The Reducing Agent Dithiothreitol Modulates the Ventilatory Responses That Occur in Freely Moving Rats during and following a Hypoxic–Hypercapnic Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Permissions, Rats, and Surgical Procedures
2.2. Protocols for Whole-Body Plethysmography Measurement of Ventilatory Parameters
2.3. Body Temperatures
2.4. Data Analyses
3. Results
3.1. Ventilatory Baseline Values and Effects of L,D-DTT
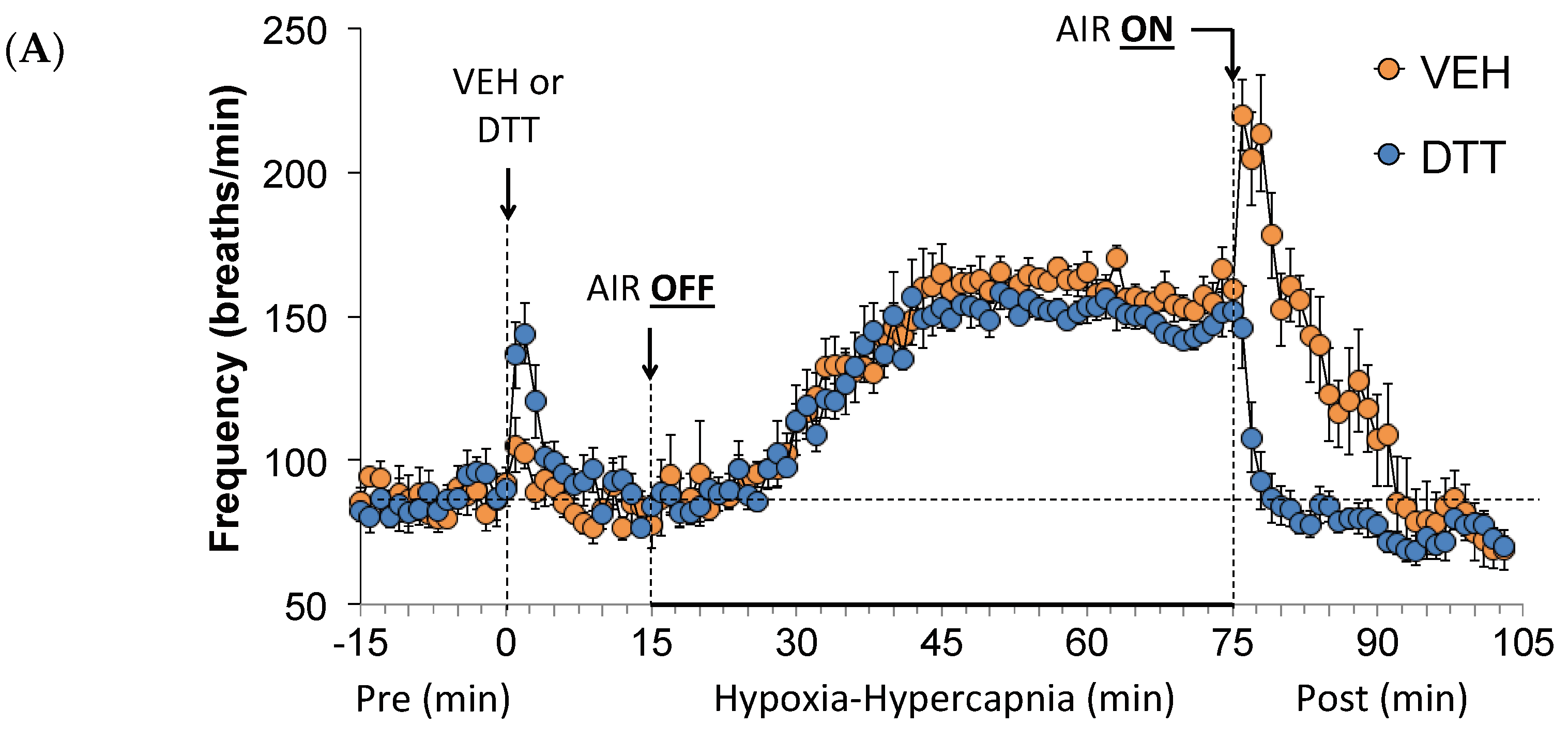
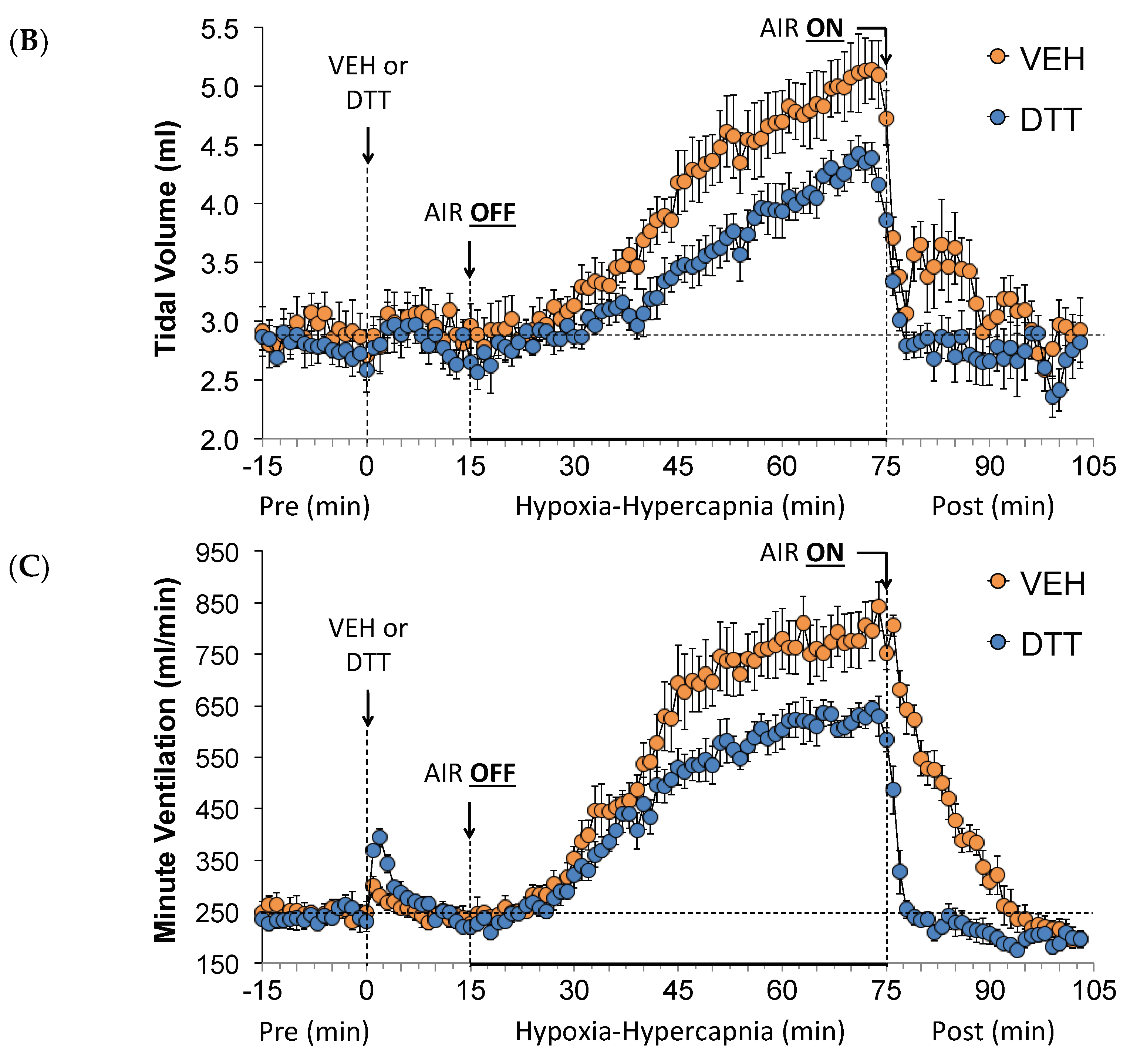
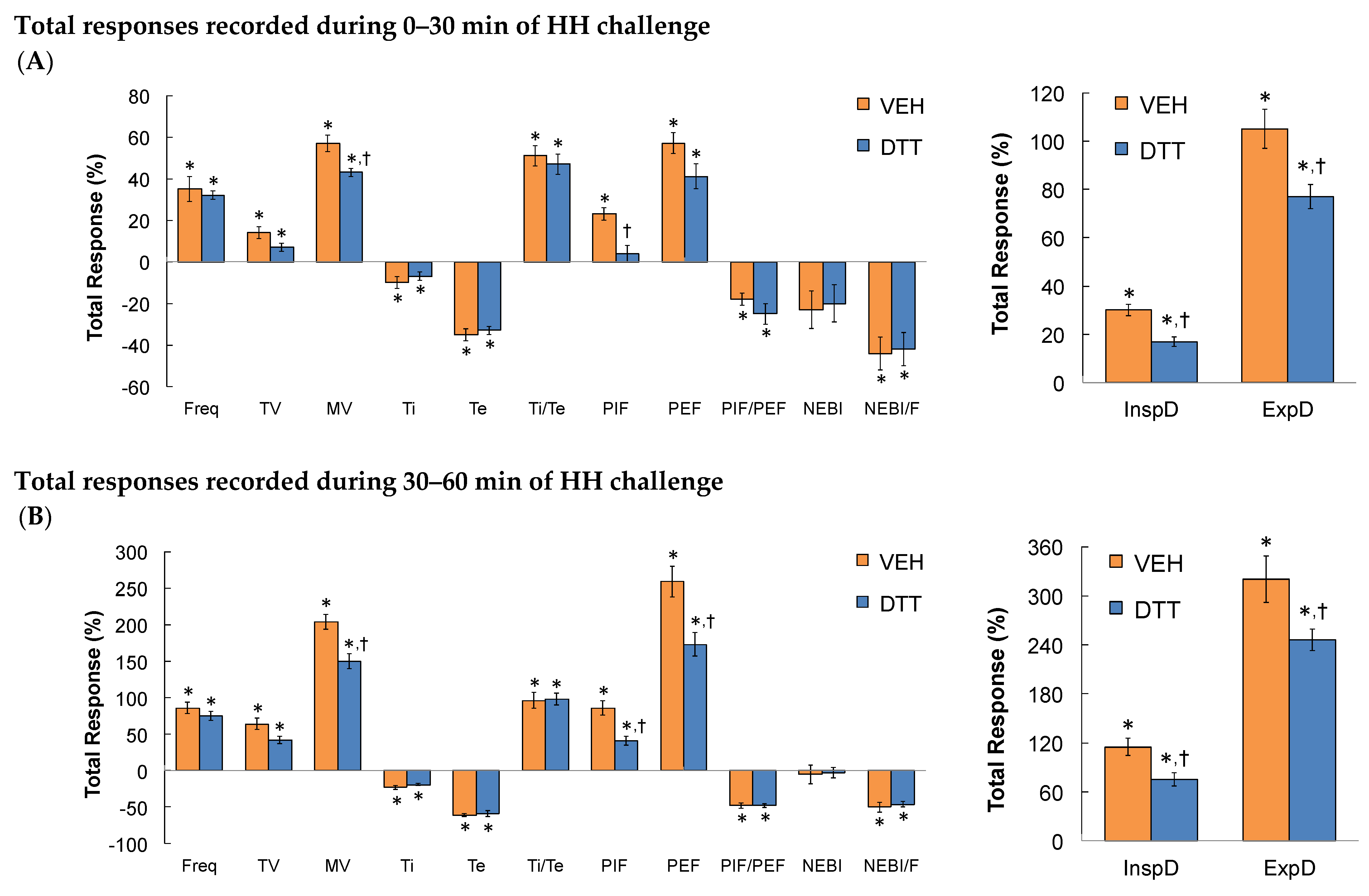
3.2. Effects of L,D-DTT on Changes in Frequency of Breathing, Tidal Volume, and Minute Ventilation
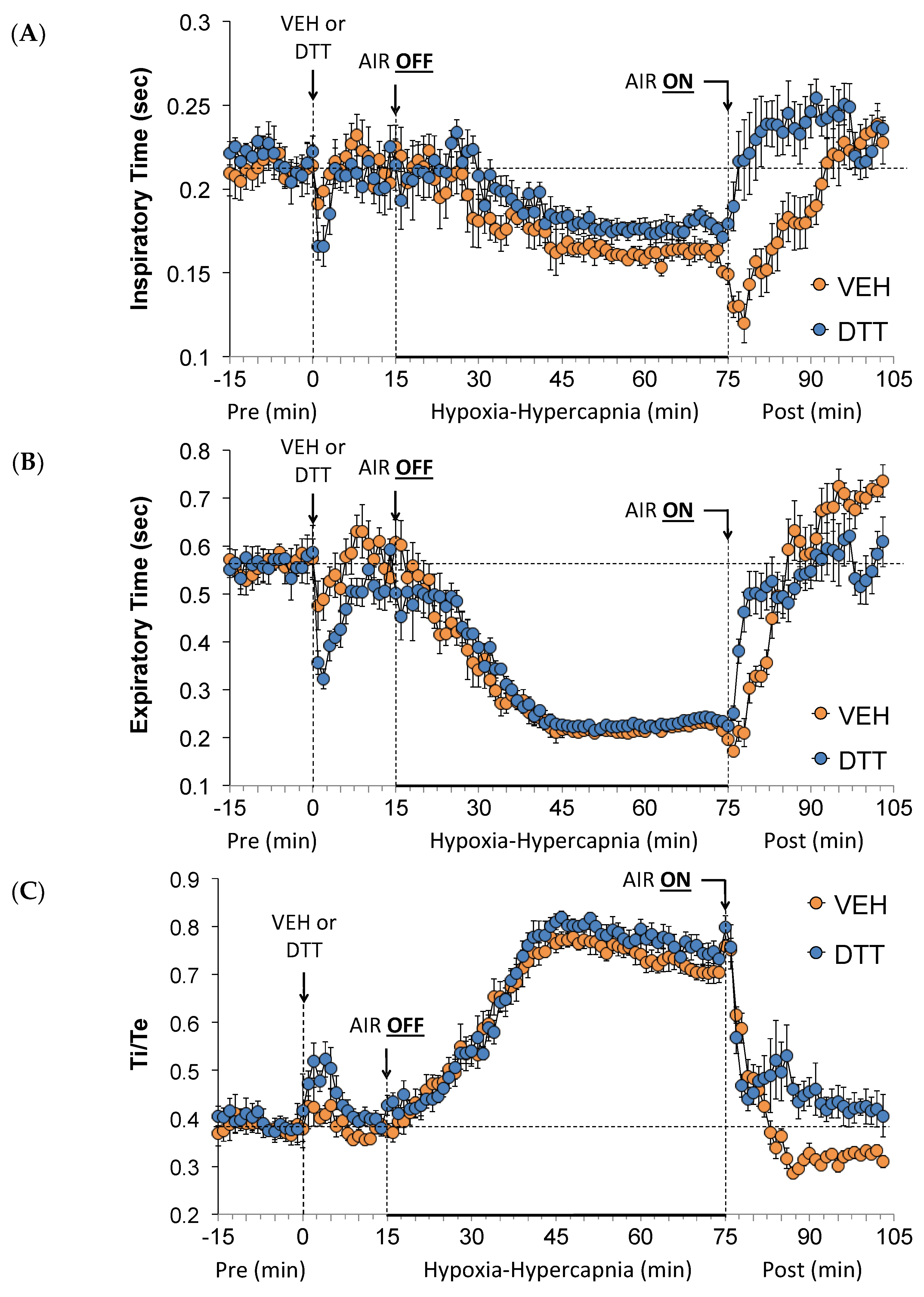
3.3. Effects of L,D-DTT on Changes in Inspiratory Time and Expiratory Time
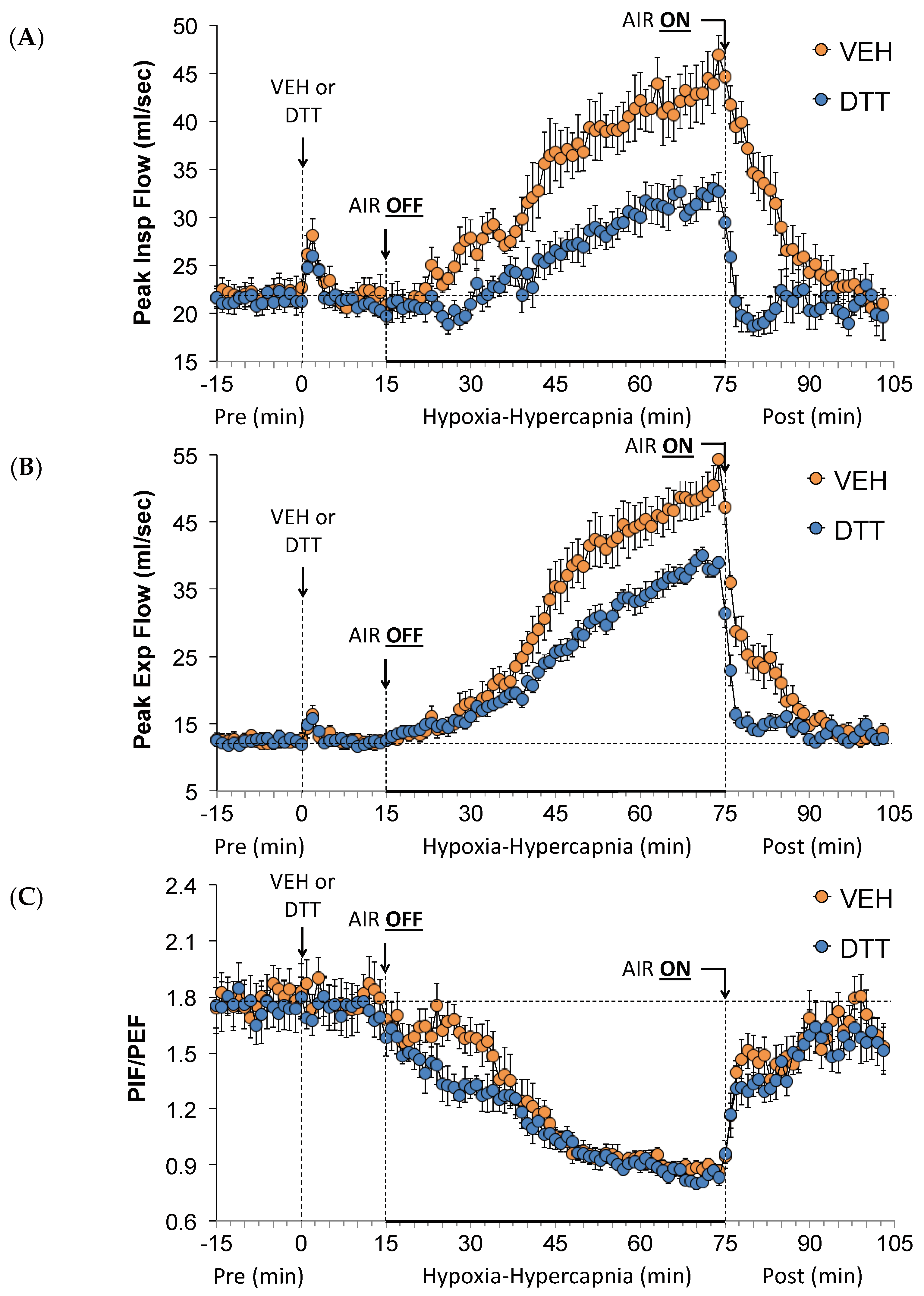
3.4. Effects of L,D-DTT on Changes in Peak Inspiratory Flow and Peak Expiratory Flow
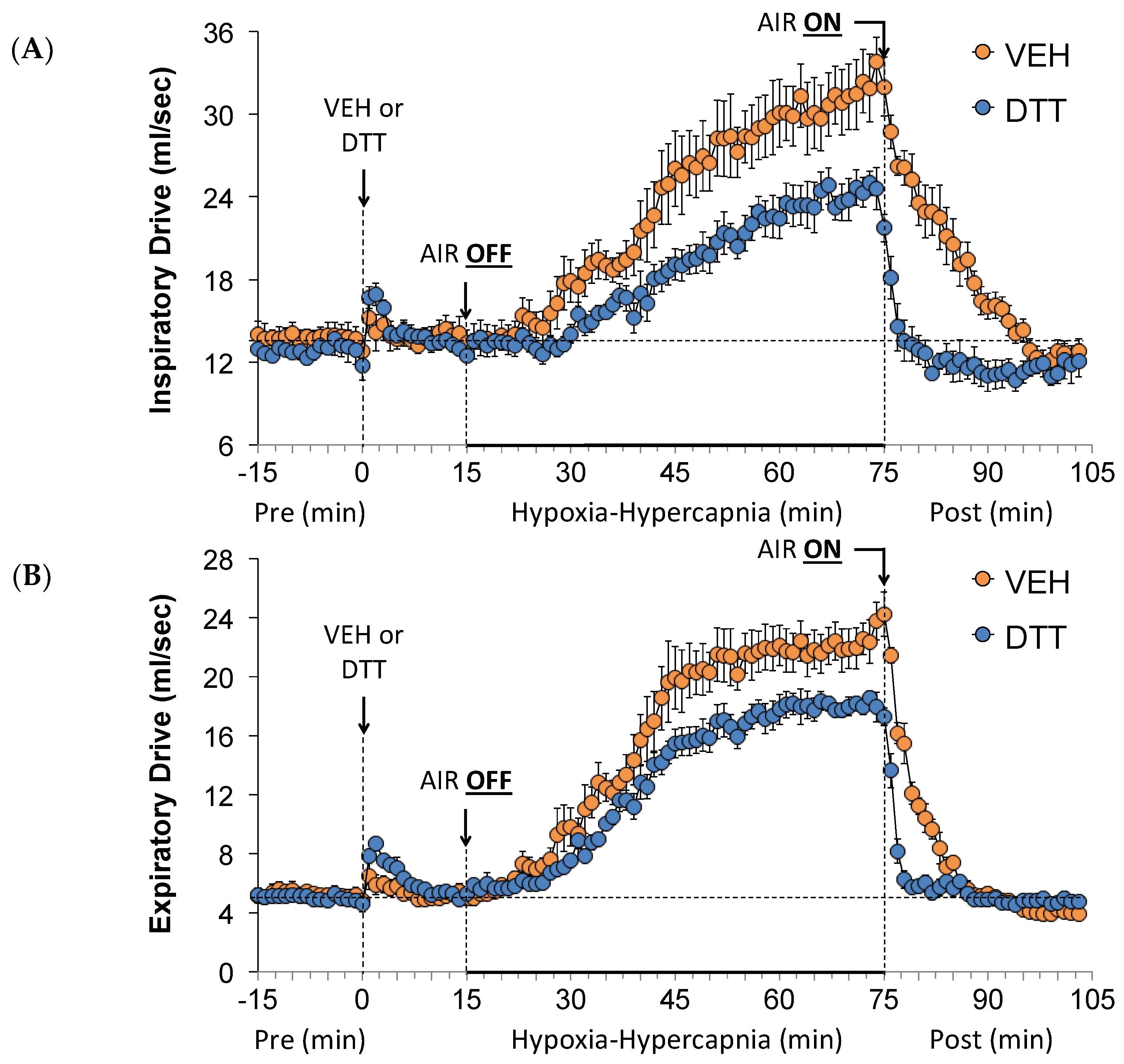
3.5. Effects of L,D-DTT on Changes Inspiratory Drive and Expiratory Drive
3.6. Effects of L,D-DTT on Changes in Non-Eupneic Breathing Index
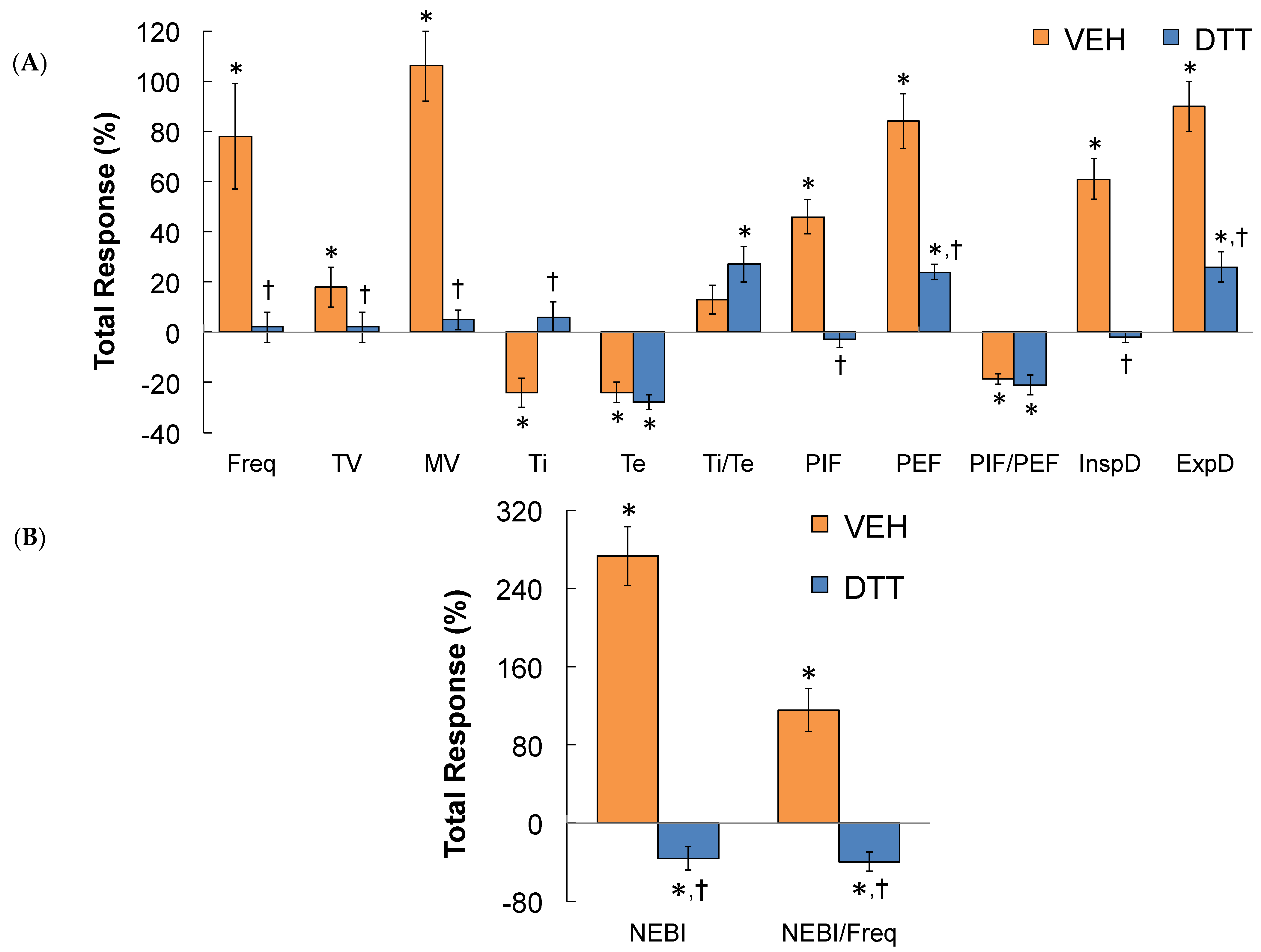
3.7. Summary of the Effects of L,D-DTT
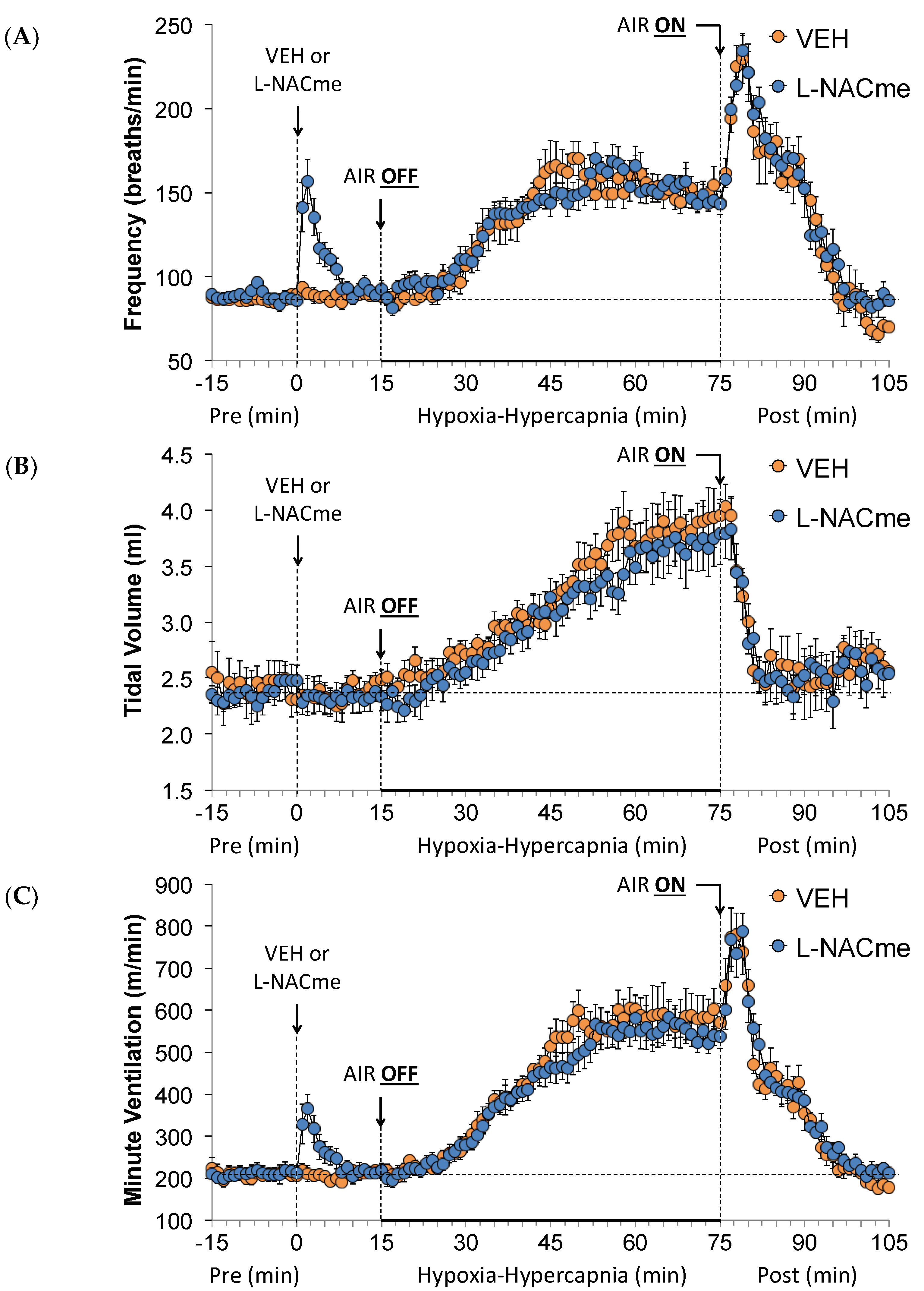
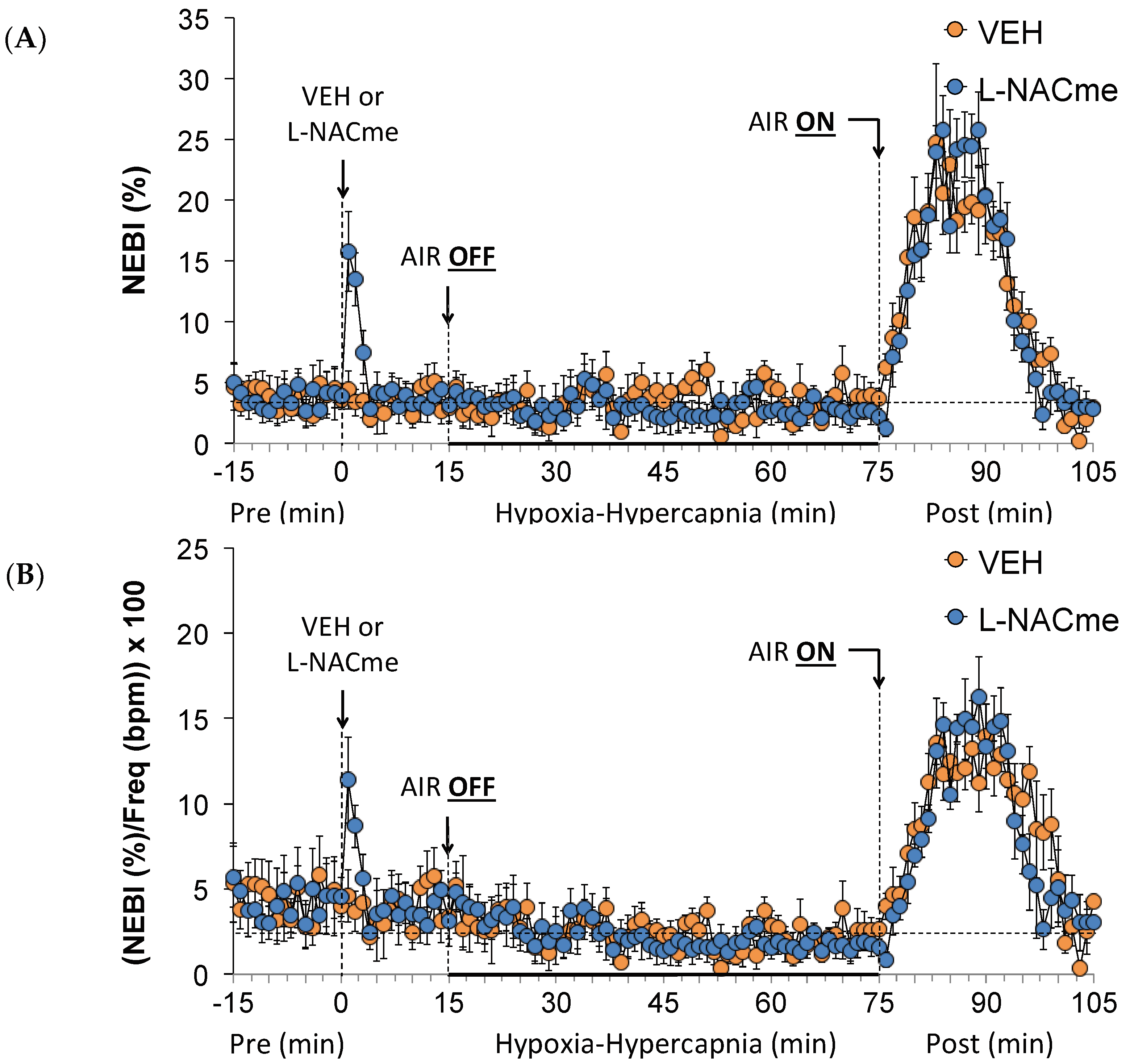
3.8. Effects of L-NACme on Ventilatory Responses
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ar, A.; Arieli, R.; Shkolnik, A. Blood-gas properties and function in the fossorial mole rat under normal and hypoxic-hypercapnic atmospheric conditions. Respir. Physiol. 1977, 30, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Mitra, J.; Cherniack, N.S. Role of substance P in hypercapnic excitation of carotid chemoreceptors. J. Appl. Physiol. 1987, 63, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Engwall, M.J.; Smith, C.A.; Dempsey, J.A.; Bisgard, G.E. Ventilatory afterdischarge and central respiratory drive interactions in the awake goat. J. Appl. Physiol. 1994, 76, 416–423. [Google Scholar] [CrossRef]
- Hein, M.S.; Schlenker, E.H.; Patel, K.P. Altered control of ventilation in streptozotocin-induced diabetic rats. Proc. Soc. Exp. Biol. Med. 1994, 207, 213–219. [Google Scholar] [CrossRef]
- Kondo, T.; Kumagai, M.; Ohta, Y.; Bishop, B. Ventilatory responses to hypercapnia and hypoxia following chronic hypercapnia in the rat. Respir. Physiol. 2000, 122, 35–43. [Google Scholar] [CrossRef]
- Campbell, A.J.; Galland, B.C.; Bolton, D.P.; Taylor, B.J.; Sayers, R.M.; Williams, S.M. Ventilatory responses to rebreathing in infants exposed to maternal smoking. Acta Paediatr. 2000, 90, 793–800. [Google Scholar] [CrossRef]
- Blain, G.M.; Smith, C.A.; Henderson, K.S.; Dempsey, J.A. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J. Physiol. 2010, 588 Pt 13, 2455–2571. [Google Scholar] [CrossRef]
- Han, F.; Subramanian, S.; Dick, T.E.; Dreshaj, I.A.; Strohl, K.P. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J. Appl. Physiol. 2001, 91, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R. Sensing hypoxia: Physiology, genetics and epigenetics. J. Physiol. 2013, 591, 2245–2257. [Google Scholar] [CrossRef]
- Getsy, P.M.; Davis, J.; Coffee, G.A.; May, W.J.; Palmer, L.A.; Strohl, K.P.; Lewis, S.J. Enhanced non-eupneic breathing following hypoxic, hypercapnic or hypoxic-hypercapnic gas challenges in conscious mice. Respir. Physiol. Neurobiol. 2014, 204, 147–159. [Google Scholar] [CrossRef]
- Getsy, P.M.; Coffee, G.A.; Lewis, S.J. The Role of Carotid Sinus Nerve Input in the Hypoxic-Hypercapnic Ventilatory Response in Juvenile Rats. Front. Physiol. 2020, 11, 613786. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.S.; Patwari, P.P.; Kenny, A.S.; Brogadir, C.D.; Stewart, T.M.; Weese-Mayer, D.E. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): Response to ventilatory challenges. Pediatr. Pulmonol. 2015, 50, 1336–1345. [Google Scholar] [CrossRef]
- Berner, J.; Shvarev, Y.; Zimmer, A.; Wickstrom, R. Hypoxic ventilatory response in Tac1−/− neonatal mice following exposure to opioids. J. Appl. Physiol. 2012, 113, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Baby, S.M.; Gruber, R.B.; Young, A.P.; MacFarlane, P.M.; Teppema, L.J.; Lewis, S.J. Bilateral carotid sinus nerve transection exacerbates morphine-induced respiratory depression. Eur. J. Pharmacol. 2018, 834, 17–29. [Google Scholar] [CrossRef] [PubMed]
- May, W.J.; Gruber, R.B.; Discala, J.F.; Puskovic, V.; Henderson, F.; Palmer, L.A.; Lewis, S.J. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic challenge. Open J. Mol. Integr. Physiol. 2013, 3, 166–180. [Google Scholar] [CrossRef] [PubMed]
- May, W.J.; Henderson, F.; Gruber, R.B.; Discala, J.F.; Young, A.P.; Bates, J.N.; Palmer, L.A.; Lewis, S.J. Morphine has latent deleterious effects on the ventilatory responses to a hypoxic-hypercapnic challenge. Open J. Mol. Integr. Physiol. 2013, 3, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Brackley, A.D.; Andrade, M.A.; Toney, G.M. Intermittent hypercapnic hypoxia induces respiratory hypersensitivity to fentanyl accompanied by tonic respiratory depression by endogenous opioids. J. Physiol. 2020, 598, 3239–3257. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Guck, D.; McCann, M.; Sternick, M.; Sonobe, T.; Tubbs, N. Severe Hypoxemia Prevents Spontaneous and Naloxone-induced Breathing Recovery after Fentanyl Overdose in Awake and Sedated Rats. Anesthesiology 2020, 132, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.W.; Khalid, F.; Baby, S.M.; May, W.J.; Young, A.P.; Bates, J.N.; Cheng, F.; Seckler, J.M.; Lewis, S.J. Glutathione ethyl ester reverses the deleterious effects of fentanyl on ventilation and arterial blood-gas chemistry while prolonging fentanyl-induced analgesia. Sci. Rep. 2021, 11, 6985. [Google Scholar] [CrossRef]
- Seckler, J.M.; Grossfield, A.; May, W.J.; Getsy, P.M.; Lewis, S.J. Nitrosyl factors play a vital role in the ventilatory depressant effects of fentanyl in unanesthetized rats. Biomed. Pharmacother. 2022, 146, 112571. [Google Scholar] [CrossRef]
- Kline, D.D.; Yang, T.; Huang, P.L.; Prabhakar, N.R. Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J. Physiol. 1998, 511, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.D.; Yang, T.; Premkumar, D.R.; Thomas, A.J.; Prabhakar, N.R. Blunted respiratory responses to hypoxia in mutant mice deficient in nitric oxide synthase-3. J. Appl. Physiol. 2000, 88, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Kline, D.D.; Prabhakar, N.R. Peripheral chemosensitivity in mutant mice deficient in nitric oxide synthase. Adv. Exp. Med. Biol. 2000, 475, 571–579. [Google Scholar] [CrossRef]
- Palmer, L.A.; May, W.J.; deRonde, K.; Brown-Steinke, K.; Gaston, B.; Lewis, S.J. Hypoxia-induced ventilatory responses in conscious mice: Gender differences in ventilatory roll-off and facilitation. Respir. Physiol. Neurobiol. 2013, 185, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.A.; May, W.J.; deRonde, K.; Brown-Steinke, K.; Bates, J.N.; Gaston, B.; Lewis, S.J. Ventilatory responses during and following exposure to a hypoxic challenge in conscious mice deficient or null in S-nitrosoglutathione reductase. Respir. Physiol. Neurobiol. 2013, 185, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Gaston, B.; May, W.J.; Sullivan, S.; Yemen, S.; Marozkina, N.V.; Palmer, L.A.; Bates, J.N.; Lewis, S.J. Essential role of hemoglobin beta-93-cysteine in posthypoxia facilitation of breathing in conscious mice. J. Appl. Physiol. 2014, 116, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Getsy, P.M.; Coffee, G.A.; Hsieh, Y.H.; Lewis, S.J. Loss of Cervical Sympathetic Chain Input to the Superior Cervical Ganglia Affects the Ventilatory Responses to Hypoxic Challenge in Freely-Moving C57BL6 Mice. Front. Physiol. 2021, 12, 619688. [Google Scholar] [CrossRef] [PubMed]
- Getsy, P.M.; Coffee, G.A.; Hsieh, Y.H.; Lewis, S.J. The superior cervical ganglia modulate ventilatory responses to hypoxia independently of preganglionic drive from the cervical sympathetic chain. J. Appl. Physiol. 2021, 131, 836–857. [Google Scholar] [CrossRef] [PubMed]
- Getsy, P.M.; Sundararajan, S.; May, W.J.; von Schill, G.C.; McLaughlin, D.K.; Palmer, L.A.; Lewis, S.J. Short-term facilitation of breathing upon cessation of hypoxic challenge is impaired in male but not female endothelial NOS knock-out mice. Sci. Rep. 2021, 11, 18346. [Google Scholar] [CrossRef]
- Getsy, P.M.; Sundararajan, S.; Lewis, S.J. Carotid sinus nerve transection abolishes the facilitation of breathing that occurs upon cessation of a hypercapnic gas challenge in male mice. J. Appl. Physiol. 2021, 131, 821–835. [Google Scholar] [CrossRef]
- Getsy, P.M.; Sundararajan, S.; May, W.J.; von Schill, G.C.; McLaughlin, D.K.; Palmer, L.A.; Lewis, S.J. Ventilatory responses during and following hypercapnic gas challenge are impaired in male but not female endothelial NOS knock-out mice. Sci. Rep. 2021, 11, 20557. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L.; Bayliss, D.A. Retrotrapezoid nucleus and central chemoreception. J. Physiol. 2008, 586, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Bayliss, D.A. Neural Control of Breathing and CO2 Homeostasis. Neuron 2015, 87, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L.; Souza, G.M.P.R.; Abbott, S.B.G.; Shi, Y.; Bayliss, D.A. The Retrotrapezoid Nucleus: Central Chemoreceptor and Regulator of Breathing Automaticity. Trends Neurosci. 2019, 42, 807–824. [Google Scholar] [CrossRef] [PubMed]
- Behn, C.; Araneda, O.F.; Llanos, A.J.; Celedón, G.; González, G. Hypoxia-related lipid peroxidation: Evidences, implications and approaches. Respir. Physiol. Neurobiol. 2007, 158, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 2, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Tawa, M.; Geddawy, A.; Shimosato, T.; Iwasaki, H.; Imamura, T.; Okamura, T. Soluble guanylate cyclase redox state under hypoxia or hypoxia/reoxygenation in isolated monkey coronary arteries. J. Pharmacol. Sci. 2014, 125, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; González-Rodríguez, P.; Ortega-Sáenz, P.; López-Barneo, J. Redox signaling in acute oxygen sensing. Redox Biol. 2017, 12, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.A.; Waypa, G.B.; Schumacker, P.T. Redox signaling during hypoxia in mammalian cells. Redox Biol. 2017, 13, 228–234. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Gyulai, L.; Dora, E.; Kovach, A.G. NAD/NADH: Redox state changes on cat brain cortex during stimulation and hypercapnia. Am. J. Physiol. 1982, 243, H619–H627. [Google Scholar] [CrossRef] [PubMed]
- Baev, V.I.; Vasil’eva, I.V.; L’vov, S.N.; Shugaleĭ, I.V. The unknown physiological role of carbon dioxide. Fiziol. Zh. Im. IM Sechenova 1995, 81, 47–52. [Google Scholar]
- Zhu, S.; Basiouny, K.F.; Crow, J.P.; Matalon, S. Carbon dioxide enhances nitration of surfactant protein A by activated alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L1025–L1031. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.B. Hypercapnia causes cellular oxidation and nitrosation in addition to acidosis: Implications for CO2 chemoreceptor function and dysfunction. J. Appl. Physiol. 2010, 108, 1786–1795. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Bauer, R.; Gedrange, T.; Walter, B.; Klinger, W.; Zwiener, U. Influence of hypoxia and hypoxia/hypercapnia upon brain and blood peroxidative and glutathione status in normal weight and growth-restricted newborn piglets. Exp. Toxicol. Pathol. 1998, 50, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.M.; Ryu, J.; Kanaan, A.; Del Carmen Rivero, M.; Dugan, L.L.; Haddad, G.G.; Ali, S.S. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am. J. Physiol. Cell Physiol. 2010, 298, C1594–C1602. [Google Scholar] [CrossRef] [PubMed]
- Acker, H.; Eyzaguirre, C.; Goldman, W.F. Redox changes in the mouse carotid body during hypoxia. Brain Res. 1985, 330, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, R.; Moya, E.A.; Iturriaga, R. Carotid body and cardiorespiratory alterations in intermittent hypoxia: The oxidative link. Eur. Respir. J. 2010, 36, 143–150. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Regulation of carotid body oxygen sensing by hypoxia-inducible factors. Pflugers Arch. 2016, 468, 71–75. [Google Scholar] [CrossRef]
- Bernardini, A.; Brockmeier, U.; Metzen, E.; Berchner-Pfannschmidt, U.; Harde, E.; Acker-Palmer, A.; Papkovsky, D.; Acker, H.; Fandrey, J. Measurement of ROS Levels and Membrane Potential Dynamics in the Intact Carotid Body Ex Vivo. Adv. Exp. Med. Biol. 2015, 860, 55–59. [Google Scholar] [CrossRef]
- Bernardini, A.; Wolf, A.; Brockmeier, U.; Riffkin, H.; Metzen, E.; Acker-Palmer, A.; Fandrey, J.; Acker, H. Carotid body type I cells engage flavoprotein and Pin1 for oxygen sensing. Am. J. Physiol. Cell Physiol. 2020, 318, C719–C731. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Alfayate, G.; Obeso, A.; Agapito, M.T.; González, C. Reduced to oxidized glutathione ratios and oxygen sensing in calf and rabbit carotid body chemoreceptor cells. J. Physiol. 2001, 537, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Sanz-Alyayate, G.; Agapito, M.T.; Obeso, A. Effects of reducing agents on glutathione metabolism and the function of carotid body chemoreceptor cells. Biol. Chem. 2004, 385, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Sanz-Alfayate, G.; Obeso, A.; Agapito, M.T. Role of glutathione redox state in oxygen sensing by carotid body chemoreceptor cells. Methods Enzymol. 2004, 381, 40–71. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Agapito, M.T.; Rocher, A.; Gonzalez-Martin, M.C.; Vega-Agapito, V.; Gomez-Niño, A.; Rigual, R.; Castañeda, J.; Obeso, A. Chemoreception in the context of the general biology of ROS. Respir. Physiol. Neurobiol. 2007, 157, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Agapito, M.T.; Sanz-Alfayate, G.; Gomez-Niño, A.; Gonzalez, C.; Obeso, A. General redox environment and carotid body chemoreceptor function. Am J. Physiol. Cell Physiol. 2009, 296, C620–C631. [Google Scholar] [CrossRef]
- Gao, L.; Arias-Mayenco, I.; Ortega-Sáenz, P.; López-Barneo, J. Using redox-sensitive fluorescent probes to record real-time reactive oxygen species production in cells from mouse carotid body slices. STAR Protoc. 2021, 2, 100535. [Google Scholar] [CrossRef]
- Read, A.D.; Bentley, R.E.; Archer, S.L.; Dunham-Snary, K.J. Mitochondrial iron-sulfur clusters: Structure, function, and an emerging role in vascular biology. Redox Biol. 2021, 47, 102164. [Google Scholar] [CrossRef]
- Roy, A.; Rozanov, C.; Mokashi, A.; Lahiri, S. Redox-based inhibition of K+ channel/current is not related to hypoxic chemosensory responses in rat carotid body. Adv. Exp. Med. Biol. 2000, 475, 645–653. [Google Scholar] [CrossRef]
- Roy, A.; Mokashi, A.; Rozanov, C.; Daudu, P.A.; Lahiri, S. Reduced glutathione, dithiothreitol and cytochrome P-450 inhibitors do not influence hypoxic chemosensory responses in the rat carotid body. Brain Res. 2001, 889, 131–137. [Google Scholar] [CrossRef]
- Cleland, W.W. Dithiothreitol, a New Protective Reagent for SH Groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Alliegro, M.C. Effects of dithiothreitol on protein activity unrelated to thiol-disulfide exchange: For consideration in the analysis of protein function with Cleland’s reagent. Anal. Biochem. 2000, 282, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Almeida, J.P.; Saldanha, C. Dithiothreitol revisited in red cells: A new head for an old hat. Clin. Hemorheol. Microcirc. 2010, 46, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mthembu, S.N.; Sharma, A.; Albericio, F.; de la Torre, B.G. Breaking a Couple: Disulfide Reducing Agents. Chembiochem 2020, 21, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Tawa, M.; Yamamizu, K.; Geddawy, A.; Shimosato, T.; Imamura, T.; Ayajiki, K.; Okamura, T. Impairment by hypoxia or hypoxia/reoxygenation of nitric oxide-mediated relaxation in isolated monkey coronary artery: The role of intracellular superoxide. J. Pharmacol. Sci. 2011, 116, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Takahata, R.; Hayaishi, O. Inhibition of sleep in rats by inorganic selenium compounds, inhibitors of prostaglandin D synthase. Proc. Natl. Acad. Sci. USA 1991, 88, 9046–9050. [Google Scholar] [CrossRef] [PubMed]
- Canini, F.; Bréjot, T.; d’Aléo, P.; Mercier, S.; Bourdon, L. NMDA receptors are involved in dithiothreitol-induced hypothermia. Eur. J. Pharmacol. 2001, 426, 179–183. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M.; Campolongo, P.; Palmery, M. Effect on rat arterial blood pressure of chemically generated peroxyl radicals and protection by antioxidants. J. Nutr. Biochem. 2004, 15, 323–327. [Google Scholar] [CrossRef]
- Heidari, R.; Taheri, V.; Rahimi, H.R.; Shirazi Yeganeh, B.; Niknahad, H.; Najibi, A. Sulfasalazine-induced renal injury in rats and the protective role of thiol-reductants. Ren. Fail. 2016, 38, 137–141. [Google Scholar] [CrossRef]
- He, J.; Guo, R.; Qiu, P.; Su, X.; Yan, G.; Feng, J. Exogenous hydrogen sulfide eliminates spatial memory retrieval impairment and hippocampal CA1 LTD enhancement caused by acute stress via promoting glutamate uptake. Neuroscience 2017, 350, 110–123. [Google Scholar] [CrossRef]
- Kumar, A.; Rani, A.; Scheinert, R.B.; Ormerod, B.K.; Foster, T.C. Nonsteroidal anti-inflammatory drug, indomethacin improves spatial memory and NMDA receptor function in aged animals. Neurobiol. Aging 2018, 70, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Farshad, O.; Niknahad, H.; Mousavi, K.; Moein, M.; Azarpira, N.; Mohammadi, H.; Jamshidzadeh, A.; Heidari, R. Oral administration of thiol-reducing agents mitigates gut barrier disintegrity and bacterial lipopolysaccharide translocation in a rat model of biliary obstruction. Curr. Res. Pharmacol. Drug Discov. 2020, 1, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Guan, X.L.; Wang, F.; Chen, J.G. N-acetylcysteine facilitates extinction of cued fear memory in rats via reestablishing basolateral amygdala glutathione homeostasis. Acta Pharmacol. Sin. 2022, 43, 260–272. [Google Scholar] [CrossRef]
- Saia, B.; Terribile, P.; Galzigna, L. Mucolytic action “in vitro” of the methyl ester of cysteine compared with the action of N-acetyl-L-cysteine. Minerva Med. 1967, 58, 3773–3775. [Google Scholar]
- Kattaya, S.A.; Akkus, O.; Slama, J. Radioprotectant and radiosensitizer effects on sterility of gamma-irradiated bone. Clin. Orthop. Relat. Res. 2008, 466, 1796–1803. [Google Scholar] [CrossRef]
- Michaelsen, J.T.; Dehnert, S.; Giustarini, D.; Beckmann, B.; Tsikas, D. HPLC analysis of human erythrocytic glutathione forms using OPA and N-acetyl-cysteine ethyl ester: Evidence for nitrite-induced GSH oxidation to GSSG. J. Chromatogr. B 2009, 877, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Tsika, D.; Rossi, R. N-Acetylcysteine ethyl ester (NACET): A novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem. Pharmacol. 2012, 84, 1522–1533. [Google Scholar] [CrossRef]
- Giustarini, D.; Galvagni, F.; Dalle Donne, I.; Milzani, A.; Severi, F.M.; Santucci, A.; Rossi, R. N-acetylcysteine ethyl ester as GSH enhancer in human primary endothelial cells: A comparative study with other drugs. Free Radic. Biol. Med. 2018, 126, 202–209. [Google Scholar] [CrossRef]
- Poopari, M.R.; Dezhahang, Z.; Xu, Y. Identifying dominant conformations of N-acetyl-L-cysteine methyl ester and N-acetyl-L-cysteine in water: VCD signatures of the amide I and the C=O stretching bands. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 131–140. [Google Scholar] [CrossRef]
- Tsikas, D.; Schwedhelm, K.S.; Surdacki, A.; Giustarini, D.; Rossi, R.; Kukoc-Modun, L.; Kedia, G.; Ückert, S. S-Nitroso-N-acetyl-L-cysteine ethyl ester (SNACET) and N-acetyl-L-cysteine ethyl ester (NACET)-Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO, H2S and GSH suppliers and as antioxidants: Results and overview. J. Pharm. Anal. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Uemura, T.; Watanabe, K.; Ko, K.; Higashi, K.; Kogure, N.; Kitajima, M.; Takayama, H.; Takao, K.; Sugita, Y.; Sakamoto, A.; et al. Protective Effects of Brain Infarction by N-Acetylcysteine Derivatives. Stroke 2018, 49, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, R.N.; Bulumulla, C.; Catchpole, T.; Takacs, A.; Christie, A.; Stefan, M.C.; Csaky, K.G. Protection of human retinal pigment epithelial cells from oxidative damage using cysteine prodrugs. Free Radic. Biol. Med. 2020, 152, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gaston, B.; Baby, S.M.; May, W.J.; Young, A.P.; Grossfield, A.; Bates, J.N.; Seckler, J.M.; Wilson, C.G.; Lewis, S.J. D-Cystine di(m)ethyl ester reverses the deleterious effects of morphine on ventilation and arterial blood gas chemistry while promoting antinociception. Sci. Rep. 2021, 11, 10038. [Google Scholar] [CrossRef] [PubMed]
- Hamelmann, E.; Schwarze, J.; Takeda, K.; Oshiba, A.; Larsen, G.L.; Irvin, C.G.; Gelfand, E.W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997, 156, 766–775. [Google Scholar] [CrossRef]
- Lomask, M. Further exploration of the Penh parameter. Exp. Toxicol. Pathol. 2006, 57 (Suppl. S2), 13–20. [Google Scholar] [CrossRef]
- Tsumuro, T.; Alejandra Hossen, M.; Kishi, Y.; Fujii, Y.; Kamei, C. Nasal congestion model in Brown Norway rats and the effects of some H1-antagonists. Int. Immunopharmacol. 2006, 6, 759–763. [Google Scholar] [CrossRef]
- Quindry, J.C.; Ballmann, C.G.; Epstein, E.E.; Selsby, J.T. Plethysmography measurements of respiratory function in conscious unrestrained mice. J. Physiol. Sci. 2016, 66, 157–164. [Google Scholar] [CrossRef]
- Henderson, F.; May, W.J.; Gruber, R.B.; Discala, J.F.; Puskovic, V.; Young, A.P.; Baby, S.M.; Lewis, S.J. Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats. Respir. Physiol. Neurobiol. 2014, 191, 95–105. [Google Scholar] [CrossRef]
- Wallenstein, S.; Zucker, C.L.; Fleiss, J.L. Some statistical methods useful in circulation research. Circ. Res. 1980, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ludbrook, J. Multiple comparison procedures updated. Clin. Exp. Pharmacol. Physiol. 1998, 25, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Multiple comparison analysis testing in ANOVA. Biochem. Med. 2011, 21, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Winer, B.J. Statistical Principles of Experimental Design; McGraw-Hill Book Co.: New York, NY, USA, 1971; pp. 752–809. [Google Scholar]
- Brock, M.W.; Mathes, C.; Gilly, W.F. Selective open-channel block of Shaker (Kv1) potassium channels by s-nitrosodithiothreitol (SNDTT). J. Gen. Physiol. 2001, 118, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Huckstepp, R.T.R.; Cardoza, K.P.; Henderson, L.E.; Feldman, J.L. Distinct parafacial regions in control of breathing in adult rats. PLoS ONE 2018, 13, e0201485. [Google Scholar] [CrossRef]
- Tosi, G.M.; Giustarini, D.; Franci, L.; Minetti, A.; Imperatore, F.; Caldi, E.; Fiorenzani, P.; Aloisi, A.M.; Sparatore, A.; Rossi, R.; et al. Superior Properties of N-Acetylcysteine Ethyl Ester over N-Acetyl Cysteine to Prevent Retinal Pigment Epithelial Cells Oxidative Damage. Int. J. Mol. Sci. 2021, 22, 600. [Google Scholar] [CrossRef]


| Parameters | Abbreviation | Units | Definition |
|---|---|---|---|
| A. Directly recorded parameters | |||
| Frequency of breaths | Freq | breaths/min | Rate of breathing |
| Inspiratory time | Ti | s | Duration of inspiration |
| Expiratory time | Te | s | Duration of expiration |
| Tidal volume | TV | mL | Volume of inspired air per breath |
| Minute ventilation | MV = freq × TV | mL/min | Total volume of air inspired per min |
| Peak inspiratory flow | PIF | mL/s | Maximum inspiratory flow |
| Peak expiratory flow | PEF | mL/s | Maximum expiratory flow |
| Non-eupneic breathing index | NEBI | % | % of non-eupneic breaths per epoch |
| B. Derived parameters | |||
| Ti/Te | Ti/Te | none | Inspiratory quotient |
| Inspiratory drive | TV/Ti | mL/s | Central urge to inhale |
| Expiratory drive | TV/Te | mL/s | Central drive to exhale |
| PIF/PEF | PIF/PEF | none | Flow balance |
| NEBI/Freq | NEBI corrected for Freq | %/(breaths/min) | Expiratory ratio |
| Phase | Time (min) | Vehicle | L,D-DTT |
|---|---|---|---|
| Pre-drug | 37.4 ± 0.1 | 37.3 ± 0.1 | |
| Post drug | +1 min | 37.3 ± 0.1 | 37.3 ± 0.1 |
| +5 min | 37.4 ± 0.1 | 37.0 ± 0.2 | |
| +15 min | 37.3 ± 0.1 | 37.0 ± 0.1 *,† | |
| HH gas challenge | +15 min | 37.3 ± 0.1 | 36.9 ± 0.1 *,† |
| +30 min | 37.2 ± 0.2 | 36.9 ± 0.1 * | |
| +45 min | 37.1 ± 0.1 | 36.9 ± 0.1 * | |
| +60 min | 37.0 ± 0.1 * | 36.9 ± 0.1 * | |
| Post-HH gas challenge | +1 min | 37.0 ± 0.2 * | 36.9 ± 0.1 * |
| +5 min | 37.2 ± 0.2 | 37.1 ± 0.2 | |
| +15 min | 37.3 ± 0.1 | 37.3 ± 0.2 | |
| +30 min | 37.4 ± 0.2 | 37.3 ± 0.1 |
| Parameter | Group | Pre | Drug Max | Pre-HH | HH Max | Pre-RA | RA Max |
|---|---|---|---|---|---|---|---|
| Freq, breaths/min | VEH | 87 ± 4 | 105 ± 9 * | 82 ± 7 | 174 ± 8 * | 160 ± 6 * | 220 ± 12 * |
| DTT | 86 ± 6 | 144 ± 10 *,† | 83 ± 4 | 151 ± 8 *,† | 150 ± 6 * | 146 ± 14 *,† | |
| TV, mL | VEH | 2.90 ± 0.20 | 3.07 ± 0.21 | 2.99 ± 0.23 | 5.14 ± 0.25 * | 4.99 ± 0.26 | 3.71 ± 0.18 * |
| DTT | 2.78 ± 0.22 | 2.98 ± 0.19 | 2.72 ± 0.12 | 4.39 ± 0.14 *,† | 4.14 ± 0.14 * | 3.34 ± 0.15 * | |
| MV, mL/min | VEH | 249 ± 19 | 299 ± 20 * | 235 ± 21 | 843 ± 48 * | 797 ± 46 * | 806 ± 21 * |
| DTT | 239 ± 17 | 394 ± 18 * | 223 ± 8 | 645 ± 25 *,† | 619 ± 26 *,† | 486 ± 46 *,† | |
| Ti, s | VEH | 0.21 ± 0.01 | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.15 ± 0.01 * | 0.15 ± 0.01 | 0.12 ± 0.01 * |
| DTT | 0.22 ± 0.01 | 0.16 ± 0.01 *,† | 0.21 ± 0.01 | 0.17 ± 0.01 * | 0.18 ± 0.01 | 0.17 ± 0.01 *,† | |
| Te, s | VEH | 0.56 ± 0.08 | 0.48 ± 0.05 | 0.57 ± 0.05 | 0.20 ± 0.02 * | 0.21 ± 0.01 * | 0.17 ± 0.01 * |
| DTT | 0.55 ± 0.07 | 0.32 ± 0.02 *,† | 0.53 ± 0.06 | 0.24 ± 0.02 * | 0.23 ± 0.01 * | 0.25 ± 0.02 *,† | |
| Ti/Te, | VEH | 0.38 ± 0.02 | 0.43 ± 0.04 * | 0.37 ± 0.02 | 0.78 ± 0.02 * | 0.76 ± 0.02 * | 0.29 ± 0.01 * |
| DTT | 0.39 ± 0.012 | 0.52 ± 0.04 * | 0.43 ± 0.02 | 0.82 ± 0.02 * | 0.80 ± 0.02 * | 0.41 ± 0.04 *,† | |
| PIF, mLs/s | VEH | 22.0 ± 1.2 | 28.1 ± 1.7 * | 21.6 ± 1.6 | 46.9 ± 2.0 * | 45.1 ± 2.7 * | 41.7 ± 2.0 * |
| DTT | 21.4 ± 1.2 | 26.0 ± 1.3 * | 20.2 ± 0.8 | 32.6 ± 2.1 *,† | 31.7 ± 1.7 *,† | 29.4 ± 1.6 *,† | |
| PEF, mLs/s | VEH | 12.5 ± 0.9 | 16.3 ± 1.3 * | 12.3 ± 0.9 | 54.4 ± 3.1 * | 50.7 ± 2.9 * | 38.0 ± 2.1 * |
| DTT | 12.4 ± 0.6 | 15.7 ± 1.2 * | 12.4 ± 0.7 | 40.0 ± 2.3 *,† | 36.1 ± 1.4 *,† | 29.4 ± 2.1 *,† | |
| PIF/PEF | VEH | 1.79 ± 0.08 | 1.90 ± 0.11 | 1.68 ± 0.10 | 0.87 ± 0.04 * | 0.95 ± 0.04 * | 38.0 ± 2.1 * |
| DTT | 1.75 ± 0.11 | 1.80 ± 0.11 | 1.58 ± 0.10 | 0.80 ± 0.03 * | 0.96 ± 0.08 * | 29.4 ± 2.1 * |
| Parameter | Group | Pre | Drug Max | Pre-HH | HH Max | Pre-RA | RA Max |
|---|---|---|---|---|---|---|---|
| TV/Ti, mL/s | VEH | 13.7 ± 0.9 | 15.2 ± 1.1 | 13.8 ± 1.1 | 33.8 ± 1.8 * | 32.6 ± 2.1 * | 28.7 ± 2.2 * |
| DTT | 12.8 ± 0.6 | 16.9 ± 0.8 * | 12.9 ± 0.5 | 25.0 ± 0.9 *,† | 24.0 ± 1.2 *,† | 18.1 ± 1.5 *,† | |
| TV/Te, mL/s | VEH | 5.3 ± 0.4 | 6.5 ± 0.6 | 5.0 ± 0.4 | 24.2 ± 1.5 * | 24.2 ± 1.5 | 3.9 ± 0.3 * |
| DTT | 5.0 ± 0.2 | 8.7 ± 0.4 *,† | 5.3 ± 0.3 | 25.0 ± 0.9 * | 17.3 ± 0.6 *,† | 4.5 ± 0.2 | |
| NEBI, % | VEH | 3.46 ± 0.23 | 0.17 ± 0.17 * | 2.75 ± 0.77 | 0.92 ± 0.49 * | 1.92 ± 1.1 | 20.72 ± 1.82 * |
| DTT | 3.39 ± 0.06 | 0.40 ± 0.19 * | 3.58 ± 1.60 | 18.4 ± 0.6 * | 1.83 ± 0.64 | 3.52 ± 0.98 † | |
| NEBI/Freq, %/bpm | VEH | 4.05 ± 0.35 | 0.23 ± 0.23 * | 3.43 ± 0.65 | 0.70 ± 0.37 * | 1.21 ± 0.70 * | 13.51 ± 0.92 * |
| DTT | 3.99 ± 0.13 | 0.44 ± 0.20 * | 3.95 ± 0.76 | 0.68 ± 0.63 * | 0.85 ± 0.32 * | 5.18 ± 0.91 † |
| Time to Return to Pre-HH Levels (min) | ||
|---|---|---|
| Parameter | Vehicle | L,D-Dithiothreitol |
| Frequency, breaths/min | 16.3 ± 1.9 | 4.1 ± 1.0 * |
| Tidal Volume, mL | 15.2 ± 3.2 | 4.5 ± 0.9 * |
| Minute Volume, mL/min | 18.3 ± 1.4 | 5.6 ± 0.8 * |
| Inspiratory Time, s | 17.4 ± 2.4 | 3.4 ± 0.8 * |
| Expiratory Time, s | 4.0 ± 1.1 | 9.2 ± 1.3 * |
| Inspiratory Time/Expiratory Time | 7.6 ± 0.8 | 13.3 ± 1.1 * |
| Tidal Volume/Inspiratory Time, mL/s | 17.8 ± 1.5 | 1.9 ± 0.6 * |
| Tidal Volume/Expiratory Time, mL/s | 7.4 ± 0.6 | 16.2 ± 1.9 * |
| Peak Inspiratory Flow, mL/s | 18.6 ± 2.5 | 2.1 ± 0.4 * |
| Peak Expiratory Flow, mL/s | 14.5 ± 2.1 | 4.7 ± 0.9 * |
| Non-Eupneic Breathing Index (NEBI), % | 14.3 ± 1.7 | N/D * |
| NEBI, %/Freq, breaths/min | 14.7 ± 1.9 | N/D * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getsy, P.M.; Coffee, G.A.; May, W.J.; Baby, S.M.; Bates, J.N.; Lewis, S.J. The Reducing Agent Dithiothreitol Modulates the Ventilatory Responses That Occur in Freely Moving Rats during and following a Hypoxic–Hypercapnic Challenge. Antioxidants 2024, 13, 498. https://doi.org/10.3390/antiox13040498
Getsy PM, Coffee GA, May WJ, Baby SM, Bates JN, Lewis SJ. The Reducing Agent Dithiothreitol Modulates the Ventilatory Responses That Occur in Freely Moving Rats during and following a Hypoxic–Hypercapnic Challenge. Antioxidants. 2024; 13(4):498. https://doi.org/10.3390/antiox13040498
Chicago/Turabian StyleGetsy, Paulina M., Gregory A. Coffee, Walter J. May, Santhosh M. Baby, James N. Bates, and Stephen J. Lewis. 2024. "The Reducing Agent Dithiothreitol Modulates the Ventilatory Responses That Occur in Freely Moving Rats during and following a Hypoxic–Hypercapnic Challenge" Antioxidants 13, no. 4: 498. https://doi.org/10.3390/antiox13040498





