Roles of O-GlcNAcylation in Mitochondrial Homeostasis and Cardiovascular Diseases
Abstract
:1. Introduction
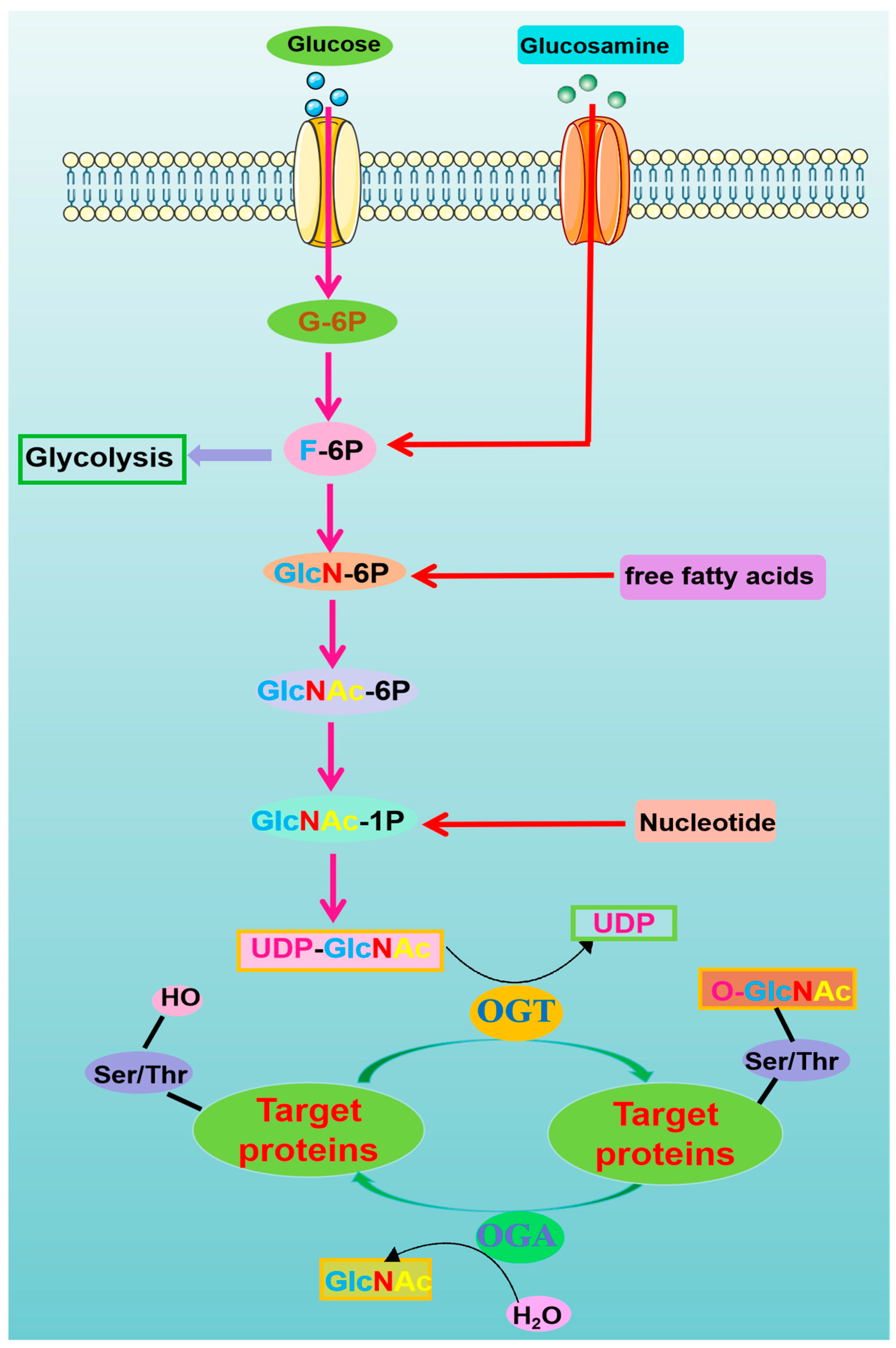
2. O-Linked β-N-Acetylglucosamine-Modification (O-GlcNAcylation)
The Modulation of O-GlcNAcylation and Its Therapeutic Implications
3. O-GlcNAcylation and Mitochondrial Homeostasis
3.1. O-GlcNAcylation of Mitochondrial Proteins
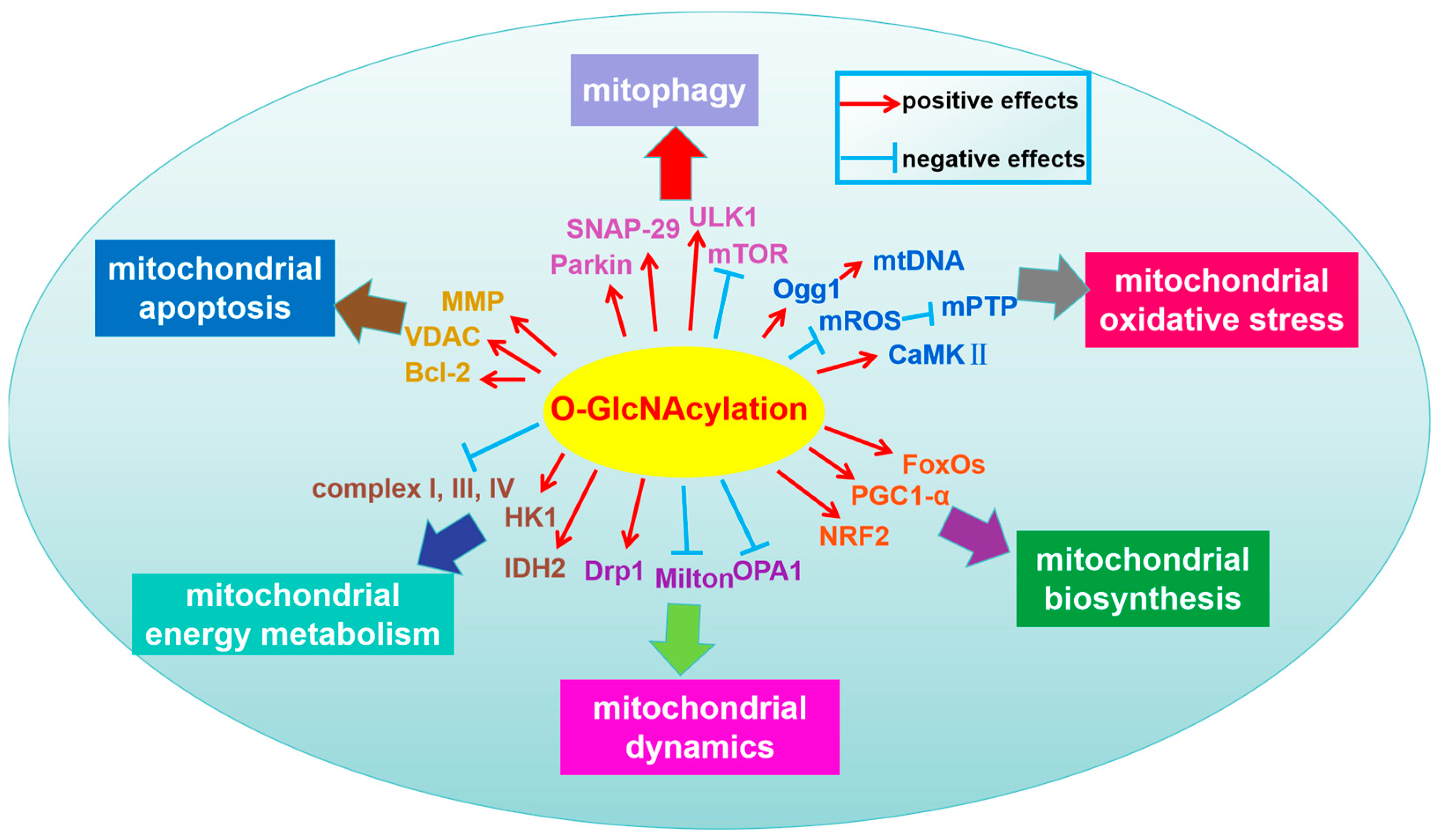
3.2. The Regulatory Mechanisms of Protein O-GlcNAcylation on Mitochondrial Homeostasis
3.2.1. Mitochondrial Dynamics (Fusion–Fission)
3.2.2. Mitochondrial Biosynthesis
3.2.3. Mitochondrial Oxidative Stress
3.2.4. Mitophagy
3.2.5. Mitochondrial Apoptosis
3.2.6. Mitochondrial Energy Metabolism
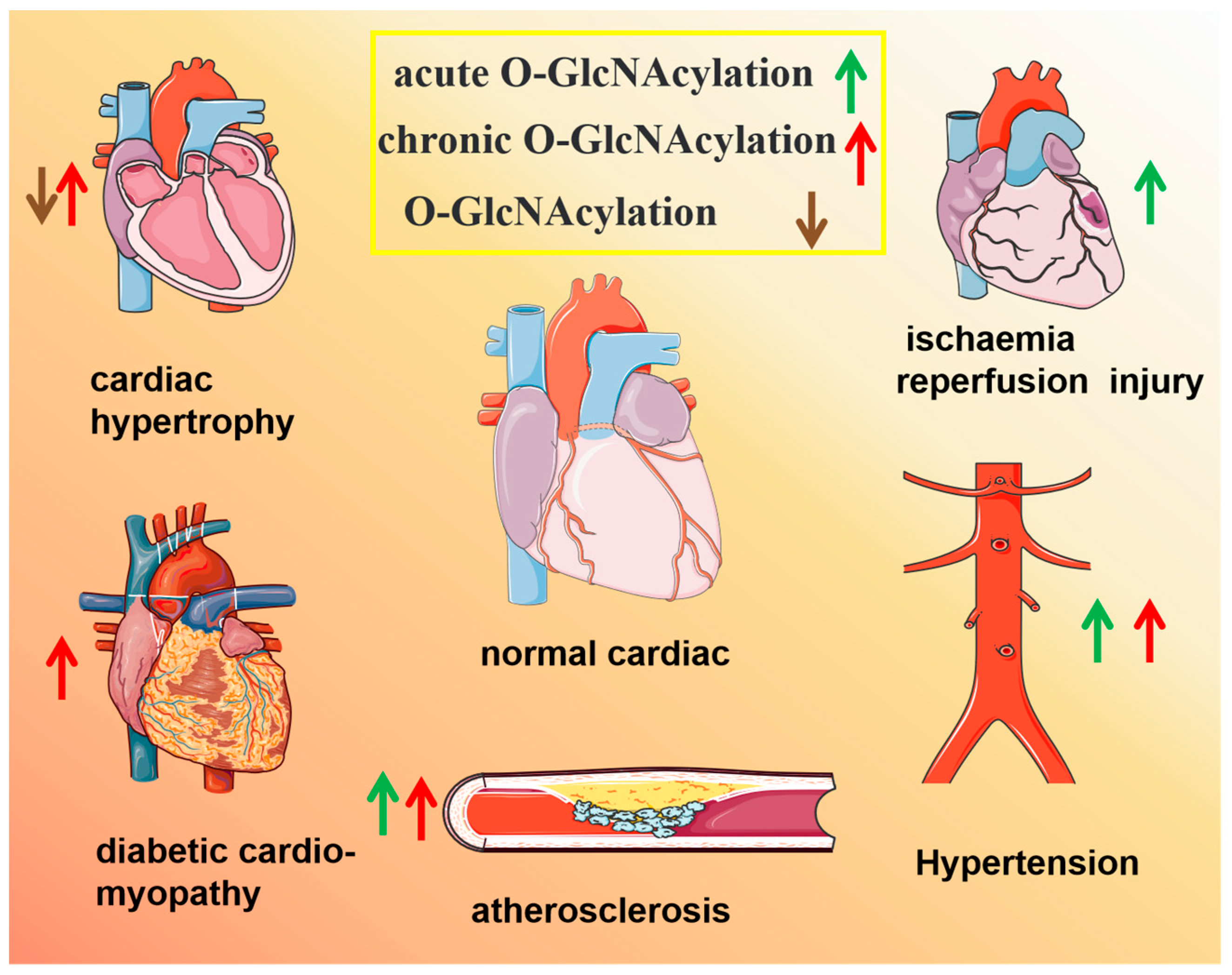
4. Role of O-GlcNAcylation in Cardiovascular Diseases (CVDs)
4.1. Cardiac Hypertrophy and Heart Failure
4.2. Diabetic Cardiomyopathy
4.3. Myocardial Ischaemia/Reperfusion (MI/R) Injury
4.4. Atherosclerosis and Coronary Heart Disease (CHD)
4.5. Hypertension and Arrhythmia
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, C.R.; Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984, 259, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Schwein, P.A.; Woo, C.M. The O-GlcNAc Modification on Kinases. ACS Chem. Biol. 2020, 15, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, L.; Ma, C.; Shi, Y.; Liu, C.; Xiao, Z.; Zhang, Y.; Tian, F.; Gao, Y.; Zhang, J.; et al. O-GlcNAcylation of Thr(12)/Ser(56) in short-form O-GlcNAc transferase (sOGT) regulates its substrate selectivity. J. Biol. Chem. 2019, 294, 16620–16633. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rellan, M.J.; Fondevila, M.F.; Dieguez, C.; Nogueiras, R. O-GlcNAcylation: A Sweet Hub in the Regulation of Glucose Metabolism in Health and Disease. Front. Endocrinol. 2022, 13, 873513. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.P.; McGreal, S.R.; Graw, S.; Tessman, R.; Koppel, S.J.; Dhakal, P.; Zhang, Z.; Machacek, M.; Zachara, N.E.; Koestler, D.C.; et al. Sustained O-GlcNAcylation reprograms mitochondrial function to regulate energy metabolism. J. Biol. Chem. 2017, 292, 14940–14962. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liao, Z.; Lu, X.; Katschinski, D.M.; Mercola, M.; Chen, J.; Heller Brown, J.; Molkentin, J.D.; Bossuyt, J.; Bers, D.M. Hyperglycemia Acutely Increases Cytosolic Reactive Oxygen Species via O-linked GlcNAcylation and CaMKII Activation in Mouse Ventricular Myocytes. Circ. Res. 2020, 126, e80–e96. [Google Scholar] [CrossRef] [PubMed]
- Huynh, V.N.; Wang, S.; Ouyang, X.; Wani, W.Y.; Johnson, M.S.; Chacko, B.K.; Jegga, A.G.; Qian, W.J.; Chatham, J.C.; Darley-Usmar, V.M.; et al. Defining the Dynamic Regulation of O-GlcNAc Proteome in the Mouse Cortex—The O-GlcNAcylation of Synaptic and Trafficking Proteins Related to Neurodegenerative Diseases. Front. Aging 2021, 2, 757801. [Google Scholar] [CrossRef] [PubMed]
- Dontaine, J.; Bouali, A.; Daussin, F.; Bultot, L.; Vertommen, D.; Martin, M.; Rathagirishnan, R.; Cuillerier, A.; Horman, S.; Beauloye, C.; et al. The intra-mitochondrial O-GlcNAcylation system rapidly modulates OXPHOS function and ROS release in the heart. Commun. Biol. 2022, 5, 349. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, P.; Ciesielski, P.; Zakrzewski, P.K.; Kozal, K.; Oracz, J.; Budryn, G.; Żyżelewicz, D.; Flament, S.; Vercoutter-Edouart, A.-S.; Bray, F.; et al. Mitochondrial O-GlcNAc Transferase Interacts with and Modifies Many Proteins and Its Up-Regulation Affects Mitochondrial Function and Cellular Energy Homeostasis. Cancers 2021, 13, 2956. [Google Scholar] [CrossRef]
- Wang, H.; Vant, J.; Wu, Y.; Sanchez, R.; Micou, M.L.; Zhang, A.; Luczak, V.; Yu, S.B.; Jabbo, M.; Yoon, S.; et al. Functional Organization of Glycolytic Metabolon on Mitochondria. bioRxiv 2023. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, P.; Yang, L.; Duan, X. Metabolomic analysis and pharmacological validation of the cerebral protective effect of 3,4-dihydroxybenzaldehyde on cerebral ischemia-reperfusion injury. Mol. Med. Rep. 2023, 27, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yue, X.; Sepulveda, H.; Burt, R.A.; Scott, D.A.; Carr, S.A.; Myers, S.A.; Rao, A. OGT controls mammalian cell viability by regulating the proteasome/mTOR/ mitochondrial axis. Proc. Natl. Acad. Sci. USA 2023, 120, e2218332120. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.H.; Goh, Y.S.; Kim, H.J.; Kim, D.H.; Kim, H.; Hwang, J.; Jung, J.S.; Kang, N.; Park, S.E.; Park, K.M.; et al. Tacrolimus Improves Therapeutic Efficacy of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Diabetic Retinopathy by Suppressing DRP1-Mediated Mitochondrial Fission. Antioxidants 2023, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.M.; Bakshi, S.; Brahma, M.K.; Potter, L.A.; Chang, S.F.; Sun, Z.; Benavides, G.A.; He, L.; Umbarkar, P.; Zou, L.; et al. Sustained Increases in Cardiomyocyte Protein O-Linked β-N-Acetylglucosamine Levels Lead to Cardiac Hypertrophy and Reduced Mitochondrial Function without Systolic Contractile Impairment. J. Am. Heart Assoc. 2023, 12, e029898. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.Y.; Wei, X.X.; Zhi, X.L.; Wang, X.H.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharmacol. 2021, 178, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zeng, Z.; Li, S.; Xie, Y.; Tong, X. The Therapeutic Strategies Targeting Mitochondrial Metabolism in Cardiovascular Disease. Pharmaceutics 2022, 14, 2760. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhao, M.; Zhou, B.; Yoshii, A.; Bugg, D.; Villet, O.; Sahu, A.; Olson, G.S.; Davis, J.; Tian, R. Mitochondrial dysfunction in macrophages promotes inflammation and suppresses repair after myocardial infarction. J. Clin. Investig. 2023, 133, e159498. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Liu, J.C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 2023, 119, 1105–1116. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zhang, Y.; Wang, M.; Li, X.; Liu, S.; Xu, D.; Bao, Y.; Jia, P.; Wu, N.; et al. The lncRNA Malat1 regulates microvascular function after myocardial infarction in mice via miR-26b-5p/Mfn1 axis-mediated mitochondrial dynamics. Redox Biol. 2021, 41, 101910. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, Å.B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Umapathi, P.; Mesubi, O.O.; Banerjee, P.S.; Abrol, N.; Wang, Q.; Luczak, E.D.; Wu, Y.; Granger, J.M.; Wei, A.C.; Reyes Gaido, O.E.; et al. Excessive O-GlcNAcylation Causes Heart Failure and Sudden Death. Circulation 2021, 143, 1687–1703. [Google Scholar] [CrossRef]
- Bolanle, I.O.; Riches-Suman, K.; Williamson, R.; Palmer, T.M. Emerging roles of protein O-GlcNAcylation in cardiovascular diseases: Insights and novel therapeutic targets. Pharmacol. Res. 2021, 165, 105467. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Ma, Z.G.; Hu, M.; Yuan, X.P.; Yuan, Y.P.; Wang, S.S.; Kong, C.Y.; Teng, T.; Tang, Q.Z. Tisp40 prevents cardiac ischemia/reperfusion injury through the hexosamine biosynthetic pathway in male mice. Nat. Commun. 2023, 14, 3383. [Google Scholar] [CrossRef] [PubMed]
- Pælestik, K.B.; Jespersen, N.R.; Jensen, R.V.; Johnsen, J.; Bøtker, H.E.; Kristiansen, S.B. Effects of hypoglycemia on myocardial susceptibility to ischemia-reperfusion injury and preconditioning in hearts from rats with and without type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 148. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, M.; Yokoe, S.; Nagatsuka, T.; Morihara, H.; Moriwaki, K.; Asahi, M. O-GlcNAcylation-induced GSK-3β activation deteriorates pressure overload-induced heart failure via lack of compensatory cardiac hypertrophy in mice. Front. Endocrinol. 2023, 14, 1122125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hart, G.W. Targeting O-GlcNAcylation to develop novel therapeutics. Mol. Asp. Med. 2021, 79, 100885. [Google Scholar] [CrossRef]
- Shi, Q.; Shen, Q.; Liu, Y.; Shi, Y.; Huang, W.; Wang, X.; Li, Z.; Chai, Y.; Wang, H.; Hu, X.; et al. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal Cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell 2022, 40, 1207–1222.e10. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, Y.X.; Zhou, Y.P.; Wei, Y.P.; Yan, Y.; Zhang, Z.J.; Jing, Z.C. Protein O-GlcNAcylation in cardiovascular diseases. Acta Pharmacol. Sin. 2023, 44, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Fahie, K.; Narayanan, B.; Zahra, F.; Reeves, R.; Fernandes, S.M.; Hart, G.W.; Zachara, N.E. Detection and Analysis of Proteins Modified by O-Linked N-Acetylglucosamine. Curr. Protoc. 2021, 1, e129. [Google Scholar] [CrossRef]
- Nie, H.; Yi, W. O-GlcNAcylation, a sweet link to the pathology of diseases. J. Zhejiang Univ. Sci. B 2019, 20, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Liu, H.-Y.; Yu, T.-J.; Lu, Q.; Zhang, F.-L.; Liu, G.-Y.; Shao, Z.-M.; Li, D.-Q. O-GlcNAcylation of MORC2 at threonine 556 by OGT couples TGF-β signaling to breast cancer progression. Cell Death Differ. 2022, 29, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Blaženović, I.; Contreras, A.J.; Pham, T.M.; Tabuloc, C.A.; Li, Y.H.; Ji, J.; Fiehn, O.A.-O.; Chiu, J.A.-O. Hexosamine biosynthetic pathway and O-GlcNAc-processing enzymes regulate daily rhythms in protein O-GlcNAcylation. Nat. Commun. 2021, 12, 4173. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Yu, L.; Wang, M.; Xu, S.; Xia, Q.; Fu, G. O-GlcNAcylation involvement in high glucose-induced cardiac hypertrophy via ERK1/2 and cyclin D2. Amino Acids 2013, 45, 339–349. [Google Scholar] [CrossRef]
- Brainard, R.E.; Facundo, H.T. Cardiac hypertrophy drives PGC-1α suppression associated with enhanced O-glycosylation. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166080. [Google Scholar] [CrossRef]
- Hilgers, R.H.; Xing, D.; Gong, K.; Chen, Y.F.; Chatham, J.C.; Oparil, S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H513–H522. [Google Scholar] [CrossRef]
- Pinho, T.S.; Correia, S.C.; Perry, G.; Ambrósio, A.F.; Moreira, P.I. Diminished O-GlcNAcylation in Alzheimer’s disease is strongly correlated with mitochondrial anomalies. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2048–2059. [Google Scholar] [CrossRef]
- Laczy, B.; Marsh, S.A.; Brocks, C.A.; Wittmann, I.; Chatham, J.C. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1715–H1727. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Cadiet, J.; Persello, A.; Prat, V.; Denis, M.; Erraud, A.; Aillerie, V.; Mevel, M.; Bigot, E.; Chatham, J.C.; et al. O-GlcNAc stimulation: A new metabolic approach to treat septic shock. Sci. Rep. 2019, 9, 18751. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhou, N.; Nan, Y.; Zhang, L.; Li, Y.; Hao, X.; Xiong, L.; Lau, W.B.; Ma, X.L.; Wang, H.; et al. Effective glycaemic control critically determines insulin cardioprotection against ischaemia/reperfusion injury in anaesthetized dogs. Cardiovasc. Res. 2014, 103, 238–247. [Google Scholar] [CrossRef]
- Gélinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, P.; Hua, F.; Hu, Y.; Xiao, F.; Liu, Q.; Huang, D.; Deng, F.; Wei, G.; Deng, W.; et al. Sevoflurane postconditioning reduces myocardial ischemia reperfusion injury-induced necroptosis by up-regulation of OGT-mediated O-GlcNAcylated RIPK3. Aging 2020, 12, 25452–25468. [Google Scholar] [CrossRef] [PubMed]
- Jo, R.; Shibata, H.; Kurihara, I.; Yokota, K.; Kobayashi, S.; Murai-Takeda, A.; Mitsuishi, Y.; Hayashi, T.; Nakamura, T.; Itoh, H. Mechanisms of mineralocorticoid receptor-associated hypertension in diabetes mellitus: The role of O-GlcNAc modification. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2023, 46, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Foetal recapitulation of nutrient surplus signalling by O-GlcNAcylation and the failing heart. Eur. J. Heart Fail. 2023, 25, 1199–1212. [Google Scholar] [CrossRef]
- Hirose, K.; Tsutsumi, Y.M.; Tsutsumi, R.; Shono, M.; Katayama, E.; Kinoshita, M.; Tanaka, K.; Oshita, S. Role of the O-linked β-N-acetylglucosamine in the cardioprotection induced by isoflurane. Anesthesiology 2011, 115, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Belke, D.D. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J. Appl. Physiol. 2011, 111, 157–162. [Google Scholar] [CrossRef]
- Ngoh, G.A.; Hamid, T.; Prabhu, S.D.; Jones, S.P. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1711–H1719. [Google Scholar] [CrossRef]
- Nie, H.; Ju, H.; Fan, J.; Shi, X.; Cheng, Y.; Cang, X.; Zheng, Z.; Duan, X.; Yi, W. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat. Commun. 2020, 11, 36. [Google Scholar] [CrossRef]
- Chatham, J.C.; Zhang, J.; Wende, A.R. Role of O-Linked N-Acetylglucosamine Protein Modification in Cellular (Patho)Physiology. Physiol. Rev. 2021, 101, 427–493. [Google Scholar] [CrossRef]
- Pagesy, P.; Bouaboud, A.; Feng, Z.; Hulin, P.; Issad, T. Short O-GlcNAcase Is Targeted to the Mitochondria and Regulates Mitochondrial Reactive Oxygen Species Level. Cells 2022, 11, 1827. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, R.; Ji, S.; Yu, S. Regulation of mitochondrial network homeostasis by O-GlcNAcylation. Mitochondrion 2022, 65, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, K.K.; Hangauer, M.J.; Smith, T.J.; Smart, B.P.; Pitcher, A.A.; Cheng, E.H.; Bertozzi, C.R.; Boyce, M. A chemical glycoproteomics platform reveals O-GlcNAcylation of mitochondrial voltage-dependent anion channel 2. Cell Rep. 2013, 5, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Taegtmeyer, H.; Wang, Z.V. Diverging consequences of hexosamine biosynthesis in cardiovascular disease. J. Mol. Cell. Cardiol. 2021, 153, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.V.; Andreadou, I.; Hausenloy, D.J.; Bøtker, H.E. The Role of O-GlcNAcylation for Protection against Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2019, 20, 404. [Google Scholar] [CrossRef] [PubMed]
- Yokoe, S.; Hayashi, T.; Nakagawa, T.; Kato, R.; Ijiri, Y.; Yamaguchi, T.; Izumi, Y.; Yoshiyama, M.; Asahi, M. Augmented O-GlcNAcylation exacerbates right ventricular dysfunction and remodeling via enhancement of hypertrophy, mitophagy, and fibrosis in mice exposed to long-term intermittent hypoxia. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2023, 46, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.G.; Aronsen, J.M.; Kvaløy, H.; Qvigstad, E.; Sjaastad, I.; Tønnessen, T.; Christensen, G.; Grønning-Wang, L.M.; Carlson, C.R. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol. Genom. 2012, 44, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Dassanayaka, S.; Brainard, R.E.; Watson, L.J.; Long, B.W.; Brittian, K.R.; DeMartino, A.M.; Aird, A.L.; Gumpert, A.M.; Audam, T.N.; Kilfoil, P.J.; et al. Cardiomyocyte Ogt limits ventricular dysfunction in mice following pressure overload without affecting hypertrophy. Basic Res. Cardiol. 2017, 112, 23. [Google Scholar] [CrossRef] [PubMed]
- Dassanayaka, S.; Brittian, K.R.; Long, B.W.; Higgins, L.A.; Bradley, J.A.; Audam, T.N.; Jurkovic, A.; Gumpert, A.M.; Harrison, L.T.; Hartyánszky, I.; et al. Cardiomyocyte Oga haploinsufficiency increases O-GlcNAcylation but hastens ventricular dysfunction following myocardial infarction. PLoS ONE 2020, 15, e0242250. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Zang, W.J. Mitochondrial homeostasis and redox status in cardiovascular diseases: Protective role of the vagal system. Free. Radic. Biol. Med. 2022, 178, 369–379. [Google Scholar] [CrossRef]
- Vasileiou, P.V.S.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.I.; Chronopoulos, E.; Passias, P.G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial Homeostasis and Cellular Senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef]
- Stram, A.R.; Payne, R.M. Post-translational modifications in mitochondria: Protein signaling in the powerhouse. Cell. Mol. Life Sci. 2016, 73, 4063–4073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, Z.; Yang, X.; Liu, J. The regulatory roles of O-GlcNAcylation in mitochondrial homeostasis and metabolic syndrome. Free. Radic. Res. 2016, 50, 1080–1088. [Google Scholar] [CrossRef]
- Tan, E.P.; Villar, M.T.; Lezi, E.; Lu, J.; Selfridge, J.E.; Artigues, A.; Swerdlow, R.H.; Slawson, C. Altering O-linked β-N-acetylglucosamine cycling disrupts mitochondrial function. J. Biol. Chem. 2014, 289, 14719–14730. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, P.; Yan, H.; Sun, H.; Liu, X.; Zhou, F.; Li, L.; Chen, Y.; Muthana, M.M.; Chen, X.; et al. Substrate specificity provides insights into the sugar donor recognition mechanism of O-GlcNAc transferase (OGT). PLoS ONE 2013, 8, e63452. [Google Scholar] [CrossRef]
- Sacoman, J.L.; Dagda, R.Y.; Burnham-Marusich, A.R.; Dagda, R.K.; Berninsone, P.M. Mitochondrial O-GlcNAc Transferase (mOGT) Regulates Mitochondrial Structure, Function, and Survival in HeLa Cells. J. Biol. Chem. 2017, 292, 4499–4518. [Google Scholar] [CrossRef]
- Trapannone, R.; Mariappa, D.; Ferenbach, A.T.; van Aalten, D.M. Nucleocytoplasmic human O-GlcNAc transferase is sufficient for O-GlcNAcylation of mitochondrial proteins. Biochem. J. 2016, 473, 1693–1702. [Google Scholar] [CrossRef]
- Mohan, R.; Jo, S.; Lockridge, A.; Ferrington, D.A.; Murray, K.; Eschenlauer, A.; Bernal-Mizrachi, E.; Fujitani, Y.; Alejandro, E.U. OGT Regulates Mitochondrial Biogenesis and Function via Diabetes Susceptibility Gene Pdx1. Diabetes 2021, 70, 2608–2625. [Google Scholar] [CrossRef]
- Gondane, A.; Poulose, N.; Walker, S.; Mills, I.G.; Itkonen, H.M. O-GlcNAc transferase maintains metabolic homeostasis in response to CDK9 inhibition. Glycobiology 2022, 32, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Cao, J.; Huang, J.; Yao, J.; Yan, G.; Xu, H.; Yang, P. Discovery and confirmation of O-GlcNAcylated proteins in rat liver mitochondria by combination of mass spectrometry and immunological methods. PLoS ONE 2013, 8, e76399. [Google Scholar] [CrossRef]
- Ma, J.; Liu, T.; Wei, A.C.; Banerjee, P.; O’Rourke, B.; Hart, G.W. O-GlcNAcomic Profiling Identifies Widespread O-Linked β-N-Acetylglucosamine Modification (O-GlcNAcylation) in Oxidative Phosphorylation System Regulating Cardiac Mitochondrial Function. J. Biol. Chem. 2015, 290, 29141–29153. [Google Scholar] [CrossRef]
- Gu, Y.; Ande, S.R.; Mishra, S. Altered O-GlcNAc modification and phosphorylation of mitochondrial proteins in myoblast cells exposed to high glucose. Arch. Biochem. Biophys. 2011, 505, 98–104. [Google Scholar] [CrossRef]
- Cabrera, J.T.; Si, R.; Tsuji-Hosokawa, A.; Cai, H.; Yuan, J.X.; Dillmann, W.H.; Makino, A. Restoration of coronary microvascular function by OGA overexpression in a high-fat diet with low-dose streptozotocin-induced type 2 diabetic mice. Diabetes Vasc. Dis. Res. 2023, 20, 14791641231173630. [Google Scholar] [CrossRef] [PubMed]
- Akinbiyi, E.O.; Abramowitz, L.K.; Bauer, B.L.; Stoll, M.S.K.; Hoppel, C.L.; Hsiao, C.P.; Hanover, J.A.; Mears, J.A. Blocked O-GlcNAc cycling alters mitochondrial morphology, function, and mass. Sci. Rep. 2021, 11, 22106. [Google Scholar] [CrossRef]
- Huang, C.W.; Rust, N.C.; Wu, H.F.; Yin, A.; Zeltner, N.; Yin, H.; Hart, G.W. Low glucose induced Alzheimer’s disease-like biochemical changes in human induced pluripotent stem cell-derived neurons is due to dysregulated O-GlcNAcylation. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 4872–4885. [Google Scholar] [CrossRef]
- Park, S.J.; Bae, J.E.; Jo, D.S.; Kim, J.B.; Park, N.Y.; Fang, J.; Jung, Y.K.; Jo, D.G.; Cho, D.H. Increased O-GlcNAcylation of Drp1 by amyloid-beta promotes mitochondrial fission and dysfunction in neuronal cells. Mol. Brain 2021, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Chen, Q.; Zheng, L.; Lou, J.; Lin, C.; Gong, J.; Zhu, Y.; Wu, Y. O-GlcNAcylation and stablization of SIRT7 promote pancreatic cancer progression by blocking the SIRT7-REGγ interaction. Cell Death Differ. 2022, 29, 1970–1981. [Google Scholar] [CrossRef]
- Parker, M.P.; Peterson, K.R.; Slawson, C. O-GlcNAcylation and O-GlcNAc Cycling Regulate Gene Transcription: Emerging Roles in Cancer. Cancers 2021, 13, 1666. [Google Scholar] [CrossRef]
- Ma, J.; Banerjee, P.; Whelan, S.A.; Liu, T.; Wei, A.C.; Ramirez-Correa, G.; McComb, M.E.; Costello, C.E.; O’Rourke, B.; Murphy, A.; et al. Comparative Proteomics Reveals Dysregulated Mitochondrial O-GlcNAcylation in Diabetic Hearts. J. Proteome Res. 2016, 15, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.S.; Ma, J.; Hart, G.W. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, 6050–6055. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Hanna, A.; Lama-Sherpa, T.; Metge, B.; Kammerud, S.C.; Benavides, G.A.; Kumar, A.; Alsheikh, H.A.; Mota, M.; Chen, D.; et al. Hedgehog Signaling Regulates Metabolism and Polarization of Mammary Tumor-Associated Macrophages. Cancer Res. 2021, 81, 5425–5437. [Google Scholar] [CrossRef] [PubMed]
- Pekkurnaz, G.; Trinidad, J.C.; Wang, X.; Kong, D.; Schwarz, T.L. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell 2014, 158, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Gawlowski, T.; Suarez, J.; Scott, B.; Torres-Gonzalez, M.; Wang, H.; Schwappacher, R.; Han, X.; Yates, J.R., 3rd; Hoshijima, M.; Dillmann, W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J. Biol. Chem. 2012, 287, 30024–30034. [Google Scholar] [CrossRef]
- Makino, A.; Suarez, J.; Gawlowski, T.; Han, W.; Wang, H.; Scott, B.T.; Dillmann, W.H. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1296–R1302. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, L.; Huo, T.; Cheng, J.; Li, X.; Huangfu, X.; Sun, S.; Wang, H.; Li, L. O-GlcNAc Transferase (OGT) Protects Cerebral Neurons from Death During Ischemia/Reperfusion (I/R) Injury by Modulating Drp1 in Mice. Neuromolecular Med. 2022, 24, 299–310. [Google Scholar] [CrossRef]
- Housley, M.P.; Udeshi, N.D.; Rodgers, J.T.; Shabanowitz, J.; Puigserver, P.; Hunt, D.F.; Hart, G.W. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J. Biol. Chem. 2009, 284, 5148–5157. [Google Scholar] [CrossRef]
- Ruan, H.B.; Han, X.; Li, M.D.; Singh, J.P.; Qian, K.; Azarhoush, S.; Zhao, L.; Bennett, A.M.; Samuel, V.T.; Wu, J.; et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab. 2012, 16, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Z.; Wang, X.; Yang, L.; Han, S.; Cao, K.; Xu, J.; Zhao, L.; Zhang, Y.; Liu, J. O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia 2016, 59, 1287–1296. [Google Scholar] [CrossRef]
- Ohashi, N.; Morino, K.; Ida, S.; Sekine, O.; Lemecha, M.; Kume, S.; Park, S.Y.; Choi, C.S.; Ugi, S.; Maegawa, H. Pivotal Role of O-GlcNAc Modification in Cold-Induced Thermogenesis by Brown Adipose Tissue Through Mitochondrial Biogenesis. Diabetes 2017, 66, 2351–2362. [Google Scholar] [CrossRef]
- Chen, P.H.; Smith, T.J.; Wu, J.; Siesser, P.F.; Bisnett, B.J.; Khan, F.; Hogue, M.; Soderblom, E.; Tang, F.; Marks, J.R.; et al. Glycosylation of KEAP1 links nutrient sensing to redox stress signaling. EMBO J. 2017, 36, 2233–2250. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; Jia, P.; Fang, Y.; Liu, T.; Song, N.; Xu, X.; Ji, J.; Ding, X. Augmented O-GlcNAc signaling via glucosamine attenuates oxidative stress and apoptosis following contrast-induced acute kidney injury in rats. Free Radic. Biol. Med. 2017, 103, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.E.; Choi, S.Y.; Kwon, O.S. OSMI-1 Enhances TRAIL-Induced Apoptosis through ER Stress and NF-κB Signaling in Colon Cancer Cells. Int. J. Mol. Sci. 2021, 22, 11073. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, M.; Li, Y.; Lu, J.; Zhou, W.; Li, X.; Shi, H.; Xu, B.; Li, S. O-GlcNAcylation of SIRT1 Protects against Cold Stress-Induced Skeletal Muscle Damage via Amelioration of Mitochondrial Homeostasis. Int. J. Mol. Sci. 2022, 23, 14520. [Google Scholar] [CrossRef] [PubMed]
- Cividini, F.; Scott, B.T.; Dai, A.; Han, W.; Suarez, J.; Diaz-Juarez, J.; Diemer, T.; Casteel, D.E.; Dillmann, W.H. O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts. J. Biol. Chem. 2016, 291, 26515–26528. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rellan, M.J.; Parracho, T.; Heras, V.; Rodriguez, A.; Fondevila, M.F.; Novoa, E.; Lima, N.; Varela-Rey, M.; Senra, A.; Chantada-Vazquez, M.D.P.; et al. Hepatocyte-specific O-GlcNAc transferase downregulation ameliorates nonalcoholic steatohepatitis by improving mitochondrial function. Mol. Metab. 2023, 75, 101776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, Z.; Zou, X.; Cao, K.; Xu, J.; Liu, J. Aging leads to elevation of O-GlcNAcylation and disruption of mitochondrial homeostasis in retina. Oxidative Med. Cell Longev. 2014, 2014, 425705. [Google Scholar] [CrossRef]
- Ngoh, G.A.; Watson, L.J.; Facundo, H.T.; Jones, S.P. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids 2011, 40, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.K.; Yoon, S.Y.; Hwang, J.S.; Shin, Y.J. O-GlcNAc Signaling Augmentation Protects Human Corneal Endothelial Cells from Oxidative Stress via AKT Pathway Activation. Curr. Eye Res. 2020, 45, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Xuefei, Y.; Dongyan, L.; Tianming, L.; Hejuan, Z.; Jianhua, F. O-linked N-acetylglucosamine affects mitochondrial homeostasis by regulating Parkin-dependent mitophagy in hyperoxia-injured alveolar type II cells injury. Respir. Res. 2023, 24, 16. [Google Scholar] [CrossRef]
- Murakami, K.; Kurotaki, D.; Kawase, W.; Soma, S.; Fukuchi, Y.; Kunimoto, H.; Yoshimi, R.; Koide, S.; Oshima, M.; Hishiki, T.; et al. OGT Regulates Hematopoietic Stem Cell Maintenance via PINK1-Dependent Mitophagy. Cell Rep. 2021, 34, 108579. [Google Scholar] [CrossRef]
- Rahman, M.A.; Cho, Y.; Hwang, H.; Rhim, H. Pharmacological Inhibition of O-GlcNAc Transferase Promotes mTOR-Dependent Autophagy in Rat Cortical Neurons. Brain Sci. 2020, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.B.; Ma, Y.; Torres, S.; Zhang, B.; Feriod, C.; Heck, R.M.; Qian, K.; Fu, M.; Li, X.; Nathanson, M.H.; et al. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017, 31, 1655–1665. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, F.; Dai, G.; Yao, Q.; Xiang, H.; Wang, L.; Xue, B.; Shan, Y.; Liu, X. Blockage of O-linked GlcNAcylation induces AMPK-dependent autophagy in bladder cancer cells. Cell Mol. Biol. Lett. 2020, 25, 17. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Q.; Li, L.; Hu, Z.; Wu, F.; Zhang, P.; Ma, Y.; Zhao, B.; Kovács, A.L.; Zhang, Z.; et al. O-GlcNAc-modification of SNAP-29 regulates autophagosome maturation. Nat. Cell Biol. 2014, 16, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Jones, S.P.; Zachara, N.E.; Ngoh, G.A.; Hill, B.G.; Teshima, Y.; Bhatnagar, A.; Hart, G.W.; Marbán, E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 2008, 117, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qin, J.; Zhao, J.; Li, J.; Li, D.; Popp, M.; Popp, F.; Alakus, H.; Kong, B.; Dong, Q.; et al. Inflammatory IFIT3 renders chemotherapy resistance by regulating post-translational modification of VDAC2 in pancreatic cancer. Theranostics 2020, 10, 7178–7192. [Google Scholar] [CrossRef]
- Ngoh, G.A.; Facundo, H.T.; Hamid, T.; Dillmann, W.; Zachara, N.E.; Jones, S.P. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ. Res. 2009, 104, 41–49. [Google Scholar] [CrossRef]
- Ngoh, G.A.; Watson, L.J.; Facundo, H.T.; Dillmann, W.; Jones, S.P. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J. Mol. Cell. Cardiol. 2008, 45, 313–325. [Google Scholar] [CrossRef]
- Marsh, S.A.; Powell, P.C.; Dell’italia, L.J.; Chatham, J.C. Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart. Life Sci. 2013, 92, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am. J. Physiol. Cell Physiol. 2008, 294, C1509–C1520. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, D.L.; Arvanitis, L.D.; Panourias, I.G.; Kitsoulis, P.; Kanavaros, P. Mitochondria-rich normal, metaplastic, and neoplastic cells show overexpression of the epitope H recognized by the monoclonal antibody H. Pathol. Res. Pract. 2005, 201, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Suarez, J.; Fricovsky, E.; Wang, H.; Scott, B.T.; Trauger, S.A.; Han, W.; Hu, Y.; Oyeleye, M.O.; Dillmann, W.H. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. J. Biol. Chem. 2009, 284, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.N.; Benavides, G.A.; Johnson, M.S.; Wani, W.; Ouyang, X.; Zou, L.; Collins, H.E.; Zhang, J.; Darley-Usmar, V.; Chatham, J.C. Acute increases in O-GlcNAc indirectly impair mitochondrial bioenergetics through dysregulation of LonP1-mediated mitochondrial protein complex turnover. Am. J. Physiol. Cell Physiol. 2019, 316, C862–C875. [Google Scholar] [CrossRef] [PubMed]
- Wende, A.R.; Schell, J.C.; Ha, C.M.; Pepin, M.E.; Khalimonchuk, O.; Schwertz, H.; Pereira, R.O.; Brahma, M.K.; Tuinei, J.; Contreras-Ferrat, A.; et al. Maintaining Myocardial Glucose Utilization in Diabetic Cardiomyopathy Accelerates Mitochondrial Dysfunction. Diabetes 2020, 69, 2094–2111. [Google Scholar] [CrossRef] [PubMed]
- Flax, J.; Wilkins, H.M.; Miller, R.; Griffith, S.; Cork, G.K.; Qiang, A.; Thompson, J.; Swerdlow, R.H.; Slawson, C. OGA Inhibition Alters Energetics and Nutrient Sensing in Alzheimer’s Disease Cytoplasmic Hybrids. J. Alzheimer’s Dis. 2020, 78, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, N.; Li, R.; Zhang, H.; Zhao, Y.; Nie, Y.; Wu, J. IDH2, a novel target of OGT, facilitates glucose uptake and cellular bioenergy production via NF-κB signaling to promote colorectal cancer progression. Cell. Oncol. 2023, 46, 145–164. [Google Scholar] [CrossRef]
- Dassanayaka, S.; Brittian, K.R.; Jurkovic, A.; Higgins, L.A.; Audam, T.N.; Long, B.W.; Harrison, L.T.; Militello, G.; Riggs, D.W.; Chitre, M.G.; et al. E2f1 deletion attenuates infarct-induced ventricular remodeling without affecting O-GlcNAcylation. Basic Res. Cardiol. 2019, 114, 28. [Google Scholar] [CrossRef]
- Toyoda, S.; Otani, N. Could the control of O-GlcNAcylation play a key role in cardiac remodeling? Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2023, 46, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Ma, H.; Ma, J.; Wang, X.; Li, D.; Xu, L. αSMA-Cre-mediated Ogt deletion leads to heart failure and vascular smooth muscle cell dysfunction in mice. Biochem. Biophys. Res. Commun. 2022, 625, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Y.; Yang, Y.; Chen, Y. Impaired intracellular calcium homeostasis enhances protein O-GlcNAcylation and promotes vascular calcification and stiffness in diabetes. Redox Biol. 2023, 63, 102720. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Bhavnani, N.; Mathias, A.; Lallo, J.; Gupta, S.; Ohanyan, V.; Ferrell, J.M.; Raman, P. Deletion of Smooth Muscle O-GlcNAc Transferase Prevents Development of Atherosclerosis in Western Diet-Fed Hyperglycemic ApoE(-/-) Mice In Vivo. Int. J. Mol. Sci. 2023, 24, 7899. [Google Scholar] [CrossRef] [PubMed]
- Kadosaka, T.; Watanabe, M.; Natsui, H.; Koizumi, T.; Nakao, M.; Koya, T.; Hagiwara, H.; Kamada, R.; Temma, T.; Karube, F.; et al. Empagliflozin attenuates arrhythmogenesis in diabetic cardiomyopathy by normalizing intracellular Ca2+ handling in ventricular cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H341–H354. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Liang, Y.; Qin, Y.; Wu, W.; Xie, M.; Zhang, Y.; Zhang, Y.; Ji, L.; Yu, H.; Li, T. Hypoxic acclimation improves cardiac redox homeostasis and protects heart against ischemia-reperfusion injury through upregulation of O-GlcNAcylation. Redox Biol. 2021, 43, 101994. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Z.; Ledee, D.; Olson, A.K. Temporal regulation of protein O-GlcNAc levels during pressure-overload cardiac hypertrophy. Physiol. Rep. 2021, 9, e14965. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Gao, F.; Jiang, T.; Liu, B.; Li, C.; Qin, X.; Zheng, Q. Hyper-O-GlcNAcylation impairs insulin response against reperfusion-induced myocardial injury and arrhythmias in obesity. Biochem. Biophys. Res. Commun. 2021, 558, 126–133. [Google Scholar] [CrossRef]
- Hegyi, B.; Fasoli, A.; Ko, C.Y.; Van, B.W.; Alim, C.C.; Shen, E.Y.; Ciccozzi, M.M.; Tapa, S.; Ripplinger, C.M.; Erickson, J.R.; et al. CaMKII Serine 280 O-GlcNAcylation Links Diabetic Hyperglycemia to Proarrhythmia. Circ. Res. 2021, 129, 98–113. [Google Scholar] [CrossRef]
- Nabeebaccus, A.A.; Zoccarato, A.; Hafstad, A.D.; Santos, C.X.; Aasum, E.; Brewer, A.C.; Zhang, M.; Beretta, M.; Yin, X.; West, J.A.; et al. Nox4 reprograms cardiac substrate metabolism via protein O-GlcNAcylation to enhance stress adaptation. JCI Insight 2017, 2, e96184. [Google Scholar] [CrossRef]
- Watson, L.J.; Long, B.W.; DeMartino, A.M.; Brittian, K.R.; Readnower, R.D.; Brainard, R.E.; Cummins, T.D.; Annamalai, L.; Hill, B.G.; Jones, S.P. Cardiomyocyte Ogt is essential for postnatal viability. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H142–H153. [Google Scholar] [CrossRef] [PubMed]
- Loaeza-Reyes, K.J.; Zenteno, E.; Moreno-Rodríguez, A.; Torres-Rosas, R.; Argueta-Figueroa, L.; Salinas-Marín, R.; Castillo-Real, L.M.; Pina-Canseco, S.; Cervera, Y.P. An Overview of Glycosylation and its Impact on Cardiovascular Health and Disease. Front. Mol. Biosci. 2021, 8, 751637. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Okolo, C.A.; Erickson, J.R.; Baldi, J.C.; Jones, P.P. Protein O-GlcNAcylation in the heart. Acta Physiol. 2021, 233, e13696. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Bers, D.M. New cardiac targets for empagliflozin: O-GlcNAcylation, CaMKII, and calcium handling. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H338–H340. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, D.; Lim, S.Y.; Erickson, J.R.; Wallace, R.S.; Lees, J.G.; Tate, M.; Kiriazis, H.; Donner, D.G.; Henstridge, D.C.; Davey, J.R.; et al. Fine-tuning the cardiac O-GlcNAcylation regulatory enzymes governs the functional and structural phenotype of the diabetic heart. Cardiovasc. Res. 2022, 118, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2023, 44, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Z.; El-Nachef, D.; Yang, X.; Ledee, D.; Olson, A.K. O-GlcNAc Transferase Promotes Compensated Cardiac Function and Protein Kinase A O-GlcNAcylation during Early and Established Pathological Hypertrophy from Pressure Overload. J. Am. Heart Assoc. 2019, 8, e011260. [Google Scholar] [CrossRef] [PubMed]
- Medford, H.M.; Porter, K.; Marsh, S.A. Immediate effects of a single exercise bout on protein O-GlcNAcylation and chromatin regulation of cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H114–H123. [Google Scholar] [CrossRef]
- Facundo, H.T.; Brainard, R.E.; Watson, L.J.; Ngoh, G.A.; Hamid, T.; Prabhu, S.D.; Jones, S.P. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2122–H2130. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; He, H.; Sun, Y.; Shen, Q.; Shi, L. Increased O-GlcNAcylation induces myocardial hypertrophy. In Vitr. Cell Dev. Biol. Anim. 2020, 56, 735–743. [Google Scholar] [CrossRef]
- Muthusamy, S.; DeMartino, A.M.; Watson, L.J.; Brittian, K.R.; Zafir, A.; Dassanayaka, S.; Hong, K.U.; Jones, S.P. MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. J. Biol. Chem. 2014, 289, 29665–29676. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Furukawa, Y.; Hayashi, T.; Nomura, A.; Yokoe, S.; Moriwaki, K.; Kato, R.; Ijiri, Y.; Yamaguchi, T.; Izumi, Y.; et al. Augmented O-GlcNAcylation attenuates intermittent hypoxia-induced cardiac remodeling through the suppression of NFAT and NF-κB activities in mice. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2019, 42, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Song, Y.; Xin, C.; Liu, L.; Hou, N.; Teng, Y.; Cheng, X.; Wang, T.; Yu, Z.; et al. PRMT5 Prevents Dilated Cardiomyopathy via Suppression of Protein O-GlcNAcylation. Circ. Res. 2021, 129, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Ducheix, S.; Magré, J.; Cariou, B.; Prieur, X. Chronic O-GlcNAcylation and Diabetic Cardiomyopathy: The Bitterness of Glucose. Front. Endocrinol. 2018, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Das, S.; Samanta, J.; Chakraborty, S.; Sengupta, A. YAP1 induces hyperglycemic stress-mediated cardiac hypertrophy and fibrosis in an AKT-FOXM1 dependent signaling pathway. Arch. Biochem. Biophys. 2022, 722, 109198. [Google Scholar] [CrossRef] [PubMed]
- De Blasio, M.J.; Huynh, N.; Deo, M.; Dubrana, L.E.; Walsh, J.; Willis, A.; Prakoso, D.; Kiriazis, H.; Donner, D.G.; Chatham, J.C.; et al. Defining the Progression of Diabetic Cardiomyopathy in a Mouse Model of Type 1 Diabetes. Front. Physiol. 2020, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Brahma, M.K.; Ha, C.M.; Pepin, M.E.; Mia, S.; Sun, Z.; Chatham, J.C.; Habegger, K.M.; Abel, E.D.; Paterson, A.J.; Young, M.E.; et al. Increased Glucose Availability Attenuates Myocardial Ketone Body Utilization. J. Am. Heart Assoc. 2020, 9, e013039. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.A.; Dell’Italia, L.J.; Chatham, J.C. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 2011, 40, 819–828. [Google Scholar] [CrossRef]
- Fricovsky, E.S.; Suarez, J.; Ihm, S.H.; Scott, B.T.; Suarez-Ramirez, J.A.; Banerjee, I.; Torres-Gonzalez, M.; Wang, H.; Ellrott, I.; Maya-Ramos, L.; et al. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R689–R699. [Google Scholar] [CrossRef]
- Clark, R.J.; McDonough, P.M.; Swanson, E.; Trost, S.U.; Suzuki, M.; Fukuda, M.; Dillmann, W.H. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 2003, 278, 44230–44237. [Google Scholar] [CrossRef]
- Ramirez-Correa, G.A.; Ma, J.; Slawson, C.; Zeidan, Q.; Lugo-Fagundo, N.S.; Xu, M.; Shen, X.; Gao, W.D.; Caceres, V.; Chakir, K.; et al. Removal of Abnormal Myofilament O-GlcNAcylation Restores Ca2+ Sensitivity in Diabetic Cardiac Muscle. Diabetes 2015, 64, 3573–3587. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.; Fricovsky, E.; Ihm, S.; Schimke, M.; Maya-Ramos, L.; Aroonsakool, N.; Ceballos, G.; Dillmann, W.; Villarreal, F.; Ramirez-Sanchez, I. Role for high-glucose-induced protein O-GlcNAcylation in stimulating cardiac fibroblast collagen synthesis. Am. J. Physiol. Cell Physiol. 2014, 306, C794–C804. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuan, P.; Yu, P.; Kong, Q.; Xu, Z.; Yan, X.; Shen, Y.; Yang, J.; Wan, R.; Hong, K.; et al. O-GlcNAc-modified SNAP29 inhibits autophagy-mediated degradation via the disturbed SNAP29-STX17-VAMP8 complex and exacerbates myocardial injury in type I diabetic rats. Int. J. Mol. Med. 2018, 42, 3278–3290. [Google Scholar] [CrossRef] [PubMed]
- Mesubi, O.O.; Rokita, A.G.; Abrol, N.; Wu, Y.; Chen, B.; Wang, Q.; Granger, J.M.; Tucker-Bartley, A.; Luczak, E.D.; Murphy, K.R.; et al. Oxidized CaMKII and O-GlcNAcylation cause increased atrial fibrillation in diabetic mice by distinct mechanisms. J. Clin. Investig. 2021, 131, e95747. [Google Scholar] [CrossRef] [PubMed]
- Zarain-Herzberg, A.; García-Rivas, G.; Estrada-Avilés, R. Regulation of SERCA pumps expression in diabetes. Cell Calcium 2014, 56, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Borst, J.M.; Bailey, L.R.J.; Shen, E.Y.; Lucena, A.J.; Navedo, M.F.; Bossuyt, J.; Bers, D.M. Hyperglycemia regulates cardiac K+ channels via O-GlcNAc-CaMKII and NOX2-ROS-PKC pathways. Basic Res. Cardiol. 2020, 115, 71. [Google Scholar] [CrossRef]
- Yokoe, S.; Asahi, M.; Takeda, T.; Otsu, K.; Taniguchi, N.; Miyoshi, E.; Suzuki, K. Inhibition of phospholamban phosphorylation by O-GlcNAcylation: Implications for diabetic cardiomyopathy. Glycobiology 2010, 20, 1217–1226. [Google Scholar] [CrossRef]
- Qin, L.; Wang, J.; Zhao, R.; Zhang, X.; Mei, Y. Ginsenoside-Rb1 Improved Diabetic Cardiomyopathy through Regulating Calcium Signaling by Alleviating Protein O-GlcNAcylation. J. Agric. Food Chem. 2019, 67, 14074–14085. [Google Scholar] [CrossRef]
- Cox, E.J.; Marsh, S.A. Exercise and diabetes have opposite effects on the assembly and O-GlcNAc modification of the mSin3A/HDAC1/2 complex in the heart. Cardiovasc. Diabetol. 2013, 12, 101. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Li, M.; Yuan, Q.; Xue, M.; Xu, F.; Chen, Y. Inhibition of ALDH2 by O-GlcNAcylation contributes to the hyperglycemic exacerbation of myocardial ischemia/reperfusion injury. Oncotarget 2017, 8, 19413–19426. [Google Scholar] [CrossRef]
- Kronlage, M.; Dewenter, M.; Grosso, J.; Fleming, T.; Oehl, U.; Lehmann, L.H.; Falcão-Pires, I.; Leite-Moreira, A.F.; Volk, N.; Gröne, H.J.; et al. O-GlcNAcylation of Histone Deacetylase 4 Protects the Diabetic Heart from Failure. Circulation 2019, 140, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Mapanga, R.F.; Joseph, D.; Symington, B.; Garson, K.L.; Kimar, C.; Kelly-Laubscher, R.; Essop, M.F. Detrimental effects of acute hyperglycaemia on the rat heart. Acta Physiol. 2014, 210, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Marchase, R.B.; Chatham, J.C. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1391–H1399. [Google Scholar] [CrossRef] [PubMed]
- Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell Physiol. 2007, 292, C178–C187. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, N.; Zhang, Z.; Marchase, R.B.; Chatham, J.C. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2227–H2236. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Belliard, A.; Mulder, P.; Bouvet, M.; Smet-Nocca, C.; Janel, S.; Lafont, F.; Beseme, O.; Amouyel, P.; Richard, V.; et al. Interplay between troponin T phosphorylation and O-N-acetylglucosaminylation in ischaemic heart failure. Cardiovasc. Res. 2015, 107, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pang, Y.; Chang, T.; Bounelis, P.; Chatham, J.C.; Marchase, R.B. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J. Mol. Cell. Cardiol. 2006, 40, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef]
- Liu, J.; Marchase, R.B.; Chatham, J.C. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J. Mol. Cell. Cardiol. 2007, 42, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, X.; Lee, S.H.; Chen, F.; Jiang, K.; Tu, Z.; Liu, Z.; Du, J.; Wang, L.; Yin, C.; et al. Diabetes Exacerbates Myocardial Ischemia/Reperfusion Injury by Down-Regulation of MicroRNA and Up-Regulation of O-GlcNAcylation. JACC Basic Transl. Sci. 2018, 3, 350–362. [Google Scholar] [CrossRef]
- Dassanayaka, S.; Jones, S.P. O-GlcNAc and the cardiovascular system. Pharmacol. Ther. 2014, 142, 62–71. [Google Scholar] [CrossRef]
- Vibjerg Jensen, R.; Johnsen, J.; Buus Kristiansen, S.; Zachara, N.E.; Bøtker, H.E. Ischemic preconditioning increases myocardial O-GlcNAc glycosylation. Scand. Cardiovasc. J. 2013, 47, 168–174. [Google Scholar] [CrossRef]
- Kristiansen, S.B.; Pælestik, K.B.; Johnsen, J.; Jespersen, N.R.; Pryds, K.; Hjortbak, M.V.; Jensen, R.V.; Bøtker, H.E. Impact of hyperglycemia on myocardial ischemia-reperfusion susceptibility and ischemic preconditioning in hearts from rats with type 2 diabetes. Cardiovasc. Diabetol. 2019, 18, 66. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, H.; Jin, Z.; Bi, S.; Yang, X.; Yi, D.; Liu, W. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur. J. Pharmacol. 2009, 613, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nature reviews. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Marston, N.A.; Giugliano, R.P.; Melloni, G.E.M.; Park, J.G.; Morrill, V.; Blazing, M.A.; Ference, B.; Stein, E.; Stroes, E.S.; Braunwald, E.; et al. Association of Apolipoprotein B-Containing Lipoproteins and Risk of Myocardial Infarction in Individuals with and without Atherosclerosis: Distinguishing between Particle Concentration, Type, and Content. JAMA Cardiol. 2022, 7, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.M.; Mao, C.; Parker, S.J.; Fu, Z.; Yu, G.; Chen, L.; Venkatraman, V.; Fu, Y.; Wang, Y.; Howard, T.D.; et al. Proteomic Architecture of Human Coronary and Aortic Atherosclerosis. Circulation 2018, 137, 2741–2756. [Google Scholar] [CrossRef]
- Floras, J.S. Hypertension, sleep apnea, and atherosclerosis. Hypertension 2009, 53, 1–3. [Google Scholar] [CrossRef]
- Cosentino, F.; Cannon, C.P.; Cherney, D.Z.I.; Masiukiewicz, U.; Pratley, R.; Dagogo-Jack, S.; Frederich, R.; Charbonnel, B.; Mancuso, J.; Shih, W.J.; et al. Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients with Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial. Circulation 2020, 142, 2205–2215. [Google Scholar] [CrossRef]
- Shrikhande, G.V.; Scali, S.T.; da Silva, C.G.; Damrauer, S.M.; Csizmadia, E.; Putheti, P.; Matthey, M.; Arjoon, R.; Patel, R.; Siracuse, J.J.; et al. O-glycosylation regulates ubiquitination and degradation of the anti-inflammatory protein A20 to accelerate atherosclerosis in diabetic ApoE-null mice. PLoS ONE 2010, 5, e14240. [Google Scholar] [CrossRef]
- Yao, D.; Xu, L.; Xu, O.; Li, R.; Chen, M.; Shen, H.; Zhu, H.; Zhang, F.; Yao, D.; Chen, Y.F.; et al. O-Linked β-N-Acetylglucosamine Modification of A20 Enhances the Inhibition of NF-κB (Nuclear Factor-κB) Activation and Elicits Vascular Protection after Acute Endoluminal Arterial Injury. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Basehore, S.E.; Bohlman, S.; Weber, C.; Swaminathan, S.; Zhang, Y.; Jang, C.; Arany, Z.; Clyne, A.M. Laminar Flow on Endothelial Cells Suppresses eNOS O-GlcNAcylation to Promote eNOS Activity. Circ. Res. 2021, 129, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Zhang, Q.; Tsuji-Hosokawa, A.; Watanabe, M.; Willson, C.; Lai, N.; Wang, J.; Dai, A.; Scott, B.T.; Dillmann, W.H.; et al. Overexpression of p53 due to excess protein O-GlcNAcylation is associated with coronary microvascular disease in type 2 diabetes. Cardiovasc. Res. 2020, 116, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.V.; Giachini, F.R.; Carneiro, F.S.; Carvalho, M.H.; Fortes, Z.B.; Webb, R.C.; Tostes, R.C. O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway. Cardiovasc. Res. 2011, 89, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sahu, S.; Chavez, R.J.; Raman, P. Trivalent chromium inhibits TSP-1 expression, proliferation, and O-GlcNAc signaling in vascular smooth muscle cells in response to high glucose in vitro. Am. J. Physiol. Cell Physiol. 2015, 308, C111–C122. [Google Scholar] [CrossRef]
- Lima, V.V.; Giachini, F.R.; Choi, H.; Carneiro, F.S.; Carneiro, Z.N.; Fortes, Z.B.; Carvalho, M.H.; Webb, R.C.; Tostes, R.C. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension 2009, 53, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Pereira, L.; Wang, L.; Han, G.; Ferguson, A.; Dao, K.; Copeland, R.J.; Despa, F.; Hart, G.W.; Ripplinger, C.M.; et al. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature 2013, 502, 372–376. [Google Scholar] [CrossRef]
- Yu, P.; Hu, L.; Xie, J.; Chen, S.; Huang, L.; Xu, Z.; Liu, X.; Zhou, Q.; Yuan, P.; Yan, X.; et al. O-GlcNAcylation of cardiac Nav1.5 contributes to the development of arrhythmias in diabetic hearts. Int. J. Cardiol. 2018, 260, 74–81. [Google Scholar] [CrossRef]
| Target Protein | Regulatory Effects on Protein | Downstream Effects | Mitochondrial Homeostasis | Refs. |
|---|---|---|---|---|
| Drp1 | upregulates Drp1 level and activity | increases mitochondrial fission | mitochondrial fusion and fission | [73,83] |
| Milton | enhances enzyme activity | reduces mitochondrial motility | mitochondrial dynamics | [82] |
| OPA1 | decreases OPA1 protein level | increases mitochondrial fragmentation, reduces the MMP and the activity of mitochondrial complex IV | mitochondrial dysfunction | [84] |
| PGC1-α | increases the activity of PGC1-α and the O-GlcNAcylation of specific transcription factors such as FoxOs | mediates gluconeogenesis | mitochondrial biogenesis and mitochondrial density | [86,87,88,89] |
| NRF2 | increases protein activation | decreases ROS levels to antioxidant response and enhance respiration | mitochondrial biosynthesis | [5,91] |
| mROS | reduces ROS generation | attenuates the formation of mPTPs and the subsequent loss of MMP | mitochondrial oxidative stress, apoptosis, respiration | [5,9,50,93,96,97,98] |
| Ogg1 | increases protein activition | leads to mtDNA damage and oxidative stress | mitochondrial oxidative stress damage | [94] |
| CaMKII | increases protein activation | impaired calcium dynamics and contractile derangements; mitochondrial calcium overload | mitochondrial dysfunction | [6,44,98] |
| mTOR | suppresses proteasome activity | mitophagy | maintains mitochondrial fitness and enhances autophagy flux | [12] |
| Parkin | increases protein activation | mitophagy | mitochondrial quality and mitochondrial homeostasis | [101,102,103] |
| ULK | increases protein activation | mitpphagy | mitochondrial homeostasis | [104] |
| SNAP-29 | increases protein activation | mittophagy | mitochondrial homeostasis | [106,110] |
| MMP | MMP upregulation | inhibits cell apoptosis | mitochondrial apoptosis | [5] |
| VDAC | increases protein activation | reduces mitochondria-related apoptosis | mitochondrial apoptosis | [52,108,109] |
| mPTP | restrains mPTP formation | reduces oxidative stress | mitochondrial apoptosis | [70,98,111], |
| Bcl-2 | increases mitochondrial Bcl-2 levels | attenuates the loss of MMP | mitochondrial apoptosis | [112,113] |
| ETC complexes complex I, complex III, complex IV | impairs activity of complexes I, III and IV | urea cycle, TCA cycle and lipid metabolism | mitochondrial dysfunction and impaired energy function of mitochondria | [8,63,69,73,115,116,117,118] |
| HK1 | enhances the mitochondrial binding of HK1 | coordinates glycolysis and mitochondrial ATP production | Mitochondrial energy metabolism | [10] |
| IDH2 | enhances the protein half-time of IDH2 | increases glycolysis and TCA cycle metabolites | Mitochondrial energy metabolism | [119] |
| O-GlcNAcylation Levels | Effect | Diseases | Refs. | |
|---|---|---|---|---|
| normalized level | positive | cardiac hypertrophy and heart failure | [143] | |
| elevated level | acute increased | negative | hypertension | [186] |
| protective | MI/R | [25,37,38,42,45,47,97,110,111,112,113,126,128,162,163,164,165,166,167,168,169,171,172,173,174] | ||
| atherosclerosis | [36,184] | |||
| chronic increased | negative | cardiac hypertrophy and heart failure | [14,26,35,44,57,121,137,138,139,140,142] | |
| diabetic cardiomyopathy | [6,78,79,129,134,135,146,147,148,149,150,151,152,154,155,156,157] | |||
| diabetic MI/R | [40,170] | |||
| atherosclerosis and coronary heart disease | [124,180,182,185] | |||
| hypertension, arrhythmia with diabetes | [125,187,188] | |||
| decreased level | protective | cardiac hypertrophy and heart failure | [41,46] | |
| diabetic cardiomyopathy | [153,158,160] | |||
| coronary heart disease with diabetes | [124,183,185] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Cui, J.; Huang, Q.; Qi, B.; Xia, Z. Roles of O-GlcNAcylation in Mitochondrial Homeostasis and Cardiovascular Diseases. Antioxidants 2024, 13, 571. https://doi.org/10.3390/antiox13050571
Qiu Z, Cui J, Huang Q, Qi B, Xia Z. Roles of O-GlcNAcylation in Mitochondrial Homeostasis and Cardiovascular Diseases. Antioxidants. 2024; 13(5):571. https://doi.org/10.3390/antiox13050571
Chicago/Turabian StyleQiu, Zhen, Jiahui Cui, Qin Huang, Biao Qi, and Zhongyuan Xia. 2024. "Roles of O-GlcNAcylation in Mitochondrial Homeostasis and Cardiovascular Diseases" Antioxidants 13, no. 5: 571. https://doi.org/10.3390/antiox13050571
APA StyleQiu, Z., Cui, J., Huang, Q., Qi, B., & Xia, Z. (2024). Roles of O-GlcNAcylation in Mitochondrial Homeostasis and Cardiovascular Diseases. Antioxidants, 13(5), 571. https://doi.org/10.3390/antiox13050571







