Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities
Abstract
:1. Introduction
2. Materials and Methods
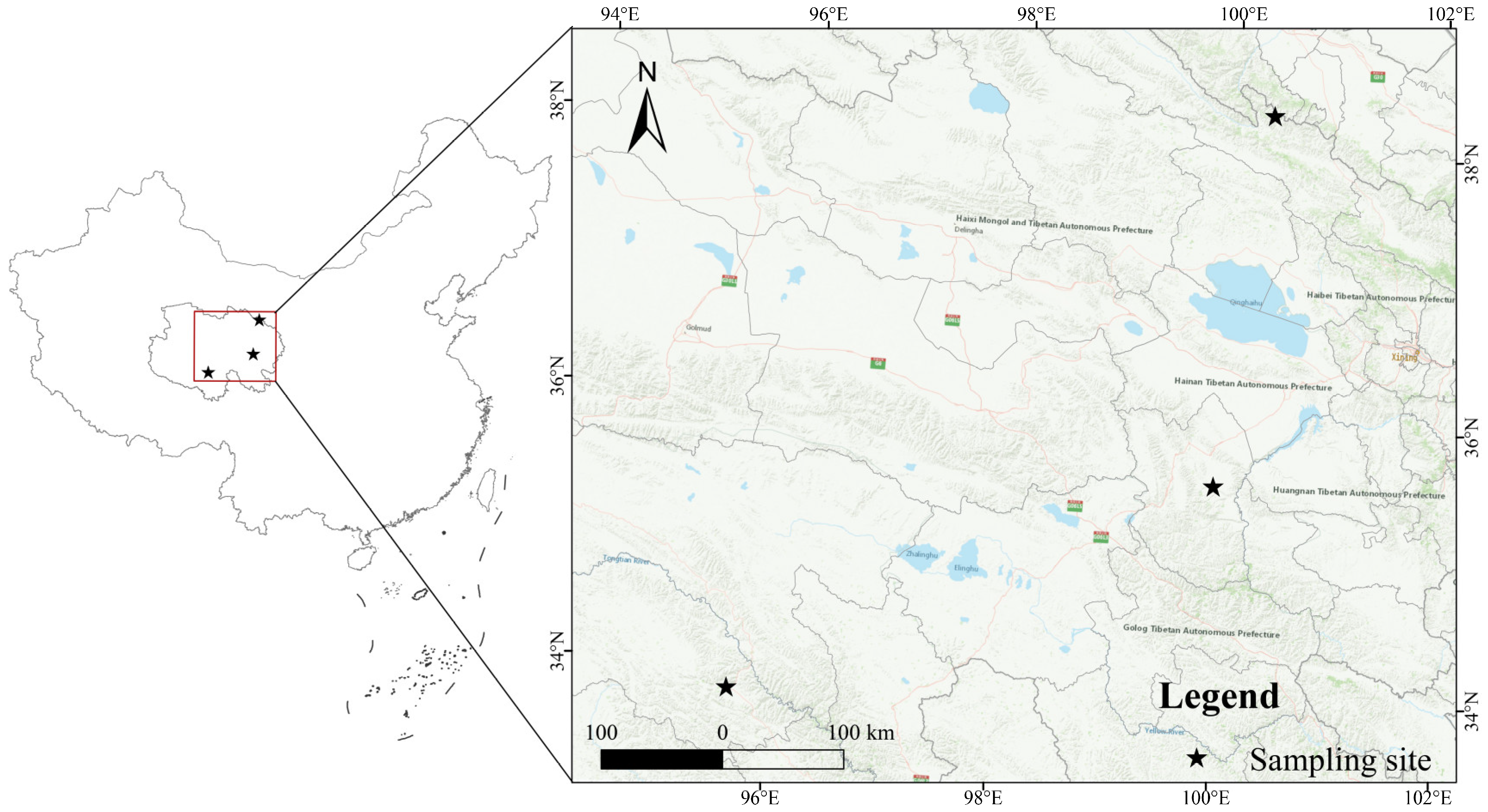
2.1. Fungal Sample Materials
2.2. In Vitro Antioxidant Activity Assay
2.3. Untargeted Metabolomics Profiling
2.3.1. Samples of Pretreatment and Metabolite Extraction
2.3.2. LC-MS/MS Analysis
2.4. Targeted Metabolomics Profiling
2.4.1. Standard Solutions and Calibration Curves
2.4.2. Samples of Pretreatment and Metabolite Extraction
2.4.3. UPLC-MS/MS Analysis
2.5. Data Preprocessing and Statistical Analysis
3. Results
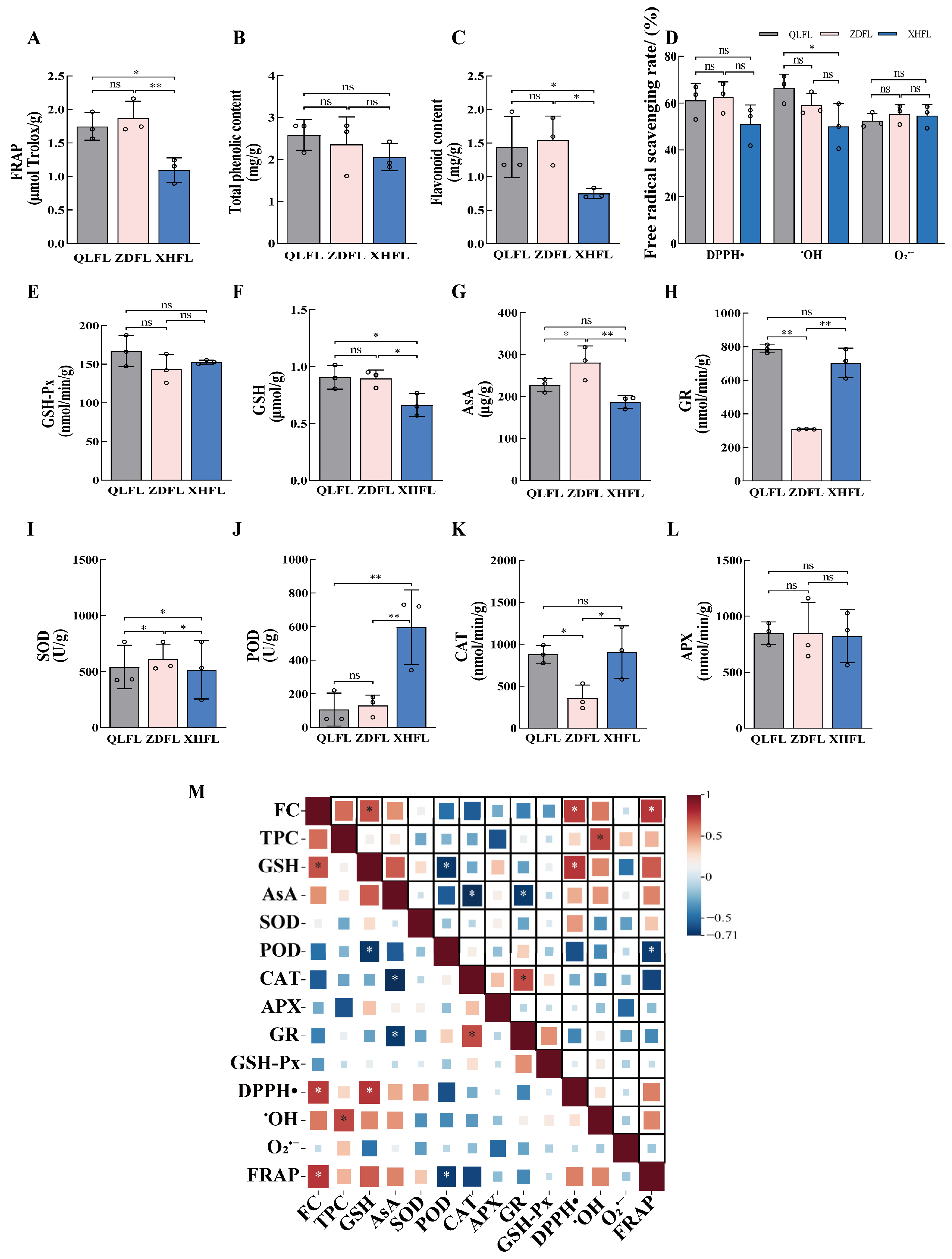
3.1. Comparative Analysis of In Vitro Antioxidant Activity
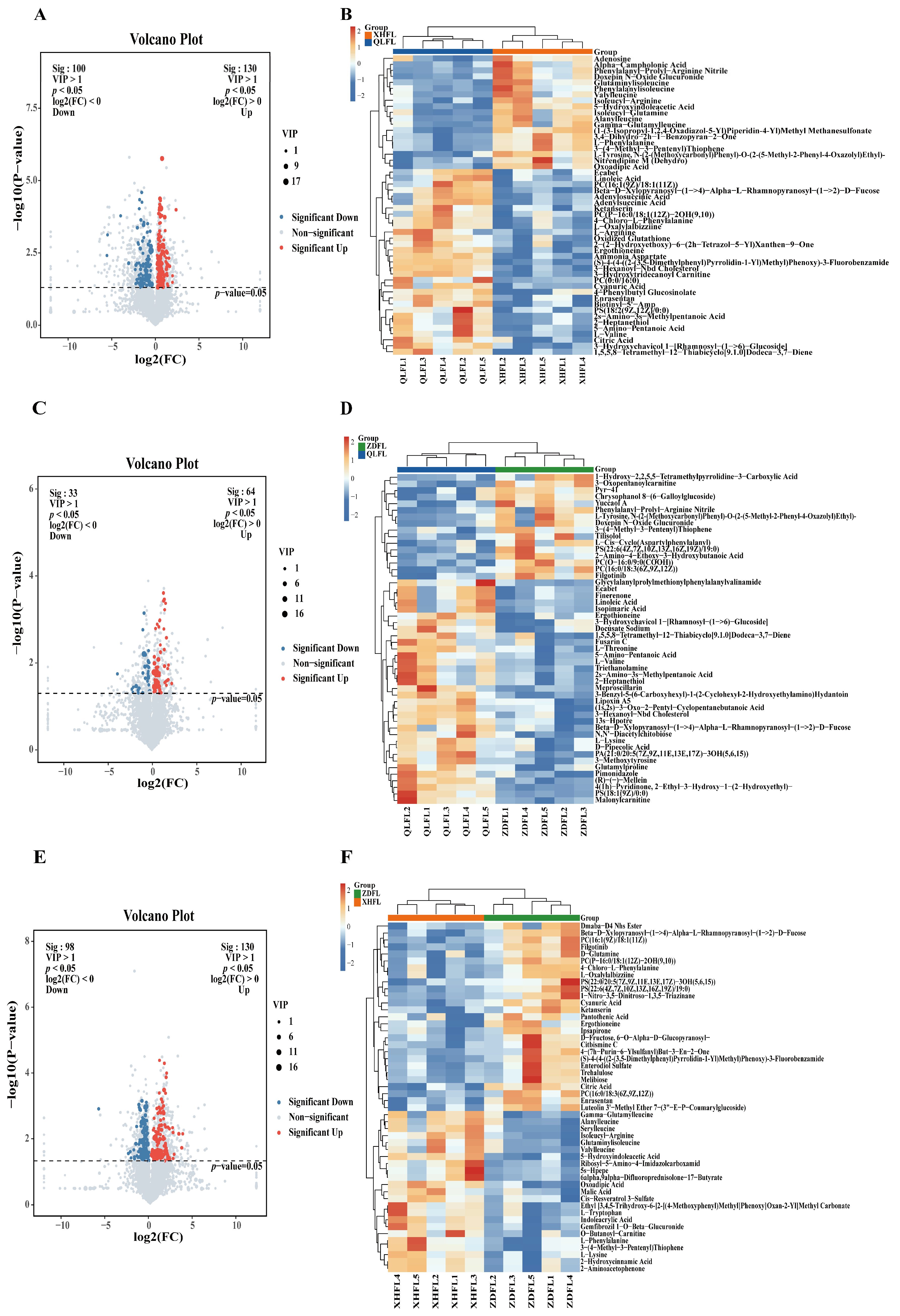
3.2. Metabolite Profiles of the F. luteovirens
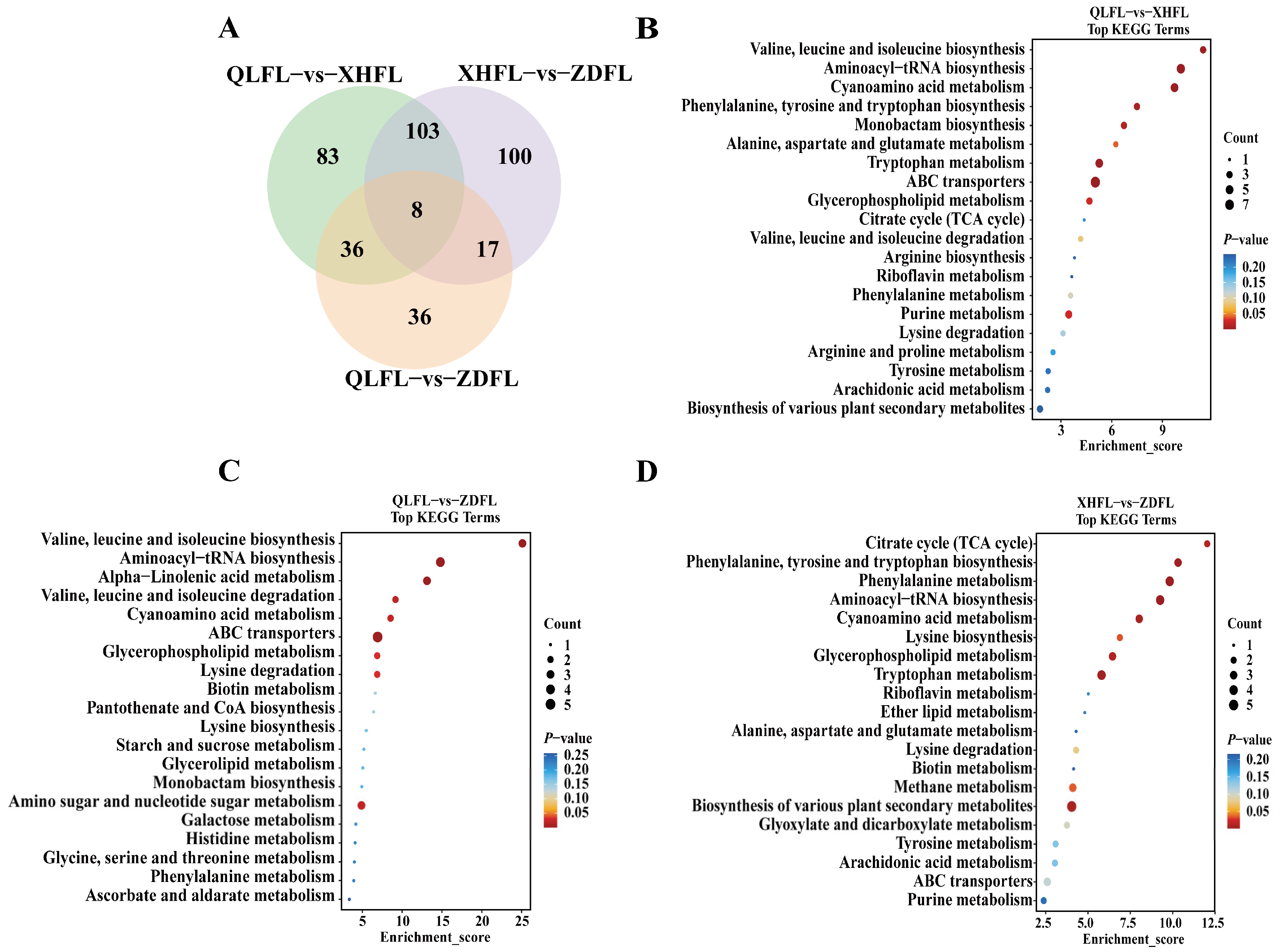
3.3. Screening of Differential Metabolites
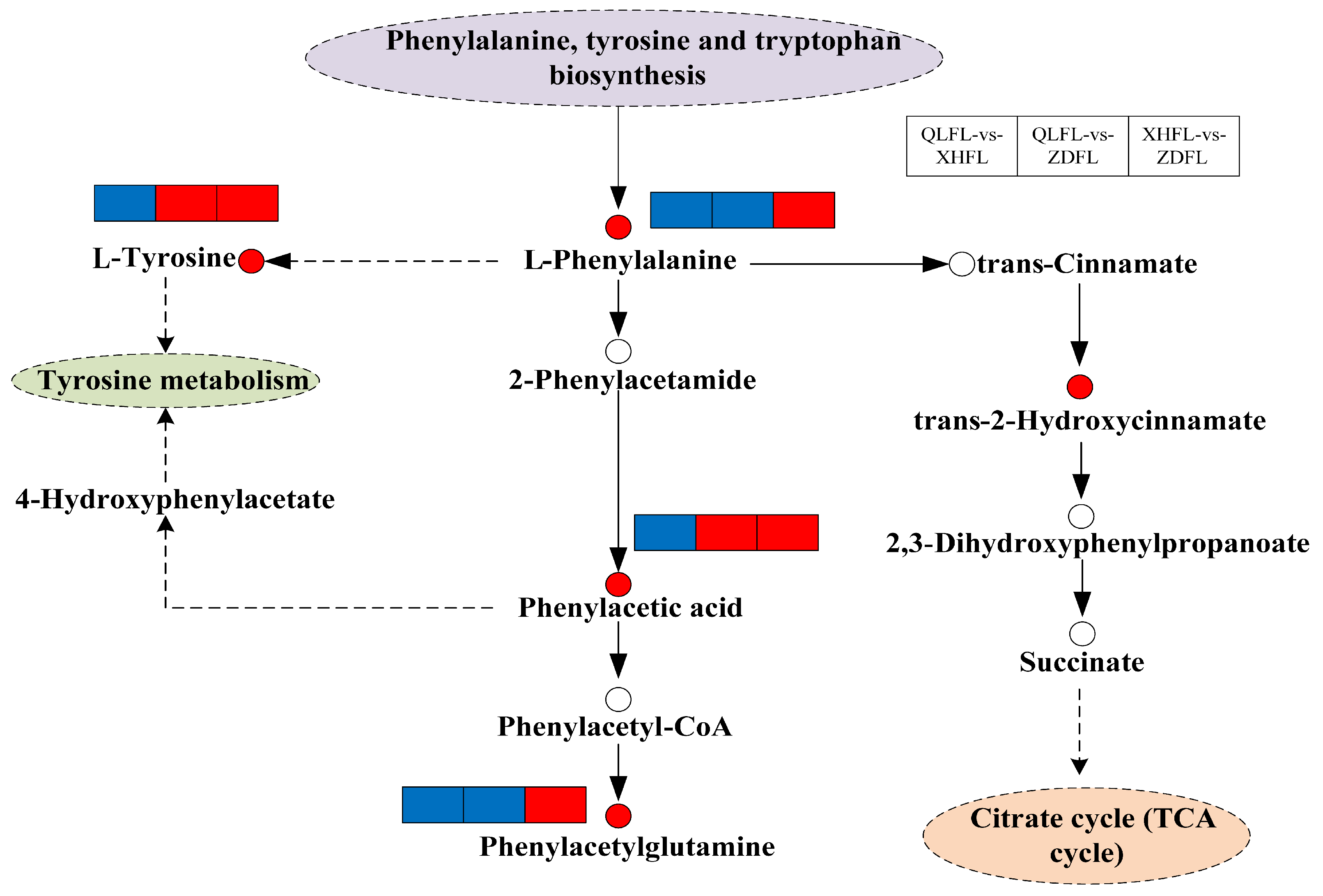
3.4. KEGG Pathway Annotation of the Differential Metabolites
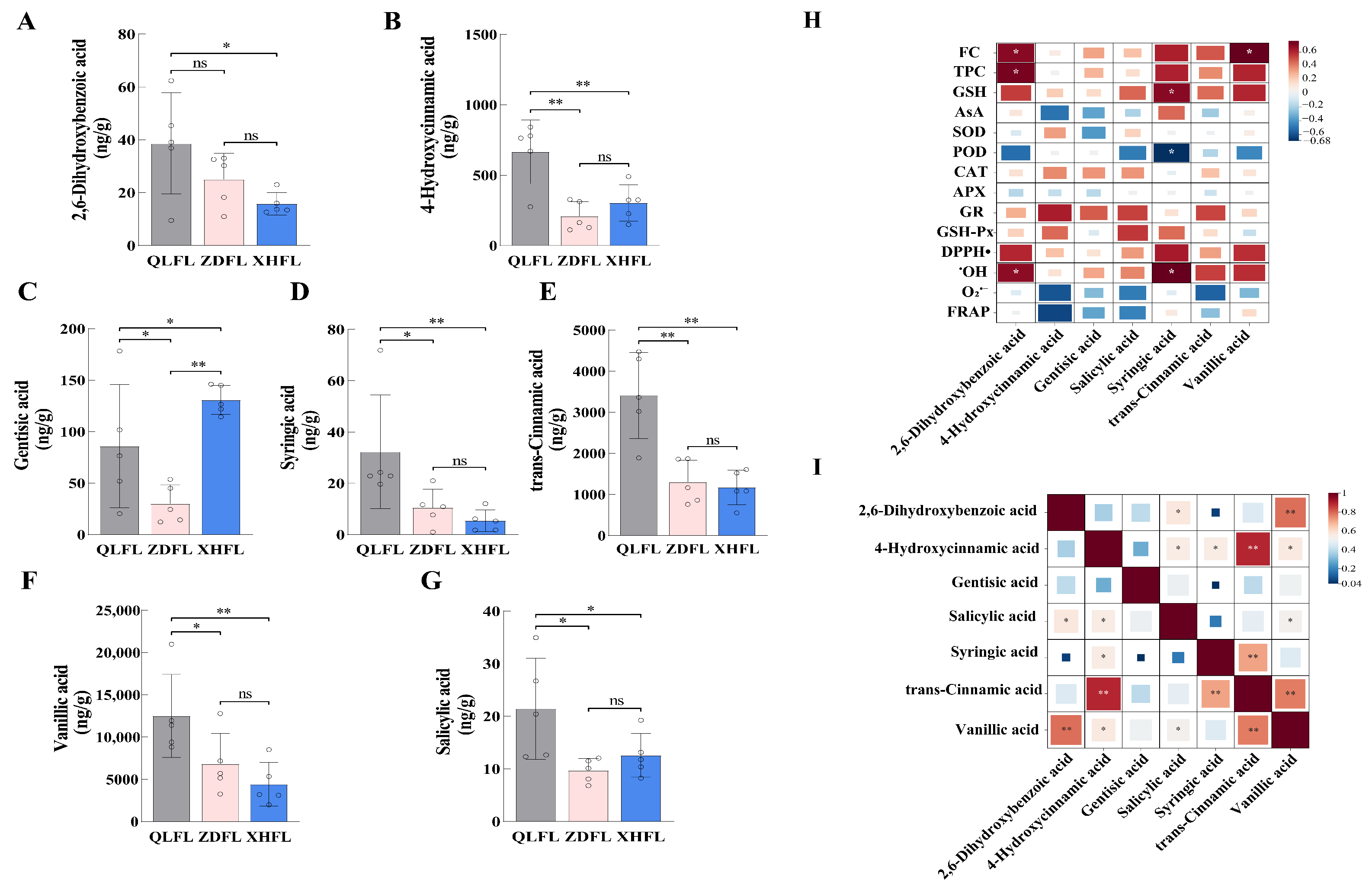
3.5. Targeted Metabolomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stajić, M.; Vukojević, J.; Knežević, A.; Laušević, S.D.; Milovanović, I. Antioxidant protective effects of mushroom metabolites. Curr. Top. Med. Chem. 2013, 13, 2660–2676. [Google Scholar] [CrossRef]
- Yu, B.P. Aging and oxidative stress: Modulation by dietary restriction. Free Radic. Biol. Med. 1996, 21, 651–668. [Google Scholar] [CrossRef]
- Hasnis, E.; Reznick, A.Z. Antioxidants and healthy aging. Isr. Med. Assoc. J. 2003, 5, 368–370. [Google Scholar]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef]
- Tripathi, R.; Gupta, R.; Sahu, M.; Srivastava, D.; Das, A.; Ambasta, R.K.; Kumar, P. Free radical biology in neurological manifestations: Mechanisms to therapeutics interventions. Environ. Sci. Pollut. Res. Int. 2022, 29, 62160–62207. [Google Scholar] [CrossRef]
- Sevindik, M.; Akgul, H.; Selamoglu, Z.; Braidy, N. Antioxidant and Antigenotoxic Potential of Infundibulicybe geotropa Mushroom Collected from Northwestern Turkey. Oxid. Med. Cell. Longev. 2020, 2020, 5620484. [Google Scholar] [CrossRef]
- Fan, H.; Bai, Q.; Yang, Y.; Shi, X.; Du, G.; Yan, J.; Shi, J.; Wang, D. The key roles of reactive oxygen species in microglial inflammatory activation: Regulation by endogenous antioxidant system and exogenous sulfur-containing compounds. Eur. J. Pharmacol. 2023, 956, 175966. [Google Scholar] [CrossRef]
- Zou, X.F.; Ji, Y.T.; Gao, G.; Zhu, X.J.; Lv, S.W.; Yan, F.; Han, S.P.; Chen, X.; Gao, C.C.; Liu, J.; et al. A novel selenium and copper-containing peptide with both superoxide dismutase and glutathione peroxidase activities. J. Microbiol. Biotechnol. 2010, 20, 88–93. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Dinçer, E.; Işık, H.; Hepokur, C.; Tutar, U.; Çelik, C. Cytotoxic, Antioxidant, Antibiofilm, and Antimicrobial Activities of Mushroom Species from Turkey. Int. J. Med. Mushrooms 2023, 25, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, D.; Hu, Q.; Ma, N.; Pei, F.; Su, A.; Ma, G. Immune regulatory functions of biologically active proteins from edible fungi. Front. Immunol. 2022, 13, 1034545. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, L.; Liu, Y.; Xiang, S.; Zhang, H.; Yi, L.; Shang, Y.; Xu, W. Identification techniques and detection methods of edible fungi species. Food Chem. 2022, 374, 131803. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Gao, Q.B.; Zhang, F.Q.; Fu, P.C.; Wang, J.L.; Yan, H.Y.; Chen, S.L. Genetic variation and phylogenetic relationships of the ectomycorrhizal Floccularia luteovirens on the Qinghai-Tibet Plateau. J. Microbiol. 2017, 55, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Lu, H.; Shu, X.; Chen, Q. Chemical characterization, antioxidant properties and anticancer activity of exopolysaccharides from Floccularia luteovirens. Carbohydr. Polym. 2020, 229, 115432. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Cao, L.; Li, W.; Zhang, Q.; Feng, R.; Zhao, Z.; Zhao, X. The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies. J. Fungi 2023, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Cao, D.; Zhang, Z.; Cheng, S.; Wei, L.; Li, S.; Liu, B. Draft Genome Assembly of Floccularia luteovirens, an Edible and Symbiotic Mushroom on Qinghai-Tibet Plateau. G3 2020, 10, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Bao, X.; Liu, B.; Li, Y.; Cao, D.; Zhang, H.; Zong, Y. Chemical Constituents and Molecular Mechanism of the Yellow Phenotype of Yellow Mushroom (Floccularia luteovirens). J. Fungi 2022, 8, 314. [Google Scholar] [CrossRef]
- Arana-Gabriel, Y.; Burrola-Aguilar, C.; Alcalá-Adán, A.; Carmen, Z.; Estrada-Zúñiga, M. Mycelial growth of the edible wild mushrooms Floccularia luteovirens in different culture mediums and pH. Agro Product. 2020, 13, 33–38. [Google Scholar] [CrossRef]
- Dang, J.; Chen, C.; Ma, J.; Dawa, Y.; Wang, Q.; Tao, Y.; Wang, Q.; Ji, T. Preparative isolation of highly polar free radical inhibitor from Floccularia luteovirens using hydrophilic interaction chromatography directed by on-line HPLC-DPPH assay. J. Chromatogr. B Anal. Technol. Biom. 2020, 1142, 122043. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Q.; Miao, R.; Lin, J.; Feng, R.; Ni, Y.; Li, W.; Yang, D.; Zhao, X. Application of omics technology in the research on edible fungi. Curr. Res. Food Sci. 2023, 6, 100430. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, D.D.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S96–S115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of untargeted metabolomics approach and molecular networking analysis to identify unique natural components in wild Morchella sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, X.; Cai, M.; Gu, H.; Hengchao, E.; Li, X.; Zhang, Y.; Lu, H.; Zhou, C. Metabolomics Analysis of Morchella sp. from Different Geographical Origins of China Using UPLC-Q-TOF-MS. Front. Nutr. 2022, 9, 865531. [Google Scholar] [CrossRef] [PubMed]
- Azofeifa, G.; Quesada, S.; Pérez, A.; Vaillant, F.; Michel, A. Effect of an In Vitro Digestion on the Antioxidant Capacity of a Microfiltrated Blackberry Juice (Rubus adenotrichos). Beverages 2018, 4, 30. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Ganesan, K.; Xu, B. New Insight into Mycochemical Profiles and Antioxidant Potential of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Deb, C.R. Nutritional and antioxidant potential of some wild edible mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Gao, W.; Li, C.; Lin, Y.; Zhao, P. AsA-GSH Cycle and Antioxidant Enzymes Play Important Roles in Cd Tolerance of Wheat. Bull. Environ. Contam. Toxicol. 2018, 101, 684–690. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Segla Koffi Dossou, S.; Xu, F.; You, J.; Zhou, R.; Li, D.; Wang, L. Widely targeted metabolome profiling of different colored sesame (Sesamum indicum L.) seeds provides new insight into their antioxidant activities. Food Res. Int. 2022, 151, 110850. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhong, L.; Yang, H.; Zhu, F.; Hou, X.; Wu, C.; Zhang, R.; Cheng, Y.J.L. Comparative analysis of antioxidant activities between dried and fresh walnut kernels by metabolomic approaches. LWT 2021, 155, 112875. [Google Scholar] [CrossRef]
- Du, Z.; Lin, W.; Yu, B.; Zhu, J.; Li, J. Integrated Metabolomic and Transcriptomic Analysis of the Flavonoid Accumulation in the Leaves of Cyclocarya paliurus at Different Altitudes. Front. Plant Sci. 2021, 12, 794137. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.; Ivanova, T.; Kuznetsova, E.; Kumachova, T. Adaptations of Malus domestica Borkh. (Rosaceae) Fruits Grown at Different Altitudes. Russ. J. Plant Physiol. 2019, 66, 922–931. [Google Scholar] [CrossRef]
- Liu, S.; Gu, X.; Jiang, Y.; Wang, L.; Xiao, N.; Chen, Y.; Jin, B.; Wang, L.; Li, W. UV-B promotes flavonoid biosynthesis in Ginkgo biloba by inducing the GbHY5-GbMYB1-GbFLS module. Hortic. Res. 2023, 10, uhad118. [Google Scholar] [CrossRef]
- Qiao, F.; Lu, Y.; Geng, G.; Zhou, L.; Chen, Z.; Wang, L.; Xie, H.; Qiu, Q.S. Flavonoid synthesis in Lamiophlomis rotata from Qinghai-Tibet Plateau is influenced by soil properties, microbial community, and gene expression. J. Plant Physiol. 2023, 287, 154043. [Google Scholar] [CrossRef]
- Zhou, S.; Yan, X.; Yang, J.; Qian, C.; Yin, X.; Fan, X.; Fang, T.; Gao, Y.; Chang, Y.; Liu, W.; et al. Variations in Flavonoid Metabolites Along Altitudinal Gradient in a Desert Medicinal Plant Agriophyllum squarrosum. Front. Plant Sci. 2021, 12, 683265. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Sun, X.; Yang, C.; Fan, Y. Integrated transcriptome and metabolome provide insight into flavonoid variation in goji berries (Lycium barbarum L.) from different areas in China. Plant Physiol. Biochem. 2023, 199, 107722. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Liu, Z.; Wang, L.; Bi, Z.; Sun, C.; Yao, P.; Zhang, J.; Bai, J.; Zeng, Y. Integrated transcriptomic and metabolomic analysis revealed altitude-related regulatory mechanisms on flavonoid accumulation in potato tubers. Food Res. Int. 2023, 170, 112997. [Google Scholar] [CrossRef]
- Kissanga, R.; Liberal, Â.; Diniz, I.; Rodrigues, A.S.B.; Baptista-Ferreira, J.L.; Batista, D.; Ivanov, M.; Soković, M.; Ferreira, I.; Fernandes, Â.; et al. Biochemical and Molecular Profiling of Wild Edible Mushrooms from Huila, Angola. Foods 2022, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutr. Biochem. 2017, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Luo, S.; Zhang, X. Dietary flavonoids: A novel strategy for the amelioration of cognitive impairment through intestinal microbiota. J. Sci. Food Agric. 2023, 103, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, Y.; Jiang, Y.; Meng, X. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 10937. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Patel, R. Unraveling the mechanisms of trans-cinnamic acid in ameliorating non-alcoholic fatty liver disease. Am. J. Transl. Res. 2023, 15, 5747–5756. [Google Scholar]
- Oke, F.; Aslim, B. Protective effect of two edible mushrooms against oxidative cell damage and their phenolic composition. Food Chem. 2011, 128, 613–619. [Google Scholar] [CrossRef]
- Karaman, M.; Jovin, E.; Malbasa, R.; Matavuly, M.; Popović, M. Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother. Res. 2010, 24, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; Martínez-Tomé, M.; Jiménez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P. Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. J. Food Prot. 2002, 65, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Choonpicharn, S.; Tanreuan, K.; Lumyong, S. Characterization of Polysaccharides from Wild Edible Mushrooms from Thailand and Their Antioxidant, Antidiabetic, and Antihypertensive Activities. Int. J. Med. Mushrooms 2020, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants 2024, 13, 620. https://doi.org/10.3390/antiox13050620
Tang C, Fan Y, Wang T, Wang J, Xiao M, He M, Chang X, Li Y, Li X. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants. 2024; 13(5):620. https://doi.org/10.3390/antiox13050620
Chicago/Turabian StyleTang, Chuyu, Yuejun Fan, Tao Wang, Jie Wang, Mengjun Xiao, Min He, Xiyun Chang, Yuling Li, and Xiuzhang Li. 2024. "Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities" Antioxidants 13, no. 5: 620. https://doi.org/10.3390/antiox13050620
APA StyleTang, C., Fan, Y., Wang, T., Wang, J., Xiao, M., He, M., Chang, X., Li, Y., & Li, X. (2024). Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants, 13(5), 620. https://doi.org/10.3390/antiox13050620






