Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives
Abstract
1. Introduction
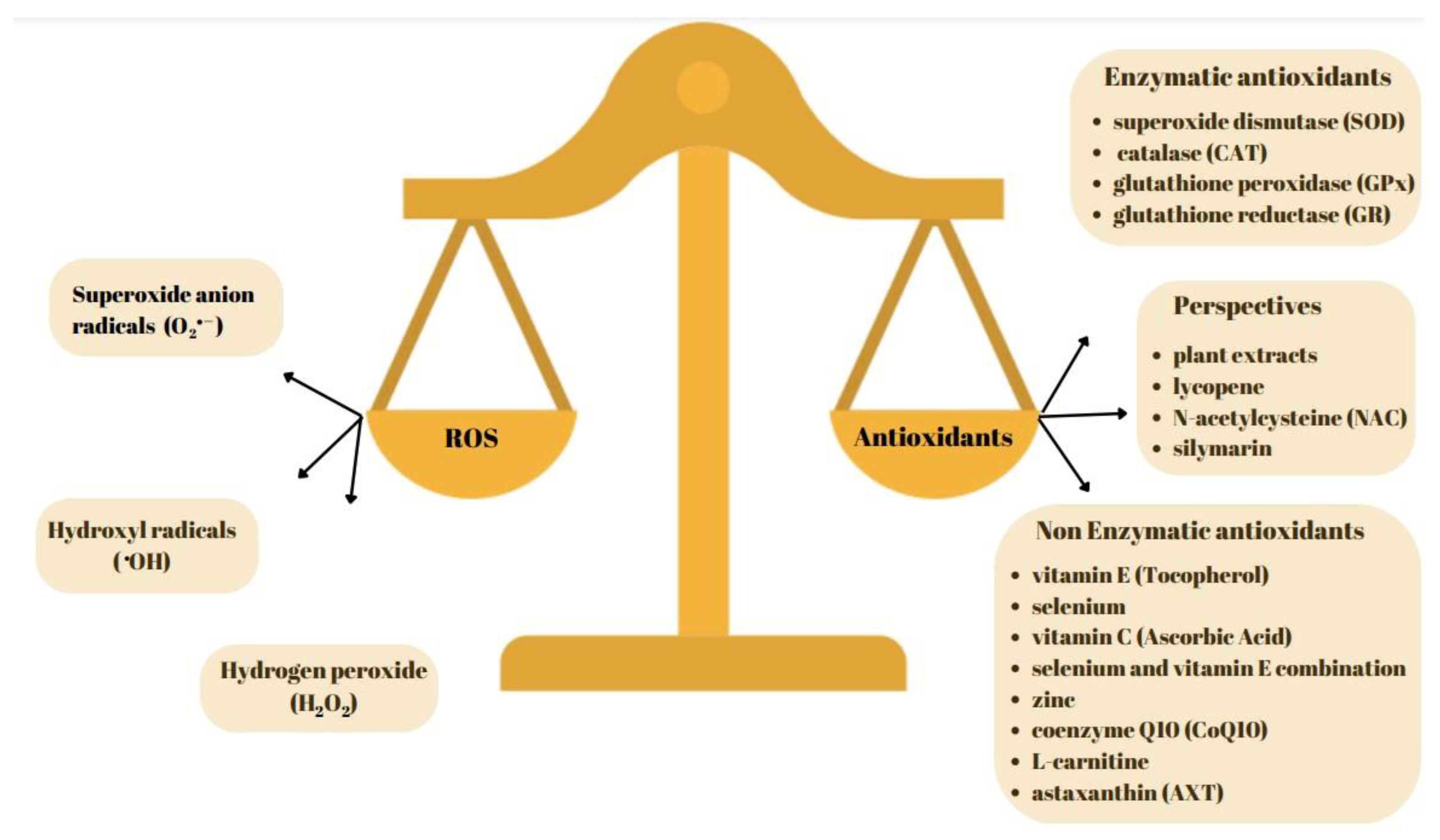
2. Free Radicals in Small Ruminant’s Semen
3. Antioxidants in Small Ruminants Semen Sample
4. The Current Status of Non-Enzymatic Antioxidant Utilization in Enhancing Seminal Material in Small Ruminants
5. The Current Status of Enzymatic Antioxidant Utilization in Enhancing Seminal Material in Small Ruminants
6. A Perspective in Exploring Various Antioxidants to Facilitate the Improvement of Sperm Quality in Rams and Bucks
7. Conclusions
- -
- Enzymatic antioxidants, such as SOD, CAT, and GPx, play crucial roles in protecting sperm from oxidative damage, thereby improving semen quality.
- -
- Supplementation of extenders with antioxidants, including SOD, CAT, and N-acetylcysteine (NAC), has shown promise in mitigating cryodamage and preserving sperm viability and motility during cryopreservation.
- -
- Natural antioxidants, such as those derived from plant extracts like silymarin, offer potential alternatives for enhancing semen cryopreservation outcomes in small ruminants.
- -
- Future research efforts should prioritize in vivo validation of antioxidant interventions and focus on achieving tangible reproductive outcomes, such as pregnancies and offspring, to assess the real-world efficacy of antioxidant supplementation.
- -
- Additionally, studies exploring the optimal dosage, timing, and interactions of antioxidants with cryoprotectants are needed to refine semen cryopreservation protocols and improve reproductive success in small ruminants.
Author Contributions
Funding
Conflicts of Interest
References
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Naveed, M.I.; Raza, S.; Fang, X.; Roy, P.K.; Bang, S.; Tanga, B.M.; Saadeldin, I.M.; Lee, S.; Cho, J. Role of antioxidants in fertility preservation of sperm—A narrative review. Anim. Biosci. 2023, 36, 385–403. [Google Scholar] [CrossRef]
- Mailloux, R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015, 4, 381–398. [Google Scholar] [CrossRef]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus 2023, 15, e42583. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; Salamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Jurado-Campos, A.; Soria-Meneses, P.J.; Arenas-Moreira, M.; Alonso-Moreno, C.; Bravo, I.; Rodríguez-Robledo, V.; Sánchez-Ajofrín, I.; Soler, J.; Garde, J.J.; Fernández-Santos, M.D.R. Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility. Antioxidants 2022, 11, 1988. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.J.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar] [PubMed] [PubMed Central]
- Durairajanayagam, D.; Agarwal, A.; Ong, C.; Prashast, P. Lycopene and male infertility. Asian J. Androl. 2014, 16, 420–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alahmar, A.T.; Sengupta, P.; Dutta, S.; Calogero, A.E. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin. Exp. Reprod. Med. 2021, 48, 150–155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical Screening, Antioxidant and Sperm Viability of Nelumbo nucifera Petal Extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Covarrubias, L.; Hernández-García, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregón, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia. 2021, 53, e13577. [Google Scholar] [CrossRef]
- Cord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar]
- Rao, A.L.; Bharani, M.; Pallavi, V. Role of antioxidants and free radicals in health and disease. Adv. Pharmacol. Toxicol. 2006, 7, 29–38. [Google Scholar]
- Pasciu, V.; Nieddu, M.; Sotgiu, F.D.; Baralla, E.; Berlinguer, F. An Overview on Assay Methods to Quantify ROS and Enzymatic Antioxidants in Erythrocytes and Spermatozoa of Small Domestic Ruminants. Animals 2023, 13, 2300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Berlinguer, F.; Pasciu, V.; Succu, S.; Cossu, I.; Caggiu, S.; Addis, D.; Castagna, A.; Fontani, V.; Rinaldi, S.; Passino, E.S. REAC Technology as Optimizer of Stallion Spermatozoa Liquid Storage. Reprod. Biol. Endocrinol. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Macías García, B.; González Fernández, L.; Ortega Ferrusola, C.; Salazar-Sandoval, C.; Morillo Rodríguez, A.; Rodríguez Martinez, H.; Tapia, J.A.; Morcuende, D.; Peña, F.J. Membrane Lipids of the Stallion Spermatozoon in Relation to Sperm Quality and Susceptibility to Lipid Peroxidation. Reprod. Domest. Anim. 2011, 46, 141–148. [Google Scholar] [CrossRef]
- Pintus, E.; Ros-Santaella, J.L. Impact of Oxidative Stress on Male Reproduction in Domestic and Wild Animals. Antioxidants 2021, 10, 1154. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.H.M.; Gadella, B.M. In Situ Detection and Localization of Lipid Peroxidation in Individual Bovine Sperm Cells. Free Radic. Biol. Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Holman, R.T. Fatty Acid Composition of Lipids of Bull, Boar, Rabbit and Human Semen. J. Reprod. Fertil. 1969, 18, 431–437. [Google Scholar] [CrossRef][Green Version]
- Nichi, M.; Goovaerts, I.G.F.; Cortada, C.N.M.; Barnabe, V.H.; De Clercq, J.B.P.; Bols, P.E.J. Roles of Lipid Peroxidation and Cytoplasmic Droplets on in vitro Fertilization Capacity of Sperm Collected from Bovine Epididymides Stored at 4 and 34 °C. Theriogenology 2007, 67, 334–340. [Google Scholar] [CrossRef]
- Perez-Patiño, C.; Barranco, I.; Li, J.; Padilla, L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J.; Parrilla, I. Cryopreservation Differentially Alters the Proteome of Epididymal and Ejaculated Pig Spermatozoa. Int. J. Mol. Sci. 2019, 20, 1791. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive Oxygen Species and Protein Modifications in Spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Woo, R.A.; Melure, K.G.; Lee, P.W. DNA dependent protein kinase acts upstream of p53 in response to DNA damage. Nature 1998, 394, 700–704. [Google Scholar] [CrossRef]
- Peña, F.J.; Flaherty, C.; Ortiz, R.J.M.; Martín, C.F.E.; Gaitskell, P.G.L.; Gil, M.C.; Ferrusola, C.O.; Flaherty, C.; Ortiz, R.J.M.; Martín, C.F.E.; et al. Redox regulation and oxidative stress: The particular case of the stallion spermatozoa. Antioxidants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Alvarez, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Boixo, J.C.; de Paz, P.; Anel, L. Multiparametric Study of Antioxidant Effect on Ram Sperm Cryopreservation—From Field Trials to Research Bench. Animals 2021, 11, 283. [Google Scholar] [CrossRef]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromation damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef]
- Allai, L.; Benmoula, A.; Silva, M.M.; Nasser, B.; Amiri, B. Supplementation of ram semen extender to improve seminal quality and fertility rate. Anim. Reprod. Sci. 2018, 192, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Durairajanayagam, D.; Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Amidi, F.; Pazhohan, A.; Shabani, N.M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, K.; Chen, C.; Hou, X.; Zhou, X. Application of antioxidants and centrifugation for cryopreservation of boar spermatozoa. Anim. Reprod. Sci. 2012, 132, 123–128. [Google Scholar] [CrossRef]
- Peris, F.P.; Soler, A.J.; Iniesta, C.M.; Martín, M.A.; Sánchez, A.I.; Medina, C.D.A.; Fernández, S.M.R.; García, Á.O.; Maroto, M.A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar] [CrossRef]
- Vahidinia, A.; Rahbar, A.R.; Shakoori Mahmoodabadi, M.M. Effect of astaxanthin, vitamin, E.; and vitamin C in combination with calorie restriction on sperm quality and quantity in male rats. J. Diet. Suppl. 2017, 14, 252–263. [Google Scholar] [CrossRef]
- Azawi, O.I.; Hussein, E.K. Effect of vitamins C or E supplementation to Tris diluent on the semen quality of Awassi rams preserved at 5 °C. Vet. Res. Forum. 2013, 4, 157–160. [Google Scholar] [PubMed] [PubMed Central]
- Gangwar, C.; Kharche, S.D.; Ranjan, R.; Kumar, S.; Goel, A.K.; Jindal, S.K.; Agrawal, S.K. Effect of Vitamin C Supplementation on Freezability of Barbari Buck Semen. Small Rumin. Res. 2015, 129, 104–107. [Google Scholar] [CrossRef]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z.; Towhidi, A.; Kohram, H.; Esmaeili, V.; Shahverdi, A.; Davachi, N.D. Fertility and flow cytometry study of frozen-thawed sperm in cryopreservation medium supplemented with soybean lecithin. Cryobiology 2016, 73, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Qureshi, M.S.; Khan, R.U. In vivo adverse effects of alpha-tocopherol on the semen quality of male bucks. J. Anim. Physiol. Anim. Nutr. 2015, 99, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, M.; Borghei-Rad, S.M.; Hezavehei, M.; Shahverdi, A.; Benson, J.D. Cryopreservation of Semen in Domestic Animals: A Review of Current Challenges, Applications, and Prospective Strategies. Animals 2022, 12, 3271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yue, D.; Yan, L.; Luo, H.; Xu, X.; Jin, X. Effect of Vitamin E supplementation on semen quality and the testicular cell membranal and mitochondrial antioxidant abilities in Aohan fine-wool sheep. Anim. Reprod. Sci. 2010, 118, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Hailing, L.; Hui, M.; Guijie, Z.; Leyan, Y.; Dubing, Y. Effect of vitamin E supplement in diet on antioxidant ability of testis in Boer goat. Anim. Reprod. Sci. 2010, 117, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Zubair, M.; Mahmood, M.; Andrabi, S.M.H.; Hameed, N.; Ahmad, E.; Saleemi, M.K. Effects of vitamins C and E in tris citric acid glucose extender on chilled semen quality of Kail ram during different storage times. Sci. Rep. 2023, 13, 18123. [Google Scholar] [CrossRef] [PubMed]
- Lukusa, K.; Lehloenya, K.C. Selenium supplementation improves testicular characteristics and semen quality of Saanen bucks. Small Rumin. Res. 2017, 151, 52–58. [Google Scholar] [CrossRef]
- Birringer, M.; Pilawa, S.; Flohe, L. Trends in selenium biochemistry. Nat. Prod. Rep. 2002, 19, 693–718. [Google Scholar] [CrossRef]
- Ali, A.B.; Bomboi, G.; Floris, B. Does vitamin E or vitamin E plus selenium improve reproductive performance of rams during hot weather? Ital. J. Anim. Sci. 2009, 8, 743–754. [Google Scholar]
- Al-Sarry, R.; Abdulameer, M.; Al-Kareem, A.; Alaa, A. The effect of adding of selenium nanoparticles to the tris extender on awassi rams semen quality preserveded at cooling and freezing storage. Biochem. Cell. Arch. 2020, 20, 543. [Google Scholar]
- Ahad, G.; Mohammad, M.M.; Manochehr, S.; Hadi, H. Influences of dietary selenium, zinc and their combination on semen characteristics and testosterone concentration in mature rams during breeding season. J. Appl. Anim. Res. 2018, 46, 813–819. [Google Scholar] [CrossRef]
- Wong, W.Y.; Merkus, H.M.; Thomas, C.M.; Menkveld, R.; Zielhuis, G.A.; Steegers-Theunissen, R.P. Effects of folic acid and zinc sulfate on male factor subfertility: A double-blind, randomized, placebo-controlled trial. Fertil. Steril. 2002, 77, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, N. Biochemistry of lycopene. J. Anim. Vet. Adv. 2012, 11, 2605–2610. [Google Scholar] [CrossRef]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Wallace Hayes, A.; Hosseini, S.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: A review. BioFactors 2019, 45, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Asadpour, R.; Mansouri, K.; Sabrivand, A.; Kazemi-Darabadi, S. Lycopene protects sperm from oxidative stress in the experimental varicocele model. Food Sci. Nutr. 2021, 9, 6806–6817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goyal, A.; Chopra, M.; Lwaleed, B.A.; Birch, B.; Cooper, A.J. The effects of dietary lycopene supplementation on human seminal plasma. BJU Int. 2007, 99, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.-L.; Tang, Z.-G. Lycopene improves sperm motility and morphology: A promising agent for treating male infertility. Eur. J. Nutr. 2020, 59, 835–836. [Google Scholar] [CrossRef]
- Williams, E.A.; Parker, M.; Robinson, A.; Pitt, S.; Pacey, A.A. A randomized placebo-controlled trial to investigate the effect of lactolycopene on semen quality in healthy males. Eur. J. Nutr. 2020, 59, 825–833. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Paál, D.; Mackovich, A.; Alimov, J.; Lukáč, N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Tvrda, E.; Mackovich, A.; Greifova, H.; Hashim, F.; Lukac, N. Antioxidant effects of lycopene on bovine sperm survival and oxidative profile following cryopreservation. Veterinární Medicína 2017, 62, 429–436. [Google Scholar] [CrossRef]
- Tripathy, A.; Ara, F.; Ghosh, P.; Ghosh, D. Effect of lycopene on testicular androgenic and anti-oxidative enzymes in cyproterone acetate-induced male infertile Wistar strain albino rats: An in vitro study. Andrologia 2020, 52, e13494. [Google Scholar] [CrossRef] [PubMed]
- Souza, H.M.; Arruda, L.C.P.; Monteiro, M.M.; Nery, I.H.A.V.; Silva, R.A.J.A.; Oliveira, A.S.; Guerra, M.M.P. The in vitro investigation of lycopene effects on post-thawed ram sperm. S. Afr. J. Anim. Sci. 2019, 49, 876–883. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lahimer, M.; Mustapha, H.; Bach, V.; Khorsi-Cauet, H.; Benkhalifa, M.; Ajina, M.; Ben Ali, H. Oxidative stress in male infertility and therapeutic approach: A mini-review. Asian Pac. J. Reprod. 2023, 12, 249–255. [Google Scholar] [CrossRef]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z. Effects of CoQ10 on the quality of ram sperm during cryopreservation in plant and animal based extenders. Anim. Reprod. Sci. 2019, 208, 106103. [Google Scholar] [CrossRef] [PubMed]
- Heydarnejad, A.; Ostadhosseini, S.; Varnosfaderani, S.R.; Jafarpour, F.; Moghimi, A.; Nasr-Esfahani, M.H. Supplementation of maturation medium with CoQ10 enhances developmental competence of ovine oocytes through improvement of mitochondrial function. Mol. Reprod. Dev. 2019, 86, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Sohail, T.; Zhang, L.; Wang, X.; Jiang, C.; Wang, J.; Sun, X.; Li, Y. Astaxanthin Improved the Quality of Hu Ram Semen by Increasing the Antioxidant Capacity and Mitochondrial Potential and Mitigating Free Radicals-Induced Oxidative Damage. Animals 2024, 14, 319. [Google Scholar] [CrossRef]
- Fang, Y.; Zhong, R.; Chen, L.; Feng, C.; Sun, H.; Zhou, D. Effects of astaxanthin supplementation on the sperm quality and antioxidant capacity of ram semen during liquid storage. Small Rumin. Res. 2015, 130, 178–182. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Kuroki, T.; Ikeda, S.; Okada, T.; Maoka, T.; Kitamura, A.; Sugimoto, M.; Kume, S. Astaxanthin ameliorates heat stress-induced impairment of blastocyst development In Vitro: –Astaxanthin colocalization with and action on mitochondria–. J. Assist. Reprod. Genet. 2013, 30, 623–631. [Google Scholar] [CrossRef]
- Farzan, M.; Chamani, M.; Varnaseri, H. The antioxidant effect of astaxanthin on quantitative and qualitative parameters of bull sperm. Indian J. Foundamental Appl. Life Sci. 2014, 4, 425–430. [Google Scholar]
- Basioura, A.; Boscos, C.; Parrilla, I.; Tsousis, G.; Tsakmakidis, I. Effect of astaxanthin on the quality of boar sperm stored at 17 C, incubated at 37 C or under in vitro conditions. Reprod. Domest. Anim. 2018, 53, 463–471. [Google Scholar] [CrossRef]
- Andrisani, A.; Donà, G.; Tibaldi, E.; Brunati, A.M.; Sabbadin, C.; Armanini, D.; Alvisi, G.; Gizzo, S.; Ambrosini, G.; Ragazzi, E.; et al. Astaxanthin Improves Human Sperm Capacitation by Inducing Lyn Displacement and Activation. Mar. Drugs 2015, 13, 5533–5551. [Google Scholar] [CrossRef]
- Najafi, D.; Taheri, R.A.; Najafi, A.; Shamsollahi, M.; Alvarez-Rodriguez, M. Effect of astaxanthin nanoparticles in protecting the post-thawing quality of rooster sperm challenged by cadmium administration. Poult. Sci. 2020, 99, 1678–1686. [Google Scholar] [CrossRef]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Donà, G.; Kožuh, I.; Brunati, A.M.; Andrisani, A.; Ambrosini, G.; Bonanni, G.; Ragazzi, E.; Armanini, D.; Clari, G.; Bordin, L. Effect of Astaxanthin on Human Sperm Capacitation. Mar. Drugs 2013, 11, 1909–1919. [Google Scholar] [CrossRef]
- Tanphaichitr, V.; Leelahagul, P. Carnitine metabolism and human carnitine deficiency. Nutrition 1993, 9, 246–254. [Google Scholar]
- Banihani, S.; Agarwal, A.; Sharma, R.; Bayachou, M. Cryoprotective effect of L-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia 2014, 46, 637–641. [Google Scholar] [CrossRef]
- Ghorbani, F.; Nasiri, Z.; Koohestanidehaghi, Y.; Lorian, K. The antioxidant roles of L-carnitine and N-acetyl cysteine against oxidative stress on human sperm functional parameters during vitrification. Clin. Exp. Reprod. Med. 2021, 48, 316–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, C.M.; Blackman, M.R.; Wang, C.; Swerdloff, R.S. The role of carnitine in the male reproductive system. Ann. N. Y. Acad. Sci. 2004, 1033, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Balagnur, K. Recent advances in cryopreservation of semen and artificial insemination in sheep: A review. Indian J. Small Rumin. 2019, 25, 134–147. [Google Scholar] [CrossRef]
- Fu, K.; Chen, X.; Guo, W.; Zhou, Z.; Zhang, Y.; Ji, T.; Yang, P.; Tian, X.; Wang, W.; Zou, Y. Effects of N Acetylcysteine on the Expression of Genes Associated with Reproductive Performance in the Goat Uterus during Early Gestation. Animals 2022, 12, 2431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seyedasgari, F.; Asadi, B.; Kim, E. The effect of different concentrations of N-acetyl cysteine on liquefaction, quality and fertility of chilled semen in dromedary camel. Small Rumin. Res. 2024, 230, 107166. [Google Scholar] [CrossRef]
- Agnieszka, P.; Wojciech, N.; Martyna, B.; Piotr, M. Effects of N-acetyl-l-cysteine and catalase on the viability and motility of chicken sperm during liquid storage. Reprod. Biol. 2015, 15, 126–129. [Google Scholar]
- Ari, U.C.; Kulaksiz, R.; Öztürkler, Y.; Lehimcioğlu, N.C.; Yıldız, S. Effect of N-Acetylcysteine (NAC) on Post-thaw Semen Quality of Tushin Rams. Kafkas Üniversitesi Vet. Fakültesi Derg. 2016, 22, 883–887. [Google Scholar]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of Oxidative Stress and Antioxidants on Semen Functions. Vet. Med. Int. 2011, 2011, 686137. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Pelzer, K.D.; Kasimanickam, V.; Swecker, W.S.; Thatcher, C.D. Association of Classical Semen Parameters, Sperm DNA Fragmentation Index, Lipid Peroxidation and Antioxidant Enzymatic Activity of Semenin Ram-Lambs. Theriogenology 2006, 65, 1407–1421. [Google Scholar] [CrossRef]
- Bucak, M.N.; Ateşşahin, A.; Yüce, A. Effect of Anti-Oxidants and Oxidative Stress Parameters on Ram Semen after the Freeze-thawing Process. Small Rumin. Res. 2008, 75, 128–134. [Google Scholar] [CrossRef]
- Maxwell, W.M.C.; Stojanov, T. Liquid Storage of Ram Semen in the Absence or Presence of Some Antioxidants. Reprod. Fertil. Dev. 1996, 8, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.D.S.; Bicudo, S.D.; Azevedo, H.C.; Sicherle, C.C.; Sousa, D.B.D.; Rodello, L. Motility and Viability of Ram Sperm Cryopreserved in a Tris-Egg yolk Extender Supplemented with Anti-Oxidants. Small Rumin. Res. 2009, 85, 85–90. [Google Scholar] [CrossRef]
- Câmara, D.R.; Mello-Pinto, M.M.C.; Pinto, L.C.; Brasil, O.O.; Nunes, J.F.; Guerra, M.M.P. Effects of Reduced Glutathione and Catalase on the Kinematics and Membrane Functionality of Sperm during Liquid Storage of Ram Semen. Small Rumin. Res. 2011, 100, 44–49. [Google Scholar] [CrossRef]
- Shi, L.; Jin, T.; Hu, Y.; Ma, Z.; Niu, H.; Ren, Y. Effects of Reduced Glutathione on Ram Sperm Parameters, Antioxidant Status, Mitochondrial Activity and the Abundance of Hexose Transporters during Liquid Storage at 5 °C. Small Rumin. Res. 2020, 189, 106139. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; Al-Damegh, M.A. Effect of Nonenzymatic Antioxidants on Sperm Motility and Survival Relative to Free Radicals and Antioxidant Enzymes of Chilled-Stored Ram Semen. Open J. Anim. Sci. 2015, 5, 50–58. [Google Scholar] [CrossRef]
- Ďuračka, M.; Benko, F.; Tvrdá, E. Molecular Markers: A New Paradigm in the Prediction of Sperm Freezability. Int. J. Mol. Sci. 2023, 24, 3379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ros-Santaella, J.L.; Pintus, E. Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants 2021, 10, 772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shokry, D.M.; Abd Eldaim, M.A.; Badr, M.R.; Khalifa, H.K.; Orabi, S.H.; Hassan, A.M.; Dohreig, R. Enhancement impact of Moringa oleifera leaves extract–base extender on cryopreservation and fertilization of Barki ram sperms: Comparative study with vitamin E and selenium combination. Ital. J. Anim. Sci. 2021, 20, 1175–1186. [Google Scholar] [CrossRef]
- El-Sheshtawy, R.I.; El-Nattat, W.S. Impact of Silymarin enriched semen extender on bull sperm preservability. Asian Pac. J. Reprod. 2017, 6, 81–84. [Google Scholar] [CrossRef]
- Ali, J.S.; Hussain, S.O.; George, R. Influence of different concentrations of Silymarin and steps of freezing on frozen semen properties of Holstein Bulls born in Iraq. Int. J. Health Sci. 2022, 6, 6767–6780. [Google Scholar] [CrossRef]
| Antioxidants | Administration Route, Dosage | Effect | Bibliographical Source |
|---|---|---|---|
| Vitamin E | Addition to extender 10 mmol/L | Significantly higher values of sperm abnormalities and acrosomal defects (37.6 ± 1.3% and 71.5 ± 1.1%) | [41] |
| Supplementation of vitamin E at a concentration of 200 IU/male in sheep diets | Positive effects on both semen quality and quantity (p < 0.05), reduced MDA levels and enhanced the activities of SOD and GSH-PX within the testicular cell membrane and mitochondria (p < 0.05) | [46] | |
| Addition of a dosage of 80 IU of vitamin E per buck | Increased activity of T-AOC) and SOD, while concurrently reducing the content of NO within the testicular environment, increased the activity of GSH-PX | [47] | |
| Vitamin C | Incorporation of vitamin C at a concentration of 56.78 μM in semen extenders | Significantly improved post-thaw motility and viability (>6%), also enhancing acrosomal integrity and hypoosmotic swelling positivity | [42] |
| Selenium | Addition of 0.2% selenium nanoparticles to a Tris extender | positive impact on most of the ram semen characteristics | [52] |
| General selenium supplementation/ Oral supplementation | Increased plasma GSH-Px concentration, elevated testosterone levels in Saanen bucks, enhanced testicular function, increased plasma concentrations of luteinizing hormone (LH) and testosterone | [49,50,51] | |
| Zinc | Diet supplemented with 17.4 and 32.4 mg Zn/kg feed in ram lambs | Enhanced testes development and sperm production | [53,54] |
| Lycopene | Lycopene supplementation in Tris–egg yolk extender at concentrations of 0.1 µM, 1 µM, and 5 µM | Increased progressive motility of cryopreserved spermatozoa after a two-hour incubation at 37 °C | [64] |
| Coenzyme Q10 | Supplementation of 2 μM coenzyme Q10 in the extenders | Enhanced sperm motility, viability, and overall semen quality in small ruminants | [68] |
| AXT | Supplementation of an optimal concentration of astaxanthin (AXT) at 3.5 µM in Hu ram semen preservation | Diminishes ROS and MDA, enhances kinematic properties, longevity, plasma membrane integrity, acrosome integrity, total antioxidant content, and mitochondrial membrane potential | [69,70] |
| NAC | Moderate concentrations of NAC, such as 0.5 mM and lower, incorporated into skim milk-based extenders | Offers a protective mechanism for ram sperm cells against OS without adversely affecting the freeze-ability of ram semen | [87] |
| Antioxidants | Administration Route, Dosage | Effect | Bibliographical Source |
|---|---|---|---|
| SOD | Incorporating SOD into extenders with concentrations of 800 U/mL or 150 μM | Enhanced protection, particularly for motility, in refrigerated ram sperm cells. | [91] |
| Combination of SOD and CAT | SOD (800 U/mL) and CAT (200 U/mL) | Increased pregnancy rates | [91] |
| CAT | CAT at concentrations of 100 and 200 U/mL to extenders | Mitigated the detrimental impact of cooling on total motility and ram sperm survival during liquid storage at 5 °C | [92,93] |
| GSH | Supplementation of ram semen extenders with 1–2 mM GSH | Improved sperm viability and reduced free radical levels after prolonged chilled storage | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berean, D.I.; Bogdan, L.M.; Cimpean, R. Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives. Antioxidants 2024, 13, 624. https://doi.org/10.3390/antiox13060624
Berean DI, Bogdan LM, Cimpean R. Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives. Antioxidants. 2024; 13(6):624. https://doi.org/10.3390/antiox13060624
Chicago/Turabian StyleBerean, Daniel Ionut, Liviu Marian Bogdan, and Raluca Cimpean. 2024. "Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives" Antioxidants 13, no. 6: 624. https://doi.org/10.3390/antiox13060624
APA StyleBerean, D. I., Bogdan, L. M., & Cimpean, R. (2024). Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives. Antioxidants, 13(6), 624. https://doi.org/10.3390/antiox13060624





