Abstract
Emerging evidence shows that the gut microbiota plays an important role in neuropathic pain (NP) via the gut–brain axis. Male rats were divided into sham, spinal nerve ligation (SNL), SNL + 200 mg GEG/kg BW (GEG200), and SNL + 600 mg GEG/kg BW (GEG600) for 5 weeks. The dosages of 200 and 600 mg GEG/kg BW for rats correspond to 45 g and 135 g raw ginger for human daily consumption, respectively. Both GEG groups mitigated SNL-induced NP behavior. GEG-supplemented animals had a decreased abundance of Rikenella, Muribaculaceae, Clostridia UCG-014, Mucispirillum schaedleri, RF39, Acetatifactor, and Clostridia UCG-009, while they had an increased abundance of Flavonifactor, Hungatella, Anaerofustis stercorihominis, and Clostridium innocuum group. Relative to sham rats, Fos and Gadd45g genes were upregulated, while Igf1, Ccl2, Hadc2, Rtn4rl1, Nfkb2, Gpr84, Pik3cg, and Abcc8 genes were downregulated in SNL rats. Compared to the SNL group, the GEG200 group and GEG600 group had increases/decreases in 16 (10/6) genes and 11 (1/10) genes, respectively. GEG downregulated Fos and Gadd45g genes and upregulated Hdac2 genes in the amygdala. In summary, GEG alleviates NP by modulating the gut microbiome and reversing a molecular neuroimmune signature.
1. Introduction
Neuropathic pain (NP) arises from damage to the peripheral or central nervous system (CNS) [1]. Nerve damage in NP leads to neuroinflammation and neuroplastic changes in the peripheral and central nervous systems (CNS) associated with sensitization and hyperexcitability [2]. The challenges of chronic NP are related to the complexity of NP symptoms (e.g., anxiety and depression), poor outcomes, and limited availability of treatment options. The most-used form of NP treatment is opioid analgesics; unfortunately, they can induce severe side effects and result in opioid use disorder [3].
Accumulating evidence suggests that the gut microbiome has a great impact on NP as an important element of the gut–brain axis via modulating neuroinflammation [4]. Gut dysbiosis has been implicated in the onset or progression of NP-associated behavior, such as pain sensitivity [5,6,7,8]. Gut dysbiosis is not only associated with marked changes in gut-associated immune cell activation in lymphoid tissues; it also exacerbates spinal inflammation/lesions, leading to impaired recovery of neurological function [9,10]. A recent systematic review with 19 eligible studies provides a rationale for targeting the microbiota for managing symptoms of neuropathy and neuroinflammation-based CNS disorders [11].
NP mechanisms include an imbalance between endogenous antioxidants and reactive oxygen species (ROS) after nerve injury, resulting in neuroimmune cross-talk [12] and neuroinflammation in the peripheral and CNS [13]. Therefore, the advancement of new, safe, and effective analgesic and anti-inflammatory alternatives is keenly desired. Ginger (Zingiber officinale Roscoe) consists of gingerols (6-gingerol, 8-gingerol, and 10-gingerol) and shogaols (6-shogaol, 8-shogaol, and 10-shogaol) that account for its anti-inflammatory properties [14]. Myriad ginger extract and its bioactive compounds have been investigated as anti-inflammatory agents; the length of their side chains influences their effectiveness [15]. Ginger and its bioactive components have been demonstrated to penetrate the blood–brain barrier via passive diffusion, suggesting the positive effects of ginger on the CNS [16].
Our previous work linked ginger’s anti-inflammatory and antioxidant properties to antinociception [17,18]. Single-dosage gingerol-enriched ginger (GEG) dietary supplementation significantly mitigated mechanical hypersensitivity in rats with spinal nerve ligation (SNL)-induced NP via, in part, (i) modulation of the gut microbiota and metabolites [17] and (ii) suppression of mRNA NF-κB and TNF-α expression in the amygdala and colon [18]. Here, we explored the effects of GEG on neuroimmune signaling with a focus on the amygdala for the following reasons. The amygdala has emerged as a key brain area for pain modulation and the affective component of pain [19], which, according to the International Association for the Study of Pain (IASP), is what defines pain [20]. Neuroplasticity in the amygdala has been linked to pain behaviors. While most research on underlying mechanisms has focused on neuronal mechanisms such as synaptic plasticity and hyperexcitability, recent evidence suggests that neuroimmune signaling contributes to pain mechanisms in the amygdala [18,21]. Finally, the amygdala has been recognized as a key region and hub for brain–gut interactions [22,23].
In the current study, we further investigated how two dosages of GEG administration via oral gavage affect 770 neuroinflammatory signature genes in the amygdala of SNL-treated animals, using the NanoString neuroinflammation panel. These neuroinflammation panels are designed to swiftly analyze important aspects of neuroimmune interactions for a thorough perspective of the complex relationship between immune and nervous systems. This neuroinflammation panel includes 23 pathways and processes that represent three core themes of neuroinflammation: stress and metabolism, immunity and inflammation, and neuropathology and neurobiology. In addition, we also conducted pain assessment and gut microbiome analysis in the cecal feces of the animals. Different from previous studies where GEG was delivered via diet at a single dosage [17,18], in the present study, GEG was given via oral gavage at two dosages (200 mg/kg and 600 mg/kg body weight daily) to evaluate if there was any response in the outcome parameters, namely, mechanical hypersensitivity, gut microbiome composition, and neuroinflammation signature gene expression.
We hypothesized that GEG administration would reduce mechanical hypersensitivity in a GEG-dose-dependent manner. Such changes in mechanical hypersensitivity would be mediated by (i) modification of the gene expression of three core themes of neuroinflammation and (ii) modulation of the gut microbiome composition with a greater abundance of beneficial microorganisms due to GEG administration. In this study, we combined a comprehensive evaluation of a neuroinflammation panel and gut microbiome abundance/composition to better understand the effects of ginger’s bioactive compounds on metabolic pathways relevant to NP in the development of personalized nutrition therapy for NP management.
2. Materials and Methods
2.1. Animals
Thirty-six male Sprague Dawley rats (4–5 weeks old, 150–180 g, Envigo, Cumberland, VA, USA) were individually housed under a 12 h light–dark cycle. All animals were given access to food and water ad libitum throughout the study period. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Texas Tech University Health Sciences Center (IACUC #20032). Food consumption, water intake, and body weights were recorded weekly.
2.2. Neuropathic Pain Induction
We employed a spinal nerve ligation (SNL) preclinical model to study the effects of ginger extract on NP progression. SNL leads to acute hypersensitivity within 1 week that persists for weeks [24] and prolonged changes in inflammatory and pronociceptive mediators, neurotransmitters, and receptor expression, leading to peripheral and central sensitization [25]. The SNL model was used to induce peripheral neuropathy in the left hind paw [2,26].
After a 5-day acclimatization, 9 animals received the sham procedure, while the remaining 27 animals received the SNL procedure. We used isoflurane [induction (3%) and maintenance (2%) of anesthesia] throughout the sham or SNL procedure. After removing the L5/L6-level paraspinal muscles and the underlying L6 transverse process, the L5 spinal nerve was separated from adjacent structures and tightly ligated with 6-0 silk thread. The paraspinal muscles were sutured closed, and the skin clipped together. Sham-operated animals served as controls for the NP model, receiving the same surgical procedure without the L5 spinal nerve ligation. After surgery, we applied ointment antibiotics (VetOne, Boise, ID, USA) to the surgery site until the staples were removed. Throughout the study period, we monitored the animals to reduce unnecessary stress or pain following the ethical guidelines of the International Association for the Study of Pain [27].
2.3. Animal Treatments
Thirty-six animals were randomly assigned into the sham + vehicle (corn oil) group (the sham group), SNL + vehicle (corn oil) group (the SNL group), SNL + 200 mg GEG/kg BW group (the SNL + GEG200 group), and SNL + 600 mg GEG/kg BW group (the SNL + GEG600 group). Both corn oil (vehicle) and GEG were administered by oral gavage for 4 weeks. All animals were given an AIN-93G diet (catalog number # D10012G, Research Diet, Inc., New Brunswick, NJ, USA). Prior studies that administered ginger extract to rats (concentrations ranging between 100 mg and 400 mg/kg BW) showed a decrease in inflammation in rats [28,29]. Thus, in this study, we tested GEG at both 200 and 600 mg/kg BW dosages in an NP model.
Ginger (Zingiber officinale) rhizomes were harvested, cleaned with water, and dried in the shade. Once dried, the ginger rhizomes were pulverized to a coarse powder form. The powdered ginger was subjected to supercritical fluid extraction to obtain a soft ginger extract standardized to 20% gingerols. Based on the results of gas chromatography–mass spectrometry, GEG consists of 18.7% 6-gingerol, 1.81% 8-gingerol, 2.86% 10-gingerol, 3.09% 6-shogoal, 0.39% 8-shogaol, and 0.41% 10-shogaol. GEG was a gift obtained from Sabinsa, Inc., East Windsor, NJ, USA.
2.4. Assessment of Pain-Related Behavior in Live Animals
We used the von Frey test to measure mechanical hypersensitivity [2]. In brief, we measured mechanical paw withdrawal thresholds (in grams) using an Electronic von Frey Aesthesiometer (IITC Life Science, Woodland Hills, CA, USA) with a plastic tip in an exclusive testing area for pain sensory assessment. The average of six measurements at least 30 s apart was calculated for each animal test.
2.5. Sample Collection
On collection day (after a 5-week feeding period), the animals were anesthetized and euthanized, and blood was drawn for plasma collection. The amygdala (right) and cecal feces were harvested, immersed in liquid nitrogen, and kept at −80 °C. We focused on the right amygdala because of previous evidence for the right hemispheric lateralization of pain plasticity and pain modulation in the amygdala [30,31,32].
2.6. RNA Isolation and Gene Expression Profiling Using Neuroinflammation Panel
We extracted total RNA from the amygdala using a Qiagen RNeasy Mini Kit (Cat # 74106, Qiagen Science, Germantown, MD, USA). We measured the purity/concentration of RNA using Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA) and stored RNA at −80 °C. We shipped amygdala RNA samples (20 ng/µL) to Cleveland Clinic, Cleveland, OH, USA, to perform gene expression profiling using the nCounter® neuroinflammation pathway panel (NanoString Technologies, Seattle, WA, USA). The neuroinflammation panel includes 757 genes covering the core pathways and processes that define neuroinflammation interactions and 13 potential housekeeping genes for normalization. RNA samples (100 ng per sample) were used for the Gene Expression Assay with a neuroinflammation panel using the nCounter MAX system, a multi-channel epifluorescence scanner with the NanoString Advanced Analysis Module plugin for quality control. We analyzed raw datasets using the ROSALIND® platform (https://rosalind.bio/ (accessed on 9 December 2022)). Sample gene transcript counts were normalized by dividing counts within a lane by the geometric mean of the normalizer probes from the same lane. Housekeeping probes for normalization were selected based on the geNorm algorithm using the NormqPCR R package (version 1.48) [33]. The abundance of various cell populations was calculated using the Nanostring Cell Type Profiling Module within ROSALIND. ROSALIND performs a filtering of Cell Type Profiling results to include results that have scores with a p-value greater than or equal to 0.05. Hypergeometric distribution was used to analyze the enrichment of pathways, gene ontology, domain structure, and other ontologies. NanoString annotation term enrichment was calculated relative to a set of background genes relevant to the experiment. Visualization was performed in R version 4.0.5 (Shake and Throw).
2.7. RNA Isolation and qRT-PCR
We validated the results of neuroinflammation gene profiling [FOS (Fos proto-oncogene-encoding proteins that form the AP-1 transcription factor complex), Gadd45g (growth arrest and DNA-damage-inducible 45 gamma), and HDAC2 (histone deacetylase 2)] using qRT-PCR. Extracted RNA was reversely transcribed into cDNA using the Maxima first-strand cDNA synthesis kit synthesis with dsDNase (Thermo Scientific, K1672, Waltham, MA, USA) on a thermal cycler Bio-rad S1000 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). qRT-PCR was performed on the Quant Studio 12K Flex real-time PCR system (Life Technologies, 4470689, Carlsbad, CA, USA) using samples cDNA for the amplification of target genes with β-actin as the control with Universal SYBR green supermix (Bio-rad Laboratories, Inc., 17251-24, Hercules, CA, USA). The following genes were tested: inflammation markers (FOS, Gadd45g, and HDAC2). The primer sequences used are below in Table 1. All gene expressions were normalized to our control β-actin. Gene expression was calculated by the following formula: 2-(ΔCT*1000) [34].

Table 1.
Primer sequences.
2.8. Gut Microbiota Profiling via 16S rRNA Amplicon Sequencing
Fecal DNA was isolated using the PowerFecal DNA isolation kit (Qiagen Inc., Germantown, MD, USA). Amplicon sequencing of the V4 variable region of the 16S rRNA gene was conducted at Molecular Research LP (Shallowater, TX, USA). Briefly, the V4 variable region was amplified using PCR primers 515F/806R. Samples were multiplexed and pooled together in equal proportions based on their molecular weight and DNA concentrations. Pooled samples were purified using calibrated Ampure XP beads, and then used in Illumina DNA library preparation. Sequencing was performed on a MiSeq. We deposited raw sequencing data under BioProject accession number PRJNA935472 in the National Center for Biotechnology Information (NCBI) BioProject database. The 16S rRNA gene sequencing data were analyzed using QIIME 2 [35]. In brief, reads were filtered, denoised, and merged. DADA2 was used to identify exact amplicon sequence variants (ASVs). For the taxonomy assignment, the Silva release 138 database was used [36,37]. To compare the relative abundance of taxa between groups, we performed compositional analysis using LOCOM, a logistic regression model for testing differential abundance in compositional microbiome data with false discovery rate control [38]. Results were regarded as significant when the p-value < 0.05, unless stated otherwise. Visualization was performed in R version 4.0.5 (codename “Shake and Throw”).
2.9. Statistical Analysis
The data were analyzed by a one-way ANOVA or two-way ANOVA (repeated measures where appropriate) followed by Tukey’s post hoc test using GraphPad Prism software version 9.0 (GraphPad Software, San Diego, CA, USA). The data were checked for normality (Gaussian distribution) before employing ANOVA. A significance level of p-value < 0.05 applies to all statistical tests. Statistical analyses for other types of data are stated in their corresponding sections above. For gut microbiota analysis, three pairwise comparisons are described as follows: SNL vs. sham, SNL + GEG200 vs. SNL, and SNL + GEG600 vs. SNL. All comparisons “Group 1 vs. Group 2” should be interpreted as “Group 1 relative to Group 2” in the text and figures.
3. Results
3.1. GEG Alleviates Mechanical Hypersensitivity in NP Rats
Figure 1 shows the effects of GEG supplementation on NP-associated mechanical hypersensitivity using the von Frey test. Relative to the sham group, the SNL group had significantly greater mechanical hypersensitivity at 1 week post-operation, which was sustained throughout the study period (Figure 1). At the end of the study (4 weeks after GEG supplementation began), both GEG SNL groups showed significantly attenuated pain sensitivity compared to untreated SNL rats, regardless of GEG dosages (Figure 1).
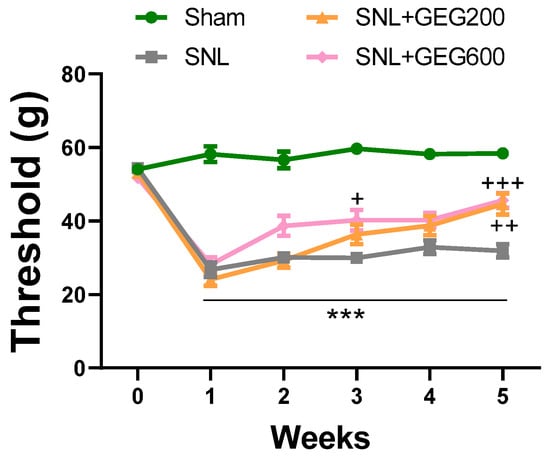
Figure 1.
Effects of GEG on mechanical hypersensitivity. Mechanical hypersensitivity of the left hind paw was assessed by an electronic von Frey anesthesiometer. Data expressed as mean ± SEM. n = 9 per group. SNL rats showed significantly decreased mechanical thresholds, indicating that SNL surgery induced pain-related hypersensitivity (*** p < 0.001 compared with sham, two-way ANOVA with Tukey’s multiple comparisons test, n = 9). SNL + GEG200-group rats and SNL + GEG600 group rats showed significantly increased mechanical thresholds (+ p < 0.05, ++ p < 0.01, +++ p < 0.001 compared with SNL, two-way ANOVA with Tukey’s multiple comparisons test, n = 9), indicating that GEG200 or GEG600 application reduced pain-related hypersensitivity.
3.2. GEG Reverses the Expression of Neuroinflammatory Markers Associated with NP
We examined the cell type, gene expression, and pathways involved in neuroinflammation and GEG effects and focused on the amygdala because it plays an important role in pain modulation [19]. To achieve this, we used the NanoString nCounter® Neuroinflammation Panel (NanoString Technologies, Inc., Seattle, WA, USA) to profile changes. One of the key outputs of the NanoString nCounter® Neuroinflammation Panel is to measure the relative abundance of 5 CNS cell types and 14 peripheral immune cell types with the unique cell-profiling feature. Based on the analysis performed within ROSALIND, three neuroinflammation cell types were identified, namely CD45+ peripheral immune cells, oligodendrocyte CNS cells, and astrocyte CNS cells (Figure 2). In general, oligodendrocytes CNS cells were the most abundant cell type (~50%) across all samples. However, the relative abundance of all three cell types was comparable between groups (ANOVA, p > 0.05).
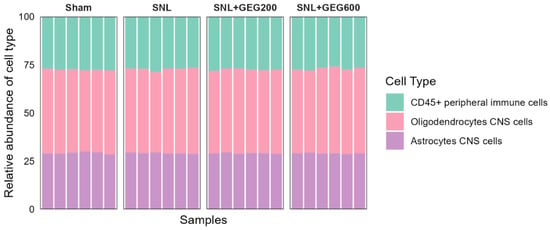
Figure 2.
Cell type profiles across groups per NanoString Neuroinflammation Panel analyzed using the ROSALIND platform. The y-axis displays the relative abundance of each cell type, while the x-axis represents samples of different groups. The colors of the bars indicate the different cell types.
Among the 770 gene expressions measured, many differentially expressed genes were identified in each comparison, i.e., SNL vs. sham, SNL + GEG200 vs. SNL, and SNL + GEG600 vs. SNL in volcano plots (Figure 3A–C) (fold change > 1.25 and p < 0.05). Ten genes (two increased and eight decreased) were differentially expressed in the amygdala of rats between the SNL and sham groups (Figure 3A). Compared to the sham group, the SNL group had higher gene expression levels of Gadd45g (growth arrest and DNA-damage-inducible 45 gamma) and Fos (FBJ osteosarcoma oncogene), while it had lower gene expression levels of Igf1, Ccl2, Hdac2, Rtn4rl1, Nfkb2, Gpr84, Pik3cg, and Abcc8 (Figure 3A).
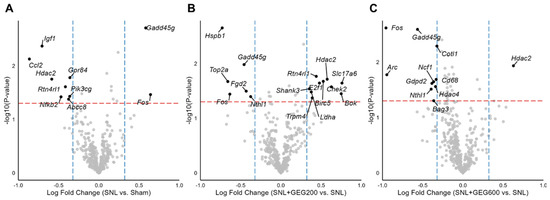
Figure 3.
Differential expression of neuroinflammation-related genes between groups. Volcano plots of (A) SNL vs. sham, (B) SNL + GEG200 vs. SNL, and (C) SNL + GEG600 vs. SNL show −log10(p-value) on the y-axis and log 2-fold change in gene expression on the x-axis. Statistical significance cutoffs are indicated by a red horizontal dashed line at a p-value of 0.05 and blue vertical dashed lines at a fold change of 1.25. Abbreviations: Abcc8, ATP-binding cassette subfamily C member 8; Arc, activity-regulated cytoskeleton-associated protein; Bag3, BAG cochaperone 3; Birc5, baculoviral IAP repeat-containing 5; Bok, BCL2 family apoptosis regulator; Ccl2, C-C motif chemokine ligand 2; Cd68, CD68 molecule; Chek2, checkpoint kinase 2; Cotl1, coactosin-like F-actin-binding protein 1; E2f1, E2F transcription factor 1; Fgd2, FYVE, RhoGEF and PH domain-containing 2; Fos, FBJ osteosarcoma oncogene; Gadd45g, growth arrest and DNA-damage-inducible 45 gamma; Gdpd2, glycerophosphodiester phosphodiesterase domain-containing 2; Gpr84, G protein-coupled receptor 84; Hdac2, histone deacetylase 2; Hdac4, histone deacetylase 4; Hspb1, heat shock protein family B (small) member 1; Igf1, insulin-like growth factor-I; Ldha, lactate dehydrogenase A; ncf1, neutrophil cytosolic factor 1; Nfkb2, nuclear factor kappa B subunit 2; Nthl1, nth-like DNA glycosylase 1; Pik3cg, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma; Rtn4rl1, reticulon 4 receptor-like 1; Shank3, SH3 and multiple ankyrin repeat domains 3; Slc17a6, solute carrier family 17 member 6; Top2a, DNA topoisomerase II alpha; Trpm4, transient receptor potential cation channel subfamily M member 4.
After identifying the molecular changes associated with SNL, we were interested to see if GEG200 and GEG600 could reverse these changes. GEG200 and GEG600 induced changes in the expression of 16 genes (Figure 3B) and 11 genes (Figure 3C), respectively. Relative to the vehicle-treated SNL group, GEG at a 200 mg/kg BW dosage led to an increase in the expression levels of 10 genes (namely, Slc17a6, Chek2, Bok, Hadc2, Birc5, Rtn4rl1, E2f1, Shank3, Ldha, and Trpm4) and downregulation of the expression levels of 6 genes (namely, Hspb1, Gadd45g, Top2a, Fgd2, Fos, and Nthl1) in the amygdala of SNL-operated animals (Figure 3B). Compared to the vehicle-treated SNL group, GEG at a 600 mg/kg BW dosage increased the expression level of 1 gene (i.e., Hadc2) and decreased the expression levels of 10 genes (i.e., Fos, Gadd45g, Cotl1, Ncf1, Cd68, Gdpd2, Nthl1, Hidac4, Bag3, and Arc) in the amygdala of SNL-treated animals (Figure 3C).
Next, we focused on the genes of common signatures between the GEG200 group and GEG600 group and those with a dose response associated with GEG concentrations. Relative to the SNL group, both the GEG200 and GEG600 groups showed downregulated Fos and Gadd45g in the amygdala of SNL rats, as shown in the log fold change of neuroinflammation (Figure 4A) and confirmed with qRT-PCR (Figure 4B). In contrast, both GEG200 and GEG600 groups showed increased Hdac2 gene expression in amygdala tissue from SNL rats compared to the vehicle-treated SNL group (Figure 4A,B). This suggests that GEG can at least partially reverse the molecular signature in the amygdala associated with neuropathic pain induced by SNL.
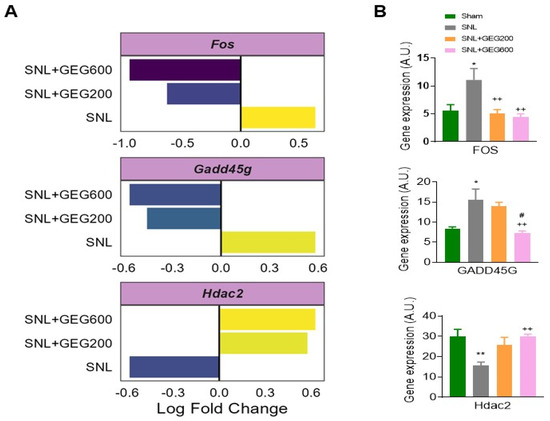
Figure 4.
Gene expression analysis of selected neuroinflammation factors, i.e., Fos, Gadd45g, and Hdac2 between groups (A); mRNA expression assessed by qRT-PCR (B). (A) Genes show differential expression between all groups, i.e., p-value < 0.05 and fold change > 1.25. Log2-fold change in gene expression is shown along the x-axis. SNL indicates fold change between SNL vs. sham, SNL + GEG200 indicates fold change between SNL + GEG200 vs. SNL, and SNL + GEG600 indicates fold change between SNL + GEG600 vs. SNL. (B) Fos, Gadd45g, and Hdac2 significantly increased in SNL rats (* p < 0.05, ** p < 0.01 one-way ANOVA, n = 9) compared to sham rats. SNL + GEG200-group rats showed decreased Fos expression (++ p < 0.01 one-way ANOVA, n = 9), but not Gadd45g and Hdac2 expression, compared to SNL rats. SNL + GEG600-group rats showed decreased Fos, Gadd45g, and Hdac2 expression (++ p < 0.01 one-way ANOVA, n = 9) compared to SNL rats. SNL + GEG600-group rats showed decreased Gadd45g expression (# p < 0.05 one-way ANOVA, n = 9) compared to SNL + 200-group rats. Abbreviation: Fos, FBJ osteosarcoma oncogene; Gadd45g, growth arrest and DNA-damage-inducible 45 gamma; Hdac2, histone deacetylase 2.
3.3. GEG-Associated Gut Microbiome Changes
The effects of GEG supplementation on the gut microbiome of animals are shown in Figure 5. The average sequencing depth per sample was ~524,000 reads. Around 51,000 non-chimeric reads were retained after filtering, denoising, and then merging. First, we examined gut microbiome alpha-diversity. Gut microbiome species (ASV) evenness and richness did not differ between the sham group and the SNL group (Figure 5A). Gut microbiome evenness and richness in GEG-supplemented groups were lower than in the vehicle-treated SNL group. Both the SNL + GEG200 and SNL + GEG600 groups showed significantly lower evenness, but only the SNL + GEG600 group showed significantly lower diversity (Figure 5A) (Wilcoxon signed-rank test, p < 0.05).
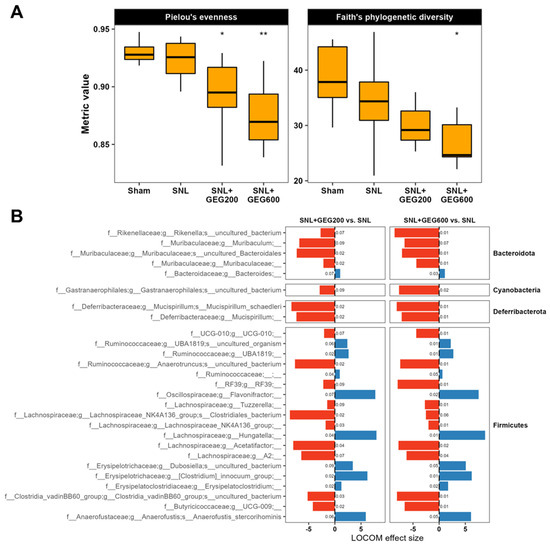
Figure 5.
Gut microbiome composition analysis in SNL rats. (A) Alpha-diversity evenness and richness are indicated across groups using box plots. Asterisks (* p < 0.05, ** p < 0.01) indicate statistical significance for SNL + GEG200 vs. SNL and SNL + GEG600 vs. SNL comparisons using the Wilcoxon signed-rank test. (B) SNL + GEG200 vs. SNL, SNL + GEG600 vs. SNL compositional microbiome analysis using LOCOM. ASVs presented are those with raw p-values < 0.05 and Benjamini–Hochberg-adjusted p-values < 0.1. LOCOM effect size is indicated on the x-axis and ASVs are indicated on the y-axis. Benjamini–Hochberg-adjusted p-values are indicated next to the corresponding bars.
Next, we aimed to find species associated with SNL and GEG treatments. We performed compositional analysis using LOCOM, a logistic regression model for testing differential abundance in compositional microbiome data with false discovery rate control. Overall, compared to the sham group, the SNL group had only minute changes in the gut microbiome composition and ASV abundance, and none of these changes were statistically significant after false discovery rate control (Benjamini–Hochberg Procedure-adjusted p-value > 0.1). Thus, we focused on changes associated with both GEG doses in the SNL groups (SNL + GEG200 vs. SNL and SNL + GEG600 vs. SNL) (Figure 5B) (Benjamini–Hochberg Procedure-adjusted p-value < 0.1). GEG supplementation significantly decreased the abundance of ASVs of f_Rikenellaceae and f_Muribaculaceae in Bacteroidota phyla; g_Gastranaerophilales in Cyanobacteria phyla; and g_Mucispirilum in Deferribacterota phyla of cecal feces of NP rats. Remarkably, relative to the vehicle-treated SNL group, both the SNL + GEG200 and SNL + GEG600 groups had an increased abundance of 10 ASVs of the taxa in Firmicutes phyla, such as UBA1819, Flavonifractor, Hungatella, Clostridium innocuum group, Erysipelatoclostridium, and Anaerofustis stercorihominis (Figure 5B). In contrast, the 17 ASVs of the taxa in Firmicutes phyla were decreased in the GEG-supplemented rats compared to the vehicle-treated SNL rats, for instance, Rikenella, Muribaculaceae, Gastranaerophilales, Clostridia UCG-010, Mucispirillum schaedleri, RF39, Acetatifactor, Clostridia, and UCG-009 (Figure 5B).
3.4. Integrated Analysis of Pain, Neuroinflammatory Markers, and the Gut Microbiome
We addressed whether NP sensitivity (mechanical hypersensitivity, VFT measurement) is associated with neuroinflammation-related genes and gut microbiome species. Because of the complexity of the collected data and identifying potential mechanisms involving the microbiome–gut–brain axis, we employed a network analysis approach based on Spearman’s correlation coefficient between all three factors, with a focus on identifying the genes and microbiome ASVs that are strongly associated with mechanical hypersensitivity (Spearman’s rank correlation coefficient > 0.6 and p < 0.01). Figure 6 shows the exploratory results of network analysis of the correlated paw withdrawal threshold (pain mechanical hypersensitivity), neuroinflammation genes, and gut microbiome ASVs in animals. For example, we found that a higher paw withdrawal threshold (less mechanical hypersensitivity) was positively correlated with the expression of Cd300lf and the abundance of Ruminococcaceae_UBA1819 and Flavonifractor (Figure 6). Moreover, a higher paw withdrawal threshold (less mechanical hypersensitivity) was negatively correlated with the abundance of UGC-010, Lachnospiraceae_FCS020_group, and Bacteroides massiliensis (Figure 6).
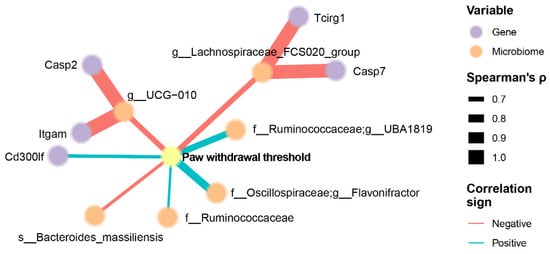
Figure 6.
Association between mechanical hypersensitivity, neuroinflammation genes of amygdala, and gut microbiome of cecal feces in GEG-supplemented NP rats. Data were obtained from male SNL rats given GEG at 200 and 600 mg/kg doses daily for 4 weeks. The pain withdrawal threshold was assessed by the von Frey Test. The neuroinflammation gene profile in the amygdala was assessed using the NanoString Neuroinflammation Panel. Gut microbiome composition was assessed by 16S rRNA amplicon sequencing. We employed a network analysis approach with a focus on identifying the genes and microbiota ASVs that are strongly associated with pain withdrawal threshold, determined by Spearman’s rank correlation coefficient >0.6 and p < 0.01. Only nodes with edges linked to pain sensitivity were retained in the network. Line thicknesses indicate the strength of the correlation.
4. Discussion
In the present study, the SNL-induced NP model was successfully employed to examine the effects of two GEG dosages on mechanical hypersensitivity, neuroinflammation/neuroimmune signature genes in the amygdala, and gut microbiome composition in male rats. Both GEG dosages via oral gavage attenuated mechanical hypersensitivity in the SNL-operated animals, independent of GEG doses, which agrees with our previous study with one GEG dosage through dietary supplementation [17,18]. The lack of GEG dose response in this study is also consistent with a previous study showing that the rhizome of Zingiber officinale roscoe (Z. officinale, ginger) at 100 and 500 mg/kg p.o. mitigated oxaliplatin-treated mechanical allodynia in mice, regardless of Zingiber officinale dosages [39]. This study shows for the first time that dietary administration of GEG modulates the neuroinflammation signature genes of the amygdala and gut microbiome composition of male rats with NP, though dose effects of GEG on gut microbiome composition are limited. The data on neuroinflammation genes and the gut microbiome provide strong evidence for the pain-mitigating effects of GEG supplementation in animals with NP through the modulation of the microbiota–CNS connection.
The current study compared, for the first time, gene expression profiles in the amygdala between SNL animals with and without GEG administration and identified two (Fos and Gadd45g) of three co-expression genes mainly involved in the mitogen-activated protein kinase (MAPK) signaling pathway [40]. Fos, an immediate early gene, is considered a general neuronal activity marker, a cause of pain-related increases in neuronal activity. The present study found Fos upregulation in the amygdala of SNL rats, which is consistent with previous work showing c-FOS gene and protein upregulation in brain regions in different NP animal models [41,42,43], as well as in the amygdala after spinal neural transection [42]. The change in c-Fos after spinal nerve injury triggers the production of TNF-α in the anterior cingulate cortex [44]; however, it remains to be determined whether this signaling mechanism is also engaged in the amygdala to contribute to mechanical hypersensitivity during the development of NP. In the present study, supplementation of GEG suppressed the gene expression of Fos in the amygdala, providing an explanation of how GEG mitigates mechanical hypersensitivity in NP.
The current study agrees with Li’s, which showed the upregulation of Gadd45A in an SNL model [45]. Gadd45g belongs to the Gadd45 family, which is known to be induced by a myriad of physiological stresses, including irradiation, ultraviolet radiation, and inflammatory cytokines [46,47,48]. The protein encoded by the Gadd45 gene responds to environmental/physiological stresses by mediating the activation of the p38/JNK pathway via MTK1/MEKK4 kinase [49,50]. Gadd45g genes are associated with neural cell injury and death, and Gadd45g is associated with core promoter lesions hypermethylated in NP development [51]. Gadd45g participates in activity-induced neurogenesis by decreasing site-specific DNA methylation in the brain [52,53]. On the other hand, Gadd45g interacts with and inhibits the kinase activity of the Cdk1/CyclinB1 complex [54], suggesting an important role in the progression from the G2 to M phase of the cell cycle [55]. In the present study, our findings of elevated Gadd45g expression in the amygdala of SNL rats agree with increased Gadd45β in the spinal dorsal horn after SNL surgery in rats [56], in the anterior cingulate cortex after sciatic nerve injury in rats [57], and increased Gadd45g protein expression in human nucleus pulposus cells isolated from advanced stages of intervertebral disc degeneration (a form of NP) [50]. In the present study, administration of GEG suppressed the gene expression of Gadd45g in the amygdala, suggesting a molecular mechanism by which GEG mitigates mechanical hypersensitivity in NP.
Hdac2 is involved in transcriptional regulation, cell cycle progression, and chronic NP development [58,59]. The reported changes in Hdac2 expression are very complex and controversial in different pain models, including arthritis pain [60], neuropathic pain [61,62,63,64,65,66], visceral pain [67,68,69], and bone cancer pain [38,70]. We found that lower Hdac2 expression in the amygdala of SNL rats agrees with previous finding that SNL procedures diminish Hdac2 occupancy in the dorsal root ganglion (DRG) of rats [59]. Excess α2δ-1 proteins produced after SNL injury directly interact with glutamate NMDA receptors to intensify synaptic NMDA receptor activity in the spinal cord, a prominent component of nerve discomfort. Because α2δ-1 upregulation after nerve damage is long lasting, gabapentinoids only temporarily relieve pain symptoms. Then, Hdac2 functions as a pivotal transcriptional repressor of NP via suppressing Cacna2d1 promoter expression in the DRG [59]. Hdac2 knockdown or conditional knockout in DRG neurons in male and female mice regularly induced long-lasting mechanical pain hypersensitivity. In the present study, we reported that GEG-supplemented SNL rats had elevated Hdac2 expression in the amygdala, further corroborating a previous study that restoring the repressive Hdac2 function and/or reducing histone acetylation at the α2δ-1 gene promoter in primary sensory neurons could lead to long-term nerve pain relief [59].
The approach of regulating gut microbiomes to affect nervous system function represents a new idea for the treatment of NP using bioactive compounds, such as GEG. Accumulating evidence from published work by our group and others [17,61,71] may indicate the gut microbiota’s impact on NP. Our findings show that “potential pro-inflammatory” taxa, such as Rikenellaceae [72], Muribaculaceae [73], Mucispirillum [74], Rikenella [75], Gastranaerophilales [76], RF39 [77], Acetatifactor [78], and UGC-009 [79], are decreased in the GEG-supplemented NP rats and support the anti-inflammatory function of GEG in pain mitigation via the modification of gut microbiome composition [74]. In the current study, GEG-treated animals had an increased abundance of Anaerofustis stercorihominis and Hungatella in cecum feces due to their anti-inflammatory potential. Anaerofustis stercorihominis has effects for treating or preventing inflammation-related diseases, such as inflammatory bowel diseases (ulcerative enteritis, gastritis, and general enteritis) and rheumatoid arthritis (WIPO). The dietary inflammatory index is inversely correlated with the relative abundance of the Hungatella group [80].
The relationship between gut microbiota and signature gene expression in the amygdala due to GEG supplementation in the context of NP is likely complex. The gut microbiome participates in the metabolism of GEG, and its modulation by GEG supplementation could influence the activity of neuroinflammation genes in the amygdala, resulting in a reduction in mechanical hypersensitivity in animals with NP status. The combination of 16S rRNA gene sequencing and signature neuroinflammation gene analysis in the amygdala can overcome the limitations of single omics to a certain extent. In the present study, the fact that a decreased hypersensitivity level was associated with increased Cd300lf gene expression in rats with chronic NP may result from its impeding role in neuroinflammation [81] and may have beneficial effects on amygdala activity in NP. Keswani et al. reported that CD300f, belonging to a family of Ig-like-encoding genes, is a potential candidate associated with cerebral malaria resistance, and the expression of CD300lf by microglia strengthens resistance to cerebral malaria by impeding neuroinflammation [81].
This study showed that increased abundance of Ruminoccaceae_UBA1819 and Flavonifractor is associated with reduced hypersensitivity in GEG-supplemented NP rats, in part, due to GEG’s anti-inflammatory response to increased Ruminoccaceae [82] and Flavonifractor [83]. Furthermore, lessened hypersensitivity was accompanied by a decreased abundance of Lachnospiraceae_FCS020_group in the NP rats, which is linked to impaired glucose metabolism and inflammation in type 1 diabetes [84] and may be due to a reduced inflammatory response thanks to GEG. Less hypersensitivity was also negatively associated with the abundance of Bacteroides massiliensis in GEG-supplemented NP rats. Bacteroides massiliensis was negatively correlated with IL-23 in rats with ulcerative colitis [85]. IL-23 is a member of the IL-12 family of cytokines with pro-inflammatory properties [86], suggesting that the beneficial effects of GEG involve mitigation of the pro-inflammatory potential of Bacteroides massiliensis [85]. The main metabolites of GEG are glucuronide or sulfate conjugates, which may indirectly interact with the gut microbiome of NP rats [87]. Glucuronide conjugates (the metabolites of GEG) likely provide a significant energy source to mammalian GI microbiomes to deactivate endobiotic and xenobiotic compounds for GI excretion [87]. In the GI tract, the microbiota prepares β-glucuronidase enzymes, which remove glucuronic acid as a carbon source, effectively reversing the actions of mammalian intestinal dysbiosis [88]. In the present study, SNL-induced dysbiosis was reversed by GEG supplementation in NP rats, likely due to the action of GEG metabolites.
We noted that this study only used males, and future studies are needed to explore if similar gut–brain interactions and GEG effects are observed in females. The present study provides the rationale for these important but more complex experiments. How the microbiome affects pain pathways is an important knowledge gap. Our study provides the basis and rationale for more research in this area to decipher underlying mechanisms and pathways. While we hypothesize that the gut microbiota plays a role in modulating pain sensitivity, this is most likely to be partial and does not fully explain the complex change in pain sensitivity as other host factors contribute significantly to the phenotype. A contribution of microbiota cannot be ruled out even though there was no detectable change because microbiota could have different functional consequences in the changed environment of the chronic pain condition. Disentangling this complex relationship is very interesting, and we hope the present study will stimulate that line of research. Incorporating cause-and-effect experiments in future studies would significantly enhance the impact of the results reported here. Furthermore, in this study, while it is reasonable to emphasize the investigation of genes (namely, Fos, Gadd45g, Hdac2) shared among distinct groups with connections between neuroimmune signaling and ginger dose response, future studies are warranted to broaden the spectrum to the other genes presented in Figure 3B, 3C that were influenced by ginger. Exploring these genes may provide further insight into the mechanisms of action of GEG.
5. Conclusions
Administration of GEG dosages to neuropathic (SNL model) animals decreased mechanical hypersensitivity (no GEG-dose response) and modified the gut microbiome composition (limited GEG dose response). GEG reversed SNL-induced signature genes of neuroinflammation with differential dose response. The data suggest the prebiotic potential of dietary ginger root intake in the management of NP.
Author Contributions
C.-L.S., J.M.S., M.M.E., G.J. and V.N. contributed to the conceptualization of the study, data interpretation, and manuscript preparation. J.M.S. performed pain behavioral assessments and data analysis, mRNA extraction for the neuroinflammation panel, and fecal DNA extraction for gut microbiome analysis. M.M.E. contributed to data analysis, interpretation, and the neuroinflammation panel and microbiome sequencing. Z.D. and V.B. provided the daily oral gavage of ginger and performed weekly food, water, and body weight measurements. G.J., V.Y., T.K. and J.L. contributed to sample collection and follow-up data interpretation. A.N.H. and S.S. contributed to manuscript editing. C.-L.S. and V.N. finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Food and Human Health (Program Code: A1343, Grant no. 2021-67017-34026, and Project Accession no. 1025432) from the USDA National Institute of Food and Agriculture (Shen and Neugebauer). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture. Support was also provided by the National Institutes of Health (NIH) grant R01NS038261 (Neugebauer).
Institutional Review Board Statement
No applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Yakhnitsa, V.; Kiritoshi, T.; Presto, P.; Neugebauer, V. Fear extinction learning ability predicts neuropathic pain behaviors and amygdala activity in male rats. Mol. Pain 2018, 14, 1744806918804441. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhou, Z.; Liang, Y.; Cheng, X.; Li, Y.; Teng, W.; Zhao, M.; Liu, C.; Guan, M.; Zhao, C. Targeting strategies for chemotherapy-induced peripheral neuropathy: Does gut microbiota play a role? Crit. Rev. Microbiol. 2019, 45, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Han, W.; Lamine, F.; Eutamene, H.; Fioramonti, J.; Bueno, L.; Theodorou, V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: A possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006, 55, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: A novel regulator of pain. J. Neural Transm. 2020, 127, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Hall, J.C.; Wang, L.; Mo, X.; Yu, Z.; Popovich, P.G. Gut dysbiosis impairs recovery after spinal cord injury. J. Exp. Med. 2016, 213, 2603–2620. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Mostacada, K.; Popovich, P.G. Gut Microbiota Are Disease-Modifying Factors After Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Boccella, S.; Guida, F.; Franzese, M.; Maione, S.; Salvatore, M. Role of gut microbiota in neuropathy and neuropathic pain states: A systematic preclinical review. Neurobiol. Dis. 2020, 170, 105773. [Google Scholar] [CrossRef] [PubMed]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: Focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol. Res. 2020, 162, 105280. [Google Scholar] [CrossRef] [PubMed]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Darcsi, A.; Kery, A.; Riethmuller, E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, R.; Ji, G.; Elmassry, M.M.; Zabet-Moghaddam, M.; Vellers, H.; Hamood, A.N.; Gong, X.; Mirzaei, P.; Sang, S.; et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J. Nutr. Biochem. 2022, 100, 108904. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Wang, R.; Yakhnitsa, V.; Santos, J.M.; Watson, C.; Kiritoshi, T.; Ji, G.; Hamood, A.N.; Neugebauer, V. Gingerol-Enriched Ginger Supplementation Mitigates Neuropathic Pain via Mitigating Intestinal Permeability and Neuroinflammation: Gut-Brain Connection. Front. Pharmacol. 2022, 13, 912609. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V. Amygdala physiology in pain. Handb. Behav. Neurosci. 2020, 26, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Presto, P.; Ji, G.; Ponomareva, O.; Ponomarev, I.; Neugebauer, V. Hmgb1 Silencing in the Amygdala Inhibits Pain-Related Behaviors in a Rat Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 11944. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.S.M.; Hoban, A.E.; Ventura-Silva, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. Bioessays 2018, 40, 1700172. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Kim, H.K.; Chung, K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol. Med. 2004, 99, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.J.; Chung, J.M.; Honore, M.; Seltzer, Z. Models of neuropathic pain in the rat. Curr. Protoc. Pharmacol. 2003, 21, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, W.; Mahimainathan, L.; Narasimhan, M.; Kiritoshi, T.; Fan, X.; Wang, J.; Green, T.A.; Neugebauer, V. 5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors. J. Neurosci. 2017, 37, 1378–1393. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; McGrath, K.C.; Nammi, S.; Heather, A.K.; Roufogalis, B.D. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin. Pharmacol. Toxicol. 2012, 110, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.F.; Abdallah, H.M.I.; Ibrahim, B.M.M.; Hegazy, R.R.; Esmail, R.S.E.; Abdel-Salam, L.O. The Carcinogenic Agent Diethylnitrosamine Induces Early Oxidative Stress, Inflammation and Proliferation in Rat Liver, Stomach and Colon: Protective Effect of Ginger Extract. Asian Pac. J. Cancer Prev. 2019, 20, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.N.; Bobnar, H.J.; Kolber, B.J. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol. 2021, 196, 101891. [Google Scholar] [CrossRef] [PubMed]
- Carrasquillo, Y.; Gereau, R.W.T. Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol. Pain 2008, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Neugebauer, V. Hemispheric lateralization of pain processing by amygdala neurons. J. Neurophysiol. 2009, 102, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.R.; Dawes, J.M.; McMahon, S.B.; Bennett, D.L.; Orengo, C.; Kohl, M. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genom. 2012, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; He, X.T.; Zhou, K.X.; Zhang, C.; Zhao, W.J.; Zhang, T.; Li, J.L.; Deng, J.P.; Dong, Y.L. The analgesic effects of triptolide in the bone cancer pain rats via inhibiting the upregulation of HDACs in spinal glial cells. J. Neuroinflamm. 2017, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale Roscoe Rhizomes Attenuate Oxaliplatin-Induced Neuropathic Pain in Mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Belluscio, L.M.; Alberca, C.D.; Pregi, N.; Canepa, E.T. Altered gene expression in hippocampus and depressive-like behavior in young adult female mice by early protein malnutrition. Genes Brain Behav. 2016, 15, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Morland, R.H.; Novejarque, A.; Spicer, C.; Pheby, T.; Rice, A.S. Enhanced c-Fos expression in the central amygdala correlates with increased thigmotaxis in rats with peripheral nerve injury. Eur. J. Pain. 2016, 20, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Watanabe, Y.; Ikeda, T.; Abe, H.; Ebihara, K.; Matsuo, H.; Nonaka, H.; Hashiguchi, H.; Nishimori, T.; Ishida, Y. Analgesic effect of milnacipran is associated with c-Fos expression in the anterior cingulate cortex in the rat neuropathic pain model. Neurosci. Res. 2009, 64, 380–384. [Google Scholar] [CrossRef]
- Yao, P.W.; Wang, S.K.; Chen, S.X.; Xin, W.J.; Liu, X.G.; Zang, Y. Upregulation of tumor necrosis factor-alpha in the anterior cingulate cortex contributes to neuropathic pain and pain-associated aversion. Neurobiol. Dis. 2019, 130, 104456. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, P.; Fan, S.; Zhai, B.; Li, S.; Li, H.; Zhang, Y.; Li, W.; Sun, G.; Han, R.; et al. miR-30a-3p can inhibit the proliferation and promote the differentiation of chicken primary myoblasts. Br. Poult. Sci. 2022, 63, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S.K.; Balliet, A.G.; Hollander, M.C.; Fornace, A.J.; Hoffman, B.; Liebermann, D.A. Hematopoietic cells from Gadd45a- and Gadd45b-deficient mice are sensitized to genotoxic-stress-induced apoptosis. Oncogene 2005, 24, 7170–7179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Gupta, M.; Hoffman, B.; Liebermann, D.A. Hematopoietic cells from gadd45a-deficient and gadd45b-deficient mice exhibit impaired stress responses to acute stimulation with cytokines, myeloablation and inflammation. Oncogene 2006, 25, 5537–5546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liebermann, D.A.; Hoffman, B. Gadd45 in stress signaling. J. Mol. Signal. 2008, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Cretu, A.; Sha, X.; Tront, J.; Hoffman, B.; Liebermann, D.A. Stress sensor Gadd45 genes as therapeutic targets in cancer. Cancer Ther. 2009, 7, 268–276. [Google Scholar] [PubMed]
- Kawaguchi, K.; Akeda, K.; Yamada, J.; Hasegawa, T.; Takegami, N.; Fujiwara, T.; Sudo, A. Expression of GADD45G and CAPRIN1 in Human Nucleus Pulposus: Implications for Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 5768. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, A.; Akeda, K.; Takebayashi, S.I.; Shimaoka, M.; Okumura, K.; Sudo, A. Genome-wide analysis of DNA methylation profile identifies differentially methylated loci associated with human intervertebral disc degeneration. PLoS ONE 2019, 14, e0222188. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Schafer, A.; Marhold, J.; Stach, D.; Swaminathan, S.K.; Handa, V.; Doderlein, G.; Maltry, N.; Wu, W.; Lyko, F.; et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 2007, 445, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Guo, J.U.; Ming, G.L.; Song, H. DNA excision repair proteins and Gadd45 as molecular players for active DNA demethylation. Cell Cycle 2009, 8, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Vairapandi, M.; Balliet, A.G.; Hoffman, B.; Liebermann, D.A. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J. Cell Physiol. 2002, 192, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.J. Cell cycle checkpoints: Preventing an identity crisis. Science 1996, 274, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Hsieh, M.C.; Ho, Y.C.; Lee, A.S.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Peng, H.Y. Growth Arrest and DNA-damage-inducible Protein 45beta-mediated DNA Demethylation of Voltage-dependent T-type Calcium Channel 3.2 Subunit Enhances Neuropathic Allodynia after Nerve Injury in Rats. Anesthesiology 2017, 126, 1077–1095. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Liu, D.; Du, J.; Guo, Y.; Deng, Y.; Hei, Z.; Li, X. Early molecular alterations in anterior cingulate cortex and hippocampus in a rodent model of neuropathic pain. Brain Res. Bull. 2021, 166, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Geranton, S.M.; Tochiki, K.K. Regulation of gene expression and pain states by epigenetic mechanisms. Prog. Mol. Biol. Transl. Sci. 2015, 131, 147–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, S.R.; Zhou, M.H.; Jin, D.; Chen, H.; Wang, L.; DePinho, R.A.; Pan, H.L. HDAC2 in Primary Sensory Neurons Constitutively Restrains Chronic Pain by Repressing alpha2delta-1 Expression and Associated NMDA Receptor Activity. J. Neurosci. 2022, 42, 8918–8935. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yamamura, S.; Essilfie-Quaye, S.; Cosio, B.; Ito, M.; Barnes, P.J.; Adcock, I.M. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J. Exp. Med. 2006, 203, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Y.; Ren, X.; Rong, L.; Huang, M.; Cao, J.; Zang, W. HDAC2, but not HDAC1, regulates Kv1.2 expression to mediate neuropathic pain in CCI rats. Neuroscience 2019, 408, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Maiaru, M.; Morgan, O.B.; Tochiki, K.K.; Hobbiger, E.J.; Rajani, K.; Overington, D.W.; Geranton, S.M. Complex regulation of the regulator of synaptic plasticity histone deacetylase 2 in the rodent dorsal horn after peripheral injury. J. Neurochem. 2016, 138, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhou, X.; Ji, T.; Chen, G. NF-kappaB p65-dependent transcriptional regulation of histone deacetylase 2 contributes to the chronic constriction injury-induced neuropathic pain via the microRNA-183/TXNIP/NLRP3 axis. J. Neuroinflamm. 2020, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Chen, D.; Hou, X.; Wang, T.; Wang, J.; Zou, W.; Song, Z.; Huang, C.; Guo, Q.; Weng, Y. Normalizing HDAC2 Levels in the Spinal Cord Alleviates Thermal and Mechanical Hyperalgesia After Peripheral Nerve Injury and Promotes GAD65 and KCC2 Expression. Front. Neurosci. 2019, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Pryce, K.D.; Serafini, R.A.; Ramakrishnan, A.; Nicolais, A.; Giosan, I.M.; Polizu, C.; Torres-Berrio, A.; Vuppala, S.; Kronman, H.; Ruiz, A.; et al. Oxycodone withdrawal induces HDAC1/HDAC2-dependent transcriptional maladaptations in the reward pathway in a mouse model of peripheral nerve injury. Nat. Neurosci. 2023, 26, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liu, C.; Wan, Y.; Yan, H.; Li, T. Effect of HDAC2/Inpp5f on neuropathic pain and cognitive function through regulating PI3K/Akt/GSK-3beta signal pathway in rats with neuropathic pain. Exp. Ther. Med. 2019, 18, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Shi, X.; Tang, Y.; Yan, Y.; Chen, L.; Chen, Y.; Gao, G.; Lin, C.; Chen, A. Contribution of Amygdala Histone Acetylation in Early Life Stress-Induced Visceral Hypersensitivity and Emotional Comorbidity. Front. Neurosci. 2022, 16, 843396. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Shao, A.; Tang, X.; Feng, M.; Wang, J.; Qiu, Y. MiR-34a affects dexmedetomidine-inhibited chronic inflammatory visceral pain by targeting to HDAC2. BMC Anesthesiol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Wang, J.; Wei, Y.Y.; Zhang, T.; Zhang, Y.; Zuo, Z.F.; Teng, X.Y.; Li, Y.Q. Histone deacetylase 2 is involved in micro-opioid receptor suppression in the spinal dorsal horn in a rat model of chronic pancreatitis pain. Mol. Med. Rep. 2018, 17, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Weng, Y.; Wang, T.; Ouyang, B.; Li, Y.; Song, Z.; Pan, Y.; Zhang, Z.; Zou, W.; Huang, C.; et al. Suppression of HDAC2 in Spinal Cord Alleviates Mechanical Hyperalgesia and Restores KCC2 Expression in a Rat Model of Bone Cancer Pain. Neuroscience 2018, 377, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Brownstone, N.; Reddy, V.; Chan, S.; Thibodeaux, Q.; Truong, A.; Bhutani, T.; Chang, H.W.; Liao, W. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101494. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, X.; Wu, R.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.; Liu, Y.; Ren, B.; Li, J.; et al. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Mathes, T.; Martens, E.C.; Kamada, N.; Nusrat, A.; Inohara, N.; Nunez, G. A specific gene-microbe interaction drives the development of Crohn’s disease-like colitis in mice. Sci. Immunol. 2019, 4, eaaw4341. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.N.; Sun, Z.F.; Li, X.Y.; Zhang, X.D.; Jin, Z.X.; Zhang, C.; Zhang, Y.; Wang, H.Y.; Huang, N.N.; Jiang, J.H.; et al. Alterations in gut microbiota are related to metabolite profiles in spinal cord injury. Neural Regen. Res. 2023, 18, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Gong, P.; Shi, R.; Chen, W.; Wang, C.; Zhang, C.; Wu, Z. Syringic Acid Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota. J. Agric. Food Chem. 2023, 71, 8458–8470. [Google Scholar] [CrossRef] [PubMed]
- Guzzardi, M.A.; La Rosa, F.; Campani, D.; Cacciato Insilla, A.; De Sena, V.; Panetta, D.; Brunetto, M.R.; Bonino, F.; Collado, M.C.; Iozzo, P. Maturation of the Visceral (Gut-Adipose-Liver) Network in Response to the Weaning Reaction versus Adult Age and Impact of Maternal High-Fat Diet. Nutrients 2021, 13, 3438. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Han, L.; Wang, B.; Shi, R.; Ye, J.; Xia, B.; Zhao, Z.; Zhao, B.; Liu, X. Leucine-Restricted Diet Ameliorates Obesity-Linked Cognitive Deficits: Involvement of the Microbiota-Gut-Brain Axis. J. Agric. Food Chem. 2023, 71, 9404–9418. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, S.; Jin, L.; Zhang, W.; Liu, N.; Wang, H.; Wang, Z.; Wei, P.; Li, F.; Yu, J.; et al. Four-week administration of nicotinemoderately impacts blood metabolic profile and gut microbiota in a diet-dependent manner. Biomed. Pharmacother. 2019, 115, 108945. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.M.; Mendes, M.M.; Oliveira, A.C.; Magalhaes, K.G.; Shivappa, N.; Hebert, J.R.; da Costa, T.H.M.; Botelho, P.B. Dietary inflammatory index and its relationship with gut microbiota in individuals with intestinal constipation: A cross-sectional study. Eur. J. Nutr. 2022, 61, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Keswani, T.; Roland, J.; Herbert, F.; Delcroix-Genete, D.; Bauderlique-Le Roy, H.; Gaayeb, L.; Cazenave, P.A.; Pied, S. Expression of CD300lf by microglia contributes to resistance to cerebral malaria by impeding the neuroinflammation. Genes Immun. 2020, 21, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020, 47, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyotylainen, T.; Hamalainen, A.M.; Peet, A.; Tillmann, V.; Poho, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, Y.N.; Lv, S.Y.; Wu, H.G.; Zhang, L.S.; Cao, Z.; Liu, H.R.; Wang, X.M.; Wu, L.Y. Gut microbiome alterations in colitis rats after moxibustion at bilateral Tianshu acupoints. BMC Gastroenterol. 2022, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a drug target for autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Pellock, S.J.; Redinbo, M.R. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J. Biol. Chem. 2017, 292, 8569–8576. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).