Differential Extraction and Preliminary Identification of Polyphenols from Ugni candollei (White Murta) Berries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Solvents
2.3. Samples
2.4. Polyphenols Extraction
2.4.1. Conventional Extraction
2.4.2. DES Extraction
2.4.3. Hot Pressurized Water Extraction (HPWE)
2.5. Polyphenol Analysis by UPLC with Orbitrap Detector
2.6. Statistical Analysis
3. Results
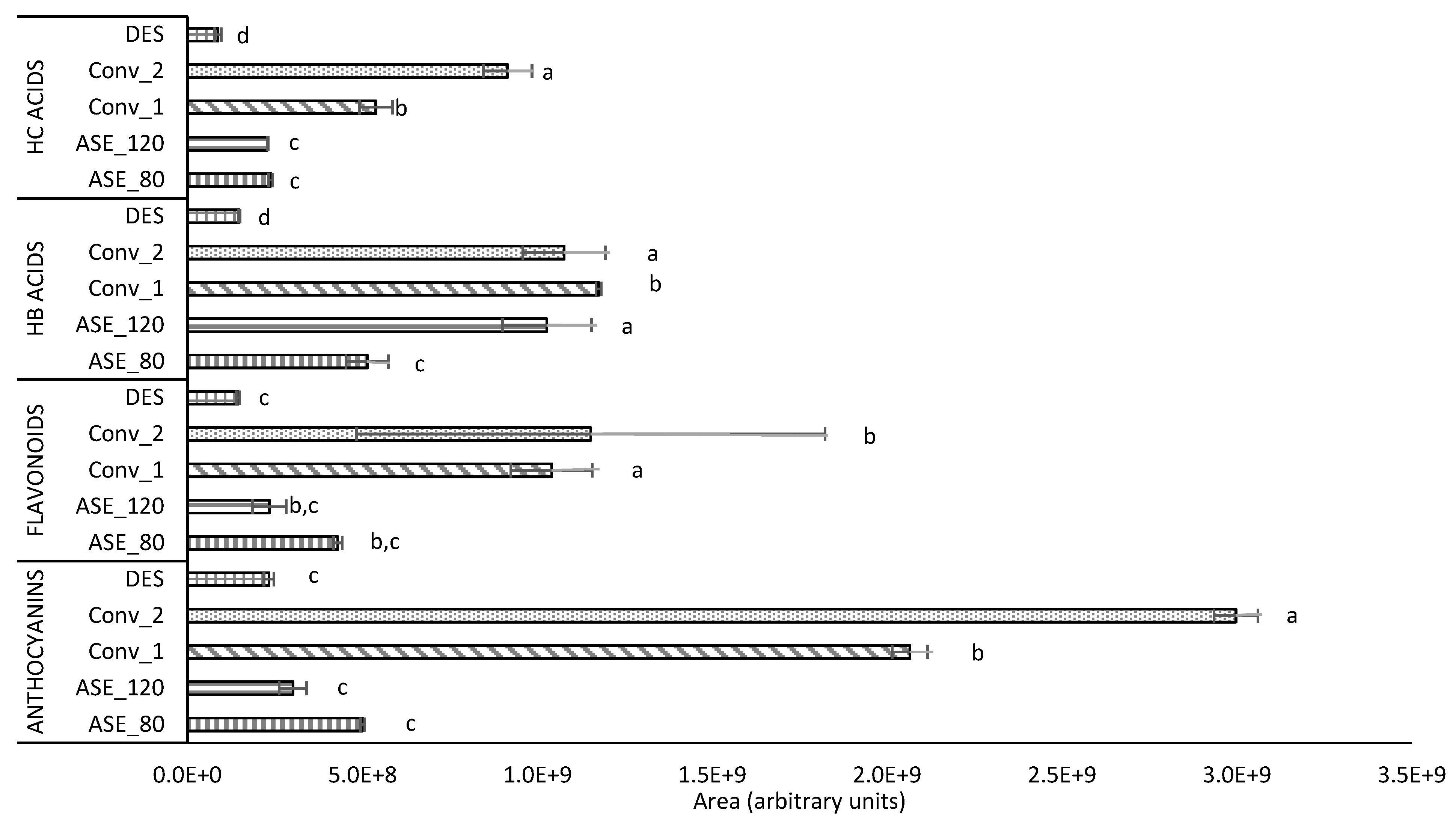
3.1. Phenolic Compounds Profile by HPLC-ESI-ORBITRAP MS Analysis
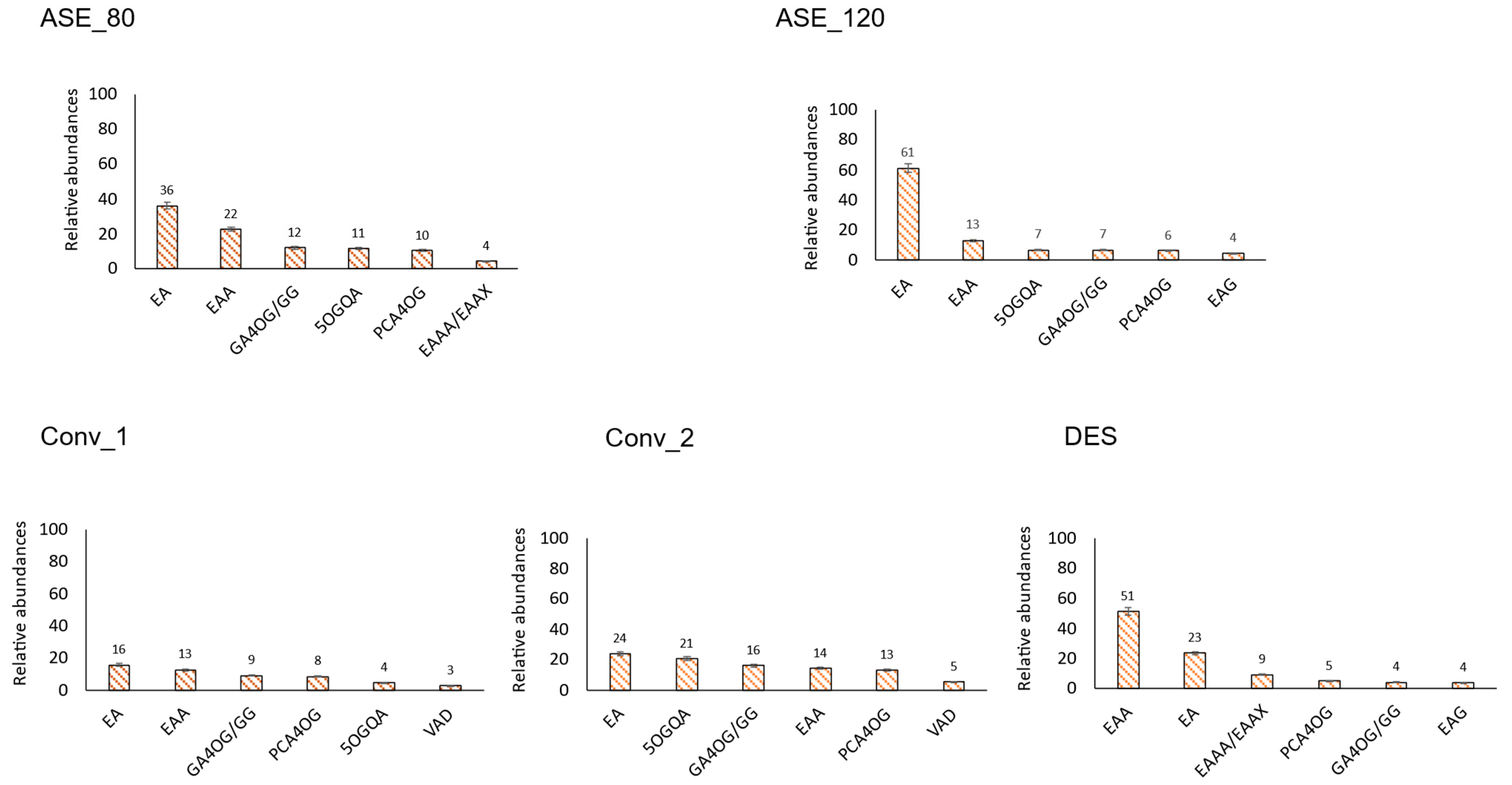
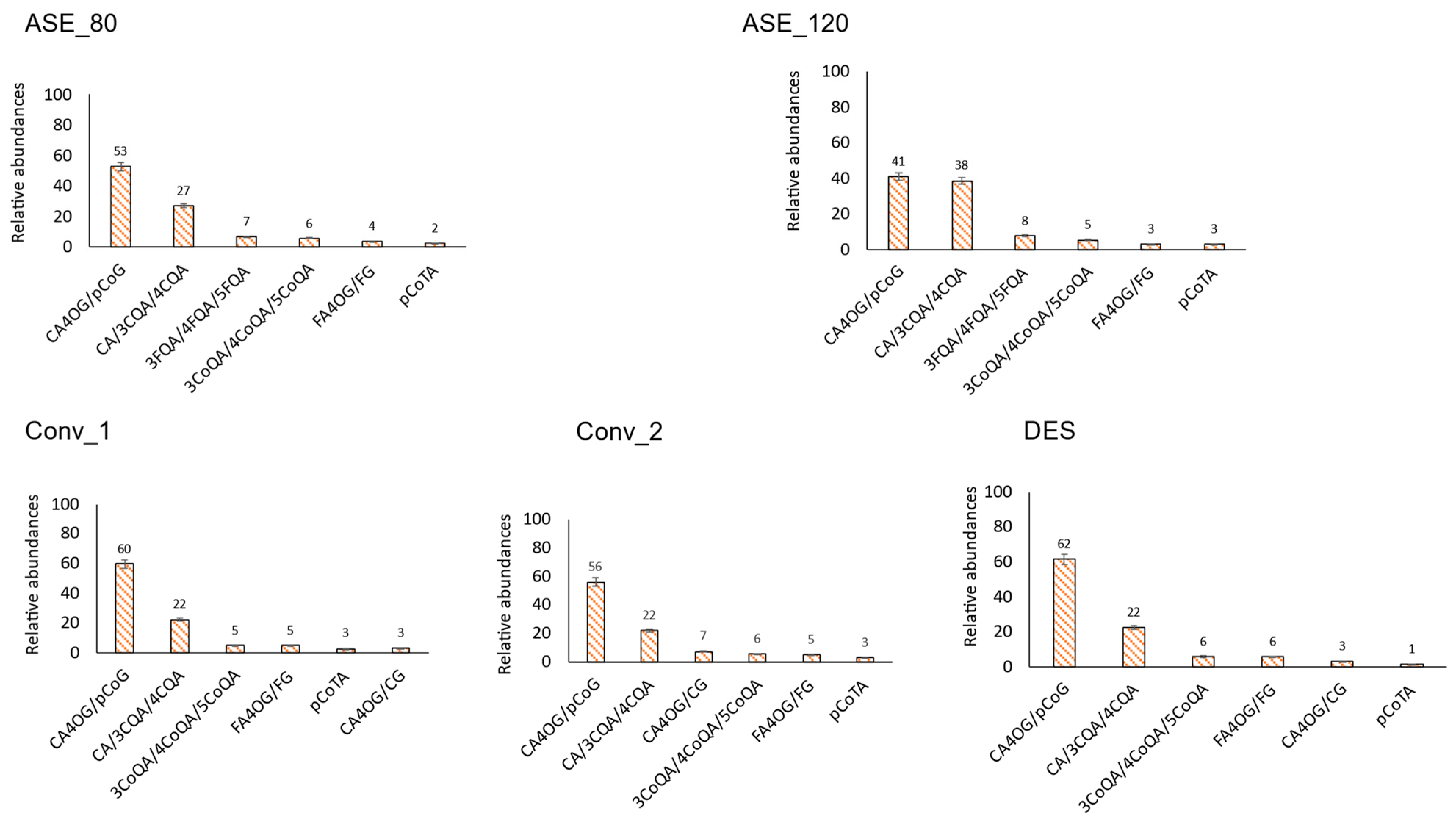
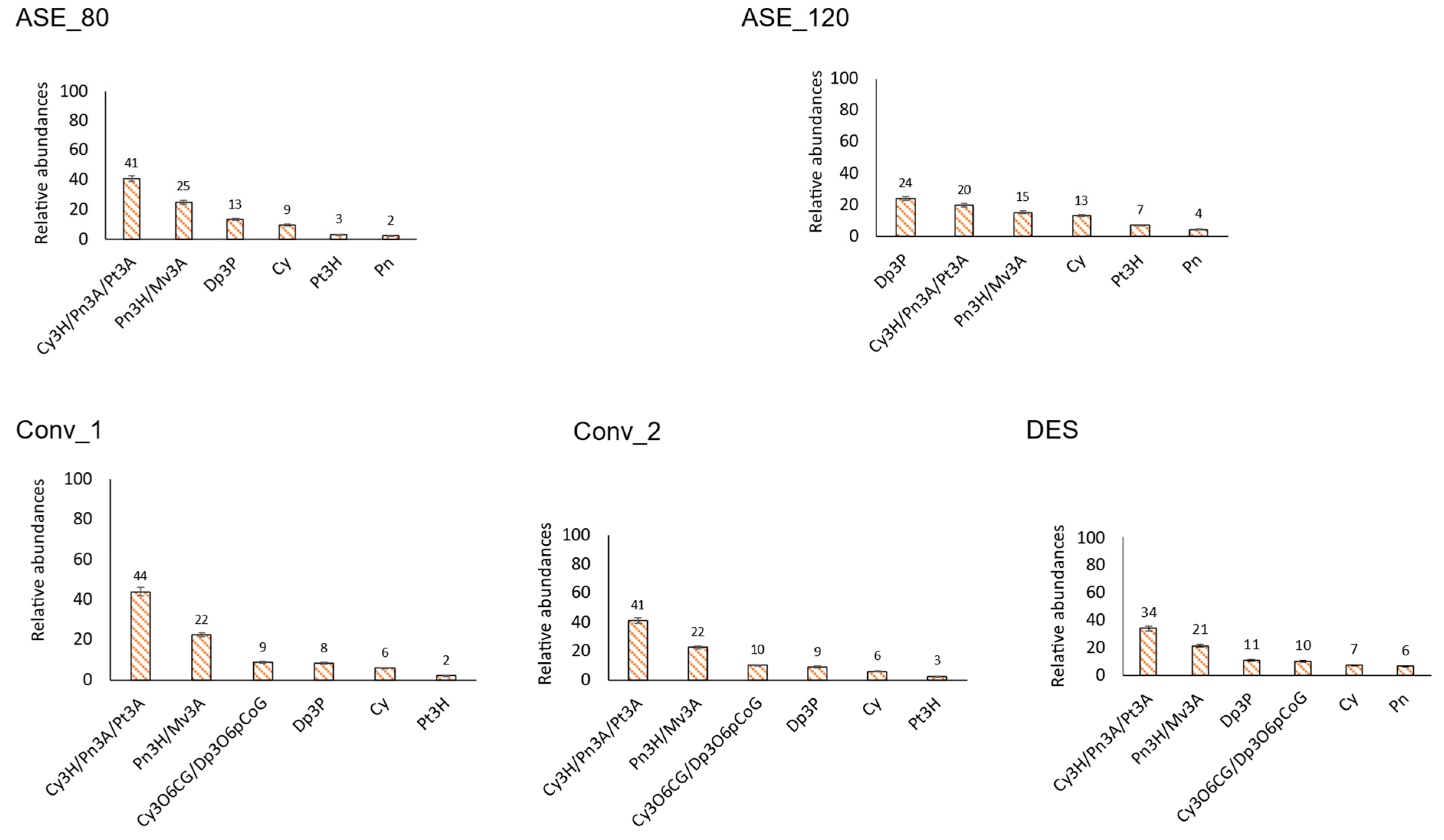
3.2. Main Individual Phenolic Compounds per Family
3.2.1. Hydroxybenzoic Acids
3.2.2. Hydroxycinnamic Acids
3.2.3. Anthocyanins
3.2.4. Other Flavonoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, C.; Langyan, S.; Echeverría, J.; Devkota, H.P.; Tewari, D.; Moosavi, M.A.; Ezzat, S.M.; Perez-Vazquez, A.; Fraga-Corral, M.; Cravotto, G.; et al. Edible fruits and berries as a source of functional polyphenols: Current scene and future perspectives. Phytochem. Rev. 2023, 10. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Villalva, M.; Martínez-García, J.J.; Jaime, L.; Santoyo, S.; Pelegrín, P.; Pérez-Jiménez, J. Polyphenols as NLRP3 inflammasome modulators in cardiometabolic diseases: A review of in vivo studies. Food Funct. 2023, 14, 9534–9553. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural polyphenols targeting senescence: A novel prevention and therapy strategy for cancer. Int. J. Mol. Sci. 2020, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Tovar, P.R.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Maqui (Aristotelia chilensis (Mol.) Stuntz) and murta (Ugni molinae Turcz): Native Chilean sources of polyphenol compounds. Mini. Rev. Org. Chem. 2018, 16, 261–276. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 Polyphenols in 452 foods and beverages: An application of the phenol-explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Otero, C.; Klagges, C.; Morales, B.; Sotomayor, P.; Escobar, J.; Fuentes, J.A.; Moreno, A.A.; Llancalahuen, F.M.; Arratia-Perez, R.; Gordillo-Fuenzalida, F.; et al. Anti-Inflammatory Chilean Endemic Plants. Pharmaceutics 2023, 15, 897. [Google Scholar] [CrossRef]
- Pérez-Arancibia, R.; Ordoñez, J.L.; Rivas, A.; Pihán, P.; Sagredo, A.; Ahumada, U.; Barriga, A.; Seguel, I.; Cárdenas, C.; Vidal, R.L.; et al. A phenolic-rich extract from Ugni molinae berries reduces abnormal protein aggregation in a cellular model of Huntington’s disease. PLoS ONE 2021, 16, e0254834. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and characterization of phenolic compounds and anthocyanins from murta (Ugni molinae Turcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef] [PubMed]
- Shene, C.; Reyes, A.K.; Villarroel, M.; Sineiro, J.; Pinelo, M.; Rubilar, M. Plant location and extraction procedure strongly alter the antimicrobial activity of murta extracts. Eur. Food Res. Technol. 2009, 228, 467–475. [Google Scholar] [CrossRef]
- Peña-Cerda, M.; Arancibia-Radich, J.; Valenzuela-Bustamante, P.; Pérez-Arancibia, R.; Barriga, A.; Seguel, I.; García, L.; Delporte, C. Phenolic composition and antioxidant capacity of Ugni molinae Turcz. leaves of different genotypes. Food Chem. 2017, 215, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Arancibia-Avila, P.; Toledo, F.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Ham, K.S.; Kang, S.G.; Vearasilp, K.; Suhaj, M. Application of Analytical Methods for the Determination of Bioactive Compounds in Some Berries. Food Anal. Methods 2013, 6, 432–444. [Google Scholar] [CrossRef]
- López, J.; Vega-Gálvez, A.; Ah-Hen, K.S.; Rodríguez, A.; Quispe-Fuentes, I.; Delporte, C.; Valenzuela-Barra, G.; Arancibia, Y.; Zambrano, A. Evaluation of the antioxidant, anti-inflammatory, and anti-tumoral properties of bioactive compounds extracted from murta berries (Ugni molinae T.) dried by different methods. Front. Plant Sci. 2023, 14, 1095179. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Barjas, G.; Quezada, A.; Bernardo, Y.; Moncada, M.; Zúñiga, E.; Wilkens, M.; Giordano, A.; Nesic, A.; Delgado, N.; Cabrera-Barjas Gcabrerab, G. Chemical composition and antibacterial activity of red murta (Ugni molinae Turcz.) seeds: An undervalued Chilean resource. J. Food Meas. Charact. 2020, 14, 1810–1821. [Google Scholar] [CrossRef]
- Castro, R.I.; Ramos, P.; Parra-Palma, C.; Morales-Quintana, L. Ugni molinae Fruit as a Source of Bioactive Compounds with Good Quality Traits. Biomed. Res. Int. 2021, 2021, 6683877. [Google Scholar] [CrossRef] [PubMed]
- Avello Lorca, C.M.; Bittner Berner, C.M.; Becerra Allende, C.J. Efectos antibacterianos de extractos de especies del género Ugni que crecen en Chile. Rev. Cuba. Plantas Med. 2013, 18, 247–257. [Google Scholar]
- Lorca, M.A.A.; Navarrete, E.P.; Berner, M.B.; Ponce, E.R. Chemical properties and assessment of the antioxidant capacity of native species from the genus Ugni. Rev. Cuba. De Plantas Med. 2016, 3, 284–297. [Google Scholar]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Waseem, M.; Majeed, Y.; Nadeem, T.; Naqvi, L.H.; Khalid, M.A.; Sajjad, M.M.; Sultan, M.; Khan, M.U.; Khayrullin, M.; Shariati, M.A.; et al. Conventional and advanced extraction methods of some bioactive compounds with health benefits of food and plant waste: A comprehensive review. Food Front. 2023, 4, 1681–1701. [Google Scholar] [CrossRef]
- Ozturk, B.; Esteban, J.; Gonzalez-Miquel, M. Deterpenation of Citrus Essential Oils Using Glycerol-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2384–2393. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Gajardo-Parra, N.F.; Lubben, M.J.; Winnert, J.M.; Leiva, Á.; Brennecke, J.F.; Canales, R.I. Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol. J. Chem. Thermodyn. 2019, 133, 272–284. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of maqui (Aristotelia chilensis) and murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, J.; Zhang, J.; Wang, Q.; Wang, F.; Qiao, Y.; Zhang, Y. Rapid determination of ten polyphenols in Kudiezi injection using ultra-performance liquid chromatography-tandem mass spectrometry in multiple reaction monitoring mode. Anal. Methods 2012, 4, 4230–4236. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four European countries. Food Res. Int. 2015, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Fara, D.C.; Yang, H.; Tämm, K.; Tamm, T.; Karelson, M. Quantitative Measures of Solvent Polarity. Chem. Rev. 2004, 104, 175–198. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Yang, J.; Ou, X.Q.; Zhang, X.; Zhou, Z.Y.; Ma, L.Y. Effect of Different Solvents on the Measurement of Phenolics and the Antioxidant Activity of Mulberry (Morus atropurpurea Roxb.) with Accelerated Solvent Extraction. J. Food Sci. 2017, 82, 605–612. [Google Scholar] [CrossRef]
- Cheng, V.J.; Bekhit, A.E.D.A.; McConnell, M.; Mros, S.; Zhao, J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012, 134, 474–482. [Google Scholar] [CrossRef]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Ko, M.J.; Cheigh, C.I.; Chung, M.S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Y.; Wang, L.; Yin, W.; Liang, J. Antioxidant activity and subcritical water extraction of anthocyanin from raspberry process optimization by response surface methodology. Food Biosci. 2021, 44, 101394. [Google Scholar] [CrossRef]
- Frosi, I.; Balduzzi, A.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of polyphenols from corn cob (Zea mays L.): Optimization of different green extraction methods and efficiency comparison. Food Bioprod. Process. 2024, 143, 212–220. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Gajardo-Parra, N.; Pérez-Correa, J.R.; Canales, R.I.; Martínez-Cifuentes, M.; Contreras-Contreras, G.; Mariotti-Celis, M.S. Enhanced Polyphenols Recovery from Grape Pomace: A Comparison of Pressurized and Atmospheric Extractions with Deep Eutectic Solvent Aqueous Mixtures. Antioxidants 2023, 12, 1446. [Google Scholar] [CrossRef]
- Čulina, P.; Cvitković, D.; Pfeifer, D.; Zorić, Z.; Repajić, M.; Garofulić, I.E.; Balbino, S.; Pedisić, S. Phenolic profile and antioxidant capacity of selected medicinal and aromatic plants: Diversity upon plant species and extraction technique. Processes 2021, 9, 2207. [Google Scholar] [CrossRef]
- Altunay, N.; Unal, Y.; Elik, A. Towards green analysis of curcumin from tea, honey and spices: Extraction by deep eutectic solvent assisted emulsification liquid-liquid microextraction method based on response surface design. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 869–881. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC-Trends Anal. Chem. 2023, 166, 117201. [Google Scholar] [CrossRef]
- Višnjevec, A.M.; Barp, L.; Lucci, P.; Moret, S. Pressurized liquid extraction for the determination of bioactive compounds in plants with emphasis on phenolics. TrAC-Trends Anal. Chem. 2024, 173, 117620. [Google Scholar] [CrossRef]
- El-Shibani, F.A.A.; Sulaiman, G.M.; Abouzied, A.S.; Al Ali, A.; Abdulkarim, A.K.; Alamami, A.D.; Asiri, M.; Mohammed, H.A. Polyphenol Fingerprint, Biological Activities, and In Silico Studies of the Medicinal Plant Cistus parviflorus L. Extract. ACS Omega 2023, 8, 48269–48279. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, S.; Mutis, A.; Palma, R.; Quiroz, A.; Seguel, I.; Scheuermann, E. Influence of genotype and harvest year on polyphenol content and antioxidant activity in murtilla (Ugni molinae Turcz) fruit. J. Soil Sci. Plant Nutr. 2013, 13, 67–78. [Google Scholar] [CrossRef]
- Augusto-Obara, T.R.; Pirce, F.; Scheuermann, E.; Spoto, M.H.F.; Vieira, T.M.F.S. Antioxidant activity and sensory analysis of murtilla (Ugni molinae Turcz.) fruit extracts in an oil model system. Grasas Y Aceites 2017, 68, e183. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.S.; Moraga, N.; Ah-Hen, K.S.; Rodríguez, A.; Vega-Gálvez, A. Dietary fibre in processed murta (Ugni molinae Turcz) berries: Bioactive components and antioxidant capacity. J. Food Sci. Technol. 2022, 59, 3093–3101. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some chilean edible berry extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.J.; Chaves, V.C.; dos Nascimento, M.V.P.S.; Calvete, E.; Li, M.; Ciraolo, E.; Ghigo, A.; Hirsch, E.; Simões, C.M.O.; Reginatto, F.H.; et al. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chem. 2018, 247, 56–65. [Google Scholar] [CrossRef]
- De Oliveira Azevedo, A.; Campos, J.J.; De Souza, G.G.; De Carvalho Veloso, C.; Duarte, I.D.G.; Braga, F.C.; De Castro Perez, A. Antinociceptive and anti-inflammatory effects of myricetin 3-O-β-galactoside isolated from Davilla elliptica: Involvement of the nitrergic system. J. Nat. Med. 2015, 69, 487–493. [Google Scholar] [CrossRef]
- Ye, S.; Ma, J.; Ye, C.; Wang, X. Neuroprotective and anti-inflammatory effects of myricetin 3-glucoside in a rat model of cerebral ischemia. Trop. J. Pharm. Res. 2020, 19, 1709–1714. [Google Scholar] [CrossRef]
- Rubilar, M.; Pinelo, M.; Ihl, M.; Scheuermann, E.; Sineiro, J.; Nuñez, M.J. Murta leaves (Ugni molinae Turcz) as a source of antioxidant polyphenols. J. Agric. Food Chem. 2006, 54, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MS n identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]

| Class/Family | ASE_80 | ASE_120 | Conv_1 | Conv_2 | DES |
|---|---|---|---|---|---|
| Hydroxycinnamic acids | 14.1 ± 3.1 | 12.8 ± 2.0 | 10.0 ± 3.4 | 16.1 ± 2.5 | 14.20 ± 0.1 |
| Hydroxybenzoic acids | 30.6 ± 1.2 | 57.3 ± 0.9 | 12.4 ± 3.1 | 18.9 ± 0.1 | 24.1 ± 1.1 |
| Flavonoids | 25.6 ± 9.7 | 13.1 ± 2.1 | 30.2 ± 5.1 | 12.3 ± 9.8 | 23.6 ± 0.3 |
| Anthocyanins | 29.8 ± 5.4 | 16.8 ± 0.8 | 47.4 ± 1.4 | 52.7 ± 7.1 | 38.1 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Jorquera, N.; Canales, R.I.; Pérez-Correa, J.R.; Pérez-Jiménez, J.; Mariotti-Celis, M.S. Differential Extraction and Preliminary Identification of Polyphenols from Ugni candollei (White Murta) Berries. Antioxidants 2024, 13, 623. https://doi.org/10.3390/antiox13060623
Fuentes-Jorquera N, Canales RI, Pérez-Correa JR, Pérez-Jiménez J, Mariotti-Celis MS. Differential Extraction and Preliminary Identification of Polyphenols from Ugni candollei (White Murta) Berries. Antioxidants. 2024; 13(6):623. https://doi.org/10.3390/antiox13060623
Chicago/Turabian StyleFuentes-Jorquera, Natalia, Roberto I. Canales, José R. Pérez-Correa, Jara Pérez-Jiménez, and María Salomé Mariotti-Celis. 2024. "Differential Extraction and Preliminary Identification of Polyphenols from Ugni candollei (White Murta) Berries" Antioxidants 13, no. 6: 623. https://doi.org/10.3390/antiox13060623
APA StyleFuentes-Jorquera, N., Canales, R. I., Pérez-Correa, J. R., Pérez-Jiménez, J., & Mariotti-Celis, M. S. (2024). Differential Extraction and Preliminary Identification of Polyphenols from Ugni candollei (White Murta) Berries. Antioxidants, 13(6), 623. https://doi.org/10.3390/antiox13060623









