Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides
Abstract
1. Introduction
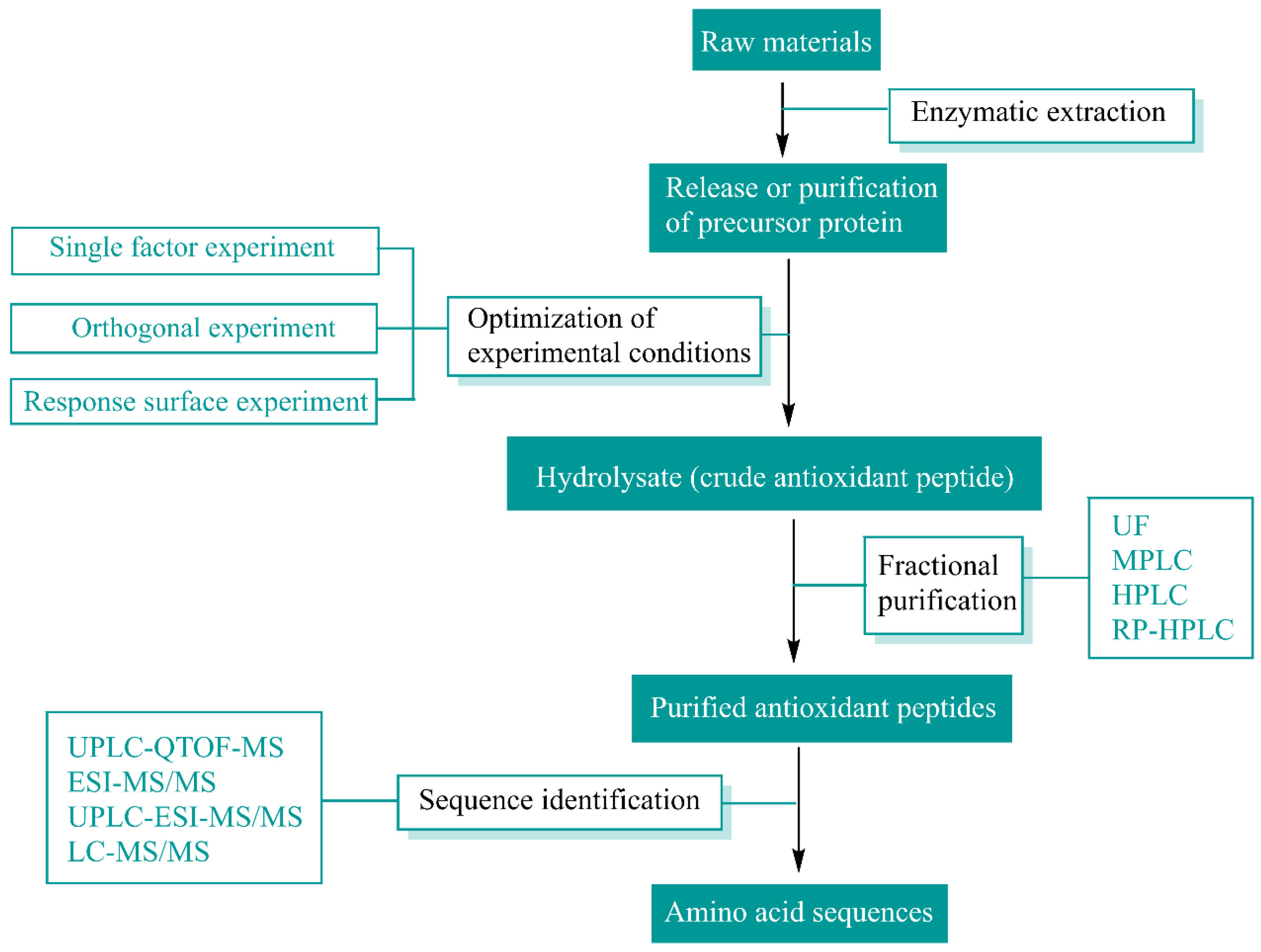
2. Sources and Preparation of Natural Antioxidant Peptides
| Source | Types of Enzymes | Methods of Purification | Methods of Identification | Peptide/Protein Hydrolysate | Reference |
|---|---|---|---|---|---|
| Purple wheat bran | Alkaline enzyme protease | sephadexed-G25 chromatography and ion-exchange chromatography | LC-MS/MS | CGFPGHC, QAC, RNF, SSC, and WF | [7] |
| Vigna radiata | Pepsin and pancreatin or thermolysin | SDS-PAGE and Native Blue-PAGE | HPLC-ESI-MS/MS | Low-molecular-weight peptides (3–30 kDa) | [8] |
| Chicken breast | Pepsin | Membrane ultrafiltration and gel filtration chromatography | LC-ESI-MS/MS | ITTNPYDY, IGWSPLGSL, ITTNPYDYHY, and LRV APEEHPTL | [9] |
| Bactrian camel milk | Trypsin | Ultrafiltration, gel filtration chromatography (HW-55F), and RP-HPLC | MALDI-TOF-MS/MS | RLDGQGRPRVWLGR, TPDNIDIWLGGIAEPQVKR and VAYSDDGENWTEYRDQGAVEGK | [10] |
| Egg white | Neutral protease | Ultrafiltration and gel filtration chromatography | UPLC-ESI-MS/MS | LAPYK, SVIRW and PKSVIRW | [11] |
| Decapterus maruadsi | Neutrase and trypsin | Ultrafiltration, gel filtration chromatography, and RP-HPLC | LC-MS/MS | KGFR | [12] |
| Agrocybe aegerita | Neutral protease | Ultrafiltration and HPLC | MS/MS | Low molecular weight peptides (<3 kDa), 3–10 kDa and >10 kDa) | [14] |
3. Measurement Methods and Techniques for Determining Antioxidant Activity
3.1. Assays Based on HAT
3.2. Assays Based on ET
3.3. Mixed-Mode Assays (HAT/ET)
| Techniques | Mechanism | Assay Method | Advantages | Limitations |
|---|---|---|---|---|
| Spectrometry | HAT | ORAC | Biologically significant and suitable for high-throughput assays. | The presence of natural pigments and fluorophores in the samples may interfere with the absorbance and fluorescence readings. |
| TRAP | ||||
| CBA | ||||
| ET | FRAP | Analysis is simple, rapid, cost-effective, and does not require special equipment. | To maintain iron solubility and drive electron transfer, experiments are performed under acidic conditions (pH 3.6); Prussian blue tends to precipitate and stain the assay tubes; and it is a non-radical method that correlates poorly with other antioxidant activity measurements [17]. | |
| CRAC | In contrast to the FRAP method, this assay requires a low redox potential to be achieved and is suitable for both lipophilic and hydrophilic systems; the reagents are more stable and readily available; it is to some extent less demanding in terms of reaction conditions (e.g., temperature, humidity, and pH); and it meets the requirements for evaluating the antioxidant capacity of endogenous and dietary molecules in vitro [20]. | Oxidative damage inflicted on biomolecules may be involved. | ||
| HAT/ET | DPPH | Low cost, reproducibility, room temperature suitability, and automation possibilities. | Sensitivity is affected by a variety of factors, such as the type of solvent used, the presence of hydrogen and metal ions, and the freshness of the DPPH reagent; there are many compounds with overlapping absorption spectra in the same wavelength range as DPPH. | |
| ABTS | Inexpensive and simple to perform; wide range of applicability, and many antioxidants are suitable; it is suitable for both hydrophilic and lipophilic systems. | Lack of biological relevance. | ||
| Chromatography | Adsorption and partition chromatography | GC | High sensitivity; wide detection range, i.e., a variety of detection methods can be used; simple process; and low cost. | The sample must be thermally stable and non-decomposable; sample processing is complicated and requires a certain level of skill. |
| Adsorption, partitioning, size exclusion, ion exchange and affinity chromatography | HPLC | High accuracy; sample preparation is simple; and a variety of detection methods can be used. | Higher column cost and susceptibility to contamination; relatively slow separation speed; and requires special operation and maintenance. | |
| Electrochemistry | Mass transport to the electrode | Cyclic voltammetry | Adequate analysis of the body’s antioxidant defense system; simple instrumentation and operation; cost-effective and efficient; can be easily applied in clinical laboratories [21]. | The determination of antioxidant activity via cyclic voltammetry may be disrupted by other forms of non-antioxidant reducing matter such as reducing sugars. |
4. Activity Evaluation Methods for Natural Antioxidant Peptides
4.1. In Vitro Chemical Methods and Their Effects
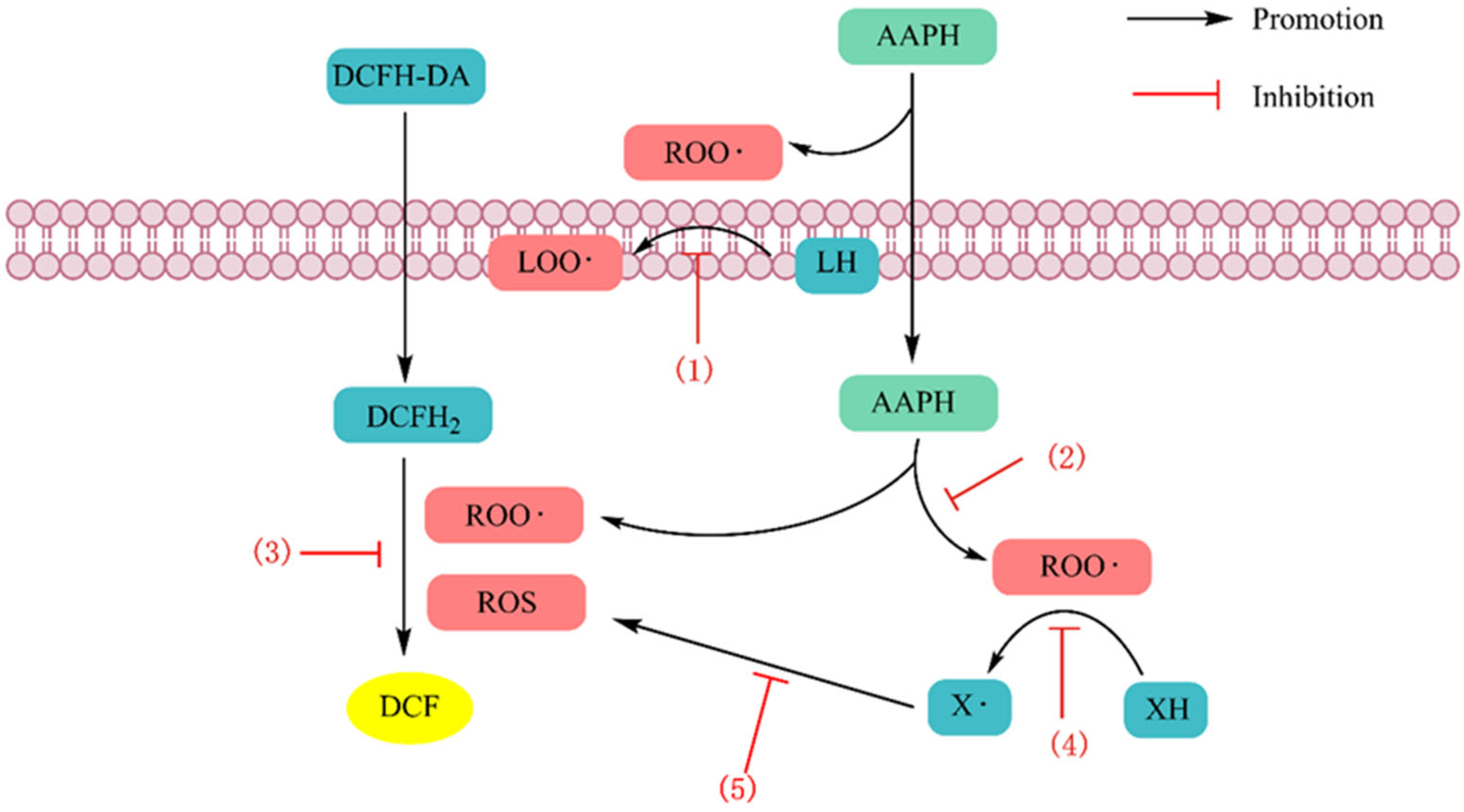
4.1.1. Scavenging Free Radicals
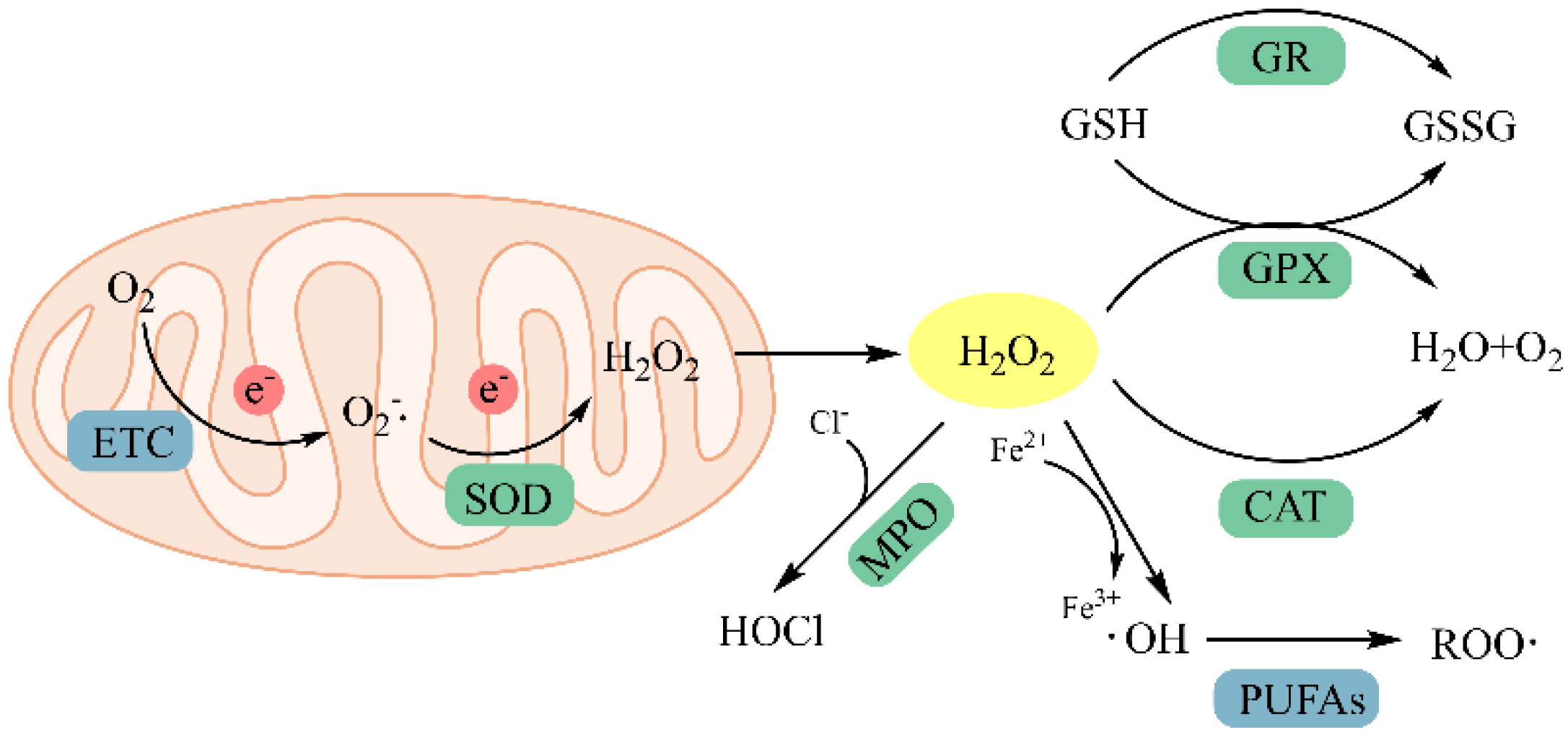
4.1.2. Chelating Metal Ions
4.1.3. Inhibition of Lipid Peroxidation
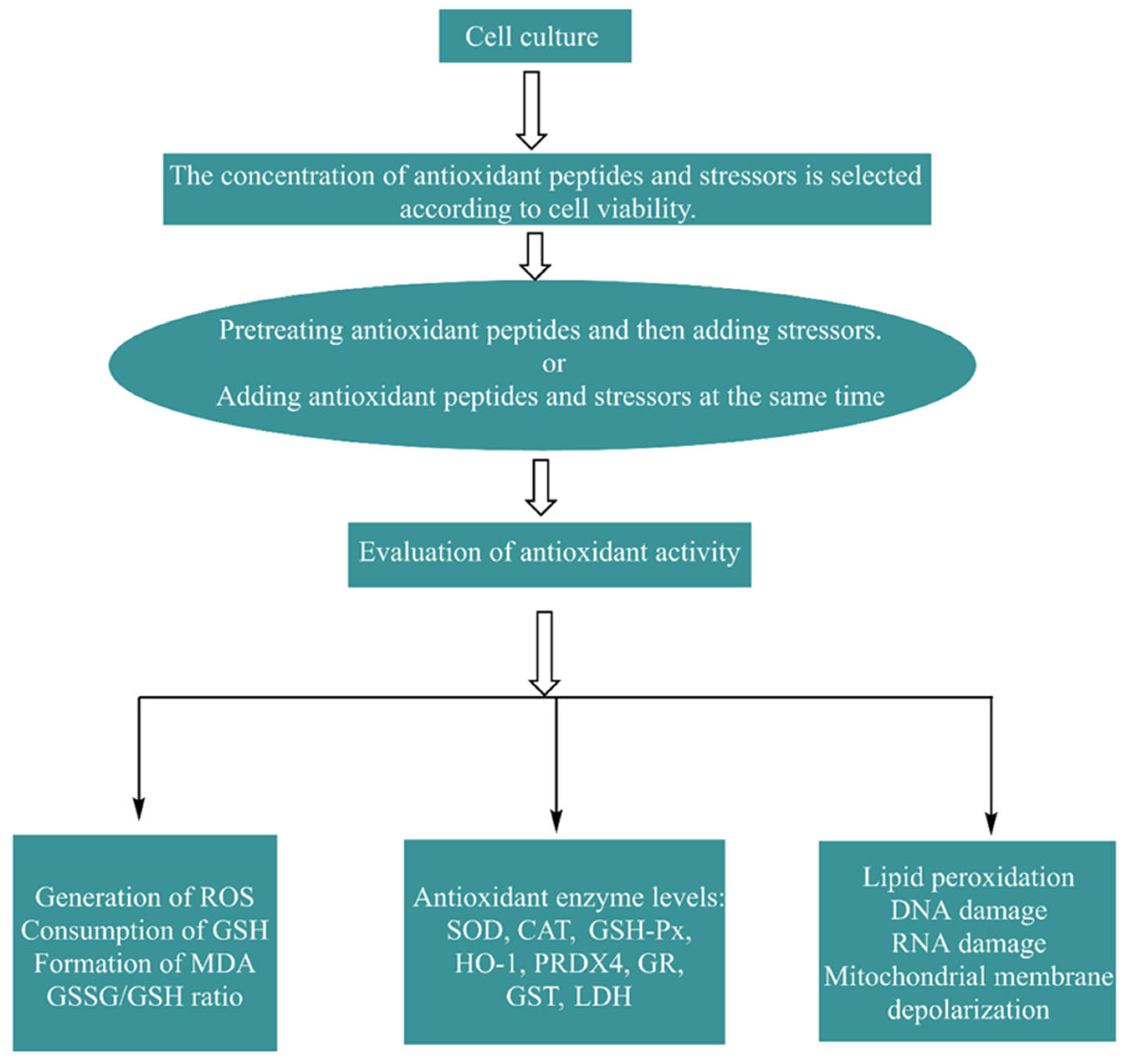
4.2. Cell Methods In Vitro and Their Effects
4.2.1. Establishment and Evaluation of the Cell Model
4.2.2. Biological Effects of Antioxidant Peptides in Cells
Inhibition of Related Oxidative Enzymes
Activation of Endogenous Antioxidant Enzymes and the Nonenzymatic Defense System
| Source | Peptide/Protein Hydrolysate | Cell Model | Biological Effect | Reference |
|---|---|---|---|---|
| Tilapia | LPGYF, PGY | HepG2 cells exposed to AAPH | The peptides not only scavenged intracellular ROS but also enhanced the activity of CAT and SOD, resulting in the activation of the cellular antioxidant defense system. | [39] |
| Moringa oleifera leaves | LALPVYN | HepG2 cells exposed to H2O2 | The peptide scavenged intracellular ROS and also enhanced endogenous antioxidant defenses, including antioxidant enzyme defenses and the glutathione system, resulting in cytoprotection via reducing MDA and ROS production in oxidatively damaged cells. | [40] |
| Hazelnut | EW, ADGF, DWDPK | HUVECs exposed to Ang II (angiotensin II) | The peptides upregulated the activities of antioxidant enzymes (CAT, SOD, and GSH-Px), downregulated LDH and MDA levels, and inhibited NOX activity and ROS production by decreasing NOX4 and p22phox levels. | [42] |
| Cottonseed meal | LGSPDVIVIR | Hepatocytes exposed to H2O2 | The peptide increased the potency of SOD and CAT activities and decreased MDA content and downregulated NOX2 mRNA expression to inhibit O2•− and ROS production. | [43] |
| Soybean | Hydrolysis products <1 kDa | Red blood cells exposed to AAPH | The peptides restored the activity of antioxidant enzymes (SOD, GSH-Px and CAT) and inhibited MDA production and GSH depletion | [44] |
| Porcine plasma | EDEQKFWGK | HepG2 cells exposed to H2O2 | This peptide decreased ROS and MDA content and increased antioxidant enzyme (SOD, CAT, GSH-Px) in order to enhance the oxidative defense system of HepG2 cells. | [45] |
| Grass carp scales | Low molecular weight peptide of 358–986 Da | HepG2 cells exposed to H2O2 | These peptides generated increases in SOD, CAT, and GPX activities and decreases in ROS levels and MDA content. | [46] |
| Euphausia superba | LKPGN, LQP | Chang liver cells exposed H2O2 | These two peptides increased the levels of antioxidant enzymes (SOD and GSH-PX) to scavenge excess ROS, increased mitochondrial membrane potential, and decreased DNA damage and MDA content. | [47] |
4.3. In Vivo Animal Methods and Their Effects
| Source | Peptide/Protein Hydrolysate | Animal Model | Biological Effect | Reference |
|---|---|---|---|---|
| Rice | Soluble rice protein hydrolysate | Oxidative stress in t-BHP-treated mice | The peptides increased the activities of antioxidant enzymes (CAT, GSH-Px, etc.) and the levels of GSH and reduced the content of MDA; they also inhibited the expression of oxidase NOX4. | [48] |
| Potatoes | Protein hydrolysate | Spontaneously hypertensive rats | The peptides activated the Nrf2-dependent antioxidant defense mechanism, which prompted the expression of endogenous antioxidant proteins (SOD1, SOD2, PRDX2, HO-1, and GSH-Px4). They also alleviated hypertension through DJ-1 and AKT signaling pathways. | [49] |
| ERJ-CP | Low-molecular-weight peptides (<1000 Da) | Oxidative stress in H2O2- treated Drosophila | These peptides prolonged the average lifespan of Drosophila; increased the levels of SOD, GSH-Px, and CAT; and decreased the content of MDA and PCO. | [50] |
| C-phycocyanin | MHLWAAK, MAQAAEYYR and MDYYFEER | Oxidative damage in H2O2- treated zebrafish embryos | These peptides inhibited ROS production, prevented MDA formation, and upregulated SOD and CAT activities | [51] |
| Yak bones | GASGPMGPR and GLPGPM | Oxidative stress in C. elegans | These peptides prolonged lifespan, enhanced the activities of SOD and CAT, and diminished the content of MDA and ROS | [52] |
| Sesame cake | Protein hydrolysate | Oxidative stress in mice | The peptides increased the activity of GSH-Px and SOD; decreased the activity of MDA and lipid peroxidation; and increased the activity of AChE to improve memory ability. | [53] |
| Manchurian walnut | Protein hydrolysate (<3 kDa) | Memory impairment in mice treated with scopolamine | The peptides inhibited ROS and increased the activity of GSH-Px and SOD and inhibited the decrease in Ach content. | [54] |
| Crimson snapper scales | Protein hydrolysate | Oxidative stress in C. elegans exposed to paraquat and ultraviolet radiation | The peptides reduced the accumulation of peroxide products (MDA and PCO); enhanced the activities of antioxidant enzymes (SOD, CAT, and GSH-Px); upregulated the expression of antioxidant genes (SOD1, SOD2, and CAT), blocked mTOR signal transduction, and activated autophagy to prolong life. | [55] |
| Stichopus variegates | Protein hydrolysate | Drosophila and D-galactose-treated senescent mice | The peptides activated SOD and GSH-Px, inhibited lipid peroxidation and protein oxidation, and upregulated the expression of anti-aging factor Klotho. | [56] |
5. Antioxidant Mechanism of Natural Antioxidant Peptides
5.1. Regulation of Redox Signal Transduction Pathways and Gene Expression
5.1.1. Nrf2 Signaling Pathway
5.1.2. NF-κB Signaling Pathway
5.1.3. Other Signaling Pathways
| Source | Peptide/Protein Hydrolysate | Oxidation Model | Antioxidant Mechanism | Reference |
|---|---|---|---|---|
| Walnut | EYWNR, FQLPR | HT22 cells exposed to H2O2 |
| [59] |
| Chinese Baijiu | PHP | HepG2 cells exposed to AAPH |
| [60] |
| Crushed rice | SGDWSDIGGR | Human embryonic lung diploid fibroblasts (2BS) cells exposed to H2O2 |
| [61] |
| Sturgeon muscle | F2 fraction | RAW264.7 cells exposed to LPS |
| [64] |
| Wheat germ | RVF | SH-SY5Y cells exposed to H2O2 |
| [65] |
| Rice | AAGALPS | HUVECs exposed to TNF- α |
| [66] |
| Rice | Soluble rice protein hydrolysate | Oxidative stress in t-BHP-treated mice |
| [48] |
| Strongylocentrotus nudus | AAVPSGASTGIYEALELR, NPLLEAFGNAK | Oxidative stress in paraquat-treated C. elegans |
| [68] |
| Sepia esculenta | DVEDLEAGLAK, EITSLAPSTM | Oxidative stress in paraquat-treated C. elegans |
| [69] |
| Acaudina leucoprocta | Protein hydrolysate | Oxidative stress in paraquat-treated C. elegans |
| [70] |
6. Structure-Activity Relationship
6.1. Molecular Weight
6.2. Amino Acid Composition and Sequence
6.3. Secondary Structure
6.4. Computer Modeling-Assisted Analysis of the Structure-Activity Relationships of Natural Antioxidant Peptides
6.4.1. Molecular Docking
6.4.2. Quantum Chemistry Calculations
6.4.3. Quantitative Structure-Activity Relationship
6.4.4. Molecular Dynamics Simulation
7. Safety Assessment, Bioavailability, and Application of Antioxidant Peptides
7.1. Safety Evaluation of Antioxidant Peptides
7.2. Bioavailability of Antioxidant Peptides
7.3. Current Applications of Antioxidant Peptides
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Roginskaya, M.; Razskazovskiy, Y. Oxidative DNA Damage and Repair: Mechanisms, Mutations, and Relation to Diseases. Antioxidants 2023, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.J.; Su, Y.W.; Wang, P.; Yang, W.; Li, R.X.; Jiao, L.X.; Zhao, R.X. Effect of Lactobacillus acidophilus fermentation on bioaccessibility: The relationship between biotransformation and antioxidant activity of apple polyphenols based on metabolomics. LWT Food Sci. Technol. 2023, 190, 115360. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Chen, J.; Chen, Y.; Ma, Y.T.; Yang, Q.S.; Fan, Y.Q.; Fu, C.M.; Limsila, B.; Li, R.; Liao, W. Extraction, structural characterization and antioxidant activity of turmeric polysaccharides. LWT Food Sci. Technol. 2022, 154, 112805. [Google Scholar] [CrossRef]
- Wen, C.T.; Zhang, J.X.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Tech. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.; Lu, Q.Y. Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran. BMC Chem. 2020, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Kusumah, J.; Hernandez, L.M.R.; de Mejia, E.G. Antioxidant Potential of Mung Bean Vigna radiata Albumin Peptides Produced by Enzymatic Hydrolysis Analyzed by Biochemical and In Silico Methods. Foods 2020, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, Y.; Zhang, L.; Zheng, J.; Guo, J.; Li, R.; Zeng, J. Purification of novel antioxidant peptides from myofibrillar protein hydrolysate of chicken breast and their antioxidant potential in chemical and H2O2-stressed cell systems. Food Funct. 2021, 12, 4897–4908. [Google Scholar] [CrossRef]
- Wali, A.; Gao, Y.H.; Ishimov, U.; Yili, A.; Aisa, H.A.; Salikhov, S. Isolation and Identification of Three Novel Antioxidant Peptides from the Bactrian Camel Milk Hydrolysates. Int. J. Pept. Res. Ther. 2020, 26, 641–650. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Liu, C.; Liu, B.; Yu, Y.; Zhang, T. Bifunctional peptides with antioxidant and angiotensin-converting enzyme inhibitory activity in vitro from egg white hydrolysates. J. Food Biochem. 2020, 44, e13347. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, X.; Wang, T.; Li, L.; Wu, Y.; Zhou, Y.; You, L. Purification and identification of antioxidant peptides from round scad (Decapterus maruadsi) hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. Toxicol. 2020, 135, 110882. [Google Scholar] [CrossRef]
- Aursuwanna, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Puthong, S.; Reamtong, O.; Karnchanatat, A. Investigating the cellular antioxidant and anti-inflammatory effects of the novel peptides in lingzhi mushrooms. Heliyon 2022, 8, e11067. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Liang, T.; Shen, Q.; Liu, J.; Lu, Y.; Tang, C.; Chen, X.; Hou, T.; Chen, Y. The optimization of production and characterization of antioxidant peptides from protein hydrolysates of Agrocybe aegerita. LWT 2020, 134, 109987. [Google Scholar] [CrossRef]
- Wen, C.T.; Zhang, J.X.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Badakné, K.; Farmani, J.; Aluko, R.E. Antioxidant peptides: Overview of production, properties, and applications in food systems. Compr. Rev. Food Sci. F 2023, 22, 46–106. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.F.; Liu, K.X.; Yang, J.Y.; Liu, S.M.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants-A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, R.; Daroit, D.J.; Correa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Yates, J.R.; Moreira, J.C.F.; Brandelli, A. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019, 49, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.Q.; Toldra, F.; Zhao, M.M.; Zhou, F.B.; Luo, D.H.; Jia, R.B.; Mora, L. In vitro and in silico analysis of potential antioxidant peptides obtained from chicken hydrolysate produced using Alcalase. Food Res. Int. 2022, 157, 111253. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, F.; Moretto, L.; Ferro, S.; Folda, A.; Scalcon, V.; Sandre, M.; Fiorese, F.; Marin, O.; Bindoli, A.; Rigobello, M.P. Insight into antioxidant properties of milk-derived bioactive peptides in vitro and in a cellular model. J. Pept. Sci. 2019, 25, e3162. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Li, J.; Song, Y.Q.; Chen, S.J.; Zhou, S.B.; Yang, X.Q. Preparation, purification, and identification of novel antioxidant peptides derived from Gracilariopsis lemaneiformis protein hydrolysates Front. Nutr. 2022, 9, 971419. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Lao, F.; Pan, X.; Wu, J.H. Food Protein-Derived Antioxidant Peptides: Molecular Mechanism, Stability and Bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.X.; Yang, M.; Zhao, B.T.; Yang, F.M. Isolation of antioxidant peptides from yak casein hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef] [PubMed]
- Kaviani, S.; Shahab, S.; Sheikhi, M.; Khaleghian, M.; Al Saud, S. Characterization of the binding affinity between some anti-Parkinson agents and Mn2+, Fe3+ and Zn2+ metal ions: A DFT insight. Inorg. Chem. Commun. 2021, 128, 108582. [Google Scholar] [CrossRef]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.N.; Tian, Y.; Kong, F.B.; Xiong, S.B.; Zhao, S.M.; Jia, D.; Manyande, A.; Du, H.Y. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef] [PubMed]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the Lipid Peroxidation Biomarkers and Methodological Aspects for Malondialdehyde Quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Chickpea chelating peptides inhibit copper-mediated lipid peroxidation. J. Sci. Food Agric. 2014, 94, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, Y.A.; Qi, Z.G.; Chen, Q.; Cao, Y.J.; Kong, Q.S. Preparation and identification of a novel peptide with high antioxidant activity from corn gluten meal. Food Chem. 2023, 424, 136389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, X.L. Purification, Identification and Evaluation of Antioxidant Peptides from Pea Protein Hydrolysates. Molecules 2023, 28, 2952. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, M.Q.; Ding, J.; Zheng, J.R.; Zhu, B.W.; Lin, S.Y. Structure-activity relationship and pathway of antioxidant shrimp peptides in a PC12 cell model. J. Funct. Foods 2020, 70, 103978. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Liceaga, A.M. Cellular antioxidant effect of bioactive peptides and molecular mechanisms underlying: Beyond chemical properties. Int. J. Food Sci. Tech. 2021, 56, 2193–2204. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.B.; Wang, S.Y.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Tech. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Zhang, X.G.; Noisa, P.; Yongsawatdigul, J. Identification and characterization of tilapia antioxidant peptides that protect AAPH-induced HepG2 cell oxidative stress. J. Funct. Foods 2021, 86, 104662. [Google Scholar] [CrossRef]
- Tao, L.; Gu, F.; Liu, Y.; Yang, M.; Wu, X.Z.; Sheng, J.; Tian, Y. Preparation of antioxidant peptides from Moringa oleifera leaves and their protection against oxidative damage in HepG2 cells. Front. Nutr. 2022, 9, 1062671. [Google Scholar] [CrossRef]
- Keoma, K.L.C.; Gabrielle Cristina, C.; Alexandre, O.; Carla Cristina, P. Oxidative Stress and Nox: Related Diseases and Main Classes of Synthetic and Natural Inhibitors. Rev. Virtual Quím. 2023, 15, 248–261. [Google Scholar] [CrossRef]
- Fang, L.; Ren, D.Y.; Wang, Z.H.; Liu, C.L.; Wang, J.; Min, W.H. Protective role of hazelnut peptides on oxidative stress injury in human umbilical vein endothelial cells. J. Food Biochem. 2018, 43, e12722. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Liu, W.B.; Wang, C.C.; Huang, Y.Y.; Dai, Y.J.; Cheng, H.H.; Jiang, G.Z. Evaluation of antioxidant capacity and immunomodulatory effects of cottonseed meal protein hydrolysate and its derivative peptides for hepatocytes of blunt snout bream (Megalobrama amblycephala). Fish. Shellfish. Immunol. 2020, 98, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Su, K.Y.; Chen, M.M.; Wang, S. Study on the antioxidant activity of peptides from soybean meal by fermentation based on the chemical method and AAPH-induced oxidative stress. Food Sci. Nutr. 2023, 11, 6634–6647. [Google Scholar] [CrossRef]
- Li, G.S.; Zhan, J.Q.; Hu, L.P.; Yuan, C.H.; Ying, X.G.; Hu, Y.Q. Identification of novel antioxidant peptide from porcine plasma hydrolysate and its effect in in vitro digestion/HepG2 cells model. J. Food Biochem. 2022, 46, e13853. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.M.; Lu, S.Z.; Li, Y.S.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z.C. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373, 131539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhao, Y.Q.; Wang, Y.M.; Zhao, W.H.; Wang, P.; Chi, C.F.; Wang, B. Antioxidant peptides from Antarctic Krill (Euphausia superba) hydrolysate: Preparation, identification and cytoprotection on H2O2-induced oxidative stress. J. Funct. Foods 2021, 86, 104701. [Google Scholar] [CrossRef]
- Han, B.K.; Park, Y.; Choi, H.S.; Suh, H.J. Hepatoprotective effects of soluble rice protein in primary hepatocytes and in mice. J. Sci. Food Agric. 2016, 96, 685–694. [Google Scholar] [CrossRef]
- Tsai, B.C.; Hsieh, D.J.; Lin, W.T.; Tamilselvi, S.; Day, C.H.; Ho, T.J.; Chang, R.L.; Viswanadha, V.P.; Kuo, C.H.; Huang, C.Y. Functional potato bioactive peptide intensifies Nrf2-dependent antioxidant defense against renal damage in hypertensive rats. Food Res. Int. 2020, 129, 108862. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, X.; Tian, Y.; Wu, D.; Du, M.; Wang, S. Protection against oxidative stress and anti-aging effect in Drosophila of royal jelly-collagen peptide. Food Chem. Toxicol. 2020, 135, 110881. [Google Scholar] [CrossRef]
- Xu, F.H.; Zhang, Y.; Qiu, Y.Z.; Yang, F.H.; Liu, G.X.; Dong, X.L.; Chen, G.; Cao, C.; Zhang, Q.; Zhang, S.S.; et al. Three novel antioxidant peptides isolated from C-phycocyanin against H2O2-induced oxidative stress in zebrafish via Nrf2 signaling pathway Front. Mar. Sci. 2022, 9, 1098091. [Google Scholar] [CrossRef]
- Wang, Y.L.; Sun, Y.D.; Wang, X.G.; Wang, Y.; Liao, L.X.; Zhang, Y.H.; Fang, B.S.; Fu, Y.S. Novel antioxidant peptides from Yak bones collagen enhanced the capacities of antiaging and antioxidant in. J. Funct. Foods 2022, 89, 104933. [Google Scholar] [CrossRef]
- Shu, Z.X.; Liu, L.Y.; Geng, P.F.; Liu, J.W.; Shen, W.Y.; Tu, M.J. Sesame cake hydrolysates improved spatial learning and memory of mice. Food Biosci. 2019, 31, 100440. [Google Scholar] [CrossRef]
- Ren, D.Y.; Zhao, F.R.; Liu, C.L.; Wang, J.; Guo, Y.; Liu, J.S.; Min, W.H. Antioxidant hydrolyzed peptides from Manchurian walnut (Juglans mandshurica Maxim.) attenuate scopolamine-induced memory impairment in mice. J. Sci. Food Agric. 2018, 98, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.X.; Chen, S.Y.; Liang, J.P.; Tang, M.Y.; Wang, S.Y. Protective effects of crimson snapper scales peptides against oxidative stress on Drosophila melanogaster and the action mechanism. Food Chem. Toxicol. 2021, 148, 111965. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Zhu, Q.Y.; Zheng, L.; Zhao, M.M.; Fan, J.Q.; Liu, S.J. Preparation of sea cucumber (Stichopus variegates) peptide fraction with desired organoleptic property and its anti-aging activity in fruit flies and D-galactose-induced aging mice. J. Funct. Foods 2020, 69, 103954. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Michalickova, D.; Hrncir, T.; Canova, N.K.; Slanar, O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur. J. Pharmacol. 2020, 873, 172973. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Shang, Y.T.; Li, S.T.; Chen, Z.; Xia, J.X.; Tian, Y.L.; Jia, Y.M.; Ma, A.J. Molecular Docking Revealed the Potential Anti-Oxidative Stress Mechanism of the Walnut Polypeptide on HT22 Cells. Foods 2023, 12, 1554. [Google Scholar] [CrossRef]
- Wu, J.H.; Sun, B.G.; Luo, X.L.; Zhao, M.M.; Zheng, F.P.; Sun, J.Y.; Li, H.H.; Sun, X.T.; Huang, M.Q. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef]
- Ren, L.K.; Yang, Y.; Fan, J.; Ma, C.M.; Bian, X.; Liu, B.X.; Wang, D.F.; Zhu, P.Y.; Fu, Y.; Zhang, N. Identification and in silico analysis of novel antioxidant peptides in broken rice protein hydrolysate and its cytoprotective effect against H2O2-induced 2BS cell model. Food Res. Int. 2022, 162, 112108. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and anti-inflammation effects of dietary phytochemicals: The Nrf2/NF-κB signalling pathway and upstream factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shu, W.; Shen, Y.; Sun, Q.; Jin, W.; Li, D.; Li, Y.; Yuan, L. Peptide fraction from sturgeon muscle by pepsin hydrolysis exerts anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages via MAPK and NF-κB pathways. Food Sci. Human. Wellness 2021, 10, 103–111. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Zhang, L.; Sun, W.; Tang, J.Q.; Lv, Z.L.; Xu, Z.; Yu, H.X. Protective effects of a wheat germ peptide (RVF) against H2O2-induced oxidative stress in human neuroblastoma cells. Biotechnol. Lett. 2014, 36, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Ju, Z.; Liu, L.; Wang, L.; Zhou, X.; Xiao, T.; Zhou, S. Rice-derived peptide AAGALPS inhibits TNF-α-induced inflammation and oxidative stress in vascular endothelial cells. Food Sci. Nutr. 2019, 8, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, R.; Zheng, M.; Li, D.; Lu, L. Protective effect of scorpion venom oligopeptides in human umbilical vein endothelial cells under benzo(a)pyrene exposure. Nat. Prod. Res. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.X.; Cheng, Q.; Peng, Q.; Yu, X.S.; Yin, X.Q.; Liang, M.; Ma, C.W.; Huang, Z.B.; Jia, W.Z. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
- Yu, X.S.; Su, Q.N.; Shen, T.Q.; Chen, Q.; Wang, Y.; Jia, W.Z. Antioxidant Peptides from Sepia esculenta Hydrolyzate Attenuate Oxidative Stress and Fat Accumulation in Caenorhabditis elegans. Mar. Drugs 2020, 18, 490. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, J.J.; Xu, C.M.; Li, Q.Q.; Ma, Y.G.; Zhao, S.L.; Zhuang, J.C.; Shen, F.; Wang, Q.Q.; Feng, F.Q.; et al. Sea cucumber (Acaudina leucoprocta) peptides extended the lifespan and enhanced antioxidant capacity via DAF-16/DAF-2/SOD-3/OLD-1/PEPT-1 in Caenorhabditis elegans. Front. Nutr. 2022, 9, 1065145. [Google Scholar] [CrossRef]
- Fan, X.K.; Han, Y.; Sun, Y.Y.; Zhang, T.; Tu, M.L.; Du, L.H.; Pan, D.D. Preparation and characterization of duck liver-derived antioxidant peptides based on LC-MS/MS, molecular docking, and machine learning. LWT Food Sci. Technol. 2023, 175, 114479. [Google Scholar] [CrossRef]
- Xia, J.A.; Song, H.D.; Huang, K.; Li, S.; Guan, X. Purification and characterization of antioxidant peptides from enzymatic hydrolysate of mungbean protein. J. Food Sci. 2020, 85, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, L.J.; Fu, Q.Q.; Zhou, G.H.; Zhang, W.G. A Review of Antioxidant Peptides Derived from Meat Muscle and By-Products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Chen, Z.; Wang, H.M.; Chen, X.D.; Yang, J.J.; Han, A.D.; Lin, D.H.; Hong, J. Underlying action mechanism of a novel antioxidant peptide derived from Allium tuberosum Rottler protein hydrolysates and its protective effects on hydrogen peroxide induced cell injury. J. Funct. Foods 2018, 40, 606–613. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.X.; Sun, Q.; Song, G.H.; Huang, J.N. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.B.; Huang, J.F.; Huan, R.; Chen, L.L.; Yi, C.P.; Liu, D.; Wang, M.; Liu, C.L.; He, H.L. New insights into the structure-activity relationships of antioxidative peptide PMRGGGGYHY. Food Chem. 2021, 337, 127678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Liu, B.Q.; Cui, B.; Wen, L.; Xu, Z.; Chen, M.L.; Wu, H. Alanine Substitution to Determine the Effect of LR5 and YR6 Rice Peptide Structure on Antioxidant and Anti-Inflammatory Activity. Nutrients 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Wang, J.P.; Liu, J.M.; John, A.; Jiang, Y.M.; Zhu, H.; Yang, B.; Wen, L.R. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem. 2022, 388, 132943. [Google Scholar] [CrossRef]
- Uno, S.; Kodama, D.; Yukawa, H.; Shidara, H.; Akamatsu, M. Quantitative analysis of the relationship between structure and antioxidant activity of tripeptides. J. Pept. Sci. 2020, 26, e3238. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, B. Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: Key sequence positions and their amino acid properties. J. Theor. Biol. 2013, 318, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure-activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Qi, L.B.; Li, D.M.; Zhong, L.M.; Wu, D.; Lin, S.Y. The regulatory mechanism of pulsed electric field (PEF) targeting at C-terminal glutamine of shrimp antioxidant peptide QMDDQ based on MD simulation. LWT Food Sci. Technol. 2021, 141, 110930. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2019, 120, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.T.; Miao, J.Y.; Li, J.L.; Li, Y.K.; Wang, X.H.; Yu, Y.; Cao, Y. Novel Antioxidant Peptides from Pearl Shell Meat Hydrolysate and Their Antioxidant Activity Mechanism. Molecules 2023, 28, 864. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Y.; Zhang, Y.; Zhang, L.; Lin, Z.; Sun, C.; Wu, H.; Zhang, M. Isolation, identification of antioxidant peptides from earthworm proteins and analysis of the structure-activity relationship of the peptides based on quantum chemical calculations. Food Chem. 2023, 431, 137137. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Fang, B.; Jiang, L.; Guo, H.; Cui, J.; Ren, F. Structure-activity relationship of a series of antioxidant tripeptides derived from β-Lactoglobulin using QSAR modeling. Dairy. Sci. Technol. 2015, 95, 451–463. [Google Scholar] [CrossRef]
- Georgoulia, P.S.; Glykos, N.M. Molecular simulation of peptides coming of age: Accurate prediction of folding, dynamics and structures. Arch. Biochem. Biophys. 2019, 664, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Xu, L.; Fan, C.; Cao, L.; Guo, X. Structural Characteristics and Antioxidant Mechanism of Donkey-Hide Gelatin Peptides by Molecular Dynamics Simulation. Molecules 2023, 28, 7975. [Google Scholar] [CrossRef]
- Gok, B.; Budama-Kilinc, Y.; Kecel-Gunduz, S. Anti-aging activity of Syn-Ake peptide by in silico approaches and in vitro tests. J. Biomol. Struct. Dyn. 2023, 1–15. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, L.; Sun, L.; Yang, Y.; Zhang, S.; Lin, S. Use of a combination of the MD simulations and NMR spectroscopy to determine the regulatory mechanism of pulsed electric field (PEF) targeting at C-terminal histidine of VNAVLH. Food Chem. 2021, 334, 127554. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Guo, M.; Cheng, C.; Li, J.; Li, Y.; Hou, Z.; Ai, Y. Structural and physicochemical characteristics, stability, toxicity and antioxidant activity of peptide-zinc chelate from coconut cake globulin hydrolysates. LWT 2023, 173, 114367. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In Vitro and In Silico Approaches to Generating and Identifying Angiotensin-Converting Enzyme I Inhibitory Peptides from Green Macroalga Ulva lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Aiello, G.; Rivardo, F.; Bartolomei, M.; Bollati, C.; Arnoldi, A.; Cruz-Chamorro, I.; Lammi, C. Antioxidant Effect Assessment and Trans Epithelial Analysis of New Hempseed Protein Hydrolysates. Antioxidants 2023, 12, 1099. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Wen, C.T.; Li, C.Z.; Duan, Y.Q.; Zhang, H.H.; Ma, H.L. Antioxidant Peptide Fractions Isolated from Wheat Germ Protein with Subcritical Water Extraction and Its Transport Across Caco-2 Cells. J. Food Sci. 2019, 84, 2139–2146. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Kusumah, J.; Bringe, N.A.; Shen, Y.; Gonzalez de Mejia, E. Peptide release, radical scavenging capacity, and antioxidant responses in intestinal cells are determined by soybean variety and gastrointestinal digestion under simulated conditions. Food Chem. 2023, 405, 134929. [Google Scholar] [CrossRef]
- Liu, H.F.; Ye, H.J.; Sun, C.Z.; Xi, H.R.; Ma, J.J.; Lai, F.R.; Wu, H. Antioxidant activity in HepG2 cells, immunomodulatory effects in RAW 264.7 cells and absorption characteristics in Caco-2 cells of the peptide fraction isolated from Dendrobium aphyllum. Int. J. Food Sci. Tech. 2018, 53, 2027–2036. [Google Scholar] [CrossRef]
- Xie, N.N.; Liu, S.S.; Wang, C.; Li, B. Stability of casein antioxidant peptide fractions during in vitro digestion/Caco-2 cell model: Characteristics of the resistant peptides. Eur. Food Res. Technol. 2014, 239, 577–586. [Google Scholar] [CrossRef]
- Liu, M.Z.; Zhang, T.; Liang, X.H.; Yuan, Q.Y.; Zeng, X.Q.; Wu, Z.; Pan, D.D.; Tao, M.X.; Guo, Y.X. Production and transepithelial transportation of casein-derived peptides and identification a novel antioxidant peptide LHSMK. LWT Food Sci. Technol. 2021, 151, 112194. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and Metabolism of Peptide WDHHAPQLR Derived from Rapeseed Protein and Inhibition of HUVEC Apoptosis under Oxidative Stress. J. Agric. Food Chem. 2018, 66, 5178–5189. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Kim, Y.-M.; Eun, J.-B. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 128339. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Salami, M.; Yarmand, M.; Emam-Djomeh, Z.; McClements, D.J. Production and characterization of functional bakery goods enriched with bioactive peptides obtained from enzymatic hydrolysis of lentil protein. J. Food Meas. Charact. 2022, 16, 3402–3409. [Google Scholar] [CrossRef]
- Karimi, N.; Zeynali, F.; Bari, M.R.; Nikoo, M.; Mohtarami, F.; Kadivar, M. Amaranth selective hydrolyzed protein influence on sourdough fermentation and wheat bread quality. Food Sci. Nutr. 2021, 9, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Miao, B.T.; Cao, H.J.; Tian, X.X.; Shen, L.J.; Yang, Z.S.; Yuan, F.L.; Ding, Y.P. Monkfish Lophius litulon Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora. Molecules 2023, 28, 245. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.F.; Jiang, Y.Q.; Ning, Z.Z.; Hu, Z.Q.; Ma, S.T.; Shao, Y.; Liu, J.B.; Zhang, T. Supplementation of egg white peptides on attenuating skin mechanical damage symptoms: A promising way to accelerate wound healing process. Food Funct. 2021, 12, 7688–7698. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fu, Y.; Dai, H.; Wang, Q.; Gao, R.; Zhang, Y. Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism. Food Sci. Human. Wellness 2022, 11, 218–229. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhao, Y.-Q.; Wang, Y.-M.; Yang, X.-R.; Chi, C.-F.; Wang, B. Gelatins and antioxidant peptides from Skipjack tuna (Katsuwonus pelamis) skins: Purification, characterization, and cytoprotection on ultraviolet-A injured human skin fibroblasts. Food Biosci. 2022, 50, 102138. [Google Scholar] [CrossRef]
- Wang, X.; Hong, H.; Wu, J. Hen collagen hydrolysate alleviates UVA-induced damage in human dermal fibroblasts. J. Funct. Foods 2019, 63, 103574. [Google Scholar] [CrossRef]

| Source | Peptide | Method | Measured Activity | Reference |
|---|---|---|---|---|
| Corn | RYLL | DPPH and ABTS radical scavenging activity | Scavenging free radicals | [34] |
| Ganoderma lucidum | DRVSIYGWG, ALLSISSF | DPPH and ABTS radical scavenging activity | Scavenging free radicals | [13] |
| Watermelon seeds | RDPEER, KELEEK, DAAGRLQE, LDDDGRL, GFAGDAPRA | DPPH and ABTS radical scavenging activity, ORAC, Fe2+ chelating ability | Scavenging free radicals; chelating metal ions | [15] |
| Feathers | LPGPILSSFPQ | ABTS and •OH radical scavenging activity, Fe2+ chelating ability, TRAP, FRAP, TBARS | Scavenging free radicals; chelating metal ions; inhibition of lipid peroxidation | [23] |
| Chicken | RWGG, YYCQ | ABTS radical scavenging activity, ORAC | Scavenging free radicals | [24] |
| Milk | YVPR, VPYPQR | DPPH and ABTS radical scavenging activity, CBA | Scavenging free radicals | [25] |
| Gracilaria lemaneiformis | LSPGEL, VYFDR, PGPTY | DPPH and ABTS radical scavenging activity, FRAP | Scavenging free radicals | [26] |
| Yak casein | RELEEL | DPPH, O2•− and •OH radical scavenging activity, FRAP, Fe2+ chelating ability | Scavenging free radicals; chelating metal ions | [28] |
| Pea | YLVN, EEHLCFR, TFY | DPPH, ABTS, O2•− and •OH radical scavenging activity, ORAC | Scavenging free radicals | [35] |
| Shrimp meat | MTTNL, MTTNI | Measurement of scavenging free radicals of DPPH and •OH via electron paramagnetic resonance spectroscopy | Scavenging free radicals | [26,36] |
| Channa argus | IVLPDEGK, PGMLGGSPPGLLGGSP, SDGSNIHFPN, SVSIRADGGEGEVTVFT. | DPPH and •OH radical scavenging activity, Fe2+ chelating ability | Scavenging free radicals; chelating metal ions | [31] |
| Types of Amino Acids | Names | Action Mechanism | Reference |
|---|---|---|---|
| Aromatic amino acids | Tyr, Trp, Phe, His | These amino acids can act as hydrogen donors (providing protons to electron-deficient free radicals) and improve ROS scavenging capacity. | [74] |
| Hydrophobic amino acids | Val, Leu, Tyr, Trp, Phe, Ile, Pro, Met | These amino acids can act as hydrogen donors and inhibit lipid peroxidation by enhancing the solubility of peptides in the lipid phase and improving the accessibility of fat-soluble ROS or polyunsaturated fatty acids. | [67] |
| Sulfur-containing amino acids | Cys, Met | These amino acids provide electrons to scavenge ROS and can be converted into GSH, and Met can be oxidized to methionine sulfoxide and has antioxidant activity. | [75] |
| Acidic amino acids | Glu, Asp | These amino acids have carboxyl and amino side chains that chelate with metal ions through electrostatic interactions. | [31,48] |
| Basic amino acids | His, Arg, Lys |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Dong, Q.; Yu, C.; Chen, H.; Zhao, Y.; Zhang, B.; Yu, P.; Chen, M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants 2024, 13, 479. https://doi.org/10.3390/antiox13040479
Xu B, Dong Q, Yu C, Chen H, Zhao Y, Zhang B, Yu P, Chen M. Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants. 2024; 13(4):479. https://doi.org/10.3390/antiox13040479
Chicago/Turabian StyleXu, Baoting, Qin Dong, Changxia Yu, Hongyu Chen, Yan Zhao, Baosheng Zhang, Panling Yu, and Mingjie Chen. 2024. "Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides" Antioxidants 13, no. 4: 479. https://doi.org/10.3390/antiox13040479
APA StyleXu, B., Dong, Q., Yu, C., Chen, H., Zhao, Y., Zhang, B., Yu, P., & Chen, M. (2024). Advances in Research on the Activity Evaluation, Mechanism and Structure-Activity Relationships of Natural Antioxidant Peptides. Antioxidants, 13(4), 479. https://doi.org/10.3390/antiox13040479







