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, T.; Ge, Y.; Cheng, Y.; Yin, L.; Pu, Y.; Chen, Z.; Liang, G. Ferritinophagy Mediated by Oxidative Stress-Driven Mitochondrial Damage Is Involved in the Polystyrene Nanoparticles-Induced Ferroptosis of Lung Injury. ACS Nano 2023, 17, 24988–25004. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Q.Z.; Liu, Y.; Xiang, Z.; Yan, H.F.; Zou, T.; Shu, Y.; Ding, X.L.; Zou, J.J.; Xu, S.; Tang, F.; et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct. Target. Ther. 2022, 7, 59. [Google Scholar] [CrossRef]
- Wu, C.; Du, M.; Yu, R.; Cheng, Y.; Wu, B.; Fu, J.; Tan, W.; Zhou, Q.; Balawi, E.; Liao, Z.B. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free Radic. Biol. Med. 2022, 178, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wei, W.; Wu, D.; Huang, F.; Li, M.; Li, W.; Yin, J.; Peng, Y.; Lu, Y.; Zhao, Q.; et al. Blockade of GCH1/BH4 Axis Activates Ferritinophagy to Mitigate the Resistance of Colorectal Cancer to Erastin-Induced Ferroptosis. Front. Cell Dev. Biol. 2022, 10, 810327. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, J.; Ralhan, I.; Ioannou, M.S. Driving factors of neuronal ferroptosis. Trends Cell Biol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.H.; Ratan, R.R. The role of iron neurotoxicity in ischemic stroke. Ageing Res. Rev. 2004, 3, 345–353. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Zhao, X.; Shao, L.; Liu, G.; Sun, C.; Xu, R.; Zhang, Z. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 2021, 93, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Jeandriens, J.; Parkes, H.G.; So, P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: Evidence of ferroptosis. Redox Biol. 2020, 32, 101494. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-S.; Gao, L.; Han, Z.; Eleuteri, S.; Shi, W.; Shen, Y.; Song, Z.-Y.; Su, M.; Yang, Q.; Qu, Y.; et al. Ferroptosis in Parkinson’s disease: Molecular mechanisms and therapeutic potential. Ageing Res. Rev. 2023, 91, 102077. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhang, X.; Zhang, Y.; Wu, Y.; Song, Y.; Wang, X.; Chen, T.; Liu, W.; Peng, B.; Yin, J.; et al. Neurotoxic A1 astrocytes promote neuronal ferroptosis via CXCL10/CXCR3 axis in epilepsy. Free Radic. Biol. Med. 2023, 195, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.S.; Wang, Y.Q.; Lin, Y.; Mao, Q.; Feng, J.F.; Gao, G.Y.; Jiang, J.Y. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2019, 25, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Schroder, N.; Figueiredo, L.S.; de Lima, M.N. Role of brain iron accumulation in cognitive dysfunction: Evidence from animal models and human studies. J. Alzheimers Dis. 2013, 34, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.; Schröder, N.; Eriksson, P.; Izquierdo, I.; Archer, T. Neonatal iron exposure induces neurobehavioural dysfunctions in adult mice. Toxicol. Appl. Pharm. 1999, 159, 25–30. [Google Scholar] [CrossRef]
- Mei, H.; Wu, D.; Yong, Z.; Cao, Y.; Chang, Y.; Liang, J.; Jiang, X.; Xu, H.; Yang, J.; Shi, X.; et al. PM(2.5) exposure exacerbates seizure symptoms and cognitive dysfunction by disrupting iron metabolism and the Nrf2-mediated ferroptosis pathway. Sci. Total Environ. 2024, 910, 168578. [Google Scholar] [CrossRef]
- Xie, R.; Zhao, W.; Lowe, S.; Bentley, R.; Hu, G.; Mei, H.; Jiang, X.; Sun, C.; Wu, Y.; Liu, Y. Quercetin alleviates kainic acid-induced seizure by inhibiting the Nrf2-mediated ferroptosis pathway. Free Radic. Biol. Med. 2022, 191, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wu, Y.; Jia, Q.; Li, J.; Li, Y.; Ma, H.; Fan, Z.; Guo, X.; Li, L.; Peng, Y.; et al. Autophagy Suppresses Ferroptosis by Degrading TFR1 to Alleviate Cognitive Dysfunction in Mice with SAE. Cell Mol. Neurobiol. 2023, 43, 3605–3622. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yuan, Q.; Du, Z.; Zhang, Q.; Yang, L.; Wang, M.; Yang, W.; Yuan, C.; Yu, J.; Wu, G.; et al. Overexpression of GPX4 attenuates cognitive dysfunction through inhibiting hippocampus ferroptosis and neuroinflammation after traumatic brain injury. Free Radic. Biol. Med. 2023, 204, 68–81. [Google Scholar] [CrossRef]
- Weiland, A.; Wang, Y.; Wu, W.; Lan, X.; Han, X.; Li, Q.; Wang, J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol. Neurobiol. 2019, 56, 4880–4893. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Xu, Y.; Sun, L.; Hashimoto, M.; Wei, J. Microglial response to aging and neuroinflammation in the development of neurodegenerative diseases. Neural Regen. Res. 2024, 19, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Aditi; Onyoni, F.; Wang, H.; Gonzales, C.; Sunyakumthorn, P.; Wu, P.; Samir, P.; Soong, L. Brain transcriptomics reveal the activation of neuroinflammation pathways during acute Orientia tsutsugamushi infection in mice. Front. Immunol. 2023, 14, 1194881. [Google Scholar] [CrossRef] [PubMed]
- Merchak, A.R.; Cahill, H.J.; Brown, L.C.; Brown, R.M.; Rivet-Noor, C.; Beiter, R.M.; Slogar, E.R.; Olgun, D.G.; Gaultier, A. The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. PLoS Biol. 2023, 21, e3002000. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Chen, P.; Wang, Y.J.; Zhou, Z.P.; Liu, X.C.; Fang, H.; Wang, C.W.; Liu, J.; Liu, R.Y.; Liu, H.K.; et al. Increased cysteinyl-tRNA synthetase drives neuroinflammation in Alzheimer’s disease. Transl. Neurodegener. 2024, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Hellani, F.; Leleu, I.; Saidi, N.; Martin, N.; Lecoeur, C.; Werkmeister, E.; Koffi, D.; Trottein, F.; Yapo-Ette, H.; Das, B.; et al. Role of astrocyte senescence regulated by the non- canonical autophagy in the neuroinflammation associated to cerebral malaria. Brain Behav. Immun. 2023, 117, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Wang, X. Inhibition of miR-429 improves neurological recovery of traumatic brain injury mice and attenuates microglial neuroinflammation. Int. Immunopharmacol. 2020, 79, 106091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, W.; Xue, J.; Chen, J.; Liu, S.; Zhang, S.; Zhang, X.; Gu, X.; Dong, Y.; Qiu, P. Neuroinflammation: The central enabler of postoperative cognitive dysfunction. Biomed. Pharmacother. 2023, 167, 115582. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, J.; Zeng, Y.; Zheng, W. Neuroinflammation in epileptogenesis: From pathophysiology to therapeutic strategies. Front. Immunol. 2023, 14, 1269241. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Li, H.Q.; Dong, M.Q.; Gu, X.Y.; Xu, S.Y.; Xia, S.N.; Bao, X.Y.; Xu, Y.; Cao, X. Infiltrating myeloid cell-derived properdin markedly promotes microglia-mediated neuroinflammation after ischemic stroke. J. Neuroinflamm. 2023, 20, 260. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Beland, L.C.; Sato, S.; Kriz, J. Microglia-derived galectin-3 in neuroinflammation; a bittersweet ligand? Med. Res. Rev. 2021, 41, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Z.; Zhou, Z.W.; Cheng, Y.; Luo, H.Y.; Li, F.J.; Xu, S.G.; Gao, L.C. The Role of Microglia in Alzheimer’s Disease From the Perspective of Immune Inflammation and Iron Metabolism. Front. Aging Neurosci. 2022, 14, 888989. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, R.; Yang, M.; Lu, M. The role of astrocyte in neuroinflammation in traumatic brain injury. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166992. [Google Scholar] [CrossRef] [PubMed]
- Skok, M. Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation. World J. Stem Cells 2021, 13, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fierro, C.; Fonte, C.; Dufourd, E.; Cazaentre, V.; Aydin, S.; Engelhardt, B.; Caspi, R.R.; Xu, B.; Martin-Blondel, G.; Spicer, J.A.; et al. Effects of a Small-Molecule Perforin Inhibitor in a Mouse Model of CD8 T Cell-Mediated Neuroinflammation. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200117. [Google Scholar] [CrossRef] [PubMed]
- Puech, C.; Badran, M.; Runion, A.R.; Barrow, M.B.; Cataldo, K.; Gozal, D. Cognitive Impairments, Neuroinflammation and Blood-Brain Barrier Permeability in Mice Exposed to Chronic Sleep Fragmentation during the Daylight Period. Int. J. Mol. Sci. 2023, 24, 9880. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, T.; Zhang, Z.; Yang, G.Y. The Function of Astrocyte Mediated Extracellular Vesicles in Central Nervous System Diseases. Front. Cell Dev. Biol. 2020, 8, 568889. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, K.; Chen, L.; Ning, J.L.; Li, X.; Yang, T.; Terrando, N.; Gu, J.; Tao, G. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J. Neuroinflamm. 2016, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Kwon, H.J.; Kim, W.; Hwang, I.K.; Choi, G.M.; Chang, I.B.; Kim, D.W.; Moon, S.M. Tat-Endophilin A1 Fusion Protein Protects Neurons from Ischemic Damage in the Gerbil Hippocampus: A Possible Mechanism of Lipid Peroxidation and Neuroinflammation Mitigation as Well as Synaptic Plasticity. Cells 2021, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Nie, L.; Ali, T.; Liu, Z.; Li, W.; Gao, R.; Zhang, Z.; Liu, J.; Dai, Z.; Xie, Y.; et al. Adiponectin deficiency accelerates brain aging via mitochondria-associated neuroinflammation. Immun. Ageing 2023, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, C.; Zhu, Y.; Chao, Z.; Sheng, Z.; Zhang, Y.; Zhao, Y. Ferrostatin-1 alleviates angiotensin II (Ang II)- induced inflammation and ferroptosis in astrocytes. Int. Immunopharmacol. 2021, 90, 107179. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.C.; Dou, B.K.; Zou, Y.X.; Han, H.Z.; Liu, D.X.; Ke, Z.J.; Wang, Z.F. Cycloastragenol upregulates SIRT1 expression, attenuates apoptosis and suppresses neuroinflammation after brain ischemia. Acta Pharmacol. Sin. 2020, 41, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, S.H.; Syam Das, S.; Thushara, A.J.; Rauf, A.A.; Indira, M. All Trans Retinoic Acid Attenuates Markers of Neuroinflammation in Rat Brain by Modulation of SIRT1 and NFkappaB. Neurochem. Res. 2018, 43, 1791–1801. [Google Scholar] [CrossRef]
- Song, H.; Ding, Z.; Chen, J.; Chen, T.; Wang, T.; Huang, J. The AMPK-SIRT1-FoxO1-NF-kappaB signaling pathway participates in hesperetin-mediated neuroprotective effects against traumatic brain injury via the NLRP3 inflammasome. Immunopharmacol. Immunotoxicol. 2022, 44, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, L.; Zhang, X.; Ying, K.; Zhou, R.; Cai, W.; Wu, X.; Jiang, H.; Xu, Q.; Miao, D.; et al. Salidroside ameliorates acetaminophen-induced acute liver injury through the inhibition of endoplasmic reticulum stress-mediated ferroptosis by activating the AMPK/SIRT1 pathway. Ecotoxicol. Environ. Saf. 2023, 262, 115331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, J.; Chen, H.; Wang, Y.; Kuai, X.; Sun, S.; Tang, Q.; Zong, F.; Chen, Q.; Wu, J.; et al. High-Dosage NMN Promotes Ferroptosis to Suppress Lung Adenocarcinoma Growth through the NAM-Mediated SIRT1-AMPK-ACC Pathway. Cancers 2023, 15, 2427. [Google Scholar] [CrossRef] [PubMed]
- Siblini, Y.; Namour, F.; Oussalah, A.; Gueant, J.L.; Chery, C. Stemness of Normal and Cancer Cells: The Influence of Methionine Needs and SIRT1/PGC-1alpha/PPAR-alpha Players. Cells 2022, 11, 3607. [Google Scholar] [CrossRef] [PubMed]
- Gurd, B.J.; Menezes, E.S.; Arhen, B.B.; Islam, H. Impacts of altered exercise volume, intensity, and duration on the activation of AMPK and CaMKII and increases in PGC-1alpha mRNA. Semin. Cell Dev. Biol. 2023, 143, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Xie, X.; Wang, N.; Wang, Y.; Zhao, J.; Chen, F.; Qu, F.; Wen, W.; Miao, J.; Cui, H. Formononetin promotes fatty acid beta-oxidation to treat non-alcoholic steatohepatitis through SIRT1/PGC-1alpha/PPARalpha pathway. Phytomedicine 2024, 124, 155285. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Hao, Z.; Qi, W.; Jiang, F.; Liu, K.; Shi, X. Aerobic exercise combined with chlorogenic acid exerts neuroprotective effects and reverses cognitive decline in Alzheimer’s disease model mice (APP/PS1) via the SIRT1/ /PGC-1alpha/PPARgamma signaling pathway. Front. Aging Neurosci. 2023, 15, 1269952. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Ding, X.; Wang, Y.; Gu, M.; Zhang, J.; Yan, S.; Li, N.; Song, Z.; Yin, J.; Lu, L.; et al. Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1alpha pathway in high-fat-diet-induced rats and insulin resistant cells. Int. J. Biol. Sci. 2019, 15, 2815–2829. [Google Scholar] [CrossRef]
- Fan, H.; Sun, Y.; Zhang, X.; Xu, Y.; Ming, Y.; Zhang, L.; Zhao, P. Malvidin promotes PGC-1alpha/Nrf2 signaling to attenuate the inflammatory response and restore mitochondrial activity in septic acute kidney injury. Chem. Biol. Interact. 2024, 388, 110850. [Google Scholar] [CrossRef]
- Zhang, X.S.; Lu, Y.; Li, W.; Tao, T.; Peng, L.; Wang, W.H.; Gao, S.; Liu, C.; Zhuang, Z.; Xia, D.Y.; et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharmacol. 2021, 178, 1114–1132. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gu, Z.T.; Li, L.; Maegele, M.; Zhou, B.Y.; Li, F.; Zhao, M.; Zhao, K.S. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol. Sin. 2017, 38, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, P.; Guo, Q.; Li, S.; Zhang, L.; Zhao, M.; Xu, H.; Yang, Y.; Poon, W.; Fei, Z. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp. Neurol. 2012, 237, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Ahrari, S.; Salimian, J.; Salehi, Z.; Karimi, M.; Emamvirdizadeh, A.; Jamalkandi, S.A.; Ghanei, M. p38 MAPK signaling in chronic obstructive pulmonary disease pathogenesis and inhibitor therapeutics. Cell Commun. Signal 2023, 21, 314. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, Y.; Wu, H.; Zhang, Q.; Liu, Q.; Li, G.; Zhao, T.; Liu, X.; Zheng, S.; Qian, Z.; et al. Forsythoside B attenuates neuro-inflammation and neuronal apoptosis by inhibition of NF-kappaB and p38-MAPK signaling pathways through activating Nrf2 post spinal cord injury. Int. Immunopharmacol. 2022, 111, 109120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y. Neuroprotective effect of berberine agonist against impairment of learning and memory skills in severe traumatic brain injury via Sirt1/p38 MAPK expression. Mol. Med. Rep. 2018, 17, 6881–6886. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Z.; Xue, H.; Gao, Q.; Zhang, Y.; Wang, C.; Zhao, P. Ferroptosis contributes to hypoxic-ischemic brain injury in neonatal rats: Role of the SIRT1/Nrf2/GPx4 signaling pathway. CNS Neurosci. Ther. 2022, 28, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, Y.P.; Chen, Y.; Sun, S.; Yan, Z.Z.; Wang, Y.H. Arc regulates brain damage and neuroinflammation via Sirt1 signaling following subarachnoid hemorrhage. Brain Res. Bull. 2023, 203, 110780. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Wang, C.; Zhang, Y.; Sun, J. Brevilin A attenuates cartilage destruction in osteoarthritis mouse model by inhibiting inflammation and ferroptosis via SIRT1/Nrf2/GPX4 signaling pathway. Int. Immunopharmacol. 2023, 124, 110924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, L.; Yang, B. Saikosaponin A alleviates Staphylococcus aureus-induced mastitis in mice by inhibiting ferroptosis via SIRT1/Nrf2 pathway. J. Cell Mol. Med. 2023, 27, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, L.; Yang, B. Diosmetin alleviates S. aureus-induced mastitis by inhibiting SIRT1/GPX4 mediated ferroptosis. Life Sci. 2023, 331, 122060. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.; Peng, H.; Zhang, M.; Wu, X.; Gui, F.; Li, W.; Ai, F.; Yu, B.; Liu, Y. Meteorin-like/Meteorin-beta protects LPS-induced acute lung injury by activating SIRT1-P53-SLC7A11 mediated ferroptosis pathway. Mol. Med. 2023, 29, 144. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, H.; Wang, B.; Wu, Z.; Wu, S.; Jiang, G.; Wang, H.; Huang, Y. Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis via regulation of SIRT1/p53 acetylation. Chem. Biol. Interact. 2023, 382, 110607. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Li, Y.; Hong, Z.; Li, S.; Liu, C. PUM2 aggravates the neuroinflammation and brain damage induced by ischemia-reperfusion through the SLC7A11-dependent inhibition of ferroptosis via suppressing the SIRT1. Mol. Cell Biochem. 2023, 478, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Rodencal, J.; Kim, N.; He, A.; Li, V.L.; Lange, M.; He, J.; Tarangelo, A.; Schafer, Z.T.; Olzmann, J.A.; Long, J.Z.; et al. Sensitization of cancer cells to ferroptosis coincident with cell cycle arrest. Cell Chem. Biol. 2024, 31, 234–248 e213. [Google Scholar] [CrossRef]
- Yan, C.; Yang, S.; Shao, S.; Zu, R.; Lu, H.; Chen, Y.; Zhou, Y.; Ying, X.; Xiang, S.; Zhang, P.; et al. Exploring the anti-ferroptosis mechanism of Kai-Xin-San against Alzheimer’s disease through integrating network pharmacology, bioinformatics, and experimental validation strategy in vivo and in vitro. J. Ethnopharmacol. 2024, 326, 117915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhao, X.D.; Shen, J.D.; Chen, S.J.; Huang, H.Y.; Zhou, X.M.; Han, Y.L.; Zhou, L.J.; Lu, X.J.; Wu, Q. Activation of SIRT1 Alleviates Ferroptosis in the Early Brain Injury after Subarachnoid Hemorrhage. Oxidative Med. Cell. Longev. 2022, 2022, 9069825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Q.; Peng, Z.; Li, X.J.; Ding, P.F.; Gao, S.; Sheng, B.; Liu, Y.; Lu, Y.; Zhuang, Z.; et al. Menaquinone-4 attenuates ferroptosis by upregulating DHODH through activation of SIRT1 after subarachnoid hemorrhage. Free Radic. Biol. Med. 2024, 210, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, J.; Peng, J.; Jiang, H.; Le, K. Resveratrol Improves Synaptic Plasticity in Hypoxic-Ischemic Brain Injury in Neonatal Mice via Alleviating SIRT1/NF-kappaB Signaling-Mediated Neuroinflammation. J. Mol. Neurosci. 2022, 72, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, F.; Yang, C.; Hou, H.; Xing, Z.; Chen, J.; Liu, L.; Peng, C.; Li, D. SIRT1 activation by 2,3,5,6-tetramethylpyrazine alleviates neuroinflammation via inhibiting M1 microglia polarization. Front. Immunol. 2023, 14, 1206513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.S.; Li, J.Y.; Li, Z.C.; Wang, L.L.; Gan, C.L.; Chen, J.; Jiang, S.Y.; Aschner, M.; Ou, S.Y.; Jiang, Y.M. Sodium Para-aminosalicylic Acid Inhibits Lead-Induced Neuroinflammation in Brain Cortex of Rats by Modulating SIRT1/HMGB1/NF-kappaB Pathway. Neurochem. Res. 2023, 48, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qian, S.; Tang, B.; Kang, P.; Zhang, H.; Shi, C. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J. Cell Mol. Med. 2023, 27, 3075–3089. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.D.; Watroba, M.; Witkowska, J.; Mikulska, A.; Sepulveda, N.; Szukiewicz, D. Interplay between Systemic Glycemia and Neuroprotective Activity of Resveratrol in Modulating Astrocyte SIRT1 Response to Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 11640. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; De Galarreta, C.M.; Cuadrado, A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, F.; Ge, W.; Meng, X.; Fan, L.; Zhang, W.; Wang, Z.; Ding, M.; Gu, S.; Xing, X.; et al. Scutellarin attenuates oxidative stress and neuroinflammation in cerebral ischemia/reperfusion injury through PI3K/Akt-mediated Nrf2 signaling pathways. Eur. J. Pharmacol. 2023, 957, 175979. [Google Scholar] [CrossRef]
- Cheng, Y.; Gao, Y.; Li, J.; Rui, T.; Li, Q.; Chen, H.; Jia, B.; Song, Y.; Gu, Z.; Wang, T.; et al. TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radic. Biol. Med. 2023, 194, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhao, F.; Gao, B.; Tan, C.; Yagishita, N.; Nakajima, T.; Wong, P.K.; Chapman, E.; Fang, D.; Zhang, D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes. Dev. 2014, 28, 708–722. [Google Scholar] [CrossRef]
- Feng, X.; Ma, W.; Zhu, J.; Jiao, W.; Wang, Y. Dexmedetomidine alleviates early brain injury following traumatic brain injury by inhibiting autophagy and neuroinflammation through the ROS/Nrf2 signaling pathway. Mol. Med. Rep. 2021, 24, 661. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Guo, H.; Jiang, H.; Liu, H.; Fu, H.; Wang, D. Forsythoside A Mitigates Alzheimer’s-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int. J. Biol. Sci. 2022, 18, 2075–2090. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hyun, D.H. The Interplay between Intracellular Iron Homeostasis and Neuroinflammation in Neurodegenerative Diseases. Antioxidants 2023, 12, 918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, P.; Wang, N.; Dong, W.; Chen, Z.; Wu, M.; Wang, Z.; Yu, Z.; Guan, D.; Wang, L.; et al. Neuroprotection of NRF2 against Ferroptosis after Traumatic Brain Injury in Mice. Antioxidants 2023, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, E.C.; Chan, J.Y.; Torti, F.M.; Torti, S.V. Nrf2 mediates the induction of ferritin H in response to xenobiotics and cancer chemopreventive dithiolethiones. J. Biol. Chem. 2003, 278, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, J.; Zhao, D.; Ruan, C.; Zhou, J.; Tan, H.; Bao, Y. Netrin-1 upregulates GPX4 and prevents ferroptosis after traumatic brain injury via the UNC5B/Nrf2 signaling pathway. CNS Neurosci. Ther. 2023, 29, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Z.; Chen, L.L.; Qu, L.; Li, H.; Wang, J.; Song, N.; Xie, J.X. Gut microbiota-induced CXCL1 elevation triggers early neuroinflammation in the substantia nigra of Parkinsonian mice. Acta Pharmacol. Sin. 2024, 45, 52–65. [Google Scholar] [CrossRef]
- Duan, C.; Jiao, D.; Wang, H.; Wu, Q.; Men, W.; Yan, H.; Li, C. Activation of the PPARgamma Prevents Ferroptosis-Induced Neuronal Loss in Response to Intracerebral Hemorrhage Through Synergistic Actions With the Nrf2. Front. Pharmacol. 2022, 13, 869300. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xu, S.; Meng, C.; Wang, Y.; Ji, L.; Li, X.; Wang, X.; Li, Q. Ferroptosis participated in hippocampal neuroinflammation damage of in offspring rats after maternal sleep deprivation. J. Neuroimmunol. 2023, 375, 578021. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Z.; Zhou, X.; Zhao, Z.; Zhao, R.; Xu, X.; Kong, X.; Ren, J.; Yao, X.; Wen, Q.; et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J. Neuroinflammation 2021, 18, 249. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhao, Y.; Luo, R.; Su, M.; Zhang, C.; Li, C.; Liu, B.; Zhou, X. Intrathecal umbilical cord mesenchymal stem cells injection alleviates neuroinflammation and oxidative stress in the cyclophosphamide-induced interstitial cystitis rats through the Sirt1/Nrf2/HO-1 pathway. Life Sci. 2023, 331, 122045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Zhu, H.Y.; Wang, X.L.; Lu, X.W.; Pan, C.L.; Xu, L.; Liu, X.; Xu, N.; Zhang, Z.Y. Oridonin Ameliorates Traumatic Brain Injury-Induced Neurological Damage by Improving Mitochondrial Function and Antioxidant Capacity and Suppressing Neuroinflammation through the Nrf2 Pathway. J. Neurotrauma 2022, 39, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, Y.Y.; Wu, Y.Q.; Zhao, C.; Wu, J.; Wang, W.J.; Meng, F.H. Koumine ameliorates neuroinflammation by regulating microglia polarization via activation of Nrf2/HO-1 pathway. Biomed. Pharmacother. 2023, 167, 115608. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ou, C.; Zhang, N.; Liu, Q.; Xiong, K.; Yu, J.; Cheng, H.; Chen, L.; Ma, M.; Xu, J.; et al. Roflumilast attenuates neuroinflammation post retinal ischemia/reperfusion injury by regulating microglia phenotype via the Nrf2/STING/NF-kappaB pathway. Int. Immunopharmacol. 2023, 124, 110952. [Google Scholar] [CrossRef]
- Xiaodan, S.; Peiyan, Z.; Hui, L.; Yan, L.; Ying, C. NRF2 participates in the suppressive tumor immune microenvironment of KRAS/KEAP1 co-mutant non-small cell lung cancer by inhibiting the STING pathway. Genes. Dis. 2023, 10, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Qu, X.; Zhao, J.; Wang, X.; Wang, Q.; Dai, J.; Zhu, C.; Li, J.; Jiang, L. Macrophage PTEN controls STING-induced inflammation and necroptosis through NICD/NRF2 signaling in APAP-induced liver injury. Cell Commun. Signal. 2023, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, D.; Zhou, W.; Han, Y.; Ju, Y.; Liu, H.; Chen, Y.; Yu, J.; Wang, L.; Wang, J.; et al. The innate immune sensor STING accelerates neointima formation via NF-kappaB signaling pathway. Int. Immunopharmacol. 2023, 121, 110412. [Google Scholar] [CrossRef]
- Bellezza, I.; Tucci, A.; Galli, F.; Grottelli, S.; Mierla, A.L.; Pilolli, F.; Minelli, A. Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012, 23, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, H.; Wang, J.; Li, F.; Li, X.; Li, T.; Wang, C.; Li, L.; Peng, R.; Liu, L.; et al. Annexin A5 ameliorates traumatic brain injury-induced neuroinflammation and neuronal ferroptosis by modulating the NF-kB/HMGB1 and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2023, 114, 109619. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-kappaB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-kappaB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.Z.; Chen, L.; Ren, M.; Li, S.; Liu, T.Y.; Chen, H.J.; Yu, H.H.; Sun, Y. CXCL8 Promotes Endothelial-to-Mesenchymal Transition of Endothelial Cells and Protects Cells from Erastin-Induced Ferroptosis via CXCR2-Mediated Activation of the NF-kappaB Signaling Pathway. Pharmaceuticals 2023, 16, 1210. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, H.; Urushihara, N.; Shizuma, K.; Ogawa, H.; Nakai, S.; Wakamatsu, T.; Takenaka, S.; Kakunaga, S. SHARPIN Enhances Ferroptosis in Synovial Sarcoma Cells via NF-kappaB- and PRMT5-Mediated PGC1alpha Reduction. Cancers 2023, 15, 3484. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Banerjee, P.; Bir, A.; Sinha, M.; Biswas, A.; Chakrabarti, S. Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer’s disease: The NF-kappaB connection. Curr. Top. Med. Chem. 2015, 15, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Wu, J.; Ding, C.C.; Lin, C.C.; Pan, S.; Bossa, N.; Xu, Y.; Yang, W.H.; Mathey-Prevot, B.; Chi, J.T. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2020, 27, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Liu, X.; Cao, Y.; Niu, H.; Lan, Z.; Li, R.; Li, F.; Sun, D.; Shi, M.; Wa, L.; et al. Deferoxamine ameliorates neurological dysfunction by inhibiting ferroptosis and neuroinflammation after traumatic brain injury. Brain Res. 2023, 1812, 148383. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhu, D.; Han, X.; Zhang, Q.; Chen, B.; Zhou, P.; Wei, Z.; Zhang, Z.; Cao, Y.; Zou, H. HMGB1: A double-edged sword and therapeutic target in the female reproductive system. Front. Immunol. 2023, 14, 1238785. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, W.; Wei, H.; Chang, C.; Yang, L.; Meng, J.; Long, T.; Xu, Q.; Zhang, C. Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury. CNS Neurosci. Ther. 2023, 29, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ju, I.G.; Choi, Y.; Park, S.; Oh, M.S. Trichosanthis Semen Suppresses Lipopolysaccharide-Induced Neuroinflammation by Regulating the NF-kappaB Signaling Pathway and HO-1 Expression in Microglia. Toxins 2021, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Liu, M.; Liu, D.N.; Shang, Y.F.; Wang, Y.H.; Du, G.H. ST2825, a Small Molecule Inhibitor of MyD88, Suppresses NF-kappaB Activation and the ROS/NLRP3/Cleaved Caspase-1 Signaling Pathway to Attenuate Lipopolysaccharide-Stimulated Neuroinflammation. Molecules 2022, 27, 2990. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Zhou, X.; Li, P.; Wang, Y.; Liu, M.; Li, F.; Cui, Y.; Huang, T.; Yao, M.; Zhang, Y.; et al. MG53 attenuates lipopolysaccharide-induced neurotoxicity and neuroinflammation via inhibiting TLR4/NF-kappaB pathway in vitro and in vivo. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109684. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Xia, J.; Pu, F.; Shi, S. MCPIP1 alleviates depressive-like behaviors in mice by inhibiting the TLR4/TRAF6/NF-kappaB pathway to suppress neuroinflammation. Mol. Med. Rep. 2024, 29, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, J.; Gao, F.; Cheng, W.; Zhang, Y.; Wei, C.; Zhang, S.; Gao, X. Engeletin alleviates cerebral ischemia reperfusion-induced neuroinflammation via the HMGB1/TLR4/NF-kappaB network. J. Cell Mol. Med. 2023, 27, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, P.; Zhu, H.; Gu, L.; Li, Y.; Feng, S.; Zeng, Z.; Chen, Q.; Zhou, B.; Xiong, X. Neutral polysaccharide from Gastrodia elata alleviates cerebral ischemia-reperfusion injury by inhibiting ferroptosis-mediated neuroinflammation via the NRF2/HO-1 signaling pathway. CNS Neurosci. Ther. 2023, 30, e14456. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, G.; Zhou, C.; Guo, H.; Hu, Z.; Hu, Q.; Li, G. Revisiting the intersection of microglial activation and neuroinflammation in Alzheimer’s disease from the perspective of ferroptosis. Chem. Biol. Interact. 2023, 375, 110387. [Google Scholar] [CrossRef] [PubMed]
- Pozniak, P.D.; White, M.K.; Khalili, K. TNF-alpha/NF-kappaB signaling in the CNS: Possible connection to EPHB2. J. Neuroimmune Pharmacol. 2014, 9, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Yu, J.; Wang, W.; Du, Z.; Gao, S.; Ma, Y.; Tang, R.; Liu, T.; Ma, S.; et al. Saikosaponin B2 ameliorates depression-induced microglia activation by inhibiting ferroptosis-mediated neuroinflammation and ER stress. J. Ethnopharmacol. 2023, 316, 116729. [Google Scholar] [CrossRef] [PubMed]
- Abdulaal, W.H.; Omar, U.M.; Zeyadi, M.; El-Agamy, D.S.; Alhakamy, N.A.; Ibrahim, S.R.M.; Almalki, N.A.R.; Asfour, H.Z.; Al-Rabia, M.W.; Mohamed, G.A.; et al. Modulation of the crosstalk between Keap1/Nrf2/HO-1 and NF-kappaB signaling pathways by Tomatidine protects against inflammation/oxidative stress-driven fulminant hepatic failure in mice. Int. Immunopharmacol. 2024, 130, 111732. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Jiang, Y.; Liang, W.; Wang, Y.; Cao, S.; Yan, H.; Gao, L.; Zhang, L. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol. Brain 2019, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Cui, H.; Zheng, S.M.; Lu, Y.; Yuan, H.W.; Zhang, L.; Wang, H.H.; Du, R.S. Electroacupuncture regulates microglial polarization via inhibiting NF-kappaB/COX2 pathway following traumatic brain injury. Brain Res. 2023, 1818, 148516. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, J.; Molotkov, A.; Mintz, A.; Mann, J.J. Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules 2021, 26, 3208. [Google Scholar] [CrossRef] [PubMed]
- Hakan, T.; Toklu, H.Z.; Biber, N.; Ozevren, H.; Solakoglu, S.; Demirturk, P.; Aker, F.V. Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood-brain barrier permeability. Neurol. Res. 2010, 32, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Xie, C.; Li, C.; Ma, L.; Zhang, Y. Anti-Ferroptotic Effects of bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Loaded with Ferrostatin-1 in Cerebral ischemia-reperfusion Injury Associate with the GPX4/COX-2 Axis. Neurochem. Res. 2023, 48, 502–518. [Google Scholar] [CrossRef]
- Moussa, N.; Dayoub, N. Exploring the role of COX-2 in Alzheimer’s disease: Potential therapeutic implications of COX-2 inhibitors. Saudi Pharm. J. 2023, 31, 101729. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Mishra, K.P.; Thakur, A.K. Inhibition of Cyclooxygenase-2 (COX-2) Initiates Autophagy and Potentiates MPTP-Induced Autophagic Cell Death of Human Neuroblastoma Cells, SH-SY5Y: An Inside in the Pathology of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 8038–8050. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Park, J.; Bui, P.T.C.; Choi, K.; Gye, M.C.; Hong, Y.C.; Kim, J.H.; Lee, Y.J. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ. Res. 2017, 158, 490–498. [Google Scholar] [CrossRef]
- Cherukuri, D.P.; Goulet, A.C.; Inoue, H.; Nelson, M.A. Selenomethionine regulates cyclooxygenase-2 (COX-2) expression through nuclear factor-kappa B (NF-kappaB) in colon cancer cells. Cancer Biol. Ther. 2005, 4, 175–180. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, Y.W.; Huang, W.S.; Lee, M.M.; Wood, W.G.; Leung, Y.M.; Tsai, H.Y. Trans-Cinnamaldehyde, An Essential Oil in Cinnamon Powder, Ameliorates Cerebral Ischemia-Induced Brain Injury via Inhibition of Neuroinflammation Through Attenuation of iNOS, COX-2 Expression and NFkappa-B Signaling Pathway. Neuromol. Med. 2016, 18, 322–333. [Google Scholar] [CrossRef]
- Yan, J.J.; Du, G.H.; Qin, X.M.; Gao, L. Baicalein attenuates the neuroinflammation in LPS-activated BV-2 microglial cells through suppression of pro-inflammatory cytokines, COX2/NF-kappaB expressions and regulation of metabolic abnormality. Int. Immunopharmacol. 2020, 79, 106092. [Google Scholar] [CrossRef]
- Haile, M.; Boutajangout, A.; Chung, K.; Chan, J.; Stolper, T.; Vincent, N.; Batchan, M.; D’Urso, J.; Lin, Y.; Kline, R.; et al. The Cox-2 Inhibitor Meloxicam Ameliorates Neuroinflammation and Depressive Behavior in Adult Mice after Splenectomy. J. Neurophysiol. Neurol. Disord. 2016, 3, 101. [Google Scholar]
- Zhou, Y.; Zhou, H.; Hua, L.; Hou, C.; Jia, Q.; Chen, J.; Zhang, S.; Wang, Y.; He, S.; Jia, E. Verification of ferroptosis and pyroptosis and identification of PTGS2 as the hub gene in human coronary artery atherosclerosis. Free Radic. Biol. Med. 2021, 171, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; He, C.; Yan, F.; Li, J.R.; Xu, H.Z.; Zhuang, J.F.; Zhou, H.; Peng, Y.C.; Fu, X.J.; et al. Selective Ferroptosis Inhibitor Liproxstatin-1 Attenuates Neurological Deficits and Neuroinflammation After Subarachnoid Hemorrhage. Neurosci. Bull. 2021, 37, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Xu, F.; Shen, J.; Xu, S. Identification of a Ferroptosis-Related Prognostic Gene PTGS2 Based on Risk Modeling and Immune Microenvironment of Early-Stage Cervical Cancer. J. Oncol. 2022, 2022, 3997562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, M.; Ma, C.; Wei, X.; Zhang, Z. BACH1 encourages ferroptosis by activating KDM4C-mediated COX2 demethylation after cerebral ischemia-reperfusion injury. Eur. J. Neurosci. 2023, 58, 2194–2214. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shu, J.; Liu, Y.; Xie, J.; Li, T.; Li, H.; Li, L. Atorvastatin attenuates ferroptosis-dependent myocardial injury and inflammation following coronary microembolization via the Hif1a/Ptgs2 pathway. Front. Pharmacol. 2022, 13, 1057583. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Zhang, B.; Xiao, C.; Sun, Z.; Gao, Y.; Dou, X. Suppression of lncRNA Gm47283 attenuates myocardial infarction via miR-706/ Ptgs2/ferroptosis axis. Bioengineered 2022, 13, 10786–10802. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, J.; Yuan, T.; Xie, L.; Zhang, L. EPC-exosomal miR-26a-5p improves airway remodeling in COPD by inhibiting ferroptosis of bronchial epithelial cells via PTGS2/PGE2 signaling pathway. Sci. Rep. 2023, 13, 6126. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Z.; Liu, H.; Li, W.; Guo, X.; Zhang, Z.; Liu, Y.; Jia, L.; Li, Y.; Ren, Y.; et al. lincRNA-Cox2 regulates NLRP3 inflammasome and autophagy mediated neuroinflammation. Cell Death Differ. 2019, 26, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, Z.; Zhu, X.; Li, F.; Chen, Q. Essential genes Ptgs2, Tlr4, and Ccr2 regulate neuro-inflammation during the acute phase of cerebral ischemic in mice. Sci. Rep. 2023, 13, 13021. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, Y.; Zhang, W.; Fang, F.; Sun, J.; Liu, M.; Li, K.; Dong, L. Long Non-coding RNA MALAT1 Inhibits Neuron Apoptosis and Neuroinflammation While Stimulates Neurite Outgrowth and Its Correlation With MiR-125b Mediates PTGS2, CDK5 and FOXQ1 in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Shu, Y.; Li, X. miR-103 Promotes Neurite Outgrowth and Suppresses Cells Apoptosis by Targeting Prostaglandin-Endoperoxide Synthase 2 in Cellular Models of Alzheimer’s Disease. Front. Cell Neurosci. 2018, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Chen, S.; Wu, P.; Xu, S.; Wang, C.; Shi, H.; Bihl, J. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res. Ther. 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.T.; Zhang, L.M.; Huang, X.N. Glycyrrhizic acid protects against temporal lobe epilepsy in young rats by regulating neuronal ferroptosis through the miR-194-5p/PTGS2 axis. Kaohsiung J. Med. Sci. 2023, 39, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, I.; Na, J.Y.; Lee, Y.; Lee, J.; Lee, J.S.; Lee, J.S. Pseudane-VII Regulates LPS-Induced Neuroinflammation in Brain Microglia Cells through the Inhibition of iNOS Expression. Molecules 2018, 23, 3196. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, M.; Chen, M.; Xu, Q.; Li, S.; Cui, Y.; Qiu, X.; Tian, W. Amelioration of Neuropathic Pain and Attenuation of Neuroinflammation Responses by Tetrahydropalmatine Through the p38MAPK/NF-kappaB/iNOS Signaling Pathways in Animal and Cellular Models. Inflammation 2022, 45, 891–903. [Google Scholar] [CrossRef]
- Wang, S.S.; Jia, J.; Wang, Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles Suppresses iNOS Expression and Ameliorates Neural Impairment in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2018, 61, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Han, L.L.; Xu, Z.X. Suppressed microRNA-96 inhibits iNOS expression and dopaminergic neuron apoptosis through inactivating the MAPK signaling pathway by targeting CACNG5 in mice with Parkinson’s disease. Mol. Med. 2018, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, C.M.; Yang, W.L.; Wang, P. Microglial CD14 activated by iNOS contributes to neuroinflammation in cerebral ischemia. Brain Res. 2013, 1506, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, C.; Lin, L.; Mu, S.; Liu, H.; Hu, X.; Chen, X.; Wang, S. TNF-alpha Impairs Pericyte-Mediated Cerebral Microcirculation via the NF-kappaB/iNOS Axis after Experimental Traumatic Brain Injury. J. Neurotrauma 2023, 40, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Adeoluwa, O.A.; Olayinka, J.N.; Adeoluwa, G.O.; Akinluyi, E.T.; Adeniyi, F.R.; Fafure, A.; Nebo, K.; Edem, E.E.; Eduviere, A.T.; Abubakar, B. Quercetin abrogates lipopolysaccharide-induced depressive-like symptoms by inhibiting neuroinflammation via microglial NLRP3/NFkappaB/iNOS signaling pathway. Behav. Brain Res. 2023, 450, 114503. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhu, T.; Chen, C.; Xia, N.; Yao, Y.; Ding, J.; Xu, P.; Li, S.; Sun, Z.; Dong, X.; et al. Miconazole exerts disease-modifying effects during epilepsy by suppressing neuroinflammation via NF-kappaB pathway and iNOS production. Neurobiol. Dis. 2022, 172, 105823. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, P.J.; Borquez, D.A.; Nunez, M.T. Inflaming the Brain with Iron. Antioxidants 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.J.; Gao, S.L.; Lin, T.K.; Chu, P.Y.; Lin, H.Y. Ferroptosis as a Major Factor and Therapeutic Target for Neuroinflammation in Parkinson’s Disease. Biomedicines 2021, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Cheng, Y.; Peng, W.; Wu, Y.; Rui, T.; Luo, C.; Zhang, J. Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol. Neurobiol. 2022, 59, 3124–3139. [Google Scholar] [CrossRef]
- Dar, H.H.; Anthonymuthu, T.S.; Ponomareva, L.A.; Souryavong, A.B.; Shurin, G.V.; Kapralov, A.O.; Tyurin, V.A.; Lee, J.S.; Mallampalli, R.K.; Wenzel, S.E.; et al. A new thiol-independent mechanism of epithelial host defense against Pseudomonas aeruginosa: iNOS/NO(*) sabotage of theft-ferroptosis. Redox Biol. 2021, 45, 102045. [Google Scholar] [CrossRef]
- Mikulska-Ruminska, K.; Anthonymuthu, T.S.; Levkina, A.; Shrivastava, I.H.; Kapralov, A.A.; Bayir, H.; Kagan, V.E.; Bahar, I. NO(●) Represses the Oxygenation of Arachidonoyl PE by 15LOX/PEBP1: Mechanism and Role in Ferroptosis. Int. J. Mol. Sci. 2021, 22, 5253. [Google Scholar] [CrossRef] [PubMed]
- Zi, Y.; Wang, X.; Zi, Y.; Yu, H.; Lan, Y.; Fan, Y.; Ren, C.; Liao, K.; Chen, H. Cigarette smoke induces the ROS accumulation and iNOS activation through deactivation of Nrf-2/SIRT3 axis to mediate the human bronchial epithelium ferroptosis. Free Radic. Biol. Med. 2023, 200, 73–86. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Zhang, L.; Wu, M.; Zhang, F.; Yang, D.; Shen, J.; Chen, J. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 2021, 173, 41–51. [Google Scholar] [CrossRef]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef]
- Bayir, H.; Kagan, V.E.; Borisenko, G.G.; Tyurina, Y.Y.; Janesko, K.L.; Vagni, V.A.; Billiar, T.R.; Williams, D.L.; Kochanek, P.M. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: Support for a neuroprotective role of iNOS. J. Cereb. Blood Flow. Metab. 2005, 25, 673–684. [Google Scholar] [CrossRef]
- Chen, X.; Pang, X.; Yeo, A.J.; Xie, S.; Xiang, M.; Shi, B.; Yu, G.; Li, C. The Molecular Mechanisms of Ferroptosis and Its Role in Blood-Brain Barrier Dysfunction. Front. Cell Neurosci. 2022, 16, 889765. [Google Scholar] [CrossRef]
- Tian, D.; Li, W.; Heffron, C.L.; Wang, B.; Mahsoub, H.M.; Sooryanarain, H.; Hassebroek, A.M.; Clark-Deener, S.; LeRoith, T.; Meng, X.J. Hepatitis E virus infects brain microvascular endothelial cells, crosses the blood-brain barrier, and invades the central nervous system. Pro. Natl. Acad. Sci. USA 2022, 119, e2201862119. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Hsu, Y.-T.; Wu, P.-Y.; Yeh, Y.-M.; Lin, P.-C.; Hsu, K.-F.; Shen, M.-R. Tight junction protein cingulin variant is associated with cancer susceptibility by overexpressed IQGAP1 and Rac1-dependent epithelial-mesenchymal transition. J. Exp. Clin. Cancer Res. 2024, 43, 65. [Google Scholar] [CrossRef]
- Wang, P.; Jin, L.; Zhang, M.; Wu, Y.; Duan, Z.; Guo, Y.; Wang, C.; Guo, Y.; Chen, W.; Liao, Z.; et al. Blood–brain barrier injury and neuroinflammation induced by SARS-CoV-2 in a lung–brain microphysiological system. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef]
- Li, W.; Tiedt, S.; Lawrence, J.H.; Harrington, M.E.; Musiek, E.S.; Lo, E.H. Circadian Biology and the Neurovascular Unit. Circ. Res. 2024, 134, 748–769. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Xu, Y.; Li, K.; Zhou, L.; Qiao, H.; Xu, Q.; Zhao, J. The Role of Ferroptosis in Blood-Brain Barrier Injury. Cell Mol. Neurobiol. 2023, 43, 223–236. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Q.; Shi, M.; Liu, Y.; Bai, H.; Chang, Y.Z. Overexpression of Mitochondrial Ferritin Enhances Blood-Brain Barrier Integrity Following Ischemic Stroke in Mice by Maintaining Iron Homeostasis in Endothelial Cells. Antioxidants 2022, 11, 1257. [Google Scholar] [CrossRef]
- You, L.; Yu, P.P.; Dong, T.; Guo, W.; Chang, S.; Zheng, B.; Ci, Y.; Wang, F.; Yu, P.; Gao, G.; et al. Astrocyte-derived hepcidin controls iron traffic at the blood-brain-barrier via regulating ferroportin 1 of microvascular endothelial cells. Cell Death Dis. 2022, 13, 667. [Google Scholar] [CrossRef]
- Rand, D.; Ravid, O.; Atrakchi, D.; Israelov, H.; Bresler, Y.; Shemesh, C.; Omesi, L.; Liraz-Zaltsman, S.; Gosselet, F.; Maskrey, T.S.; et al. Endothelial Iron Homeostasis Regulates Blood-Brain Barrier Integrity via the HIF2alpha-Ve-Cadherin Pathway. Pharmaceutics 2021, 13, 311. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Ge, Y.; Chen, J.; Ma, J.; Wang, C.; Sun, M.; Wang, L.; Yao, S.; Yao, C. beta-amyloid protein induces mitophagy-dependent ferroptosis through the CD36/PINK/PARKIN pathway leading to blood-brain barrier destruction in Alzheimer’s disease. Cell Biosci. 2022, 12, 69. [Google Scholar] [CrossRef]
- Liu, Q.; Song, T.; Chen, B.; Zhang, J.; Li, W. Ferroptosis of brain microvascular endothelial cells contributes to hypoxia-induced blood-brain barrier injury. FASEB J. 2023, 37, e22874. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Li, Y.; Li, X.; Lv, E.; Zhang, X.; Shi, Y.; Wang, Y. Neuroprotective effect of Dl-3-n-butylphthalide against ischemia-reperfusion injury is mediated by ferroptosis regulation via the SLC7A11/GSH/GPX4 pathway and the attenuation of blood-brain barrier disruption. Front. Aging Neurosci. 2023, 15, 1028178. [Google Scholar] [CrossRef]
- Fang, J.; Yuan, Q.; Du, Z.; Fei, M.; Zhang, Q.; Yang, L.; Wang, M.; Yang, W.; Yu, J.; Wu, G.; et al. Ferroptosis in brain microvascular endothelial cells mediates blood-brain barrier disruption after traumatic brain injury. Biochem. Biophys. Res. Commun. 2022, 619, 34–41. [Google Scholar] [CrossRef]
- Meng, S.; Cao, H.; Huang, Y.; Shi, Z.; Li, J.; Wang, Y.; Zhang, Y.; Chen, S.; Shi, H.; Gao, Y. ASK1-K716R reduces neuroinflammation and white matter injury via preserving blood-brain barrier integrity after traumatic brain injury. J. Neuroinflamm. 2023, 20, 244. [Google Scholar] [CrossRef]
- Villalba, N.; Ma, Y.; Gahan, S.A.; Joly-Amado, A.; Spence, S.; Yang, X.; Nash, K.R.; Yuan, S.Y. Lung infection by Pseudomonas aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice. J. Neuroinflamm. 2023, 20, 127. [Google Scholar] [CrossRef]
- Liao, Y.C.; Wang, J.W.; Guo, C.; Bai, M.; Ran, Z.; Wen, L.M.; Ju, B.W.; Ding, Y.; Hu, J.P.; Yang, J.H. Cistanche tubulosa alleviates ischemic stroke-induced blood-brain barrier damage by modulating microglia-mediated neuroinflammation. J. Ethnopharmacol. 2023, 309, 116269. [Google Scholar] [CrossRef]
- Erickson, M.A.; Shulyatnikova, T.; Banks, W.A.; Hayden, M.R. Ultrastructural Remodeling of the Blood-Brain Barrier and Neurovascular Unit by Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2023, 24, 1640. [Google Scholar] [CrossRef]
- Larochelle, J.; Yang, C.; Liu, L.; Candelario-Jalil, E. An Unexplored Role for MMP-7 (Matrix Metalloproteinase-7) in Promoting Gut Permeability After Ischemic Stroke. Stroke 2022, 53, 3238–3242. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, Y.; Li, Z.; Yong, V.W.; Xue, M. Extracellular matrix metalloproteinase inducer in brain ischemia and intracerebral hemorrhage. Front. Immunol. 2022, 13, 986469. [Google Scholar] [CrossRef]
- Isik, S.; Yeman Kiyak, B.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Yu, H.; Chang, Q.; Sun, T.; He, X.; Wen, L.; An, J.; Feng, J.; Zhao, Y. Metabolic reprogramming and polarization of microglia in Parkinson’s disease: Role of inflammasome and iron. Ageing Res. Rev. 2023, 90, 102032. [Google Scholar] [CrossRef]
- Liu, S.; Gao, X.; Zhou, S. New Target for Prevention and Treatment of Neuroinflammation: Microglia Iron Accumulation and Ferroptosis. ASN Neuro 2022, 14, 17590914221133236. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, R.; Lv, M.; Xu, X.; Liu, W.; Li, X.; Gao, Y.; Zhao, Z.; Zhang, Z.; Li, Y.; et al. ACSL4 promotes microglia-mediated neuroinflammation by regulating lipid metabolism and VGLL4 expression. Brain Behav. Immun. 2023, 109, 331–343. [Google Scholar] [CrossRef]
- Wu, Y.; Eisel, U.L.M. Microglia-Astrocyte Communication in Alzheimer’s Disease. J. Alzheimers Dis. 2023, 95, 785–803. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood-Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef]
- Juric, D.M.; Krzan, M.; Lipnik-Stangelj, M. Histamine and astrocyte function. Pharmacol. Res. 2016, 111, 774–783. [Google Scholar] [CrossRef]
- Blackburn, D.; Sargsyan, S.; Monk, P.N.; Shaw, P.J. Astrocyte function and role in motor neuron disease: A future therapeutic target? Glia 2009, 57, 1251–1264. [Google Scholar] [CrossRef]
- Murphy-Royal, C.; Ching, S.; Papouin, T. A conceptual framework for astrocyte function. Nat. Neurosci. 2023, 26, 1848–1856. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Y.; Wu, M.; Li, N.; Yan, C.; Guo, H.; Li, Q.; Li, Q.; Wang, Q. Gemfibrozil Alleviates Cognitive Impairment by Inhibiting Ferroptosis of Astrocytes via Restoring the Iron Metabolism and Promoting Antioxidant Capacity in Type 2 Diabetes. Mol. Neurobiol. 2024, 61, 1187–1201. [Google Scholar] [CrossRef]
- Xu, S.X.; Xie, X.H.; Yao, L.; Wang, W.; Zhang, H.; Chen, M.M.; Sun, S.; Nie, Z.W.; Nagy, C.; Liu, Z. Human in vivo evidence of reduced astrocyte activation and neuroinflammation in patients with treatment-resistant depression following electroconvulsive therapy. Psychiatry Clin. Neurosci. 2023, 77, 653–664. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Mekhaeil, M.; Conroy, M.J.; Dev, K.K. Elucidating the Therapeutic Utility of Olaparib in Sulfatide-Induced Human Astrocyte Toxicity and Neuroinflammation. J. Neuroimmune Pharmacol. 2023, 18, 592–609. [Google Scholar] [CrossRef]
- Nakano-Kobayashi, A.; Canela, A.; Yoshihara, T.; Hagiwara, M. Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway. Proc. Natl. Acad. Sci. USA 2023, 120, e2303809120. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, X.M.; Wang, Q.; Du, T.T.; Zhang, M.X.; Wang, H.Z.; Li, R.P.; Liang, K.; Gao, Y.; Zhou, S.Y.; et al. Astrocyte-derived SerpinA3N promotes neuroinflammation and epileptic seizures by activating the NF-kappaB signaling pathway in mice with temporal lobe epilepsy. J. Neuroinflamm. 2023, 20, 161. [Google Scholar] [CrossRef]
- Yin, S.; Ma, X.Y.; Sun, Y.F.; Yin, Y.Q.; Long, Y.; Zhao, C.L.; Ma, J.W.; Li, S.; Hu, Y.; Li, M.T.; et al. RGS5 augments astrocyte activation and facilitates neuroinflammation via TNF signaling. J. Neuroinflamm. 2023, 20, 203. [Google Scholar] [CrossRef]
- Davaanyam, D.; Lee, H.; Seol, S.I.; Oh, S.A.; Kim, S.W.; Lee, J.K. HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain. Exp. Mol. Med. 2023, 55, 2402–2416. [Google Scholar] [CrossRef]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic. Biol. Med. 2019, 133, 169–178. [Google Scholar] [CrossRef]
- Chen, J.; Qu, X.; Li, Z.; Zhang, D.; Hou, L. Peak Neutrophil-to-Lymphocyte Ratio Correlates with Clinical Outcomes in Patients with Severe Traumatic Brain Injury. Neurocrit. Care 2019, 30, 334–339. [Google Scholar] [CrossRef]
- Li, F.; Weng, G.; Zhou, H.; Zhang, W.; Deng, B.; Luo, Y.; Tao, X.; Deng, M.; Guo, H.; Zhu, S.; et al. The neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and neutrophil-to-high-density-lipoprotein ratio are correlated with the severity of Parkinson’s disease. Front. Neurol. 2024, 15, 1322228. [Google Scholar] [CrossRef]
- Xu, C.; Cai, L.; Yi, T.; Yi, X.; Hu, Y. Neutrophil-to-lymphocyte ratio is associated with stroke progression and functional outcome in patients with ischemic stroke. Brain Behav. 2023, 13, e3261. [Google Scholar] [CrossRef]
- Nikaido, Y.; Midorikawa, Y.; Furukawa, T.; Shimoyama, S.; Takekawa, D.; Kitayama, M.; Ueno, S.; Kushikata, T.; Hirota, K. The role of neutrophil gelatinase-associated lipocalin and iron homeostasis in object recognition impairment in aged sepsis-survivor rats. Sci. Rep. 2022, 12, 249. [Google Scholar] [CrossRef]
- Liu, Y.W.; Li, S.; Dai, S.S. Neutrophils in traumatic brain injury (TBI): Friend or foe? J. Neuroinflamm. 2018, 15, 146. [Google Scholar] [CrossRef]
- Wang, K.; Wang, M.; Liao, X.; Gao, S.; Hua, J.; Wu, X.; Guo, Q.; Xu, W.; Sun, J.; He, Y.; et al. Locally organised and activated Fth1(hi) neutrophils aggravate inflammation of acute lung injury in an IL-10-dependent manner. Nat. Commun. 2022, 13, 7703. [Google Scholar] [CrossRef]
- Semple, B.D.; Trivedi, A.; Gimlin, K.; Noble-Haeusslein, L.J. Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain. Neurobiol. Dis. 2015, 74, 263–280. [Google Scholar] [CrossRef]
- Garcia-Culebras, A.; Duran-Laforet, V.; Pena-Martinez, C.; Ballesteros, I.; Pradillo, J.M.; Diaz-Guzman, J.; Lizasoain, I.; Moro, M.A. Myeloid cells as therapeutic targets in neuroinflammation after stroke: Specific roles of neutrophils and neutrophil-platelet interactions. J. Cereb. Blood Flow. Metab. 2018, 38, 2150–2164. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, P.; Hao, X.; Sun, X.; Feng, H.; Chen, Z. Neutrophil Extracellular Traps (NETs): A New Therapeutic Target for Neuroinflammation and Microthrombosis After Subarachnoid Hemorrhage? Transl. Stroke Res. 2023, 14, 443–445. [Google Scholar] [CrossRef]
- Wang, D.; Yin, K.; Zhang, Y.; Lu, H.; Hou, L.; Zhao, H.; Xing, M. Fluoride induces neutrophil extracellular traps and aggravates brain inflammation by disrupting neutrophil calcium homeostasis and causing ferroptosis. Environ. Pollut. 2023, 331, 121847. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sheng, X.; Dong, X.; Zhou, W.; Li, Y.; Ma, X.; Song, Y.; Dai, H.; Du, Y. Neutrophil extracellular traps mediate TLR9/Merlin axis to resist ferroptosis and promote triple negative breast cancer progression. Apoptosis 2023, 28, 1484–1495. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Zhou, Y.; Qu, M.; Wang, Y.; Guo, K.; Shen, R.; Sun, Z.; Cata, J.P.; Yang, S.; et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int. J. Biol. Sci. 2022, 18, 3337–3357. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Xiong, X.; Yan, W.; Liu, M.; Xu, H.; He, Q. Mitochondrial general control of amino acid synthesis 5 like 1 promotes nonalcoholic steatohepatitis development through ferroptosis-induced formation of neutrophil extracellular traps. Clin. Transl. Med. 2023, 13, e1325. [Google Scholar] [CrossRef] [PubMed]
- Galicia, G.; Boulianne, B.; Pikor, N.; Martin, A.; Gommerman, J.L. Secondary B cell receptor diversification is necessary for T cell mediated neuro-inflammation during experimental autoimmune encephalomyelitis. PLoS ONE 2013, 8, e61478. [Google Scholar] [CrossRef] [PubMed]
- Hartlehnert, M.; Borsch, A.L.; Li, X.; Burmeister, M.; Gerwien, H.; Schafflick, D.; Heming, M.; Lu, I.N.; Narayanan, V.; Strecker, J.K.; et al. Bcl6 controls meningeal Th17-B cell interaction in murine neuroinflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2023174118. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Mrdjen, D.; Utz, S.G.; Greter, M.; Schreiner, B.; Becher, B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 2019, 4, eaau8380. [Google Scholar] [CrossRef]
- Rayasam, A.; Kijak, J.A.; Dallmann, M.; Hsu, M.; Zindl, N.; Lindstedt, A.; Steinmetz, L.; Harding, J.S.; Harris, M.G.; Karman, J.; et al. Regional Distribution of CNS Antigens Differentially Determines T-Cell Mediated Neuroinflammation in a CX3CR1-Dependent Manner. J. Neurosci. 2018, 38, 7058–7071. [Google Scholar] [CrossRef] [PubMed]
- Harrer, C.; Otto, F.; Pilz, G.; Haschke-Becher, E.; Trinka, E.; Hitzl, W.; Wipfler, P.; Harrer, A. The CXCL13/CXCR5-chemokine axis in neuroinflammation: Evidence of CXCR5+CD4 T cell recruitment to CSF. Fluids Barriers CNS 2021, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Manivasagam, S.; Smith, B.C.; Sim, J.; Vollmer, L.L.; Daniels, B.P.; Russell, J.H.; Klein, R.S. Astrocyte-T cell crosstalk regulates region-specific neuroinflammation. Glia 2020, 68, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Luoqian, J.; Yang, W.; Ding, X.; Tuo, Q.Z.; Xiang, Z.; Zheng, Z.; Guo, Y.J.; Li, L.; Guan, P.; Ayton, S.; et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol. Immunol. 2022, 19, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.C.; Meyer, C.; Bhatia, U.; Yshii, L.; Kleffner, I.; Bauer, J.; Troscher, A.R.; Schulte-Mecklenbeck, A.; Herich, S.; Schneider-Hohendorf, T.; et al. CD8(+) T cell-mediated endotheliopathy is a targetable mechanism of neuro-inflammation in Susac syndrome. Nat. Commun. 2019, 10, 5779. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shen, Y.; Wang, Y.; Huang, Y.; Wang, J.; Zhu, Q.; Tong, G.; Yu, K.; Cao, W.; Wang, Q.; et al. Ferroptosis participates in neuron damage in experimental cerebral malaria and is partially induced by activated CD8(+) T cells. Mol. Brain 2022, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Dorothee, G.; Hunot, S.; Martin, E.; Monnet, Y.; Duchamp, M.; Dong, Y.; Legeron, F.P.; Leboucher, A.; Burnouf, S.; et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 2017, 140, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Yshii, L.; Pasciuto, E.; Bielefeld, P.; Mascali, L.; Lemaitre, P.; Marino, M.; Dooley, J.; Kouser, L.; Verschoren, S.; Lagou, V.; et al. Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat. Immunol. 2022, 23, 878–891. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Jia, B.; Li, J.; Li, Q.; Luo, C. The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders. Antioxidants 2024, 13, 395. https://doi.org/10.3390/antiox13040395
Xu Y, Jia B, Li J, Li Q, Luo C. The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders. Antioxidants. 2024; 13(4):395. https://doi.org/10.3390/antiox13040395
Chicago/Turabian StyleXu, Yejia, Bowen Jia, Jing Li, Qianqian Li, and Chengliang Luo. 2024. "The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders" Antioxidants 13, no. 4: 395. https://doi.org/10.3390/antiox13040395
APA StyleXu, Y., Jia, B., Li, J., Li, Q., & Luo, C. (2024). The Interplay between Ferroptosis and Neuroinflammation in Central Neurological Disorders. Antioxidants, 13(4), 395. https://doi.org/10.3390/antiox13040395






