Ziziphus jujuba Miller Ethanol Extract Restores Disrupted Intestinal Barrier Function via Tight Junction Recovery and Reduces Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extracts of Sample
2.2. Preparation of Standard Solutions and Samples
2.3. Cell Culture and Treatments
2.4. Cell Viability Assay
2.5. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radical Cation Assay
2.6. ABTS (2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) Radical Cation Assay
2.7. RNA Extraction and Quantitative PCR
2.8. Measurement of Trans-Epithelial Electrical Resistance (TEER) and Epithelial Permeability Assay
2.9. Immunofluorescence (IF) Staining
2.10. Protein Extraction and Western Blotting
2.11. Experimental Animals
2.12. Dextran Sodium Sulfate (DSS)-Induced Colitis and Treatment
2.13. Disease Activity Index (DAI) and Scoring
2.14. Endoscopy and Hematoxylin and Eosin (H&E) Staining
2.15. Cytokine Detection Using Enzyme-Linked Immunosorbent (ELISA) Assay
2.16. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.17. Statistical Analysis
3. Results
3.1. ZJB Improved the Disruption of the Intestinal Epithelial Barrier by IL-6
3.1.1. Cytotoxic Effects of IL-6 and ZJB in Caco2 Cells
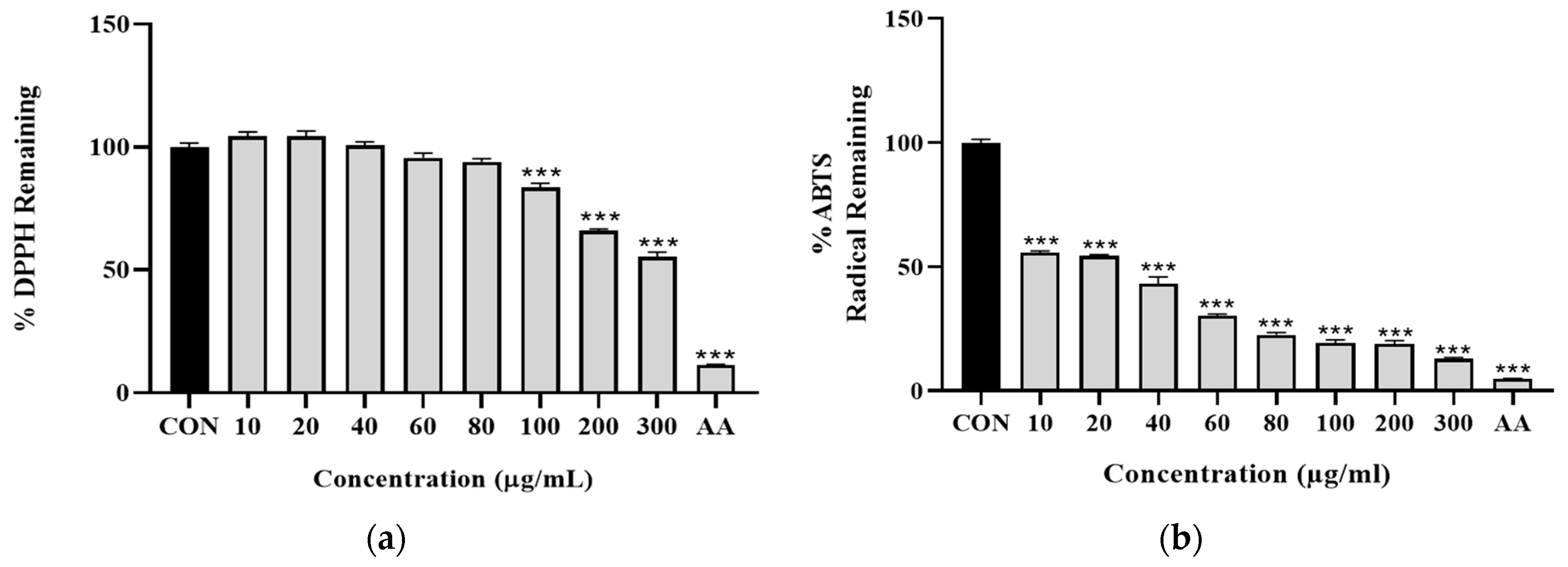
3.1.2. ZJB Has an Antioxidant Effect
3.1.3. Inflammatory Cytokines and Chemokines Are Regulated by ZJB
3.1.4. ZJB Maintains the Function of Intestinal Epithelial Cell Monolayers
3.1.5. ZJB Prevents Morphological Disruption of TJ Proteins Induced by IL-6
3.1.6. ZJB Prevents the Reduction in TJ Proteins Induced by IL-6
3.2. ZJB Protected against Impairment of the Intestinal Barrier and TJs in DSS-Induced Mice
3.2.1. ZJB Restores Weight Loss and Colon Shortening Caused by DSS
3.2.2. ZJB Reduces Cellular Infiltration and Improves Damaged Colon Tissues
3.2.3. ZJB Regulates the Expression of TJ Protein in DSS-Induced Mice
3.2.4. ZJB Inhibits Pro-Inflammatory Cytokine Secretion in DSS-Induced Colitis Mice
3.3. Analysis of LC–MS/MS to Identify Active Compounds in ZJB
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, K.R. Epidemiology: Rising in the east. Nature 2016, 540, S100–S102. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D modulates intestinal microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Park, K.; Bass, D. Inflammatory bowel disease-attributable costs and cost-effective strategies in the United States: A review. Inflamm. Bowel Dis. 2011, 17, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. WJG 2014, 20, 1165. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Huang, Y.; Su, Y.; Qin, R.; Wang, L.; Zhang, Z.; Huang, W.; Fan, X.; Yao, Y.; Wang, H. Mechanism by which oleracein E alleviates TNBS-induced ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 854–864. [Google Scholar] [CrossRef]
- Kim, J.M. Inflammatory bowel diseases and inflammasome. Korean J. Gastroenterol. 2011, 58, 300–310. [Google Scholar] [CrossRef]
- Yang, Q.; Xing, M.; Wang, K.; Wei, Q.; Zhao, J.; Wang, Y.; Ji, K.; Song, S. Application of Fucoidan in Caco-2 Model Establishment. Pharmaceuticals 2022, 15, 418. [Google Scholar] [CrossRef]
- Jing, W.; Dong, S.; Luo, X.; Liu, J.; Wei, B.; Du, W.; Yang, L.; Luo, H.; Wang, Y.; Wang, S. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol. Res. 2021, 164, 105358. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 210. [Google Scholar]
- Ke, F.; Yadav, P.K.; Ju, L.Z. Herbal medicine in the treatment of ulcerative colitis. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2012, 18, 3. [Google Scholar]
- Lee, H.J.; Nagappan, A.; Park, H.S.; Hong, G.E.; Yumnam, S.; Raha, S.; Saralamma, V.V.G.; Lee, W.S.; Kim, E.H.; Kim, G.S. Flavonoids isolated from Citrus platymamma induce mitochondrial-dependent apoptosis in AGS cells by modulation of the PI3K/AKT and MAPK pathways. Oncol. Rep. 2015, 34, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-E.; Kim, J.-A.; Nagappan, A.; Yumnam, S.; Lee, H.-J.; Kim, E.-H.; Lee, W.-S.; Shin, S.-C.; Park, H.-S.; Kim, G.-S. Flavonoids identified from Korean Scutellaria baicalensis Georgi inhibit inflammatory signaling by suppressing activation of NF-κB and MAPK in RAW 264.7 cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 912031. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Wu, W.-H.; Chien, S.-P.; Liu, C.-T.; Liu, M.-Y. Dietary Ziziphus jujuba fruit attenuates colitis-associated tumorigenesis: A pivotal role of the NF-κB/IL-6/JAK1/STAT3 pathway. Nutr. Cancer 2020, 72, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-H.; Wu, C.-S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and antioxidant activity of flavonoids extracted from Xinjiang jujube (Ziziphus jujube Mill.) leaves with ultra-high pressure extraction technology. Molecules 2018, 24, 122. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Matsuoka, K.; Yoshida, A.; Naganuma, M.; Hisamatsu, T.; Yajima, T.; Inoue, N.; Okamoto, S.; Iwao, Y.; Ogata, H. 5-Aminosalicylic acid aggravates colitis mimicking exacerbation of ulcerative colitis. Intest. Res. 2018, 16, 635–640. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Karomah, A.H.; Rafi, M.; Septaningsih, D.A.; Ilmiawati, A.; Syafitri, U.D.; Aminah, N.S.; Insanu, M.; Rohman, A. UHPLC-Q-Orbitrap HRMS-based Untargeted Metabolomics of Sida rhombifolia Leaves and Stem Extracts. HAYATI J. Biosci. 2023, 30, 770–778. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Dautz, C.; Bode-Böger, S.M. Improved method for the determination of cyclic guanosine monophosphate (cGMP) in human plasma by LC–MS/MS. J. Chromatogr. B 2010, 878, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (muscadine grapes) as determined by HPLC-DAD-ESI-MS n. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef]
- Liu, R.; Cai, Z.; Xu, B. Characterization and quantification of flavonoids and saponins in adzuki bean (Vigna angularis L.) by HPLC–DAD–ESI–MSn analysis. Chem. Cent. J. 2017, 11, 93. [Google Scholar] [CrossRef]
- Arun, P.K.; Rajesh, S.S.; Sundaram, S.M.; Sivaraman, T.; Brindha, P. Structural characterizations of lead anticancer compounds from the methanolic extract of. Bangl. J. Pharmacol. 2014, 9, 452–465. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Roblot, F.; Cavé, A.; de, Q. Paulo, M.; Fournet, A. Pessoine and spinosine, two catecholic berbines from Annona spinescens. J. Nat. Prod. 1996, 59, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.-D.; Li, P.; Qi, L.-W.; Li, H.-J.; Yi, L.; Wang, W.; Wang, Y.-Q. Characterization of flavonoid metabolites in rat plasma, urine, and feces after oral administration of Semen Ziziphi Spinosae extract by HPLC-diode-array detection (DAD) and ion-trap mass spectrometry (MSn). Chem. Pharm. Bull. 2009, 57, 144–148. [Google Scholar] [CrossRef]
- Camilleri, Á.; Madsen, K.; Spiller, R.; Van Meerveld, B.; Verne, G. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Chen, X.; Luo, D.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. L-theanine attenuates porcine intestinal tight junction damage induced by LPS via p38 MAPK/NLRP3 signaling in IPEC-J2 cells. Food Chem. Toxicol. 2023, 178, 113870. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Mu, S.; Han, Y.; Chen, Y.; Kuang, Z.; Wu, X.; Luo, Y.; Tong, C.; Zhang, Y.; Yang, Y.; et al. Gpr174 Knockout Alleviates DSS-Induced Colitis via Regulating the Immune Function of Dendritic Cells. Front. Immunol. 2022, 13, 841254. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; He, J.; Xie, K. Zonula Occludens Proteins Signaling in Inflammation and Tumorigenesis. Int. J. Biol. Sci. 2023, 19, 3804. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Song, G.; Hu, H.; Xu, Y.; Zeng, P.; Lin, S.; Yang, J.; Jiang, J.; Song, X.; Luo, Y.; et al. Intestine epithelial cell-derived extracellular vesicles alleviate inflammation induced by Clostridioides difficile TcdB through the activity of TGF-beta1. Mol. Cell Toxicol. 2022, 19, 509–519. [Google Scholar] [CrossRef]
- Sałaga, M.; Zatorski, H.; Sobczak, M.; Chen, C.; Fichna, J. Chinese herbal medicines in the treatment of IBD and colorectal cancer: A review. Curr. Treat. Options Oncol. 2014, 15, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Jang, M.H.; Jang, B.; Bae, S.J.; Bak, S.B.; Lee, S.M.; Yun, U.-J.; Lee, J.H.; Park, S.M.; Jung, D.H.; et al. Jageum-Jung, the herbal pharmaceuticals, inhibits the hepatic fibrogenesis as mediated with TGF-β1/smad signaling. Mol. Cell. Toxicol. 2022, 18, 243–251. [Google Scholar] [CrossRef]
- Pinton, P. Computational models in inflammatory bowel disease. Clin. Transl. Sci. 2022, 15, 824–830. [Google Scholar] [CrossRef]
- Riemschneider, S.; Hoffmann, M.; Slanina, U.; Weber, K.; Hauschildt, S.; Lehmann, J. Indol-3-Carbinol and Quercetin Ameliorate Chronic DSS-Induced Colitis in C57BL/6 Mice by AhR-Mediated Anti-Inflammatory Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 2262. [Google Scholar] [CrossRef]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.-E.; Conklin, L.S.; Centola, M.; Li, X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.N.; Brudzewsky, D.; Gad, M.; Claesson, M.H. Chemokines involved in protection from colitis by CD4+CD25+ regulatory T cells. Inflamm. Bowel Dis. 2006, 12, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R.; Thomas, D.G.; Selvendran, S. The Role of Specific Chemokines in the Amelioration of Colitis by Appendicitis and Appendectomy. Biomolecules 2018, 8, 59. [Google Scholar] [CrossRef]
- Han, X.; Fink, M.P.; Uchiyama, T.; Yang, R.; Delude, R.L. Increased iNOS activity is essential for pulmonary epithelial tight junction dysfunction in endotoxemic mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L259–L267. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Lee, H.D.; Lee, S.; Lee, A.Y. Taraxacum coreanum Nakai extract attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier dysfunction in Caco-2 cells. J. Ethnopharmacol. 2024, 319, 117105. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, W.; Li, M.; He, D.; Han, Y.; Liu, M.; Lu, M. Association between CCL5, CCL11, and CCL17 polymorphisms and atopic dermatitis risk: A systematic review and meta-analysis. Medicine 2024, 103, e36897. [Google Scholar] [CrossRef]
- Rodrı́guez-Juan, C.; Pérez-Blas, M.; Valeri, A.P.; Aguilera, N.; Arnaiz-Villena, A.; Pacheco-Castro, A.; Martı́n-Villa, J.M. Cell surface phenotype and cytokine secretion in Caco-2 cell cultures: Increased RANTES production and IL-2 transcription upon stimulation with IL-1β. Tissue Cell 2001, 33, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Noh, E.-M.; Lee, S.-H.; Lee, S.; Kim, D.H.; Lee, N.H.; Kim, S.-Y.; Park, M.H. Momordica charantia extracts obtained by ultrasound-assisted extraction inhibit the inflammatory pathways. Mol. Cell. Toxicol. 2024, 20, 67–74. [Google Scholar] [CrossRef]
- Pott, J.; Kabat, A.M.; Maloy, K.J. Intestinal epithelial cell autophagy is required to protect against TNF-induced apoptosis during chronic colitis in mice. Cell Host Microbe 2018, 23, 191–202.e194. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, H.; Hu, X.; Dong, W. Hesperetin ameliorates DSS-induced colitis by maintaining the epithelial barrier via blocking RIPK3/MLKL necroptosis signaling. Eur. J. Pharmacol. 2020, 873, 172992. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight junction proteins and signaling pathways in cancer and inflammation: A functional crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, T.; Pathak, D.; Chopra, H. An updated review of Ziziphus jujube: Major focus on its phytochemicals and pharmacological properties. Pharmacol. Res. Mod. Chin. Med. 2023, 8, 100297. [Google Scholar] [CrossRef]
- Van Crombruggen, K.; Van Nassauw, L.; Demetter, P.; Cuvelier, C.; Timmermans, J.P.; Lefebvre, R.A. Influence of soluble guanylate cyclase inhibition on inflammation and motility disturbances in DSS-induced colitis. Eur. J. Pharmacol. 2008, 579, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Serio, R. Preventive effects of guanosine on intestinal inflammation in 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology 2019, 27, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, J.A.; Waldman, S.A. The Guanylate Cyclase C-cGMP Signaling Axis Opposes Intestinal Epithelial Injury and Neoplasia. Front. Oncol. 2018, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Aszodi, A.; Seidler, U.; Ruth, P.; Hofmann, F.; Fassler, R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 1996, 274, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.E.; Snook, A.E.; Li, P.; Stoecker, B.A.; Kim, G.W.; Magee, M.S.; Garcia, A.V.; Valentino, M.A.; Hyslop, T.; Schulz, S.; et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS ONE 2012, 7, e31686. [Google Scholar] [CrossRef]
- Han, X.; Mann, E.; Gilbert, S.; Guan, Y.; Steinbrecher, K.A.; Montrose, M.H.; Cohen, M.B. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS ONE 2011, 6, e16139. [Google Scholar] [CrossRef]












| Gene | Forward | Reverse |
|---|---|---|
| TNF-α | 5′-ACATACTGACCCACGGCTTC-3′ | 5′-GCACTCACCTCTTCCCTCTG-3′ |
| CCL5 | 5′-TGCTGCTTTGCCTACATTG-3′ | 5′-CACTTGGCGGTTCTTTCG-3′ |
| CCL17 | 5′-CTGATGAGCCTCAGGTGACA-3′ | 5′-CCAGGATGCTCTCAGTCACA-3′ |
| GAPDH | 5′-GAAGGTGAAGGTCGGAGT-3′ | 5′-CATGGGTGGAATCATATTGGAA-3′ |
| Peak No. | Compound | Retention Time (min) | Formula | [M + H]+ | MS/MS |
|---|---|---|---|---|---|
| 1 | Guanosine | 3.51 | C10H13N5O5 | 284 | 152, 135, 110, 55 |
| 2 | Guanosine 3′,5′-cyclic monophosphate (cGMP) | 4.59 | C10H12N5O7P− | 346 | 346, 152 |
| 3 | Delphinidin-3,5-diglucoside | 13.42 | C27H31O17+ | 627 | 303, 149 |
| 4 | Vitexin 4″-O-glucoside | 21.96 | C27H30O15 | 595 | 432, 314, 284 |
| 5 | Spinosine | 24.6 | C19H21NO4 | 327 | 327, 192 |
| 6 | Swertisin | 31.3 | C22H22O10 | 446 | 326 |
| 7 | Sinapaldehyde | 32.04 | C11H12O4 | 209 | 191, 177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.J.; Kim, M.J.; Lee, H.J.; Lee, W.-Y.; Yang, J.-H.; Kim, H.H.; Shim, M.S.; Heo, J.W.; Son, J.D.; Kim, W.H.; et al. Ziziphus jujuba Miller Ethanol Extract Restores Disrupted Intestinal Barrier Function via Tight Junction Recovery and Reduces Inflammation. Antioxidants 2024, 13, 575. https://doi.org/10.3390/antiox13050575
Yang YJ, Kim MJ, Lee HJ, Lee W-Y, Yang J-H, Kim HH, Shim MS, Heo JW, Son JD, Kim WH, et al. Ziziphus jujuba Miller Ethanol Extract Restores Disrupted Intestinal Barrier Function via Tight Junction Recovery and Reduces Inflammation. Antioxidants. 2024; 13(5):575. https://doi.org/10.3390/antiox13050575
Chicago/Turabian StyleYang, Ye Jin, Min Jung Kim, Ho Jeong Lee, Won-Yung Lee, Ju-Hye Yang, Hun Hwan Kim, Min Sub Shim, Ji Woong Heo, Jae Dong Son, Woo H. Kim, and et al. 2024. "Ziziphus jujuba Miller Ethanol Extract Restores Disrupted Intestinal Barrier Function via Tight Junction Recovery and Reduces Inflammation" Antioxidants 13, no. 5: 575. https://doi.org/10.3390/antiox13050575
APA StyleYang, Y. J., Kim, M. J., Lee, H. J., Lee, W. -Y., Yang, J. -H., Kim, H. H., Shim, M. S., Heo, J. W., Son, J. D., Kim, W. H., Kim, G. S., Lee, H. -J., Kim, Y. -W., Kim, K. Y., & Park, K. I. (2024). Ziziphus jujuba Miller Ethanol Extract Restores Disrupted Intestinal Barrier Function via Tight Junction Recovery and Reduces Inflammation. Antioxidants, 13(5), 575. https://doi.org/10.3390/antiox13050575








