The Mitochondrial-Derived Peptide MOTS-c Alleviates Radiation Pneumonitis via an Nrf2-Dependent Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Design
2.2. Immunohistochemistry Staining
2.3. Histological Analysis
2.4. Collection and Analysis of Bronchoalveolar Lavage Fluid
2.5. RNA-Sequence Data Acquirement
2.6. Cell Cultures and Reagents
2.7. Biochemical Indexes Analysis
2.8. Quantitative Real-Time PCR
2.9. Western Blot
2.10. Immunofluorescence Staining
2.11. TUNEL Staining
2.12. Flow Cytometry
2.13. Detection of Cytosolic ROS and Mitochondrial ROS in MLE-12 Cells
2.14. Mitochondrial Membrane Potential
2.15. Adenosine Triphosphate Content
2.16. Intracellular Calcium Concentration in MLE-12 Cells
2.17. Enzyme-Linked Immunosorbent Assay
2.18. Preparation of Primary AT II Cells
2.19. Statistical Analysis
3. Results
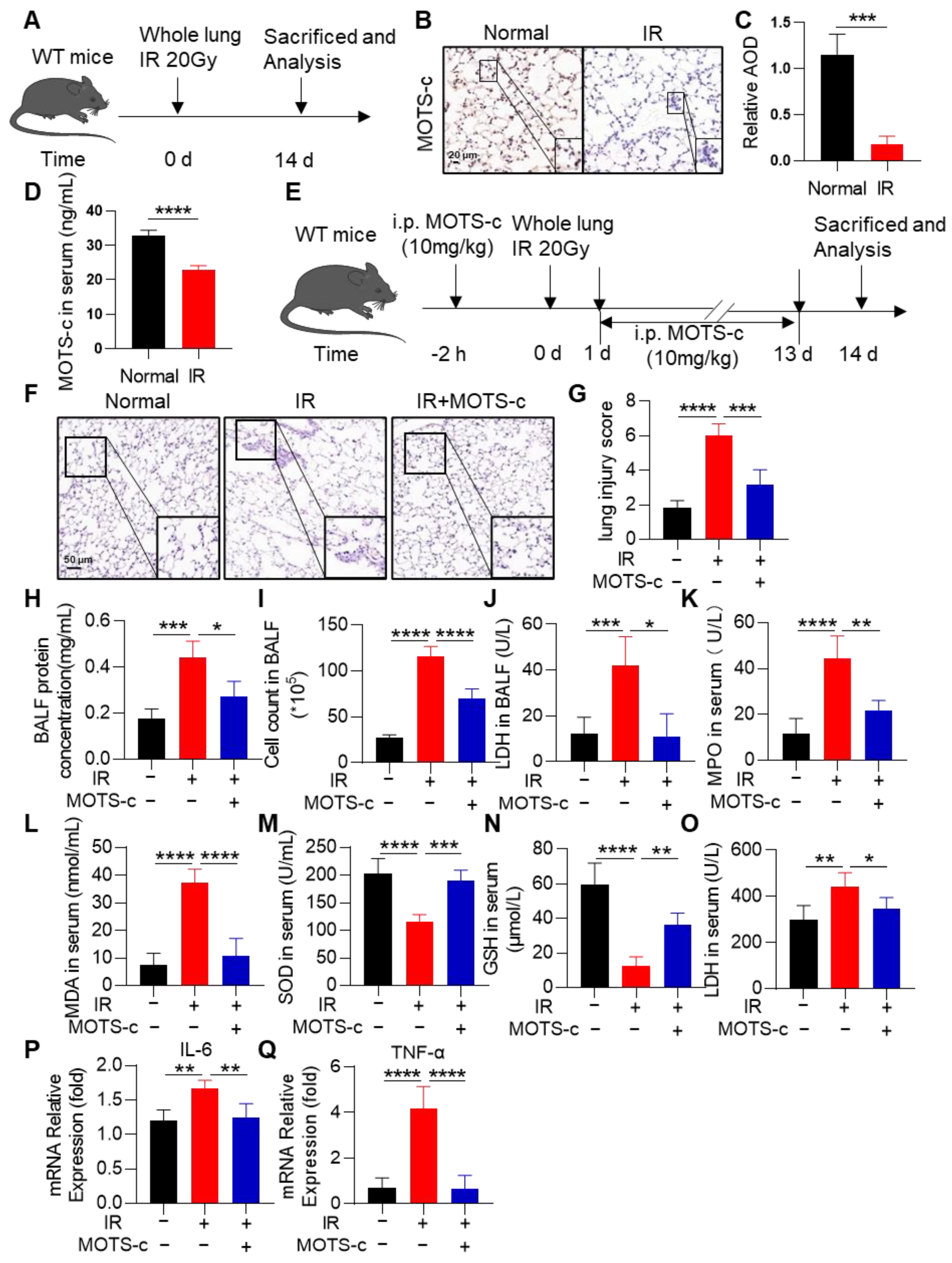
3.1. MOTS-c Alleviated the Oxidant Damage, Inflammation, and Lung Tissue Injury in Irradiated Mice
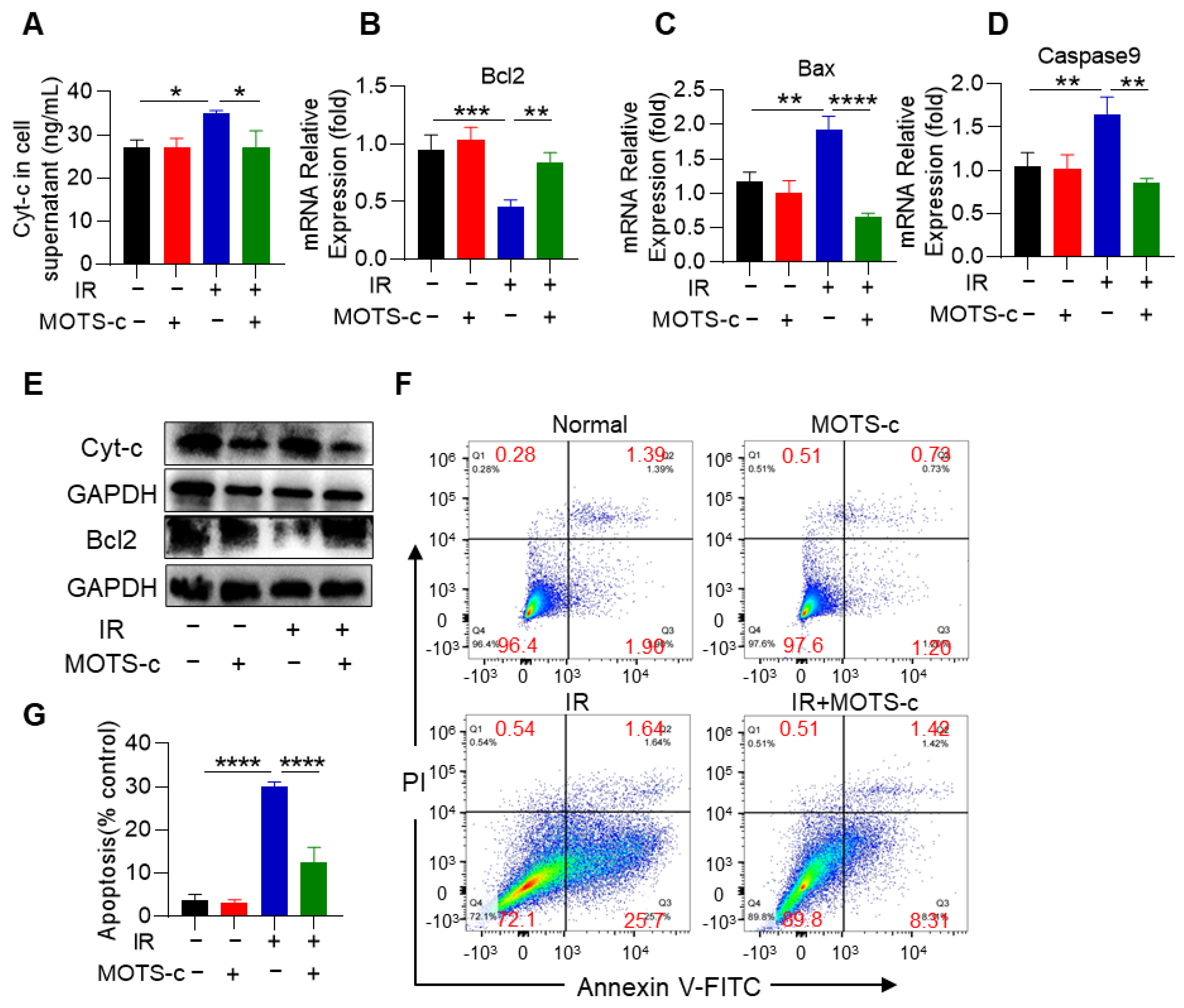
3.2. MOTS-c Prevented the Apoptosis of Alveolar Epithelial Cells in RP Mice
3.3. MOTS-c Alleviates Radiation-Induced Oxidative Stress and Inflammation in MLE-12 Cells
3.4. MOTS-c Reduced the Radiation-Induced Apoptosis of MLE-12 Cells
3.5. MOTS-c Relieved Radiation-Induced Mitochondrial Damage in MLE-12 Cells
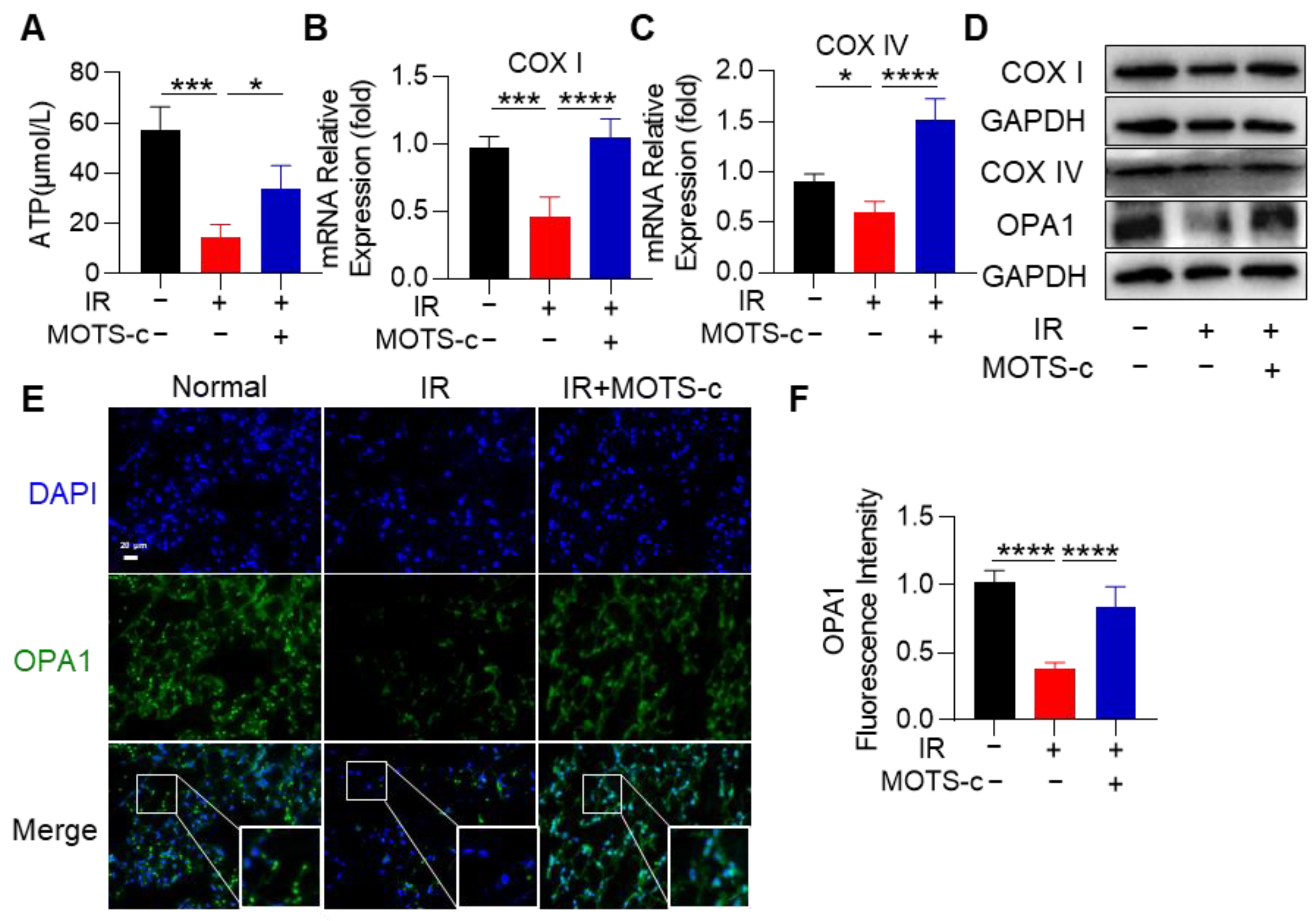
3.6. MOTS-c Mitigated Mitochondrial Injury in RP Mice
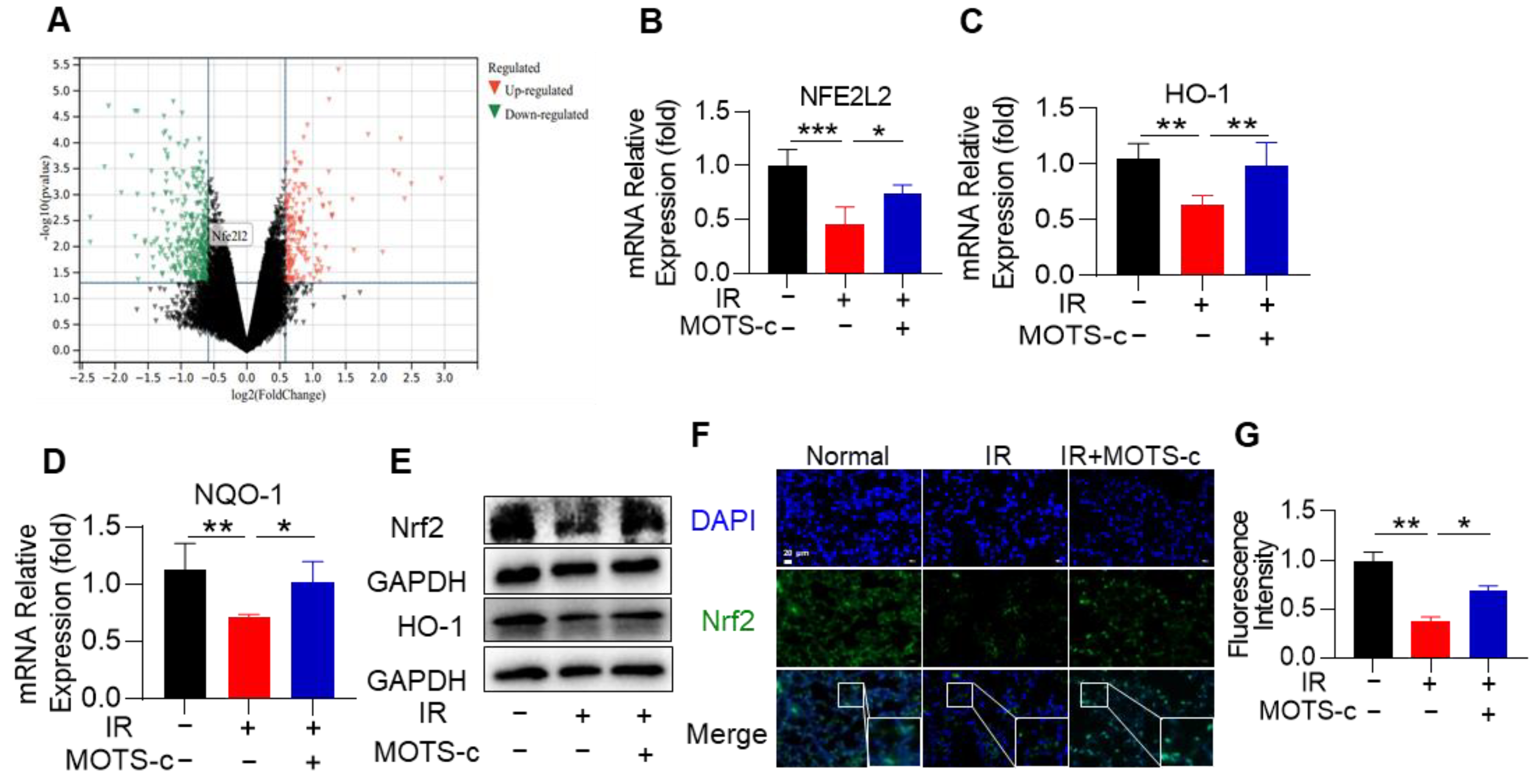
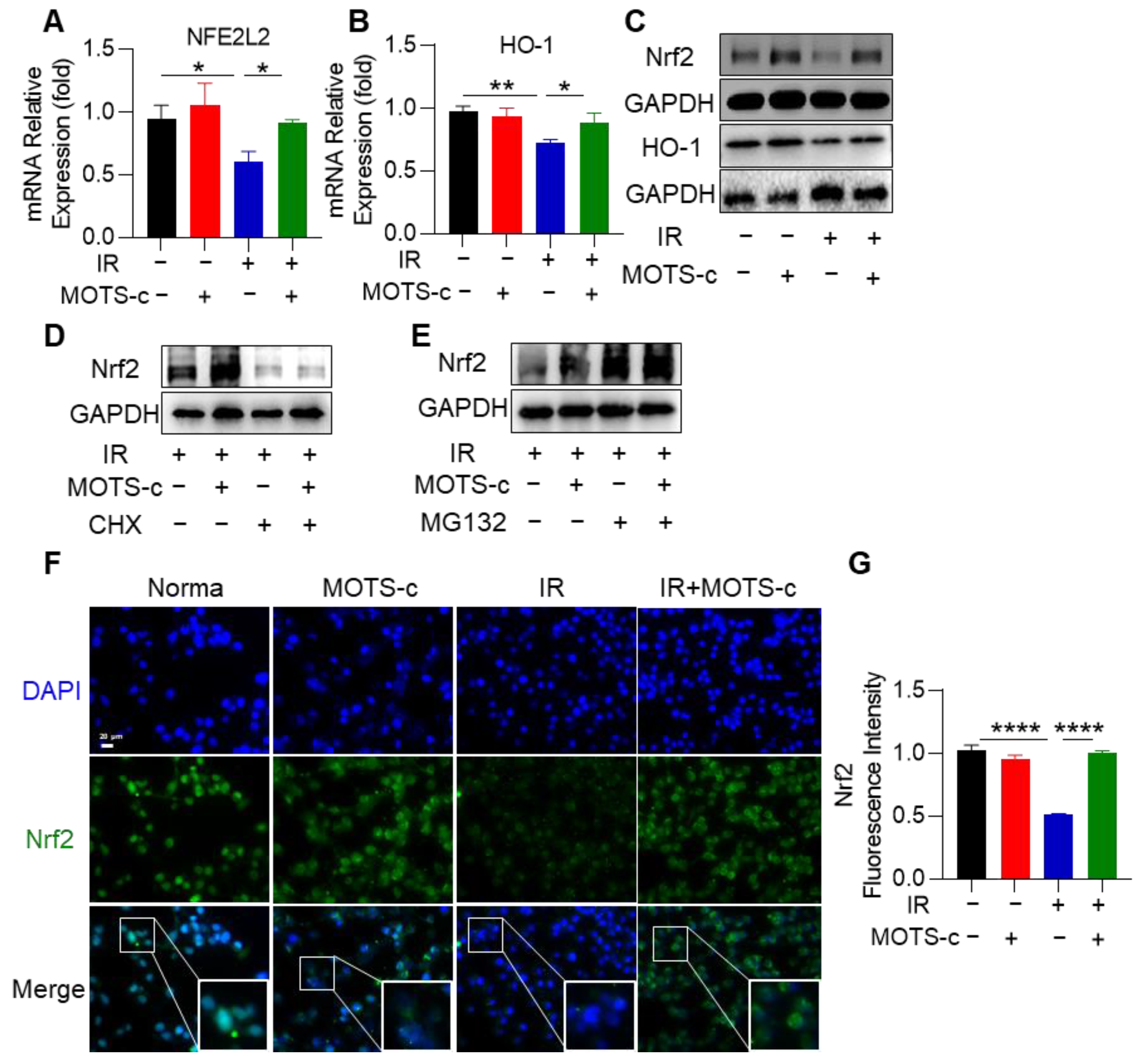
3.7. MOTS-c Increased Nrf2 Content and Induced Its Nucleus Translocation in RP Mice
3.8. MOTS-c Increased Nrf2 Content and Induced Its Nucleus Translocation in MLE-12 Cells
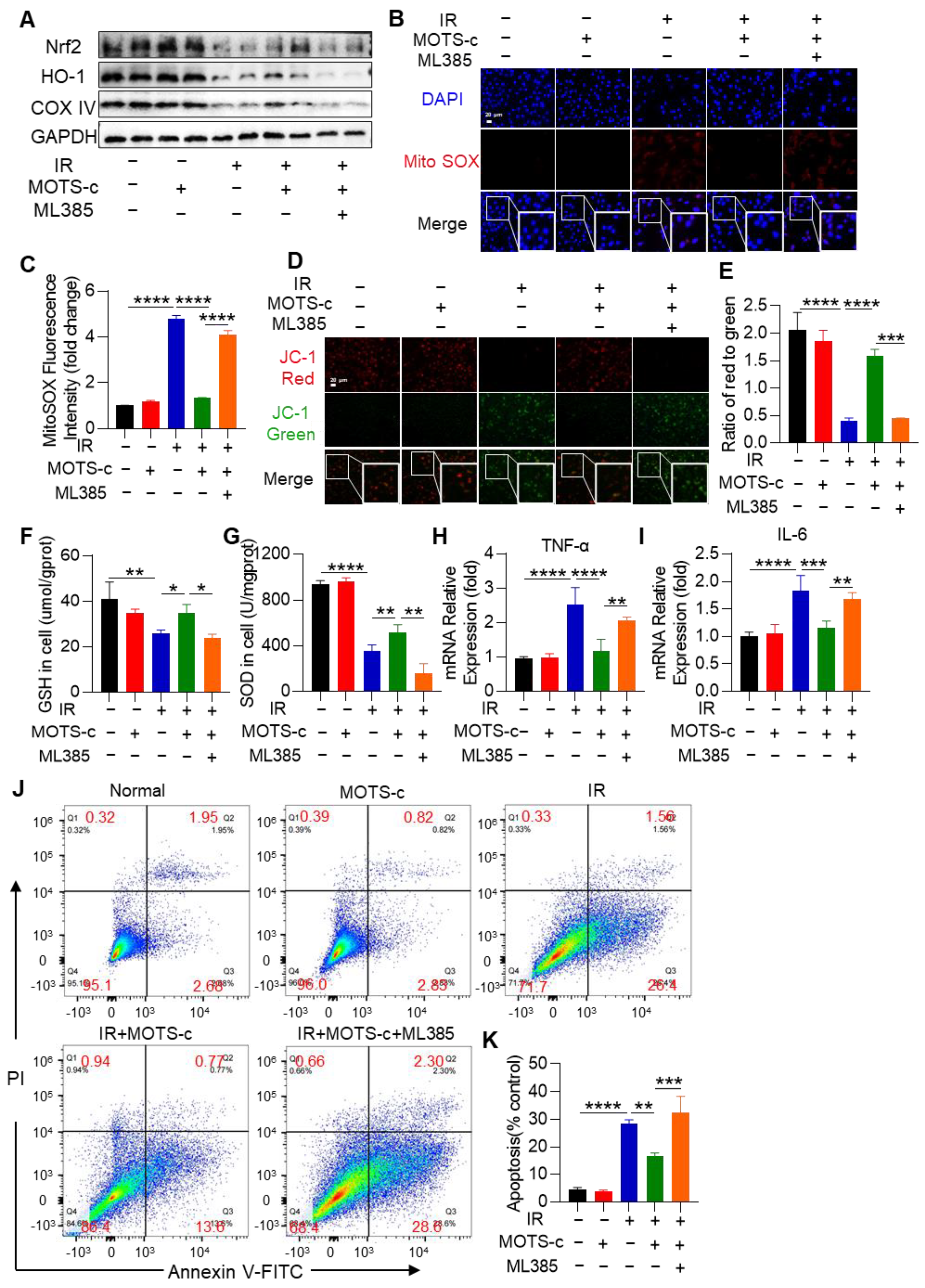
3.9. Inhibition of Nrf2 Abolished the Protective Function of MOTS-c in MLE-12 Cells
3.10. Nrf2 Deficiency Abolished the Protective Function of MOTS-c in RP Mice
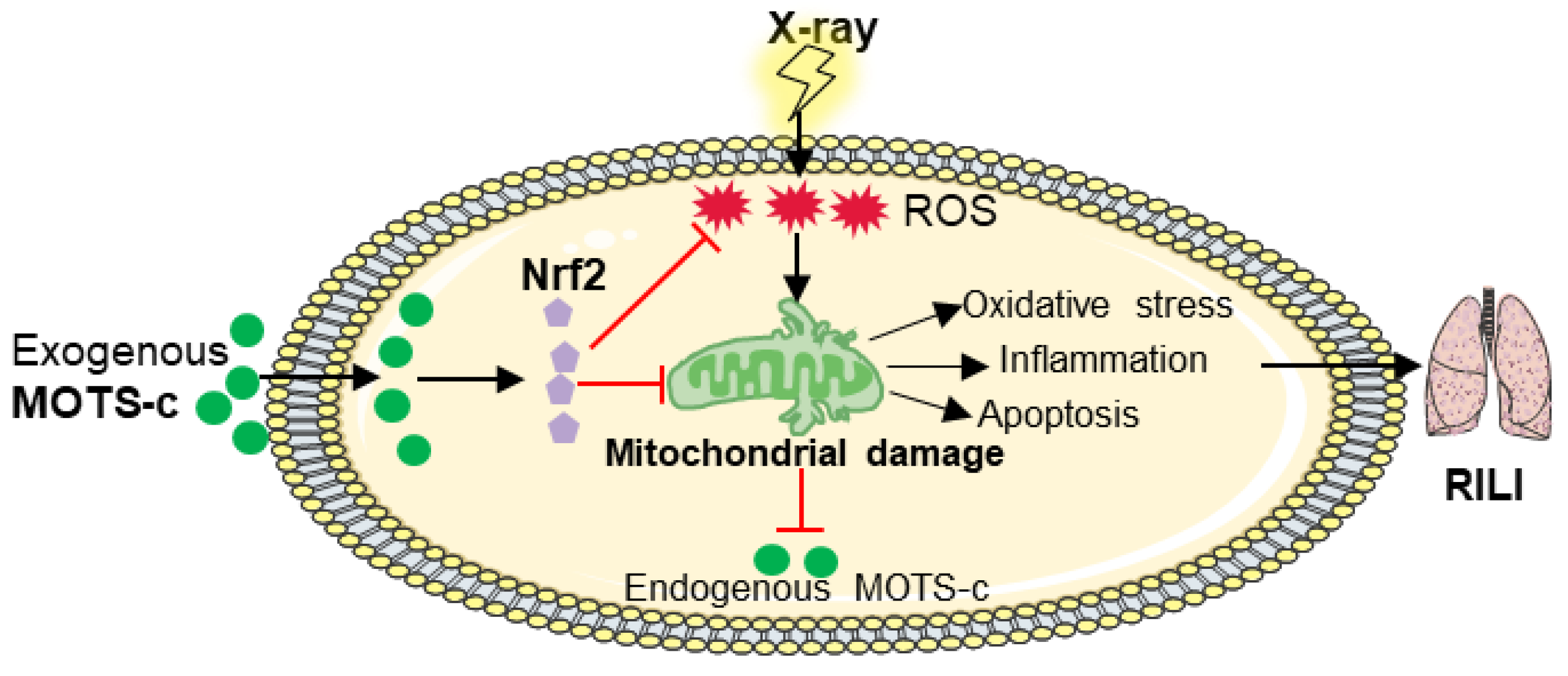
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Z.; Jiang, X.; Wang, Y.; Yang, Z.; Mao, Y.; Wu, Z.; Li, G.; Chen, H. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3beta pathway via c-MET in radiation-induced lung injury. J. Exp. Clin. Cancer Res. 2022, 41, 128. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Du, L.; Ma, N.; Zhang, P.; Wang, Y.; Han, Y.; Huang, X.; Zhang, Q.; Tan, X.; Lei, X.; et al. Monophosphoryl lipid A ameliorates radiation-induced lung injury by promoting the polarization of macrophages to the M1 phenotype. J. Transl. Med. 2022, 20, 597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Ren, Y.; Lei, Y.; Yang, S.; Shi, Y.; Peng, H.; Yang, W.; Guo, T.; Yu, Y.; et al. Mitochondrial-derived peptides in cardiovascular disease: Novel insights and therapeutic opportunities. J. Adv. Res. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Guan, C.; Luo, N.; Chen, H.; Li, A.; Zhuang, H.; Yang, J.; Diao, H.; Zeng, S.; et al. Mitochondrial micropeptide MOXI promotes fibrotic gene transcription by translocation to the nucleus and bridging N-acetyltransferase 14 with transcription factor c-Jun. Kidney Int. 2023, 103, 886–902. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Mehta, H.H.; Wan, J.; Kuehnemann, C.; Chen, J.; Hu, J.-F.; Hoffman, A.R.; Cohen, P. Mitochondrial peptides modulate mitochondrial function during cellular senescence. Aging 2018, 10, 1239–1256. [Google Scholar] [CrossRef]
- Zhong, P.; Peng, J.; Hu, Y.; Zhang, J.; Shen, C. Mitochondrial derived peptide MOTS-c prevents the development of heart failure under pressure overload conditions in mice. J. Cell Mol. Med. 2022, 26, 5369–5378. [Google Scholar] [CrossRef]
- Benayoun, B.A.; Lee, C. MOTS-c: A Mitochondrial-Encoded Regulator of the Nucleus. Bioessays 2019, 41, e1900046. [Google Scholar] [CrossRef]
- Yu, W.D.; Kim, Y.J.; Cho, M.J.; Seok, J.; Kim, G.J.; Lee, C.H.; Ko, J.J.; Kim, Y.S.; Lee, J.H. The mitochondrial-derived peptide MOTS-c promotes homeostasis in aged human placenta-derived mesenchymal stem cells in vitro. Mitochondrion 2021, 58, 135–146. [Google Scholar] [CrossRef]
- Wang, M.; Wang, G.; Pang, X.; Ma, J.; Yuan, J.; Pan, Y.; Fu, Y.; Laher, I.; Li, S. MOTS-c repairs myocardial damage by inhibiting the CCN1/ERK1/2/EGR1 pathway in diabetic rats. Front. Nutr. 2022, 9, 1060684. [Google Scholar] [CrossRef]
- Mohtashami, Z.; Singh, M.K.; Salimiaghdam, N.; Ozgul, M.; Kenney, M.C. MOTS-c, the Most Recent Mitochondrial Derived Peptide in Human Aging and Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 11991. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Miller, B.; Kumagai, H.; Silverstein, A.R.; Flores, M.; Yen, K. Mitochondrial-derived peptides in aging and age-related diseases. Geroscience 2021, 43, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Woodhead, J.S.T.; Hedges, C.P.; Zeng, N.; Wan, J.; Kumagai, H.; Lee, C.; Cohen, P.; Cameron-Smith, D.; Mitchell, C.J.; et al. Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging 2020, 12, 5244–5258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Ye, Z.; Jiang, Y.; Xu, C.; Ruan, B.; Yang, Y.; Lei, X.; Xiang, A.; Lu, H.; Zhu, Z.; et al. MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA. Mol. Immunol. 2017, 92, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 2010, 1802, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Rustamov, N.; Roh, Y.-S. The Roles of NFR2-Regulated Oxidative Stress and Mitochondrial Quality Control in Chronic Liver Diseases. Antioxidants 2023, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic. Biol. Med. 2022, 179, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Narasimhan, M.; Shanmugam, G.; Milash, B.; Hoidal, J.R.; Rajasekaran, N.S. Differential regulation of miRNA and mRNA expression in the myocardium of Nrf2 knockout mice. BMC Genom. 2017, 18, 509. [Google Scholar] [CrossRef]
- Gao, L.; Kumar, V.; Vellichirammal, N.N.; Park, S.-Y.; Rudebush, T.L.; Yu, L.; Son, W.-M.; Pekas, E.J.; Wafi, A.M.; Hong, J.; et al. Functional, proteomic and bioinformatic analyses of Nrf2- and Keap1- null skeletal muscle. J. Physiol. 2020, 598, 5427–5451. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Q.; Wu, C.; Qiu, Y.B.; Wu, Y.X.; Chen, J.L.; Huang, J.F.; Chen, D.; Pang, Q.F. Heme oxygenase-1 attenuates seawater drowning-induced acute lung injury through a reduction in inflammation and oxidative stress. Int. Immunopharmacol. 2019, 74, 105634. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Garasa, S.; Rodriguez, I.; Solorzano, J.L.; Barbes, B.; Yanguas, A.; Teijeira, A.; Etxeberria, I.; Aristu, J.J.; Halin, C.; et al. Intercellular Adhesion Molecule-1 and Vascular Cell Adhesion Molecule Are Induced by Ionizing Radiation on Lymphatic Endothelium. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Pan, Y.; He, J.; Zhong, H.; Wu, Y.; Ji, C.; Liu, L.; Cui, X. The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacol. Res. 2022, 175, 105987. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Huang, K.; Xu, S.; Garcia, J.G.N.; Wang, C.; Cai, H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020, 36, 101638. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef]
- Qiu, Y.B.; Wan, B.B.; Liu, G.; Wu, Y.X.; Chen, D.; Lu, M.D.; Chen, J.L.; Yu, R.Q.; Chen, D.Z.; Pang, Q.F. Nrf2 protects against seawater drowning-induced acute lung injury via inhibiting ferroptosis. Respir. Res. 2020, 21, 232. [Google Scholar] [CrossRef]
- Ares, G.R. cGMP induces degradation of NKCC2 in the thick ascending limb via the ubiquitin-proteasomal system. Am. J. Physiol. Renal Physiol. 2019, 316, F838–F846. [Google Scholar] [CrossRef] [PubMed]
- Wood, N.J.; Jenkinson, H.F.; Davis, S.A.; Mann, S.; O’Sullivan, D.J.; Barbour, M.E. Chlorhexidine hexametaphosphate nanoparticles as a novel antimicrobial coating for dental implants. J. Mater. Sci. Mater. Med. 2015, 26, 201. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Zhang, Y.-R.; Jiang, F.-J.; He, S.; Zhang, Y.-L.; Chen, D.; Tong, Y.; Nie, Y.-J.; Pang, Q.-F. 4-OI ameliorates bleomycin-induced pulmonary fibrosis by activating Nrf2 and suppressing macrophage-mediated epithelial-mesenchymal transition. Inflamm. Res. 2023, 72, 1133–1145. [Google Scholar] [CrossRef]
- Zhang, M.; Qian, J.; Xing, X.; Kong, F.M.; Zhao, L.; Chen, M.; Lawrence, T.S. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin. Cancer Res. 2008, 14, 1868–1876. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, 877. [Google Scholar] [CrossRef]
- Li, X.; Gong, Y.; Li, D.; Xiang, L.; Ou, Y.; Jiang, L.; Shu, P.; Liu, X.; Guo, F.; Qin, D.; et al. Low-Dose Radiation Therapy Promotes Radiation Pneumonitis by Activating NLRP3 Inflammasome. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 804–814. [Google Scholar] [CrossRef]
- Flockerzi, E.; Schanz, S.; Rübe, C.E. Even low doses of radiation lead to DNA damage accumulation in lung tissue according to the genetically-defined DNA repair capacity. Radiother. Oncol. 2014, 111, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Drishya, S.; Dhanisha, S.S.; Raghukumar, P.; Guruvayoorappan, C. Amomum subulatum mitigates experimental thoracic radiation-induced lung injury by regulating antioxidant status and inflammatory responses. Food Funct. 2023, 14, 1545–1559. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Yu, H.; Dong, L.; Yu, R.; Li, Q.; Song, J.; Chen, H.; Zhang, H.; Pu, J.; et al. Activation of Nrf2/ARE pathway by Anisodamine (654-2) for Inhibition of cellular aging and alleviation of Radiation-Induced lung injury. Int. Immunopharmacol. 2023, 124, 110864. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, G.; Deng, S.; Wang, Q. Oxidative stress levels and dynamic changes in mitochondrial gene expression in a radiation-induced lung injury model. J. Radiat. Res. 2019, 60, 204–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Rabbani, Z.N.; Jackson, I.L.; Vujaskovic, Z. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gollapudi, S. Susceptibility of naive and subsets of memory T cells to apoptosis via multiple signaling pathways. Autoimmun. Rev. 2007, 6, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, J.G. First steps in the acquisition of Spanish stress. J. Child. Lang. 1988, 15, 273–292. [Google Scholar] [CrossRef]
- Cen, M.; Ouyang, W.; Zhang, W.; Yang, L.; Lin, X.; Dai, M.; Hu, H.; Tang, H.; Liu, H.; Xia, J.; et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021, 41, 101936. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Du, L.; Yu, W.; Wang, Y.; Ma, N.; Qu, B. GSTP1 as a novel target in radiation induced lung injury. J. Transl. Med. 2021, 19, 297. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Tesei, A. The Role of Mesenchymal Stem Cells in Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2019, 20, 3876. [Google Scholar] [CrossRef] [PubMed]
- Cogno, N.; Bauer, R.; Durante, M. An Agent-Based Model of Radiation-Induced Lung Fibrosis. Int. J. Mol. Sci. 2022, 23, 13920. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jing, Y.; Chen, Q.; Abbas, A.B.; Hu, J.; Xu, H. The intraperitoneal administration of MOTS-c produces antinociceptive and anti-inflammatory effects through the activation of AMPK pathway in the mouse formalin test. Eur. J. Pharmacol. 2020, 870, 172909. [Google Scholar] [CrossRef] [PubMed]
- Xinqiang, Y.; Quan, C.; Yuanyuan, J.; Hanmei, X. Protective effect of MOTS-c on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2020, 80, 106174. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gidlund, E.K.; Witasp, A.; Qureshi, A.R.; Soderberg, M.; Thorell, A.; Nader, G.A.; Barany, P.; Stenvinkel, P.; von Walden, F. Reduced skeletal muscle expression of mitochondrial-derived peptides humanin and MOTS-C and Nrf2 in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2019, 317, F1122–F1131. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhu, S.; Wang, H.; Wang, L.; Du, T.; Ye, Z.; Zhai, D.; Zhu, Z.; Tian, X.; Lu, Z.; et al. MOTS-c inhibits Osteolysis in the Mouse Calvaria by affecting osteocyte-osteoclast crosstalk and inhibiting inflammation. Pharmacol. Res. 2019, 147, 104381. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Han, X.; Zhou, D.; Zhong, J.; Liu, L.; Wang, Y.; Ni, J.; Shen, X.; Liang, C.; Fang, H. BIG1 mediates sepsis-induced lung injury by modulating lipid raft-dependent macrophage inflammatory responses. Acta Biochim. Biophys. Sin. 2021, 53, 1088–1097. [Google Scholar] [CrossRef]
- Weng, F.B.; Zhu, L.F.; Zhou, J.X.; Shan, Y.; Tian, Z.G.; Yang, L.W. MOTS-c accelerates bone fracture healing by stimulating osteogenesis of bone marrow mesenchymal stem cells via positively regulating FOXF1 to activate the TGF-beta pathway. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2459. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Shan, Y.; Ye, F.; Zhang, X.; Cheng, J.; Wang, X.; Zhao, Y.; Dan, G.; Chen, M.; et al. The Mitochondrial-Derived Peptide (MOTS-c) Interacted with Nrf2 to Defend the Antioxidant System to Protect Dopaminergic Neurons Against Rotenone Exposure. Mol. Neurobiol. 2023, 60, 5915–5930. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, K.; Jiang, T.; Zhao, G.J. MOTS-c attenuates endothelial dysfunction via suppressing the MAPK/NF-kappaB pathway. Int. J. Cardiol. 2018, 268, 40. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Feng, M.; Peng, J.; Du, X.; Chu, H.; Chen, X. The Mitochondrial-Derived Peptide MOTS-c Attenuates Oxidative Stress Injury and the Inflammatory Response of H9c2 Cells Through the Nrf2/ARE and NF-kappaB Pathways. Cardiovasc. Eng. Technol. 2022, 13, 651–661. [Google Scholar] [CrossRef] [PubMed]




| Criteria | Score = 0 | Score = 1 | Score = 2 | Score = 3 | Score = 4 |
|---|---|---|---|---|---|
| Inflammation | Absent | Mild | Moderate | Severe | Very severe |
| Edema and hemorrhage | None | <10% | 10–30% | 30–50% | >50% |
| Alveolar septal thickening | <15 μm | 15–30 μm | 30–45 μm | 45–60 μm | >60 μm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Huang, J.; Zhang, Y.; Jiang, F.; Li, S.; He, S.; Sun, J.; Chen, D.; Tong, Y.; Pang, Q.; et al. The Mitochondrial-Derived Peptide MOTS-c Alleviates Radiation Pneumonitis via an Nrf2-Dependent Mechanism. Antioxidants 2024, 13, 613. https://doi.org/10.3390/antiox13050613
Zhang Y, Huang J, Zhang Y, Jiang F, Li S, He S, Sun J, Chen D, Tong Y, Pang Q, et al. The Mitochondrial-Derived Peptide MOTS-c Alleviates Radiation Pneumonitis via an Nrf2-Dependent Mechanism. Antioxidants. 2024; 13(5):613. https://doi.org/10.3390/antiox13050613
Chicago/Turabian StyleZhang, Yanli, Jianfeng Huang, Yaru Zhang, Fengjuan Jiang, Shengpeng Li, Shuai He, Jiaojiao Sun, Dan Chen, Ying Tong, Qingfeng Pang, and et al. 2024. "The Mitochondrial-Derived Peptide MOTS-c Alleviates Radiation Pneumonitis via an Nrf2-Dependent Mechanism" Antioxidants 13, no. 5: 613. https://doi.org/10.3390/antiox13050613
APA StyleZhang, Y., Huang, J., Zhang, Y., Jiang, F., Li, S., He, S., Sun, J., Chen, D., Tong, Y., Pang, Q., & Wu, Y. (2024). The Mitochondrial-Derived Peptide MOTS-c Alleviates Radiation Pneumonitis via an Nrf2-Dependent Mechanism. Antioxidants, 13(5), 613. https://doi.org/10.3390/antiox13050613







