Microplastics and Oxidative Stress—Current Problems and Prospects
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- Plastic particles, which are common environmental contaminants and exist in the size characteristic of MPs (from 0.1 to 5000 µm);
- (2)
- The evaluation of the direct and indirect impacts of oxidative stress in humans and animals.
3. Results and Discussion
3.1. Oxidative Stress: Mechanisms and Implications
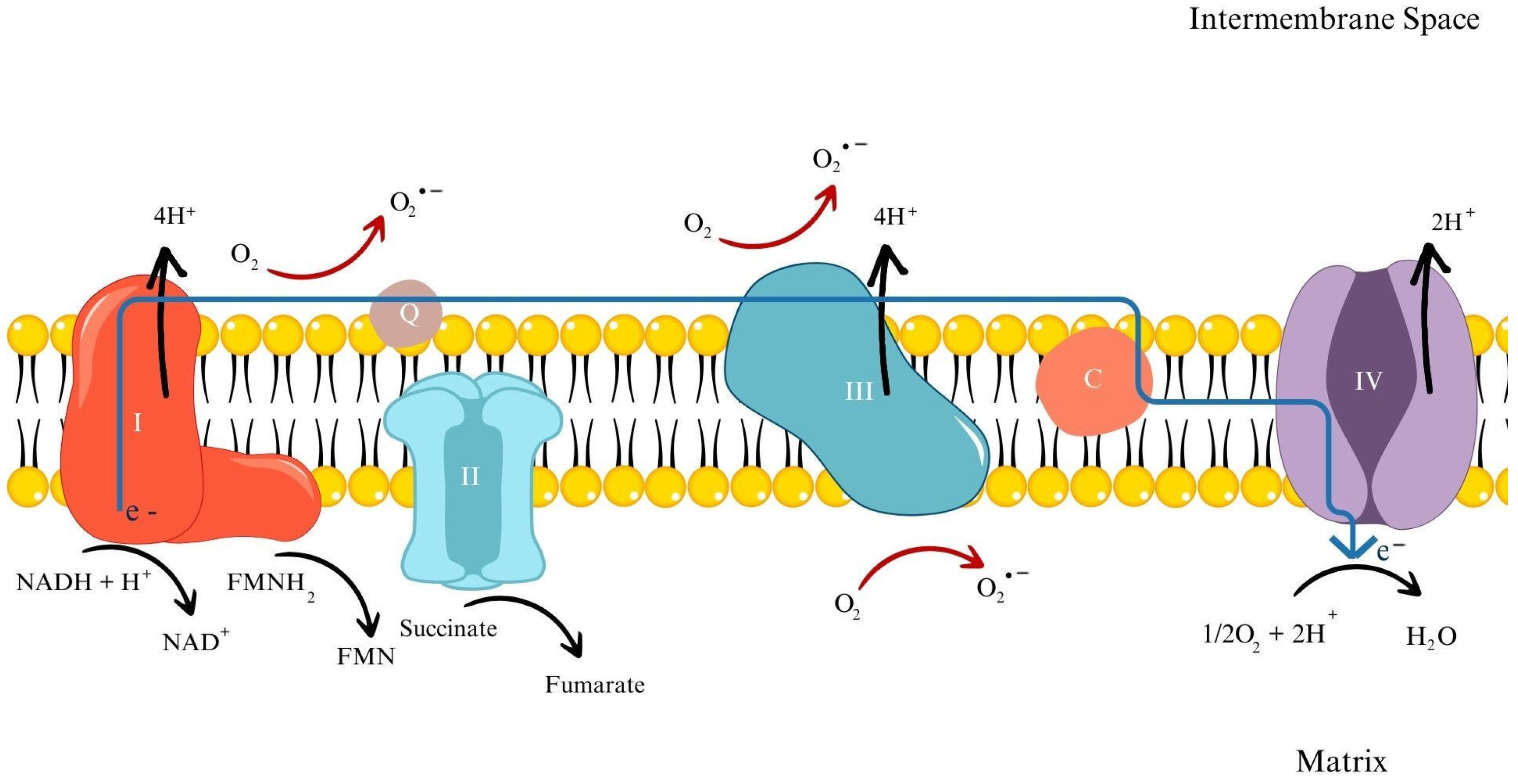
3.1.1. Mechanisms of ROS Generation
3.1.2. Defense Mechanisms against ROS Overproduction
3.2. Consequences of Microplastics—Induced Oxidative Stress
3.2.1. Formation of Oxidative Stress Due to the Effects of MPs
3.2.2. Causes of the Negative Impact of MPs on Organisms
3.3. Research Approaches and Methodologies
3.3.1. Measurement of Reactive Oxygen Species
3.3.2. Oxidative Damage Assessment–LPO Peroxidation
3.3.3. Oxidative Damage Assessment—DNA Damage
3.3.4. Oxidative Status Assessment—Enzymatic Induction of Oxidative Stress
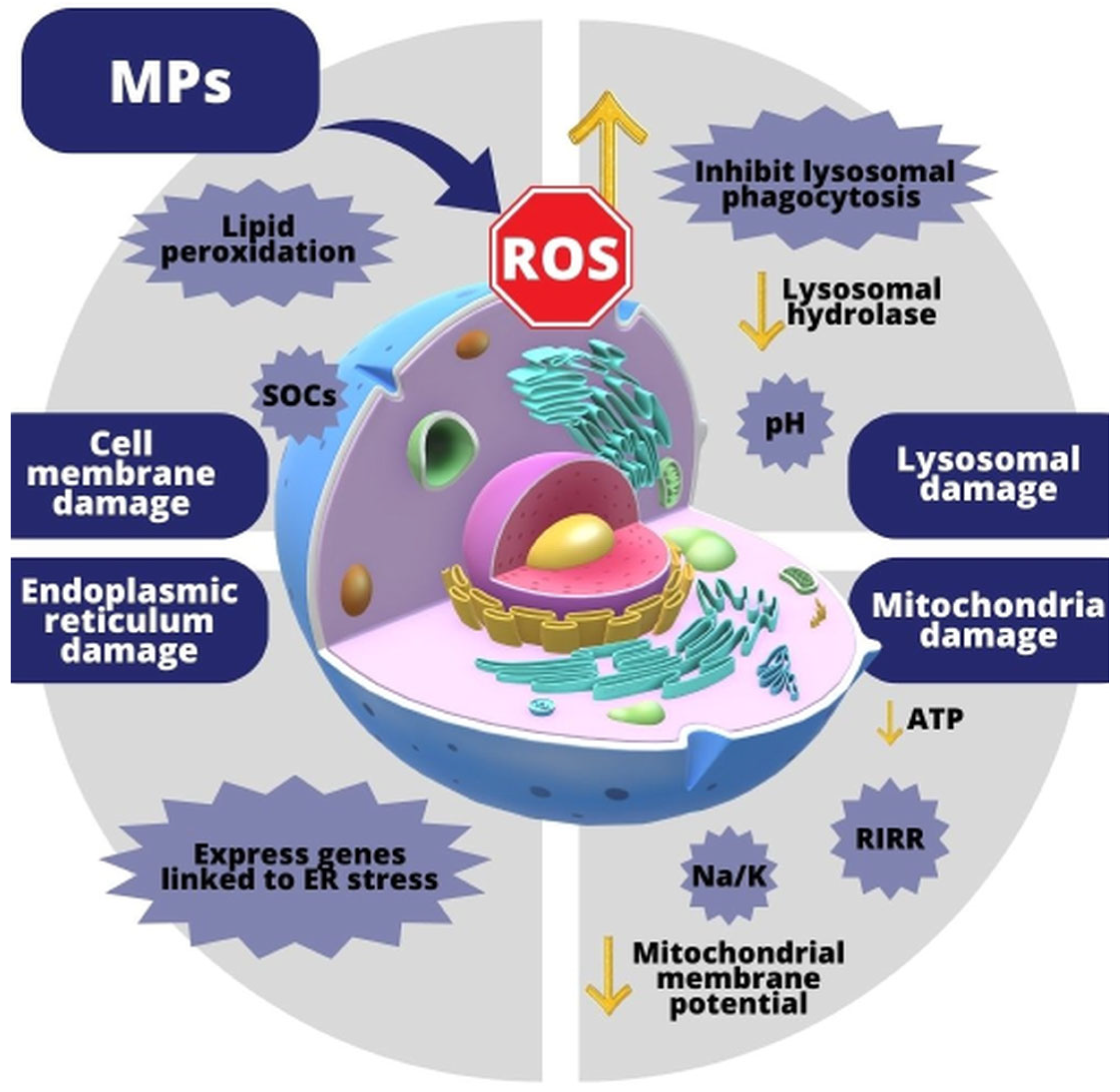
3.4. Effects of MP-Induced Oxidative Stress on Cells
3.4.1. Effect of MPs on the Cell Membrane
3.4.2. Effect of MPs on Lysosomes
3.4.3. Effect of MPs on Mitochondria
3.4.4. Effect of MPs on an Endoplasmic Reticulum
3.5. Effects of MP-Induced Oxidative Stress on Tissues and Organs
3.6. Effects of MP-Induced Oxidative Stress on Organisms
3.6.1. Inhibition of Growth and Reduction in Body Size
3.6.2. Negative Effects on Reproduction and Developmental Changes
3.6.3. Shorter Lifespan
3.7. Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadac-Czapska, K.; Jutrzenka Trzebiatowska, P.; Knez, E.; Zaleska-Medynska, A.; Grembecka, M. Microplastics in Food—A Critical Approach to Definition, Sample Preparation, and Characterisation. Food Chem. 2023, 418, 135985. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Knez, E.; Grembecka, M. Food and Human Safety: The Impact of Microplastics. Crit. Rev. Food Sci. Nutr. 2024, 64, 3502–3521. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Shyu, D.J.H.; Domínguez, R.; Lorenzo, J.M.; Pereira, J.A.M.; Câmara, J.S. Polystyrene Microplastic Particles in the Food Chain: Characteristics and Toxicity—A Review. Sci. Total Environ. 2023, 892, 164531. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Jeon, J.H.; Jeong, J.; Kim, G.; Lee, S.; Kim, S.; Maruthupandy, M.; Lee, K.; Yang, S.I.; Cho, W.-S. Size- and Oxidative Potential-Dependent Toxicity of Environmentally Relevant Expanded Polystyrene Styrofoam Microplastics to Macrophages. J. Hazard. Mater. 2023, 459, 132295. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sözener, Z.; Cevhertas, L.; Nadeau, K.; Akdis, M.; Akdis, C.A. Environmental Factors in Epithelial Barrier Dysfunction. J. Allergy Clin. Immunol. 2020, 145, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Environment: Intake through the Food Web, Human Exposure and Toxicological Effects. Toxics 2021, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as Carriers of Inorganic and Organic Contaminants in the Environment: A Review of Recent Progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Rainieri, S.; Barranco, A. Microplastics, a Food Safety Issue? Trends Food Sci. Technol. 2019, 84, 55–57. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, S.; Liu, Y.; Feng, Z.; Mu, C.; Zhang, T. The Combined Effects of Microplastics and Bisphenol-A on the Innate Immune System Response and Intestinal Microflora of the Swimming Crab Portunus Trituberculatus. Aquat. Toxicol. 2024, 268, 106855. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yan, Z.; Shen, R.; Huang, Y.; Ren, H.; Zhang, Y. Enhanced Reproductive Toxicities Induced by Phthalates Contaminated Microplastics in Male Mice (Mus musculus). J. Hazard. Mater. 2021, 406, 124644. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.E.; Zucker, I. Interactions of Microplastics and Organic Compounds in Aquatic Environments: A Case Study of Augmented Joint Toxicity. Chemosphere 2022, 289, 133212. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.C.; Monnolo, A.; Del Piano, F.; Mattace Raso, G.; Meli, R. The Pressing Issue of Micro- and Nanoplastic Contamination: Profiling the Reproductive Alterations Mediated by Oxidative Stress. Antioxidants 2022, 11, 193. [Google Scholar] [CrossRef]
- Ding, R.; Ma, Y.; Li, T.; Sun, M.; Sun, Z.; Duan, J. The Detrimental Effects of Micro-and Nano-Plastics on Digestive System: An Overview of Oxidative Stress-Related Adverse Outcome Pathway. Sci. Total Environ. 2023, 878, 163144. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of Marine Mussels Mytilus Spp. to Polystyrene Microplastics: Toxicity and Influence on Fluoranthene Bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-P38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Liu, L.; Zhang, Q.; Xu, S.; Guo, M. Polystyrene Microplastics Induced Inflammation with Activating the TLR2 Signal by Excessive Accumulation of ROS in Hepatopancreas of Carp (Cyprinus Carpio). Ecotoxicol. Environ. Saf. 2023, 251, 114539. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D. Oxidative Stress. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 45–48. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Niki, E. Oxidative Stress and Antioxidants: Distress or Eustress? Free Radic. Biol. Med. 2018, 124, 564. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; O’Neill, L.A.J. The Role of the Electron Transport Chain in Immunity. FASEB J. 2021, 35, e21974. [Google Scholar] [CrossRef]
- Mazat, J.-P.; Devin, A.; Ransac, S. Modelling Mitochondrial ROS Production by the Respiratory Chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron Homeostasis and Iron-Regulated ROS in Cell Death, Senescence and Human Diseases. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yang, W.; Li, X.; Lin, T.; Lun, Y.; Lin, F.; Lv, S.; Yan, G.; Liu, J.; Shen, J.; et al. A Trifunctional Enzyme with Glutathione S-Transferase, Glutathione Peroxidase and Superoxide Dismutase Activity. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2008, 1780, 869–872. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Brioukhanov, A.L.; Netrusov, A.I. Catalase and Superoxide Dismutase: Distribution, Properties, and Physiological Role in Cells of Strict Anaerobes. Biochemistry 2004, 69, 949–962. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Gao, Y.; Yin, P.; Pan, C.; Liu, W.; Zhou, Z.; Wang, J. Curcumin Protects Human Adipose-Derived Mesenchymal Stem Cells against Oxidative Stress-Induced Inhibition of Osteogenesis. J. Pharmacol. Sci. 2016, 132, 192–200. [Google Scholar] [CrossRef]
- Saleem, U.; Sabir, S.; Niazi, S.G.; Naeem, M.; Ahmad, B. Role of Oxidative Stress and Antioxidant Defense Biomarkers in Neurodegenerative Diseases. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 311–322. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene Nanoplastics Deteriorate LPS-Modulated Duodenal Permeability and Inflammation in Mice via ROS Drived-NF-ΚB/NLRP3 Pathway. Chemosphere 2022, 307, 135662. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chang, X.; Hu, M.; Fang, J.K.-H.; Sokolova, I.M.; Huang, W.; Xu, E.G.; Wang, Y. Is Microplastic an Oxidative Stressor? Evidence from a Meta-Analysis on Bivalves. J. Hazard. Mater. 2022, 423, 127211. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene Microplastics-Induced Cardiotoxicity in Chickens via the ROS-Driven NF-ΚB-NLRP3-GSDMD and AMPK-PGC-1α Axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef]
- Zou, H.; Qu, H.; Bian, Y.; Sun, J.; Wang, T.; Ma, Y.; Yuan, Y.; Gu, J.; Bian, J.; Liu, Z. Polystyrene Microplastics Induce Oxidative Stress in Mouse Hepatocytes in Relation to Their Size. Int. J. Mol. Sci. 2023, 24, 7382. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, M.; Song, Y.; Lu, S.; Hu, J.; Cao, C.; Xie, B.; Shi, H.; He, D. Polystyrene (Nano)Microplastics Cause Size-Dependent Neurotoxicity, Oxidative Damage and Other Adverse Effects in Caenorhabditis elegans. Environ. Sci. Nano 2018, 5, 2009–2020. [Google Scholar] [CrossRef]
- Tidjani, A. Comparison of Formation of Oxidation Products during Photo-Oxidation of Linear Low Density Polyethylene under Different Natural and Accelerated Weathering Conditions. Polym. Degrad. Stab. 2000, 68, 465–469. [Google Scholar] [CrossRef]
- Gillen, K.T.; Bernstein, R.; Celina, M. Non-Arrhenius Behavior for Oxidative Degradation of Chlorosulfonated Polyethylene Materials. Polym. Degrad. Stab. 2005, 87, 335–346. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. Springerplus 2013, 2, 398. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and Effects of Microplastics on Cells and Tissue of the Blue Mussel Mytilus edulis L. after an Experimental Exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Geys, J.; Coenegrachts, L.; Vercammen, J.; Engelborghs, Y.; Nemmar, A.; Nemery, B.; Hoet, P.H.M. In Vitro Study of the Pulmonary Translocation of Nanoparticles. Toxicol. Lett. 2006, 160, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yan, X.; Feng, L.; Jiang, S.; Lu, Z.; Xie, H.; Sun, S.; Chen, J.; Li, C. Challenge for the Detection of Microplastics in the Environment. Water Environ. Res. 2021, 93, 5–15. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene Microplastics Cause Granulosa Cells Apoptosis and Fibrosis in Ovary through Oxidative Stress in Rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to Polystyrene Microplastics Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-B.; Kang, H.-M.; Lee, M.-C.; Kim, D.-H.; Han, J.; Hwang, D.-S.; Souissi, S.; Lee, S.-J.; Shin, K.-H.; Park, H.G.; et al. Adverse Effects of Microplastics and Oxidative Stress-Induced MAPK/Nrf2 Pathway-Mediated Defense Mechanisms in the Marine Copepod Paracyclopina Nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio Rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, C.; Zhou, J.; Shen, M.; Wang, X.; Fu, Z.; Jin, Y. Effects of Polystyrene Microplastics on the Composition of the Microbiome and Metabolism in Larval Zebrafish. Chemosphere 2019, 217, 646–658. [Google Scholar] [CrossRef]
- McCubrey, J.A.; LaHair, M.M.; Franklin, R.A. Reactive Oxygen Species-Induced Activation of the MAP Kinase Signaling Pathways. Antioxid. Redox Signal 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, B. The Role of Nrf2 and MAPK Pathways in PFOS-Induced Oxidative Stress in Zebrafish Embryos. Toxicol. Sci. 2010, 115, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of Polystyrene Microplastics in Juvenile Eriocheir Sinensis and Oxidative Stress Effects in the Liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Fleury, J.-B.; Baulin, V.A. Microplastics Destabilize Lipid Membranes by Mechanical Stretching. Proc. Natl. Acad. Sci. USA 2021, 118, e2104610118. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.-G. Lipid Peroxidation and Its Toxicological Implications. Toxicol. Res. 2011, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics Cause Neurotoxicity, Oxidative Damage and Energy-Related Changes and Interact with the Bioaccumulation of Mercury in the European Seabass, Dicentrarchus Labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Montalbetti, E.; Isa, V.; Vencato, S.; Louis, Y.; Montano, S.; Lavorano, S.; Maggioni, D.; Galli, P.; Seveso, D. Short-Term Microplastic Exposure Triggers Cellular Damage through Oxidative Stress in the Soft Coral Coelogorgia palmosa. Mar. Biol. Res. 2022, 18, 495–508. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, J.; Li, F.; Ji, C.; Wu, H. Evidence-Based Meta-Analysis of the Genotoxicity Induced by Microplastics in Aquatic Organisms at Environmentally Relevant Concentrations. Sci. Total Environ. 2021, 783, 147076. [Google Scholar] [CrossRef]
- Maity, S.; Guchhait, R.; De, S.; Pramanick, K. High Doses of Nano-Polystyrene Aggravate the Oxidative Stress, DNA Damage, and the Cell Death in Onions. Environ. Pollut. 2023, 316, 120611. [Google Scholar] [CrossRef]
- Shen, R.; Yang, K.; Cheng, X.; Guo, C.; Xing, X.; Sun, H.; Liu, D.; Liu, X.; Wang, D. Accumulation of Polystyrene Microplastics Induces Liver Fibrosis by Activating CGAS/STING Pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef]
- He, T.; Qu, Y.; Yang, X.; Liu, L.; Xiong, F.; Wang, D.; Liu, M.; Sun, R. Research Progress on the Cellular Toxicity Caused by Microplastics and Nanoplastics. J. Appl. Toxicol. 2023, 43, 1576–1593. [Google Scholar] [CrossRef]
- Malinowska, K.; Bukowska, B.; Piwoński, I.; Foksiński, M.; Kisielewska, A.; Zarakowska, E.; Gackowski, D.; Sicińska, P. Polystyrene Nanoparticles: The Mechanism of Their Genotoxicity in Human Peripheral Blood Mononuclear Cells. Nanotoxicology 2022, 16, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and Cytotoxic Effects of Polyethylene Microplastics on Human Peripheral Blood Lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of Microplastics on the Accumulation and Chronic Toxic Effects of Cadmium in Zebrafish (Danio Rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics Effects in Scrobicularia Plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants Bioavailability and Toxicological Risk from Microplastics to Marine Mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.-D.H. Antioxidants and Molecular Damage in Nile Tilapia (Oreochromis Niloticus) after Exposure to Microplastics. Environ. Sci. Pollut. Res. 2020, 27, 14581–14588. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, P.; Maharjan, A.; Acharya, M.; Lee, D.; Kusma, S.; Gautam, R.; Kwon, J.-T.; Kim, C.; Kim, K.; Kim, H.; et al. Polytetrafluorethylene Microplastic Particles Mediated Oxidative Stress, Inflammation, and Intracellular Signaling Pathway Alteration in Human Derived Cell Lines. Sci. Total Environ. 2023, 897, 165295. [Google Scholar] [CrossRef] [PubMed]
- Boháčková, J.; Havlíčková, L.; Semerád, J.; Titov, I.; Trhlíková, O.; Beneš, H.; Cajthaml, T. In Vitro Toxicity Assessment of Polyethylene Terephthalate and Polyvinyl Chloride Microplastics Using Three Cell Lines from Rainbow Trout (Oncorhynchus Mykiss). Chemosphere 2023, 312, 136996. [Google Scholar] [CrossRef]
- Liu, T.; Hou, B.; Wang, Z.; Yang, Y. Polystyrene Microplastics Induce Mitochondrial Damage in Mouse GC-2 Cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, J.; Ning, B.; Fan, L.; Sun, T.; Fang, Y.; Wu, J.; Li, S.; Duan, C.; Zhang, Y.; et al. Effects of Bisphenol A and Nanoscale and Microscale Polystyrene Plastic Exposure on Particle Uptake and Toxicity in Human Caco-2 Cells. Chemosphere 2020, 254, 126788. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Li, Y.; Feng, Y.; Wang, Y.; Cheng, W. Low-Dose of Polystyrene Microplastics Induce Cardiotoxicity in Mice and Human-Originated Cardiac Organoids. Environ. Int. 2023, 179, 108171. [Google Scholar] [CrossRef] [PubMed]
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P.; et al. Specific Uptake and Genotoxicity Induced by Polystyrene Nanobeads with Distinct Surface Chemistry on Human Lung Epithelial Cells and Macrophages. PLoS ONE 2015, 10, e0123297. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; da Silva Brito, W.A.; Singer, D.; Mühl, M.; Berner, J.; Saadati, F.; Wolff, C.; Miebach, L.; Wende, K.; Bekeschus, S. Short- and Long-Term Polystyrene Nano- and Microplastic Exposure Promotes Oxidative Stress and Divergently Affects Skin Cell Architecture and Wnt/Beta-Catenin Signaling. Part. Fibre Toxicol. 2023, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Magara, G.; Khan, F.R.; Pinti, M.; Syberg, K.; Inzirillo, A.; Elia, A.C. Effects of Combined Exposures of Fluoranthene and Polyethylene or Polyhydroxybutyrate Microplastics on Oxidative Stress Biomarkers in the Blue Mussel (Mytilus edulis). J. Toxicol. Environ. Health A 2019, 82, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Chen Wongworawat, Y.; Filippova, M.; Williams, V.M.; Filippov, V.; Duerksen-Hughes, P.J. Chronic Oxidative Stress Increases the Integration Frequency of Foreign DNA and Human Papillomavirus 16 in Human Keratinocytes. Am. J. Cancer Res. 2016, 6, 764–780. [Google Scholar] [PubMed]
- Cohen, S. Reactive Oxygen Species and Serous Epithelial Ovarian Adenocarcinoma. Cancer Res. J. 2016, 4, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ubezio, P.; Civoli, F. Flow Cytometric Detection of Hydrogen Peroxide Production Induced by Doxorubicin in Cancer Cells. Free Radic. Biol. Med. 1994, 16, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Peshavariya, H.M.; Dusting, G.J.; Selemidis, S. Analysis of Dihydroethidium Fluorescence for the Detection of Intracellular and Extracellular Superoxide Produced by NADPH Oxidase. Free Radic. Res. 2007, 41, 699–712. [Google Scholar] [CrossRef]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and Clinical Application of a New, Fast Method for the Detection of Hydroperoxides in Serum. Panminerva Med. 2002, 44, 37–40. [Google Scholar] [PubMed]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Buwono, N.R.; Risjani, Y.; Soegianto, A. Oxidative Stress Responses of Microplastic-Contaminated Gambusia Affinis Obtained from the Brantas River in East Java, Indonesia. Chemosphere 2022, 293, 133543. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. Oxidative Stress and Human Diseases: Origin, Link, Measurement, Mechanisms, and Biomarkers. Crit. Rev. Clin. Lab. Sci. 2009, 46, 241–281. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, S.S.; de Souza-Neto, F.P.; Ramalho, L.N.Z.; Derossi, D.R.; Guarnier, F.A.; da Silva, C.F.N.; Melo, G.P.; Simão, A.N.C.; Cecchini, R.; Cecchini, A.L. Systemic Oxidative Profile after Tumor Removal and the Tumor Microenvironment in Melanoma Patients. Cancer Lett. 2015, 361, 226–232. [Google Scholar] [CrossRef]
- Blasi, M.A.; Maresca, V.; Roccella, M.; Roccella, F.; Sansolini, T.; Grammatico, P.; Balestrazzi, E.; Picardo, M. Antioxidant Pattern in Uveal Melanocytes and Melanoma Cell Cultures. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3012–3016. [Google Scholar] [PubMed]
- Yu, R.; Zhao, G.; Christman, J.W.; Xiao, L.; van Breemen, R.B. Method Development and Validation for Ultra-High Pressure Liquid Chromatography/Tandem Mass Spectrometry Determination of Multiple Prostanoids in Biological Samples. J. AOAC Int. 2013, 96, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Nishikawa, T.; Nakajima, T.; Okada, Y.; Yamaguchi, K.; Mitsuyoshi, H.; Yasui, K.; Minami, M.; Iwai, M.; Kagawa, K.; et al. Oxidative Stress Is Closely Associated with Tumor Angiogenesis of Hepatocellular Carcinoma. J. Gastroenterol. 2011, 46, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E. Lipid Peroxidation and Antioxidant Status in Colorectal Cancer. World J. Gastroenterol. 2005, 11, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Facundo, H.T.F.; Brandt, C.T.; Owen, J.S.; Lima, V.L.M. Elevated Levels of Erythrocyte-Conjugated Dienes Indicate Increased Lipid Peroxidation in Schistosomiasis Mansoni Patients. Braz. J. Med. Biol. Res. 2004, 37, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Nouroozzadeh, J.; Tajaddinisarmadi, J.; Wolff, S.P. Measurement of Plasma Hydroperoxide Concentrations by the Ferrous Oxidation-Xylenol Orange Assay in Conjunction with Triphenylphosphine. Anal. Biochem. 1994, 220, 403–409. [Google Scholar] [CrossRef]
- Gonzalez-Hunt, C.P.; Wadhwa, M.; Sanders, L.H. DNA Damage by Oxidative Stress: Measurement Strategies for Two Genomes. Curr. Opin. Toxicol. 2018, 7, 87–94. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.; Di Mascio, P.; Wagner, J.R. Formation and Repair of Oxidatively Generated Damage in Cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Anson, R.M.; Mason, P.A.; Bohr, V.A. Gene-Specific and Mitochondrial Repair of Oxidative DNA Damage. Methods Mol. Biol. 2006, 314, 155–181. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Cadet, J.; Mőller, L.; Poulsen, H.E.; Viña, J. Are We Sure We Know How to Measure 8-Oxo-7,8-Dihydroguanine in DNA from Human Cells? Arch. Biochem. Biophys. 2004, 423, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shi, W.; Tang, Y.; Han, Y.; Du, X.; Zhou, W.; Zhang, W.; Sun, C.; Liu, G. The Toxic Impacts of Microplastics (MPs) and Polycyclic Aromatic Hydrocarbons (PAHs) on Haematic Parameters in a Marine Bivalve Species and Their Potential Mechanisms of Action. Sci. Total Environ. 2021, 783, 147003. [Google Scholar] [CrossRef]
- Lin, W.; Jiang, R.; Hu, S.; Xiao, X.; Wu, J.; Wei, S.; Xiong, Y.; Ouyang, G. Investigating the Toxicities of Different Functionalized Polystyrene Nanoplastics on Daphnia Magna. Ecotoxicol. Environ. Saf. 2019, 180, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Camenzind, M.; Fent, K. Silica Nanoparticles Induce Endoplasmic Reticulum Stress Response, Oxidative Stress and Activate the Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway. Toxicol. Rep. 2014, 1, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Paraiso, K.H.T.; Van Der Kooi, K.; Messina, J.L.; Smalley, K.S.M. Measurement of Constitutive MAPK and PI3K/AKT Signaling Activity in Human Cancer Cell Lines. Methods Enzymol. 2010, 484, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Kefaloyianni, E.; Gaitanaki, C.; Beis, I. ERK1/2 and P38-MAPK Signalling Pathways, through MSK1, Are Involved in NF-ΚB Transactivation during Oxidative Stress in Skeletal Myoblasts. Cell Signal 2006, 18, 2238–2251. [Google Scholar] [CrossRef]
- Xu, B.; Lang, L.-M.; Lian, S.; Guo, J.-R.; Wang, J.-F.; Yang, H.-M.; Li, S.-Z. Oxidation Stress-Mediated MAPK Signaling Pathway Activation Induces Neuronal Loss in the CA1 and CA3 Regions of the Hippocampus of Mice Following Chronic Cold Exposure. Brain Sci. 2019, 9, 273. [Google Scholar] [CrossRef]
- Naderi, J.; Hung, M.; Pandey, S. Oxidative Stress-Induced Apoptosis in Dividing Fibroblasts Involves Activation of P38 MAP Kinase and over-Expression of Bax: Resistance of Quiescent Cells to Oxidative Stress. Apoptosis 2003, 8, 91–100. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen Free Radicals and Iron in Relation to Biology and Medicine: Some Problems and Concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A Simple Method for Clinical Assay of Superoxide Dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, V.B. Free Radicals in Cell Biology. Int. Rev. Cytol. 2004, 237, 57–89. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Masiak, R.; Szpinda, M.; Mila-Kierzenkowska, C.; Woźniak, B.; Makarewicz, R.; Szpinda, A. Oxidative Stress Markers in Prostate Cancer Patients after HDR Brachytherapy Combined with External Beam Radiation. Oxid. Med. Cell Longev. 2012, 2012, 789870. [Google Scholar] [CrossRef]
- Arthur, J.R. The Glutathione Peroxidases. Cell. Mol. Life Sci. 2001, 57, 1825–1835. [Google Scholar] [CrossRef]
- Güner, G.; İşlekel, H.; Oto, Ö.; Hazan, E.; Açikel, Ü. Evaluation of Some Antioxidant Enzymes in Lung Carcinoma Tissue. Cancer Lett. 1996, 103, 233–239. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Strzelczyk, J.K.; Wielkoszyński, T.; Krakowczyk, Ł.; Adamek, B.; Zalewska-Ziob, M.; Gawron, K.; Kasperczyk, J.; Wiczkowski, A. The Activity of Antioxidant Enzymes in Colorectal Adenocarcinoma and Corresponding Normal Mucosa. Acta Biochim. Pol. 2012, 59, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ridderström, M.; Mannervik, B. Human Glutathione Transferase A4-4: An Alpha Class Enzyme with High Catalytic Efficiency in the Conjugation of 4-Hydroxynonenal and Other Genotoxic Products of Lipid Peroxidation. Biochem. J. 1998, 330, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Roušar, T. Detection of Oxidative Stress Induced by Nanomaterials in Cells—The Roles of Reactive Oxygen Species and Glutathione. Molecules 2021, 26, 4710. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, G.F.; Pérez-Pomeda, I.; Sanchís, J.; Rossini, C.; Farré, M.; Barceló, D. Cytotoxic Effects of Commonly Used Nanomaterials and Microplastics on Cerebral and Epithelial Human Cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Galloway, T.S. Ingestion of Nanoplastics and Microplastics by Pacific Oyster Larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Alavehzadeh, A.; Ramezani, M.; Pourahmad, J. Differences in Sensitivity of Human Lymphocytes and Fish Lymphocytes to Polyvinyl Chloride Microplastic Toxicity. Toxicol. Ind. Health 2022, 38, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, M.; Salmistraro, N.; Porro, D.; Pinsino, A.; Colangelo, A.M.; Gaglio, D. Polystyrene Micro and Nano-Particles Induce Metabolic Rewiring in Normal Human Colon Cells: A Risk Factor for Human Health. Chemosphere 2022, 303, 134947. [Google Scholar] [CrossRef] [PubMed]
- Saenen, N.D.; Witters, M.S.; Hantoro, I.; Tejeda, I.; Ethirajan, A.; Van Belleghem, F.; Smeets, K. Polystyrene Microplastics of Varying Sizes and Shapes Induce Distinct Redox and Mitochondrial Stress Responses in a Caco-2 Monolayer. Antioxidants 2023, 12, 739. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Olga, V.; Xue, Y.; Lv, S.; Diao, X.; Zhang, Y.; Han, Q.; Zhou, H. The Potential Effects of Microplastic Pollution on Human Digestive Tract Cells. Chemosphere 2022, 291, 132714. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, K.-F.; Lin, K.-Y.A.; Chen, J.-K.; Jiang, X.-Y.; Lin, C.-H. The Nephrotoxic Potential of Polystyrene Microplastics at Realistic Environmental Concentrations. J. Hazard. Mater. 2022, 427, 127871. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and Sub-Chronic Effects of Microplastics (3 and 10 Μm) on the Human Intestinal Cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 5833. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Chandrasekaran, N.; Gopinath, P.M.; Mukherjee, A. Exposure to Polystyrene Nanoplastics Impairs Lipid Metabolism in Human and Murine Macrophages in Vitro. Ecotoxicol. Environ. Saf. 2022, 238, 113612. [Google Scholar] [CrossRef] [PubMed]
- Watson, H. Biological Membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular Internalization and Release of Polystyrene Microplastics and Nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef] [PubMed]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of Polycations: Relationship of Molecular Weight and the Hydrolytic Theory of the Mechanism of Toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Qiu, Z.; Cui, Z.; Li, N.; Li, X.; Wang, Y.; Zhang, H.; Zhao, C. Effects of Polyethylene Microplastics on Cell Membranes: A Combined Study of Experiments and Molecular Dynamics Simulations. J. Hazard. Mater. 2022, 429, 128323. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Han, J.; Liu, X.; Li, K.; Lai, W.; Bian, L.; Yan, J.; Xi, Z. Exposure to Polypropylene Microplastics via Oral Ingestion Induces Colonic Apoptosis and Intestinal Barrier Damage through Oxidative Stress and Inflammation in Mice. Toxics 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.E.; Zoncu, R. The Lysosome as a Cellular Centre for Signalling, Metabolism and Quality Control. Nat. Cell Biol. 2019, 21, 133–142. [Google Scholar] [CrossRef]
- Deng, J.; Ibrahim, M.S.; Tan, L.Y.; Yeo, X.Y.; Lee, Y.A.; Park, S.J.; Wüstefeld, T.; Park, J.W.; Jung, S.; Cho, N.J. Microplastics Released from Food Containers Can Suppress Lysosomal Activity in Mouse Macrophages. J. Hazard. Mater. 2022, 435, 128980. [Google Scholar] [CrossRef]
- Fiorentino, I.; Gualtieri, R.; Barbato, V.; Mollo, V.; Braun, S.; Angrisani, A.; Turano, M.; Furia, M.; Netti, P.A.; Guarnieri, D.; et al. Energy Independent Uptake and Release of Polystyrene Nanoparticles in Primary Mammalian Cell Cultures. Exp. Cell Res. 2015, 330, 240–247. [Google Scholar] [CrossRef]
- Florance, I.; Ramasubbu, S.; Mukherjee, A.; Chandrasekaran, N. Polystyrene Nanoplastics Dysregulate Lipid Metabolism in Murine Macrophages in Vitro. Toxicology 2021, 458, 152850. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Ciacci, C.; Fabbri, R.; Balbi, T.; Salis, A.; Damonte, G.; Cortese, K.; Caratto, V.; Monopoli, M.P.; Dawson, K.; et al. Interactions of Cationic Polystyrene Nanoparticles with Marine Bivalve Hemocytes in a Physiological Environment: Role of Soluble Hemolymph Proteins. Environ. Res. 2016, 150, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bexiga, M.G.; Anguissola, S.; Boya, P.; Simpson, J.C.; Salvati, A.; Dawson, K.A. Time Resolved Study of Cell Death Mechanisms Induced by Amine-Modified Polystyrene Nanoparticles. Nanoscale 2013, 5, 10868. [Google Scholar] [CrossRef] [PubMed]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative Diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, X.; Liu, Q.; Zhou, N.; Zhu, S.; Li, Z.; Li, X.; Yao, J.; Zhang, L. The Impact of Polystyrene Microplastics on Cardiomyocytes Pyroptosis through NLRP3/Caspase-1 Signaling Pathway and Oxidative Stress in Wistar Rats. Environ. Toxicol. 2021, 36, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, X.; Qi, X.; Liu, X.; Zhang, Y.; Qiao, S.; Lin, H. Di-(2-Ethylhexyl) Phthalate and Microplastics Induced Neuronal Apoptosis through the PI3K/AKT Pathway and Mitochondrial Dysfunction. J. Agric. Food Chem. 2022, 70, 10771–10781. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-Induced ROS Release: An Update and Review. Biochim. Biophys. Acta (BBA)—Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Lee, S.E.; Yi, Y.; Moon, S.; Yoon, H.; Park, Y.S. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites 2022, 12, 897. [Google Scholar] [CrossRef]
- Malinowska, K.; Sicińska, P.; Michałowicz, J.; Bukowska, B. The Effects of Non-Functionalized Polystyrene Nanoparticles of Different Diameters on the Induction of Apoptosis and MTOR Level in Human Peripheral Blood Mononuclear Cells. Chemosphere 2023, 335, 139137. [Google Scholar] [CrossRef] [PubMed]
- Félix, L.; Carreira, P.; Peixoto, F. Effects of Chronic Exposure of Naturally Weathered Microplastics on Oxidative Stress Level, Behaviour, and Mitochondrial Function of Adult Zebrafish (Danio Rerio). Chemosphere 2023, 310, 136895. [Google Scholar] [CrossRef] [PubMed]
- Herrala, M.; Huovinen, M.; Järvelä, E.; Hellman, J.; Tolonen, P.; Lahtela-Kakkonen, M.; Rysä, J. Micro-Sized Polyethylene Particles Affect Cell Viability and Oxidative Stress Responses in Human Colorectal Adenocarcinoma Caco-2 and HT-29 Cells. Sci. Total Environ. 2023, 867, 161512. [Google Scholar] [CrossRef]
- Pan, L.; Yu, D.; Zhang, Y.; Zhu, C.; Yin, Q.; Hu, Y.; Zhang, X.; Yue, R.; Xiong, X. Polystyrene Microplastics-Triggered Mitophagy and Oxidative Burst via Activation of PERK Pathway. Sci. Total Environ. 2021, 781, 146753. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic Reticulum Stress Signalling—From Basic Mechanisms to Clinical Applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- Haeri, M.; Knox, B.E. Endoplasmic Reticulum Stress and Unfolded Protein Response Pathways: Potential for Treating Age-Related Retinal Degeneration. J. Ophthalmic Vis. Res. 2012, 7, 45–59. [Google Scholar] [PubMed]
- Wang, W.; Guan, J.; Feng, Y.; Nie, L.; Xu, Y.; Xu, H.; Fu, F. Polystyrene Microplastics Induced Nephrotoxicity Associated with Oxidative Stress, Inflammation, and Endoplasmic Reticulum Stress in Juvenile Rats. Front. Nutr. 2022, 9, 1059660. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Q.; Cui, J.; Bao, B.; Deng, X.; Liu, L.; Guo, M. Polystyrene Microplastics Induce Endoplasmic Reticulum Stress, Apoptosis and Inflammation by Disrupting the Gut Microbiota in Carp Intestines. Environ. Pollut. 2023, 323, 121233. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guan, J.; Feng, Y.; Liu, S.; Zhao, Y.; Xu, Y.; Xu, H.; Fu, F. Polystyrene Microplastics Induced Ovarian Toxicity in Juvenile Rats Associated with Oxidative Stress and Activation of the PERK-EIF2α-ATF4-CHOP Signaling Pathway. Toxics 2023, 11, 225. [Google Scholar] [CrossRef]
- Saemi-Komsari, M.; Pashaei, R.; Abbasi, S.; Esmaeili, H.R.; Dzingelevičienė, R.; Shirkavand Hadavand, B.; Pasalari Kalako, M.; Szultka-Mlynska, M.; Gadzała-Kopciuch, R.; Buszewski, B.; et al. Accumulation of Polystyrene Nanoplastics and Triclosan by a Model Tooth-Carp Fish, Aphaniops hormuzensis (Teleostei: Aphaniidae). Environ. Pollut. 2023, 333, 121997. [Google Scholar] [CrossRef]
- da Silva Brito, W.A.; Mutter, F.; Wende, K.; Cecchini, A.L.; Schmidt, A.; Bekeschus, S. Consequences of Nano and Microplastic Exposure in Rodent Models: The Known and Unknown. Part. Fibre Toxicol. 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hosny, M.; Eltaweil, A.S.; Omar, S.; Elgarahy, A.M.; Farghali, M.; Yap, P.-S.; Wu, Y.-S.; Nagandran, S.; Batumalaie, K.; et al. Microplastic Sources, Formation, Toxicity and Remediation: A Review. Environ. Chem. Lett. 2023, 21, 2129–2169. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Espinosa, C.; García Beltrán, J.M.; Esteban, M.A.; Cuesta, A. In Vitro Effects of Virgin Microplastics on Fish Head-Kidney Leucocyte Activities. Environ. Pollut. 2018, 235, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, T.; Lv, W.; Wang, H.; Chen, H.; Xu, Q.; Cai, H.; Dai, J. Intratracheal Administration of Polystyrene Microplastics Induces Pulmonary Fibrosis by Activating Oxidative Stress and Wnt/β-Catenin Signaling Pathway in Mice. Ecotoxicol. Environ. Saf. 2022, 232, 113238. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Deng, B.; Kang, Z.; Araujo, P.; Mjøs, S.A.; Liu, R.; Lin, J.; Yang, T.; Qu, Y. Tissue Accumulation of Polystyrene Microplastics Causes Oxidative Stress, Hepatopancreatic Injury and Metabolome Alterations in Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2023, 256, 114871. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Sadasivam, N.; Kim, Y.-J.; Radhakrishnan, K.; Kim, D.-K. Oxidative Stress, Genomic Integrity, and Liver Diseases. Molecules 2022, 27, 3159. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Z.; Huang, H. Effect of Microplastics on Antioxidant Enzyme System in Juvenile Red Crucian Carp. Environ. Sci. Technol. China 2019, 42, 23–27. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Adverse Outcome Pathways Potentially Related to Hazard Identification of Microplastics Based on Toxicity Mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in Female Fertility: A Role in Oxidative Stress and Aging. Antioxid. Redox Signal 2020, 32, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Almeida, H.; Castro, J.P. (In)Fertility and Oxidative Stress: New Insights into Novel Redox Mechanisms Controlling Fundamental Reproductive Processes. Oxid. Med. Cell Longev. 2020, 2020, 4674896. [Google Scholar] [CrossRef] [PubMed]
- Jeyavani, J.; Sibiya, A.; Bhavaniramya, S.; Mahboob, S.; Al-Ghanim, K.A.; Nisa, Z.; Riaz, M.N.; Nicoletti, M.; Govindarajan, M.; Vaseeharan, B. Toxicity Evaluation of Polypropylene Microplastic on Marine Microcrustacean Artemia Salina: An Analysis of Implications and Vulnerability. Chemosphere 2022, 296, 133990. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Besseling, E.; Foekema, E.M.; Kamermans, P.; Koelmans, A.A. Effects of Nanopolystyrene on the Feeding Behavior of the Blue Mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 2012, 31, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic Effects of Microplastic on Marine Microalgae Skeletonema Costatum: Interactions between Microplastic and Algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens. Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoon, H.; Choi, J.S.; Jung, Y.-J.; Park, J.-W. Chronic Effects of Nano and Microplastics on Reproduction and Development of Marine Copepod Tigriopus Japonicus. Ecotoxicol. Environ. Saf. 2022, 243, 113962. [Google Scholar] [CrossRef]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive Toxicity of Polystyrene Microplastics: In Vivo Experimental Study on Testicular Toxicity in Mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene Microplastics Induced Male Reproductive Toxicity in Mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene Microplastics Induced Female Reproductive Toxicity in Mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, Y.; Rihan, N.; Jiang, Q.; Liu, X.; Zhao, Y.; Che, X. Polystyrene Nanoplastics Decrease Nutrient Accumulation, Disturb Sex Hormones, and Inhibit Reproductive Development in Juvenile Macrobrachium Nipponense. Sci. Total Environ. 2023, 891, 164481. [Google Scholar] [CrossRef] [PubMed]
- Jaafarzadeh Haghighi Fard, N.; Mohammadi, M.J.; Jahedi, F. Effects of Nano and Microplastics on the Reproduction System: In Vitro and in Vivo Studies Review. Food Chem. Toxicol. 2023, 178, 113938. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Wang, S.; Xie, J.; Han, Q.; Chen, M. Comparing the Effects of Polystyrene Microplastics Exposure on Reproduction and Fertility in Male and Female Mice. Toxicology 2022, 465, 153059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, C.-Y.; Duan, J.-X.; Li, Q.; Yang, H.-H.; Sun, C.-C.; Zhang, J.; Luo, X.-Q.; Liu, S.-K. Vasoactive Intestinal Peptide Suppresses the NLRP3 Inflammasome Activation in Lipopolysaccharide-Induced Acute Lung Injury Mice and Macrophages. Biomed. Pharmacother. 2020, 121, 109596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene Microplastics Cause Tissue Damages, Sex-Specific Reproductive Disruption and Transgenerational Effects in Marine Medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Cheng, J. Exposure to Polystyrene Microplastics Impairs Gonads of Zebrafish (Danio rerio). Chemosphere 2021, 263, 128161. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Zhao, H.; Cai, J.; Sultan, Y.; Fang, H.; Zhang, B.; Ma, J. Effects of Polyvinyl Chloride Microplastics on Reproduction, Oxidative Stress and Reproduction and Detoxification-Related Genes in Daphnia Magna. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109269. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of Microplastic Toxicity to Embryonic Development of the Sea Urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Afreen, V.; Hashmi, K.; Nasir, R.; Saleem, A.; Khan, M.I.; Akhtar, M.F. Adverse Health Effects and Mechanisms of Microplastics on Female Reproductive System: A Descriptive Review. Environ. Sci. Pollut. Res. 2023, 30, 76283–76296. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the Mass of Microplastics Ingested—A Pivotal First Step towards Human Health Risk Assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M. Ingested Microplastics: Do Humans Eat One Credit Card per Week? J. Hazard. Mater. Lett. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; de Jersey, A.M.; Lavers, J.L.; Rodemann, T.; Rivers-Auty, J. Identifying Laboratory Sources of Microplastic and Nanoplastic Contamination from the Air, Water, and Consumables. J. Hazard. Mater. 2024, 465, 133276. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, A.; Zubrowska-Sudol, M.; Krasinski, A.; Sudol, M. Cross-Contamination as a Problem in Collection and Analysis of Environmental Samples Containing Microplastics—A Review. Sustainability 2021, 13, 12123. [Google Scholar] [CrossRef]
- Jeyavani, J.; Sibiya, A.; Gopi, N.; Mahboob, S.; Riaz, M.N.; Vaseeharan, B. Dietary Consumption of Polypropylene Microplastics Alter the Biochemical Parameters and Histological Response in Freshwater Benthic Mollusc Pomacea paludosa. Environ. Res. 2022, 212, 113370. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhu, X.; Duan, Y.; Huang, J.; Nan, Y.; Zhang, J. Toxic Effects of Nitrite and Microplastics Stress on Histology, Oxidative Stress, and Metabolic Function in the Gills of Pacific White Shrimp, Litopenaeus vannamei. Mar. Pollut. Bull. 2023, 187, 114531. [Google Scholar] [CrossRef]
- Yedier, S.; Yalçınkaya, S.K.; Bostancı, D. Exposure to Polypropylene Microplastics via Diet and Water Induces Oxidative Stress in Cyprinus Carpio. Aquat. Toxicol. 2023, 259, 106540. [Google Scholar] [CrossRef]
- Kelpsiene, E.; Ekvall, M.T.; Lundqvist, M.; Torstensson, O.; Hua, J.; Cedervall, T. Review of Ecotoxicological Studies of Widely Used Polystyrene Nanoparticles. Environ. Sci. Process Impacts 2022, 24, 8–16. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and Biotechnological Perspectives on Microbial Degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Li, Y.; Tao, L.; Wang, Q.; Wang, F.; Li, G.; Song, M. Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects. Environ. Health 2023, 1, 249–257. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-Sized Microplastics and Pigmented Particles in Bottled Mineral Water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Yhome, N.; Muralidaran, Y.; Rajagopal, S.; Mishra, P. Nanoplastics Toxicity Specific to Liver in Inducing Metabolic Dysfunction—A Comprehensive Review. Genes 2023, 14, 590. [Google Scholar] [CrossRef] [PubMed]
| Cells | MP Type | MP Size | MP Shape | Dose | Exposure Time | Association (OS vs. Endpoints) | Reference |
|---|---|---|---|---|---|---|---|
| Human cells | |||||||
| A549 cells | PTFE | 6 and 31.7 μm | Fragments | 10, 100, 500, and 1000 μg/mL | 24 h | ROS increase (6 µm, 10 μg/mL); ROS decrease (31.7 µm, 1000 μg/mL); Increase secretion of IL-6 (6 µm, 1000 μg/mL; 31.7 µm, 10, 1000 μg/mL) | [68] |
| Caco-2 cells | PS | 0.3, 0.5, 1, 3, and 6 μm | Spheres | 20, 50, 70, 90, and 120 μg/mL | 24 h | ROS increase (20, 50, 70, 90, and 120 μg/L); Increased mitochondrial membrane potential (20, 50, 70, 90, and 120 μg/L); Cytotoxicity (0.3 µm, 20, 50, 70, 90, 120 μg/mL; 0.5 µm, 120 µg/mL, 1µm, 90, 120 µg/mL; 3 µm, 70, 90, 120 µg/mL; 6 µm, 50, 70, 90, and 120 μg/mL) | [71] |
| Caco-2 cells | PS | 0.1 and 5 µm | Spheres | 1, 10, 40, 80, and 200 µg/mL | 12 h | ROS increase (200 µg/mL) | [120] |
| Caco-2 cells | PS | 0.2 and 2 μm | Spheres | 10, and 100 µg/mL | 24 h | Decrease in intracellular H2O2 levels (0.2 µm, 10 µg/mL); Differential expressions of redox-related genes, including HMOX1, CAT, and GPx1 (2 µm, 100 µg/mL) | [119] |
| Caco-2 cells | PS | 8.9 µm and 1.14 µm | Fibers/ Fragments | 10 and 100 µg/mL | 24 h | Decrease in intracellular H2O2 levels (10 and 100 µg/mL) | [119] |
| Caco-2 cells | PTFE | 6 and 31.7 μm | Fragments | 10, 100, 500, and 1000 μg/mL | 24 h | ROS increase (6 µm; 100 μg/mL); ROS decrease (31.7 µm, 1000 μg/mL); Nitric oxide induction (6 µm, 500 μg/mL; 31.7 µm, 1000 μg/mL) | [68] |
| CCD841CoN | PS | 0.1, 0.5, 1, and 5 µm | Spheres | 12.5, 25, 50, and 100 μg/mL | 1 h | ROS increase (0.5 µm, 100 μg/mL) | [121] |
| CCD841CoN | PS | 0.1, 0.5, 1, and 5 µm | Spheres | 12.5, 25, 50, and 100 μg/mL | 0.5 h | ROS decrease (0.1 µm, 50 and 100 μg/mL; 0.5 µm, 25, 50, and 100 μg/mL; 5 µm, 50 and 100 μg/mL) | [121] |
| HaCaT cells | PTFE | 6 and 31.7 μm | Fragments | 10, 100, 500, and 1000 μg/mL | 24 h | ROS decrease (6 µm, 500 and 1000 μg/mL; 31.7 µm, 1000 μg/mL); Nitric oxide induction (6 µm, 10, 100, and 1000 μg/mL; 31.7 µm, 10 μg/mL) | [68] |
| HeLa cells | PE | 3–16 µm | Spheres | from 0.01 to 10 μg/mL | 24 h | Cytotoxicity (0.05, 0.1, 1, and 10 μg/mL) | [115] |
| HeLa cells | PS | 10 µm | Spheres | from 0.01 to 10 μg/mL | 24 h | ROS increase (10 μg/mL); Cytotoxicity (0.05, 0.1, 1, and 10 μg/mL) | [115] |
| HEK293 cells | PS | 3.15–3.93 µm | Spheres | 300 ng/mL | - | Decreased activity of HMOX1 (300 ng/mL); Cytotoxicity (300 ng/mL) | [122] |
| HIEC-6 | PS | 0.1, 0.5, 1, and 5 µm | Spheres | 12.5, 25, 50, and 100 μg/mL | 4 h | ROS increase (0.1 µm, 100 μg/mL; 0.5 µm, 100 μg/mL; 5 µm, 25, 50, and 100 μg/mL) | [121] |
| HIEC-6 | PS | 0.1, 0.5, 1, and 5 µm | Spheres | 12.5, 25, 50, and 100 μg/mL | 8 h | ROS increase (0.1 µm, 100 μg/mL; 0.5 µm, 25, 50, and 100 μg/mL; 5 µm, 100 μg/mL) | [121] |
| HIEC-6 | PS | 0.1, 0.5, 1, and 5 µm | Spheres | 12.5, 25, 50, and 100 μg/mL | 24 h | ROS increase (0.1 µm, 50 and 100 μg/mL) | [121] |
| HT-29 cells | PS | 3 and 10 µm | Spheres | 800 and 1600 MPs/mL | 7 d | ROS decrease (3 µm, 1600 MPs/mL; 10 µm, 1600 MPs/mL); ROS increase (3 µm, 800 MPs/mL; 10 µm, 800 MPs/mL) | [123] |
| HT-29 cells | PS | 3 and 10 µm | Spheres | 800 and 1600 MPs/mL | 14 and 21 d | ROS decrease (800 and 1600 MPs/mL) | [123] |
| HT-29 cells | PS | 3 and 10 µm | Spheres | 800 and 1600 MPs/mL | 28 d | ROS decrease (3 µm, 1600 MPs/mL) ROS increase (3 µm, 800 MPs/mL; 10 µm, 800 MPs/mL; 10 µm, 1600 MPs/mL) | [123] |
| HT-29 cells | PS | 3 and 10 µm | Spheres | 800 and 1600 MPs/mL | 48 d | ROS increase (3 µm, 800 and 1600 MPs/mL; 10 µm, 800 and 1600 MPs/mL | [123] |
| Human lymphocytes | PVC | 0.16–1.82 μm | Spheres | 24, 48, and 96 μg/mL | 1 h | ROS increase (48 and 96 μg/mL); Increased activity of GSSG (24, 48, and 96 μg/mL); Decreased activity of GSH (24, 48, and 96 μg/mL); Mitochondrial membrane potential collapse (24, 48, and 96 μg/mL) | [117] |
| Human lymphocytes | PVC | 0.16–1.82 μm | Spheres | 24, 48, and 96 μg/mL | 2 and 3 h | ROS increase (24, 48, and 96 μg/mL); Increased activity of GSSG (24, 48, and 96 μg/mL); Decreased activity of GSH (24, 48, and 96 μg/mL); Mitochondrial membrane potential collapse (24, 48, and 96 μg/mL) | [117] |
| Human lymphocytes | PVC | 0.16–1.82 μm | Spheres | 12, 25, 50, and 100 μg/mL | 3 h | Cytotoxicity (25, 50, and 100 μg/mL) | [117] |
| Human macrophage | PS | 0.2 µm | Spheres | 100 µg/mL | 24 h | The accumulation of lipids droplets in the cytoplasm (100 μg/mL) | [124] |
| Human-originated cardiac organoids | PS | 1 µm | Spheres | 0.025, 0.25, and 2.5 µg/mL | 24 h | Decrease in ATP content (0.025, 0.25, and 2.5 µg/mL); SOD reduction (0.025, 0.25, and 2.5 µg/mL); Cytotoxicity (0.25 and 2.5 µg/mL) | [72] |
| Jurkat cells | PTFE | 6 and 31.7 μm | Fragments | 10, 100, 500, and 1000 μg/mL | 48 h | ROS increase (6 µm, 10 μg/mL; 31.7 µm, 10 and 100 μg/mL); Nitric oxide induction (31.7 µm, 10 and 1000 μg/mL) | [68] |
| THP-1 cells | PTFE | 6 and 31.7 μm | Fragments | 10, 100, 500, and 1000 μg/mL | 48 h | ROS increase (6 µm, 10, 100, 500, and 1000 μg/mL; 31.7 µm, 10 and 500 μg/mL); Nitric oxide induction (6 µm, 500 and 1000 μg/mL) | [68] |
| THP-1 cells | PS | 0.5–1 μm and 8–10 μm | Fragments | 62.5, 125, and 250 μg/mL | 24 h and 72 h | Activation of NLRP3 inflammasome (250 μg/mL); Increased levels of IL-1β and MIP-1β (62.5, 125, and 250 μg/mL) | [5] |
| THP-1 cells | PS | 0.5 μm and 3 μm | Spheres | 62.5, 125, and 250 μg/mL | 24 h and 72 h | Activation of NLRP3 inflammasome (250 μg/mL) Increased levels of IL-1β and MIP-1β (62.5, 125, and 250 μg/mL) | [5] |
| T98G cells | PE | 3–16 µm | Spheres | from 0.01 to 10 μg/mL | 24 h | ROS increase (0.05, 0.1 μg/mL); Cytotoxicity (0.05, 0.1, 1, and 10 μg/mL) | [115] |
| T98G cells | PS | 10 µm | Spheres | from 0.01 to 10 μg/mL | 24 h | ROS increase (0.05, 0.1, 1, and 10 μg/mL); Cytotoxicity (0.05, 0.1, 1, and 10 μg/mL) | [115] |
| U937 cells | PTFE | 6 and 31.7 μm | Fragments | 10, 100, 500, and 1000 μg/mL | 48 h | ROS increase (6 µm, 100, 500, and 1000 μg/mL) | [68] |
| Animal cells | |||||||
| GC-2 cells | PS | 5 µm | Spheres | 25 mg/mL | 6 h | ROS increase (25 mg/mL); Decrease in ATP content (25 mg/mL) | [70] |
| GC-2 cells | PS | 5 µm | Spheres | 25 mg/mL | 12 h | ROS increase (25 mg/mL) | [70] |
| GC-2 cells | PS | 5 µm | Spheres | 25 mg/mL | 18 h | ROS increase (25 mg/mL) | [70] |
| GC-2 cells | PS | 5 µm | Spheres | 25 mg/mL | 24 h | ROS increase (25 mg/mL); Reduction in mitochondrial membrane potential (25 mg/mL); Activation of the mitochondrial autophagy pathway PINK1/Parkin (25 mg/mL) | [70] |
| RTG-2 cells | PVC | 25 and 90 μm | Spheres | 1 mg/mL | 24 h | ROS increase (1 mg/mL) | [69] |
| RTgill-W1 cells | PVC | 25 and 90 μm | Spheres | 1 mg/mL | 24 h | ROS increase (1 mg/mL) | [69] |
| RTL-W1 cells | PVC | 25 and 90 μm | Spheres | 1 mg/mL | 24 h | ROS increase (1 mg/mL) | [69] |
| Skin cells mouse (fibroblasts, keratinocyte) | PS | 0.2, 1, 2 and 6 µm | Spheres | 100 µg/mL | 24 h | ROS increase (100 µg/mL) | [74] |
| Tissues/Organs | MP Type | MP Size | MP Shape | Dose | Exposure Time | Association (OS vs. Endpoints) | Reference |
|---|---|---|---|---|---|---|---|
| Mice | |||||||
| Mice intestinal tract | PP | 8 and 10 µm | Fragments | 0.1, 1.0, and 10 mg/mL | 28 d | Increased activity of MDA (0.1, 1.0, and 10 mg/mL) and GSSG (1.0, and 10 mg/mL); Decreased activity of CAT, SOD, GSH, and GPx (0.1, 1.0, and 10 mg/mL); Activation of the TLR4/NFκB inflammatory signal pathway (0.1, 1.0, and 10 mg/mL) | [129] |
| Mice liver tissue | PS | 0.5 and 5 μm | Spheres | 10 mg/L | 3 m | Decreased activity of SOD, GPx, and CAT (10 mg/L); Reduction in the expression of proteins related to oxidative stress, SIRT3, and SOD2 (10 mg/L) | [37] |
| Mice liver tissue | PS | 5 µm | Spheres | 0.01 mg/day (1 × 105 MPs) | 28 d | Increased activity of CAT, GPx, and AChE (0.01 mg/day) | [158] |
| Mice liver tissue | PS | 5 µm | Spheres | 0.1 mg/day (1 × 106 MPs) and 0.5 mg/day (5 × 106 MPs) | 28 d | Decreased activity of CAT (0.1 mg/day and 0.5 mg/day); Increased activity of SOD, GPx, and AChE (0.1 mg/day and 0.5 mg/day) | [158] |
| Mice liver tissue | PS | 20 µm | Spheres | 0.01 mg/day (2 × 103 MPs) | 28 d | Increased activity of SOD, GPx, and AChE (0.01 mg/day) | [158] |
| Mice liver tissue | PS | 20 µm | Spheres | 0.1 mg/day (2 × 104 MPs) and 0.5 mg/day (1 × 105 MPs) | 28 d | Decreased activity of CAT (0.1 mg/day and 0.5 mg/day); Increased activity of SOD, GPx, and AChE (0.1 mg/day and 0.5 mg/day) | [158] |
| Mice lung tissue | PS | 5 μm | Spheres | 1.25 and 6.25 mg/kg | 3 times a week for 3 w | Decreased activity of SOD (6.25 mg/kg) and GPx (1.25 and 6.25 mg/kg) | [156] |
| Mice myocardial tissue | PS | 500 μm | Spheres | 0.5, 5, and 50 mg/L | 90 d | Increased levels of MDA (5 and 50 mg/L); Decreased activity of SOD, GPx, and CAT (5 and 50 mg/L); Morphological changes in mitochondria (5 and 50 mg/L) | [138] |
| Other | |||||||
| Clam digestive gland (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 3 d | Increased activity of GPx (1 mg/L) | [65] |
| Clam digestive gland (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 14 d | Increased activity of SOD (1 mg/L) | [65] |
| Clam digestive gland (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 21 d | Increased activity of SOD (1 mg/L); Decreased activity of CAT, GPx, and GST (1 mg/L) | [65] |
| Clam gills (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 3 d | Increased activity of CAT and GPx (1 mg/L) | [65] |
| Clam gills (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 7 d | Increased activity of SOD (1 mg/L) | [65] |
| Clam gills (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 14 d | Increased activity of SOD and GST (1 mg/L) | [65] |
| Clam gills (Scrobicularia plana) | PS | 20 µm | Spheres | 1 mg/L | 21 d | Increased activity of SOD and GPx (1 mg/L) | [65] |
| Crab liver tissue (Eriocheir sinensis) | PS | 0.5 µm | Spheres | 40 and 400 μg/L | 7 d | Increased activity of SOD, GSH, GPx, and GOT (40 and 400 μg/L); Decreased activity of CAT, AChE, GPT, GST, and MDA (40 and 400 μg/L) | [53] |
| Crab liver tissue (Eriocheir sinensis) | PS | 0.5 µm | Spheres | 4000 and 40,000 μg/L | 7 d | Increased activity of MDA (4000 and 40,000 μg/L); Decreased activity of CAT, SOD, AChE, GOT, GPT, GPx, GSH, and GST (4000 and 40,000 μg/L) | [53] |
| Shrimp (Litopenaeus vannamei) liver tissues | PS | 2 μm | Spheres | 0.02 mg/L | 8 d | Increased levels of SOD and GPx (0.02 mg/L); Decreased activity of CAT (0.02 mg/L) | [157] |
| Shrimp (Litopenaeus vannamei) pancreas tissues | PS | 2 μm | Spheres | 0.2 and 1 mg/L | 8 d | Increased levels of MDA, SOD, and GPx (0.2 and 1 mg/L); Decreased activity of CAT (0.2 and 1 mg/L) | [157] |
| Zebrafish brain (Danio rerio) | EP | <200 µm | Fragments | 0.1 and 1 mg/L | 21 d | Increased activity of CAT, GSH, and GSSG (1 mg/L); Decreased of LDH (1 mg/L) | [143] |
| Zebrafish gut (Danio rerio) | PS | 5 µm | Spheres | 50 μg/L and 500 μg/L | 21 d | Increased activity of CAT, SOD, and D-lactate (50 μg/L and 500 μg/L); Decreased activity of DAO (50 μg/L and 500 μg/L) | [42] |
| Zebrafish liver (Danio rerio) | PS | 5 µm | Spheres | 20 µg/L (2.9 × 102 particles/mL) | 7 d | Increased activity of CAT (20 µg/L) | [49] |
| Zebrafish liver (Danio rerio) | PS | 5 µm | Spheres | 200 µg/L (2.9 × 103 particles/mL) and 2000 µg/L (2.9 × 104 particles/mL) | 7 d | Increased activity of CAT and SOD (200 µg/L and 2000 µg/L) | [49] |
| Zebrafish liver (Danio rerio) | EP | <200 µm | Fragments | 0.1 and 1 mg/L | 21 d | Increased activity of CAT and SOD (1 mg/L); Decreased activity of GPx and GST (1 mg/L); Decreased mitochondrial membrane potential (1 mg/L) | [143] |
| Organism | MP Type | MP Size | MP Shape | Dose | Exposure Time | Association (OS vs. Endpoints) | Reference |
|---|---|---|---|---|---|---|---|
| Benthic mollusc (Pomacea paludosa) | PP | 11.86–44.62 μm | Spheres | 250, 500, and 750 mg/kg | 28 d | ROS increase (250, 500, and 750 mg/kg); Lipid peroxidation (250, 500, and 750 mg/kg); Impairs the biochemical parameters of CAT and GPx (250, 500, and 750 mg/kg); Reduced GSH and GST (250, 500, and 750 mg/kg) | [1] |
| Caenorhabditis elegans | PS | 0.5, 1, 2, and 5 µm | Spheres | 1 mg/L | 3 d | Increase in gst-4p: GFP expression (1 mg/L) | [2] |
| Coral (Coelogorgia palmosa) | PE | 180–212 µm | Spheres | 50–70 mg/L | 2 d | Increased activity of CAT, SOD, and GSR (50–70 mg/L); Lipid peroxidation (50–70 mg/L) | [3] |
| Daphnia magna | carboxylate-modified PS | 0.3 µm | Spheres | 1 mg/L | 2 d | Increased activity of SOD (1 mg/L); Decreased activity of GSH (1 mg/L); Increased levels of MDA (1 mg/L); Reduction in AChE (1 mg/L) | [4] |
| Larval zebrafish (Danio rerio) | PS | 5 and 50 µm | Spheres | 100 and 1000 µg/L | 7 d | Decreased activity of GSH (100 and 1000 µg/L); Decreased activity of CAT (1000 µg/L) | [5] |
| Marine copepod (Paracyclopina nana) | PS | 0.5 µm | Spheres | 20 mg/mL | 1 d | Increased activity of GSR, SOD, GST, and GPx (20 mg/mL) | [6] |
| Marine copepod (Paracyclopina nana) | PS | 6 µm | Spheres | 20 mg/mL | 1 d | Increased activity of SOD, GST, and GPx (20 mg/mL) | [6] |
| Marine copepod (Tigriopus japonicus) | PS | 2 µm | Spheres | 0.5 μg/L and 100 mg/L | 30 d | ROS increase (0.5 μg/L and 100 mg/L) | [7] |
| Marine microcrustacean (Artemia salina) | PS | 11.86–44.62 μm | Spheres | 1, 25, 50, 75, and 100 μg/mL | 2 d | Increased activity of SOD, CAT, GST, and GSH (1, 25, 50, 75, and 100 μg/mL); Reduction in AChE activity (1, 25, 50, 75, and 100 μg/mL) | [8] |
| Monogonont rotifer (Brachionus koreanus) | PS | 0.5 μm | Spheres | 10 μg/mL | 1 d | ROS increase (10 μg/mL); Increased activity of SOD, GSR, and GST (10 μg/mL); Decreased activity of GSH (10 μg/mL) | [9] |
| Monogonont rotifer (Brachionus koreanus) | PS | 6 μm | Spheres | 10 μg/mL | 1 d | ROS increase (10 μg/mL); Increased activity of GST (10 μg/mL); Decreased activity of GSH and SOD (10 μg/mL) | [9] |
| Nematode (Caenorhabditis elegans) | PS | 1 μm | Spheres | 1 mg/L | 3 d | Induced oxidative stress (1 mg/L); Enhanced the expression of GST-4 (1 mg/L) | [2] |
| Sex | Organism | MPs Type | MPs Size | MPs Shape | Dose | Exposure Time | Association (OS vs. Endpoints) | Reference |
|---|---|---|---|---|---|---|---|---|
| Mammals–Female | Rats | PS | 0.5 μm | Spheres | 1, 5, and 25 μg/mL (0.015, 0.15, and 1.5 mg/d) | 90 d | Increased levels of MDA (0.015, 0.15, and 1.5 mg/d); Decreased the level of SOD (0.15 and 1.5 mg/d), GPx, and CAT (0.015, 0.15, and 1.5 mg/d); Fibrosis and granulosa cells apoptosis of ovary (5 and 25 μg/mL) | [1] |
| Mice | PS | 0.8 μm | Spheres | 30 mg/kg/d | 35 d | Increased level of ROS in oocytes (30 mg/kg/d); Reduced level of MDA (30 mg/kg/d); Increased IL-6 concentration in ovaries (30 mg/kg/d); Decreased viability of oocytes (30 mg/kg/d); Induced inflammation of ovaries (30 mg/kg/d) | [2] | |
| Mammals–Male | Mice | PS | 5 μm | Spheres | 0.1, 1, and 10 μg/mL (0.7, 7, and 70 μg/d) | 35 d | Decreased expression of Nrf2 in the medium and high dose groups (7 and 70 μg/d); Inflammatory reaction in testicular tissue—increased factor IL-1β (7 and 70 μg/d); Decrease in number of viable epididymis (70 μg/d); Destroyed testis tissue structure (0.7, 7, and 70 μg/d) | [3] |
| Mice | PS | 0.5, 4, and 10 μm | Spheres | 1 mg/mL (1 mg/d) | 28 d | Inflammatory reaction in testis—increased factors TNF-α and IL-6 (1 mg/d); Decreased testosterone level (1 mg/d); Abnormal sperm morphology (1 mg/d); Decreased consumption of food by tested animals (1 mg/d) | [4] | |
| Mice | PS | 5–5.9 μm | Spheres | 0.01, 0.1, 1, and 100 mg/d | 42 d | Activation of p38 MAPK (0.01, 0.1, 1, and 100 mg/d); Increased level of Casp-3, TNF-α, IL-1β, and IL-6 in the testicular tissue (0.01, 0.1, 1, and 100 mg/d); Decreased concentration of testosterone (0.01, 0.1, 1, and 100 mg/d); Reduced the activity of enzymes LDH and SDH (0.01, 0.1, 1, and 100 mg/d); Decreased in number of spermatogenic cells (0.01, 0.1, 1, and 100 mg/d) | [5] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. https://doi.org/10.3390/antiox13050579
Kadac-Czapska K, Ośko J, Knez E, Grembecka M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants. 2024; 13(5):579. https://doi.org/10.3390/antiox13050579
Chicago/Turabian StyleKadac-Czapska, Kornelia, Justyna Ośko, Eliza Knez, and Małgorzata Grembecka. 2024. "Microplastics and Oxidative Stress—Current Problems and Prospects" Antioxidants 13, no. 5: 579. https://doi.org/10.3390/antiox13050579
APA StyleKadac-Czapska, K., Ośko, J., Knez, E., & Grembecka, M. (2024). Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants, 13(5), 579. https://doi.org/10.3390/antiox13050579








