Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies
Abstract
1. Introduction
2. Stroke
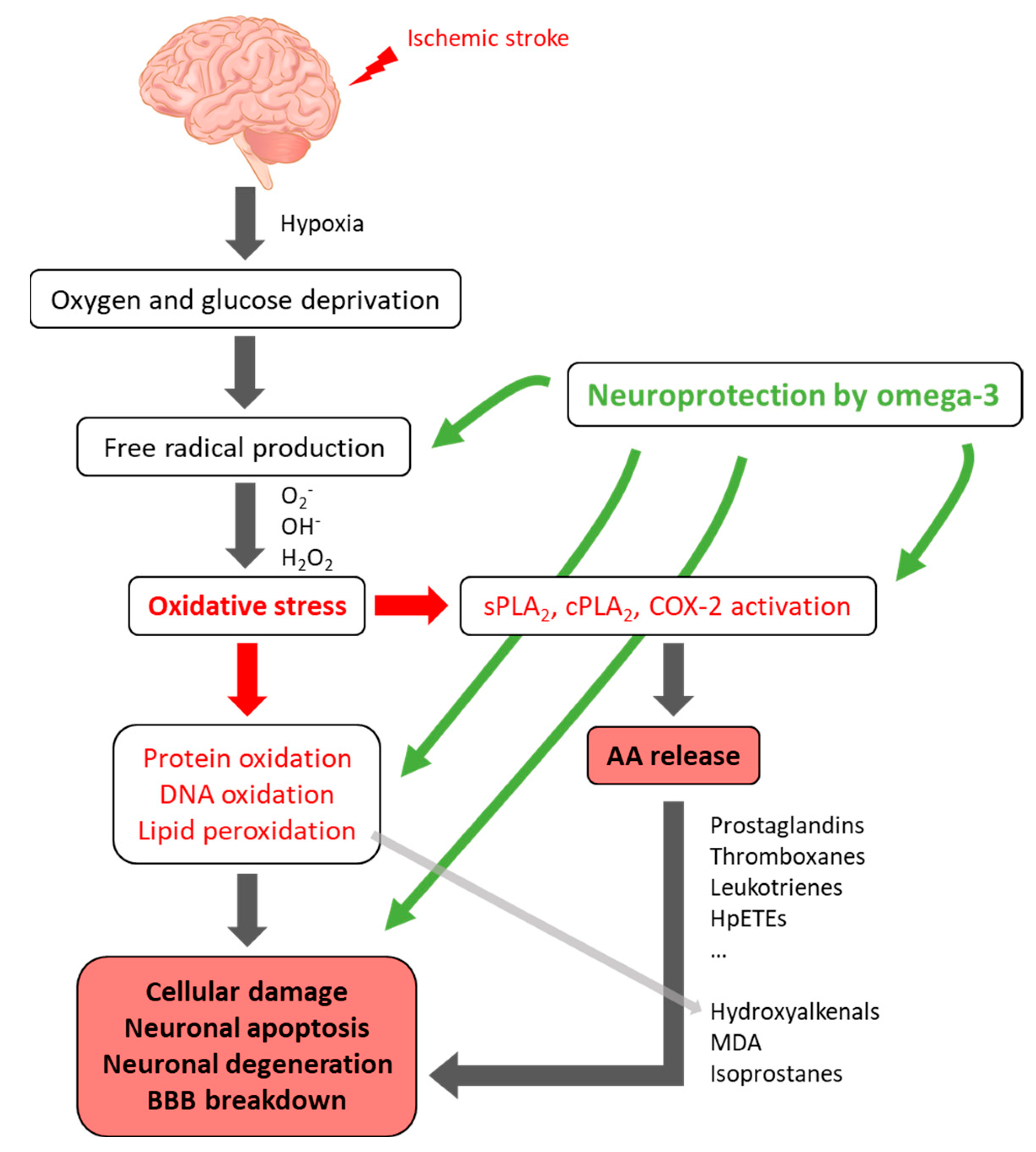
3. Oxidative Stress and Ischemic Stroke
4. Lipids and Ischemic Stroke
4.1. Lipids and the Brain
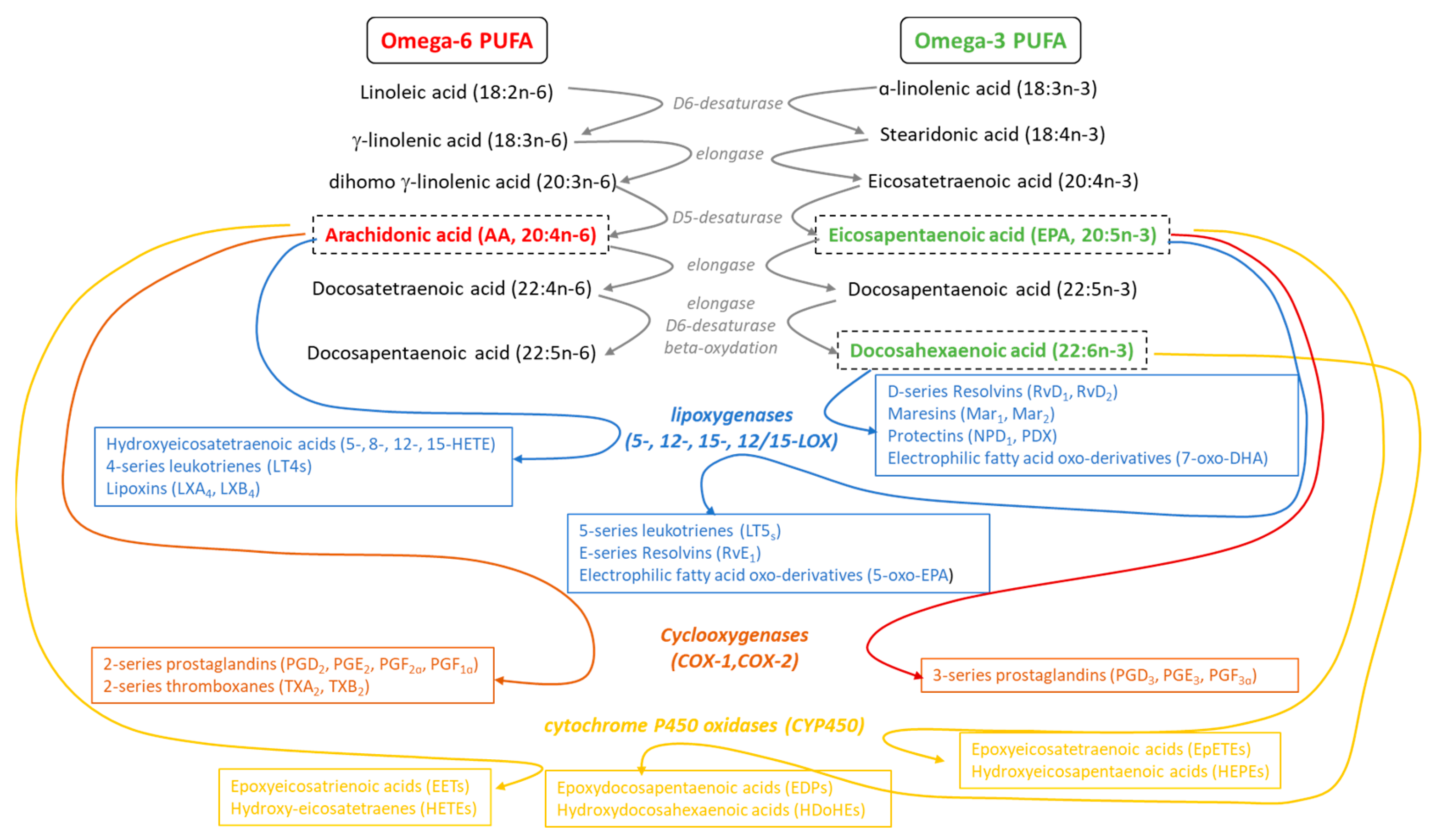
4.1.1. Enzymatic Metabolism of PUFAs
4.1.2. Peroxidation of Lipids
4.2. Dysregulation of Lipid Metabolism in the Pathogenesis of Stroke
4.3. Lipids as Neuroprotective Agents for Ischemic Stroke
4.3.1. Omega 3 PUFAs
4.3.2. PUFA Derivatives from Enzymatic Pathways
4.3.3. Delivery of Omega-3 PUFAs to the Brain
4.4. Lipid Biomarkers in Ischemic Stroke
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators; Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; You, S.; Zheng, D.; Zhang, Z.; Cao, Y.; Chang, J. Global, regional, and national temporal trends of diet-related ischemic stroke mortality and disability from 1990 to 2019. Int. J. Stroke 2024, 17474930241237932. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Sasaki, T.; Kassell, N.F.; Nakagomi, T.; Lehman, R.M.; Hongo, K. Immunohistochemical demonstration of increase in prostaglandin F2-alpha after recirculation in global ischemic rat brains. Acta Neuropathol. 1987, 75, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Usui, M.; Asano, T.; Takakura, K. Identification and quantitative analysis of hydroxy-eicosatetraenoic acids in rat brains exposed to regional ischemia. Stroke 1987, 18, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.G.; Cornet, S.; Spinnewyn, B.; Demerlé-Pallardy, C.; Auguet, M.; Chabrier, P.E. BN 80933 inhibits F2-isoprostane elevation in focal cerebral ischaemia and hypoxic neuronal cultures. Neuroreport 2000, 11, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Yokota, C.; Kaji, T.; Kuge, Y.; Inoue, H.; Tamaki, N.; Minematsu, K. Temporal and topographic profiles of cyclooxygenase-2 expression during 24 h of focal brain ishemia in rats. Neurosci. Lett. 2004, 357, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.D.; Mathers, C.D.; Ezzati, M.; Jamison, D.T.; Murray, C.J. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006, 367, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- GBD 2017 US Neurological Disorders Collaborators; Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; et al. Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [CrossRef]
- GBD 2021 Nervous System Disorders Collaborators; Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Deuschl, G.; Beghi, E.; Fazekas, F.; Varga, T.; Christoforidi, K.A.; Sipido, E.; Bassetti, C.L.; Vos, T.; Feigin, V.L. The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 2020, 5, e551–e567. [Google Scholar] [CrossRef] [PubMed]
- Kissela, B.M.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Adeoye, O.; Flaherty, M.L.; Khatri, P.; Ferioli, S.; De Los Rios La Rosa, F.; et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology 2012, 79, 1781–1787. [Google Scholar] [CrossRef]
- Béjot, Y.; Daubail, B.; Jacquin, A.; Durier, J.; Osseby, G.V.; Rouaud, O.; Giroud, M. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: The Dijon Stroke Registry. J. Neurol. Neurosurg. Psychiatry 2014, 85, 509–513. [Google Scholar] [CrossRef]
- Burke, J.F.; Skolarus, L.E. Are More Young People Having Strokes?—A Simple Question with an Uncertain Answer. JAMA Neurol. 2017, 74, 639–641. [Google Scholar] [CrossRef]
- Scott, C.A.; Li, L.; Rothwell, P.M. Diverging Temporal Trends in Stroke Incidence in Younger vs Older People: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 1036–1048. [Google Scholar] [CrossRef]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.C.; Mendis, S.; Mathers, C.D. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009, 8, 345–354. [Google Scholar] [CrossRef]
- Owolabi, M.O.; Ugoya, S.; Platz, T. Racial disparity in stroke risk factors: The Berlin-Ibadan experience; a retrospective study. Acta Neurol. Scand. 2009, 119, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bravata, D.M.; Wells, C.K.; Gulanski, B.; Kernan, W.N.; Brass, L.M.; Long, J.; Concato, J. Racial disparities in stroke risk factors: The impact of socioeconomic status. Stroke 2005, 36, 1507–1511. [Google Scholar] [CrossRef]
- González, H.M.; Tarraf, W.; Rodríguez, C.J.; Gallo, L.C.; Sacco, R.L.; Talavera, G.A.; Heiss, G.; Kizer, J.R.; Hernandez, R.; Davis, S.; et al. Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am. Heart J. 2016, 176, 134–144. [Google Scholar] [CrossRef]
- Silva, G.S.; Koroshetz, W.J.; González, R.G.; Schwamm, L.H. Causes of Ischemic Stroke. In Acute Ischemic Stroke; González, R., Hirsch, J., Lev, M., Schaefer, P., Schwamm, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Adams, R.; Alberts, M.J.; Appel, L.J.; Brass, L.M.; Bushnell, C.D.; Culebras, A.; Degraba, T.J.; Gorelick, P.B.; Guyton, J.R.; et al. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline. Stroke 2006, 37, 1583–1633. [Google Scholar] [CrossRef]
- Sacco, R.L.; Adams, R.; Albers, G.; Alberts, M.J.; Benavente, O.; Furie, K.; Goldstein, L.B.; Gorelick, P.; Halperin, J.; Harbaugh, R.; et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-sponsored by the Council on Cardiovascular Radiology and Intervention: The American Academy of Neurology affirms the value of this guideline. Stroke 2006, 37, 577–617. [Google Scholar] [CrossRef]
- Donnan, G.A. International Journal of Stroke. Editorial. Int. J. Stroke 2008, 3, 157. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of stroke subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef]
- Unnithan, A.K.A.; Das, J.M.; Mehta, P. Hemorrhagic Stroke—Abstract—Europe PMC. 2022. Available online: https://europepmc.org/article/nbk/nbk559173?utm_medium=email&utm_source=transaction&client=bot&client=bot (accessed on 17 April 2023).
- Chen, S.; Zeng, L.; Hu, Z. Progressing haemorrhagic stroke: Categories, causes, mechanisms and managements. J. Neurol. 2014, 261, 2061–2078. [Google Scholar] [CrossRef]
- Ojaghihaghighi, S.; Vahdati, S.S.; Mikaeilpour, A.; Ramouz, A. Comparison of neurological clinical manifestation in patients with hemorrhagic and ischemic stroke. World J Emerg Med. 2017, 8, 34–38. [Google Scholar] [CrossRef]
- Montaño, A.; Hanley, D.F.; Hemphill, J.C., 3rd. Hemorrhagic stroke. Handb. Clin. Neurol. 2021, 176, 229–248. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging neuroprotective strategies for the treatment of ischemic stroke: An overview of clinical and preclinical studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Pandey, V.; Phanithi, P.B. The interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: An epigenetic connection. Mol. Cell. Neurosci. 2017, 82, 176–194. [Google Scholar] [CrossRef]
- Ren, J.X.; Li, C.; Yan, X.L.; Qu, Y.; Yang, Y.; Guo, Z.N. Crosstalk between oxidative stress and ferroptosis/oxytosis in ischemic stroke: Possible targets and molecular mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6643382. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.; Chaurasia, B.; Garg, K.; Deora, H.; Umana, G.E.; Palmisciano, P.; Scalia, G.; Lu, B. Molecular mechanisms of oxidative stress in stroke and cancer. Brain Disord. 2022, 5, 100029. [Google Scholar] [CrossRef]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007, 182, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Briones-Valdivieso, C.; Briones, F.; Orellana-Urzúa, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Novel Multi-Antioxidant Approach for Ischemic Stroke Therapy Targeting the Role of Oxidative Stress. Biomedicines 2024, 12, 501. [Google Scholar] [CrossRef]
- Cadenas, E. Basic mechanisms of antioxidant activity. Biofactors 1997, 6, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M. Telser Free radicals and antioxidants in normal physiological functions and human disease. J. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S. Brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats: Efficiency of Sonchus asper. Behav. Brain Funct. 2012, 8, 21. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, M.; Tanaka Ki Miyazaki, I.; Fujita, N.; Higashi, Y.; Asanuma, M.; Ogawa, N. The dopamine agonist cabergoline provides neuroprotection by activation of the glutathione system and scavenging free radicals. Neurosci. Res. 2002, 43, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M.; Bonneil, M.; Clément, M.; Dumont, O.; Durand, G.; Lafont, H.; Nalbone, G.; Piciotti, M. Function of dietary polyunsaturated fatty acids in the nervous system. Prostaglandins Leukot. Essent. Fat. Acids 1993, 48, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Belkouch, M.; Hachem, M.; Elgot, A.; Lo Van, A.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Bernoud-Hubac, N.; Lagarde, M. How the plasma lysophospholipid and unesterified fatty acid pools supply the brain with docosahexaenoic acid. Prostaglandins Leukot. Essent. Fat. Acids 2019, 142, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Phillis, J.W.; Horrocks, L.A.; Farooqui, A.A. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: Their role and involvement in neurological disorders. Brain Res. Rev. 2006, 52, 201–243. [Google Scholar] [CrossRef]
- Smith, M.L.; Murphy, R.C. The eicosanoids: Cyclooxygenase, lipoxygenase and epoxygenase pathways. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgeway, N.D., McLeod, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 260–296. [Google Scholar]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Lands, W.E.; Samuelsson, B. Phospholipid precursors of prostaglandins. Biochim. Biophys. Acta. 1968, 164, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Svensson, J.; Samuelsson, B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc. Natl. Acad. Sci. USA 1974, 71, 3824–3828. [Google Scholar] [CrossRef]
- Salomon, R.G.; Miller, D.B. Levuglandins: Isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv. Prostaglandin Thromboxane Leukot. Res. 1985, 15, 323–326. [Google Scholar]
- Kulmacz, R.J.; van der Donk, W.A.; Tsai, A.L. Comparison of the properties of prostaglandin H synthase-1 and -2. Prog. Lipid Res. 2003, 42, 377–404. [Google Scholar] [CrossRef]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenases: Structural and functional insights. J. Lipid Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Guichardant, M.; Chen, P.; Liu, M.; Lo Van, A.; Jouvène, C.; Bernoud-Hubac, N.; Véricel, E.; Lagarde, M. Double lipoxygenation of polyunsaturated fatty acids of nutritional interest. Prostaglandins Leukot. Essent. Fat. Acids 2020, 162, 102185. [Google Scholar] [CrossRef]
- Cipollina, C.; Salvatore, S.R.; Muldoon, M.F.; Freeman, B.A.; Schopfer, F.J. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS ONE. 2014, 9, e94836. [Google Scholar] [CrossRef]
- Capdevila, J.H.; Falck, J.R. The arachidonic acid monooxygenase: From biochemical curiosity to physiological/pathophysiological significance. J. Lipid Res. 2018, 59, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Konkel, A.; Fischer, R.; Schunck, W.H. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010, 62, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A.; Caldwell, S.E.; Mills, K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 1995, 30, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J., 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Reich, E.E.; Zackert, W.E.; Brame, C.J.; Chen, Y.; Roberts, L.J., 2nd; Hachey, D.L.; Montine, T.J.; Morrow, J.D. Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acid. Biochemistry 2000, 39, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Awad, J.A.; Boss, H.J.; Blair, I.A.; Roberts, L.J., 2nd. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA 1992, 89, 10721–10725. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Minton, T.A.; Mukundan, C.R.; Campbell, M.D.; Zackert, W.E.; Daniel, V.C.; Badr, K.F.; Blair, I.A.; Roberts, L.J., 2nd. Free radical-induced generation of isoprostanes in vivo. Evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 1994, 269, 4317–4326. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.D.; Awad, J.A.; Wu, A.; Zackert, W.E.; Daniel, V.C.; Roberts, L.J., 2nd. Nonenzymatic free radical-catalyzed generation of thromboxane-like compounds (isothromboxanes) in vivo. J. Biol. Chem. 1996, 271, 23185–23190. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J., 2nd; Montine, T.J.; Markesbery, W.R.; Tapper, A.R.; Hardy, P.; Chemtob, S.; Dettbarn, W.D.; Morrow, J.D. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 1998, 273, 13605–13612. [Google Scholar] [CrossRef]
- Brame, C.J.; Salomon, R.G.; Morrow, J.D.; Roberts, L.J., 2nd. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 1999, 274, 13139–13146. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Davies, S.S.; Boutaud, O.; Montine, T.J.; Roberts, L.J., 2nd. Formation of highly reactive gamma-ketoaldehydes (neuroketals) as products of the neuroprostane pathway. J. Biol. Chem. 2001, 276, 30964–30970. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Roberts, L.J., 2nd. Identification of oxidized derivatives of neuroketals. Biochemistry 2002, 41, 11466–11471. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Fay, L.B.; Armarnath, V.; Guichardant, M.; Bacot, S.; Davies, S.S.; Roberts, L.J., 2nd; Lagarde, M. Covalent binding of isoketals to ethanolamine phospholipids. Free Radic. Biol Med. 2004, 37, 1604–1611. [Google Scholar] [CrossRef]
- Esterbauer, H.; Benedetti, A.; Lang, J.; Fulceri, R.; Fauler, G.; Comporti, M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim. Biophys. Acta 1986, 876, 154–166. [Google Scholar] [CrossRef]
- Van Kuijk, F.J.; Holte, L.L.; Dratz, E.A. 4-Hydroxyhexenal: A lipid peroxidation product derived from oxidized docosahexaenoic acid. Biochim. Biophys. Acta 1990, 1043, 116–118. [Google Scholar] [CrossRef]
- Murthi, K.K.; Friedman, L.R.; Oleinick, N.L.; Salomon, R.G. Formation of DNA-protein cross-links in mammalian cells by levuglandin E2. Biochemistry 1993, 32, 4090–4097. [Google Scholar] [CrossRef]
- Davies, S.S.; Amarnath, V.; Montine, K.S.; Bernoud-Hubac, N.; Boutaud, O.; Montine, T.J.; Roberts, L.J., 2nd. Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002, 16, 715–717. [Google Scholar] [CrossRef]
- Bacot, S.; Bernoud-Hubac, N.; Baddas, N.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Lagarde, M.; Guichardant, M. Covalent binding of hydroxy-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J. Lipid Res. 2003, 44, 917–926. [Google Scholar] [CrossRef]
- Brame, C.J.; Boutaud, O.; Davies, S.S.; Yang, T.; Oates, J.A.; Roden, D.; Roberts, L.J., 2nd. Modification of proteins by isoketal-containing oxidized phospholipids. J. Biol. Chem. 2004, 279, 13447–13451. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Alam, D.A.; Lefils, J.; Davies, S.S.; Amarnath, V.; Guichardant, M.; Roberts, L.J., 2nd; Lagarde, M. Low concentrations of reactive gamma-ketoaldehydes prime thromboxane-dependent human platelet aggregation via p38-MAPK activation. Biochim. Biophys. Acta 2009, 1791, 307–313. [Google Scholar] [CrossRef]
- Davies, S.S.; Bodine, C.; Matafonova, E.; Pantazides, B.G.; Bernoud-Hubac, N.; Harrison, F.E.; Olson, S.J.; Montine, T.J.; Amarnath, V.; Roberts, L.J., 2nd. Treatment with a γ-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J. Alzheimers Dis. 2011, 27, 49–59. [Google Scholar] [CrossRef]
- Rodriguez de Turco, E.B.; Belayev, L.; Liu, Y.; Busto, R.; Parkins, N.; Bazan, N.G.; Ginsberg, M.D. Systemic fatty acid responses to transient focal cerebral ischemia: Influence of neuroprotectant therapy with human albumin. J. Neurochem. 2002, 83, 515–524. [Google Scholar] [CrossRef]
- Golovko, S.A.; Golovko, M.Y. Plasma Unesterified Fatty-Acid Profile Is Dramatically and Acutely Changed under Ischemic Stroke in the Mouse Model. Lipids 2018, 53, 641–645. [Google Scholar] [CrossRef]
- Lauritzen, I.; Heurteaux, C.; Lazdunski, M. Expression of group II phospholipase A2 in rat brain after severe forebrain ischemia and in endotoxic shock. Brain Res. 1994, 651, 353–356. [Google Scholar] [CrossRef]
- Estevez, A.Y.; Phillis, J.W. The phospholipase A2 inhibitor, quinacrine, reduces infarct size in rats after transient middle cerebral artery occlusion. Brain Res. 1997, 752, 203–208. [Google Scholar] [CrossRef]
- Sairanen, T.; Ristimäki, A.; Karjalainen-Lindsberg, M.L.; Paetau, A.; Kaste, M.; Lindsberg, P.J. Cyclooxygenase-2 is induced globally in infarcted human brain. Ann. Neurol. 1998, 43, 738–747. [Google Scholar] [CrossRef]
- Nogawa, S.; Zhang, F.; Ross, M.E.; Iadecola, C. Cyclo-oxygenase-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 1997, 17, 2746–2755. [Google Scholar] [CrossRef]
- Futaki, N.; Yoshikawa, K.; Hamasaka, Y.; Arai, I.; Higuchi, S.; Iizuka, H.; Otomo, S. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen. Pharmacol. 1993, 24, 105–110. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Isakson, P.C.; Seibert, K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 3228–3232. [Google Scholar] [CrossRef]
- Fagan, S.C.; Castellani, D.; Gengo, F.M. Prostanoid concentrations in human CSF following acute ischaemic brain infarction. Clin. Exp. Pharmacol. Physiol. 1986, 13, 629–632. [Google Scholar] [CrossRef]
- Kawano, T.; Anrather, J.; Zhou, P.; Park, L.; Wang, G.; Frys, K.A.; Kunz, A.; Cho, S.; Orio, M.; Iadecola, C. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat. Med. 2006, 12, 225–229. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, A.S.; Zhuang, H.; Maruyama, T.; Narumiya, S.; Doré, S. Stimulation of prostaglandin E2-EP3 receptors exacerbates stroke and excitotoxic injury. J. Neuroimmunol. 2007, 184, 172–179. [Google Scholar] [CrossRef][Green Version]
- Saleem, S.; Ahmad, A.S.; Maruyama, T.; Narumiya, S.; Doré, S. PGF2α FP receptor contributes to brain damage following transient focal brain ischemia. Neurotox. Res. 2009, 15, 62–70. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Kiwak, K.J.; Hekimian, K.; Levine, L. Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science 1984, 224, 886–889. [Google Scholar] [CrossRef]
- Kiwak, K.J.; Moskowitz, M.A.; Levine, L. Leukotriene production in gerbil brain after ischemic insult, subarachnoid hemorrhage, and concussive injury. J. Neurosurg. 1985, 62, 865–869. [Google Scholar] [CrossRef]
- Samuelsson, B.; Hammarström, S. Leukotrienes: A novel group of biologically active compounds. Vitam. Horm. 1982, 39, 1–30. [Google Scholar] [CrossRef]
- Ström, J.O.; Strid, T.; Hammarström, S. Disruption of the alox5ap gene ameliorates focal ischemic stroke: Possible consequence of impaired leukotriene biosynthesis. BMC Neurosci. 2012, 13, 146. [Google Scholar] [CrossRef]
- Kelly, P.J.; Morrow, J.D.; Ning, M.; Koroshetz, W.; Lo, E.H.; Terry, E.; Milne, G.L.; Hubbard, J.; Lee, H.; Stevenson, E.; et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 2008, 39, 100–104. [Google Scholar] [CrossRef]
- Zeiger, S.L.; Musiek, E.S.; Zanoni, G.; Vidari, G.; Morrow, J.D.; Milne, G.J.; McLaughlin, B. Neurotoxic lipid peroxidation species formed by ischemic stroke increase injury. Free Radic. Biol. Med. 2009, 47, 1422–1431. [Google Scholar] [CrossRef][Green Version]
- Musiek, E.S.; Breeding, R.S.; Milne, G.L.; Zanoni, G.; Morrow, J.D.; McLaughlin, B. Cyclopentenone isoprostanes are novel bioactive products of lipid oxidation which enhance neurodegeneration. J. Neurochem. 2006, 97, 1301–1313. [Google Scholar] [CrossRef]
- Seet, R.C.; Lee, C.Y.; Chan, B.P.; Sharma, V.K.; Teoh, H.L.; Venketasubramanian, N.; Lim, E.C.; Chong, W.L.; Looi, W.F.; Huang, S.H.; et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke 2011, 42, 2326–2329. [Google Scholar] [CrossRef]
- Lee, W.C.; Wong, H.Y.; Chai, Y.Y.; Shi, C.W.; Amino, N.; Kikuchi, S.; Huang, S.H. Lipid peroxidation dysregulation in ischemic stroke: Plasma 4-HNE as a potential biomarker? Biochem. Biophys. Res. Commun. 2012, 425, 842–847. [Google Scholar] [CrossRef]
- Guo, J.M.; Liu, A.J.; Zang, P.; Dong, W.Z.; Ying, L.; Wang, W.; Xu, P.; Song, X.R.; Cai, J.; Zhang, S.Q.; et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013, 23, 915–930. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Sapira, V.; Ionescu, A. Evaluation of oxidative stress in patients with acute ischemic stroke. Rom. J. Intern. Med. 2013, 51, 97–106. [Google Scholar]
- Elsayed, W.M.; Abdel-Gawad, E.H.A.; Mesallam, D.I.; El-Serafy, T.S. The relationship between oxidative stress and acute ischemic stroke severity and functional outcome. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 74. [Google Scholar] [CrossRef]
- Menon, B.; Ramalingam, K.; Kumar, R. Evaluating the Role of Oxidative Stress in Acute Ischemic Stroke. J. Neurosci. Rural Pract. 2020, 11, 156–159. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Tu, X.; Shen, H.; Qiu, H.; Chen, H.; He, J. High Serum Levels of Malondialdehyde and 8-OHdG are both Associated with Early Cognitive Impairment in Patients with Acute Ischaemic Stroke. Sci. Rep. 2017, 7, 9493. [Google Scholar] [CrossRef]
- Carrié, I.; Clément, M.; de Javel, D.; Francès, H.; Bourre, J.M. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [CrossRef]
- Joffre, C.; Grégoire, S.; De Smedt, V.; Acar, N.; Bretillon, L.; Nadjar, A.; Layé, S. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot. Essent. Fat. Acids 2016, 114, 1–10. [Google Scholar] [CrossRef]
- Peet, M.; Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005, 65, 1051–1059. [Google Scholar] [CrossRef]
- Gao, X.; Chen, H.; Fung, T.T.; Logroscino, G.; Schwarzschild, M.A.; Hu, F.B.; Ascherio, A. Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 2007, 86, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 Fatty Acids and their Role in Central Nervous System—A Review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Mishina, M.; Kim, K.; Kominami, S.; Mizunari, T.; Kobayashi, S.; Katayama, Y. Impact of polyunsaturated fatty acid consumption prior to ischemic stroke. Acta Neurol. Scand. 2013, 127, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Kotlega, D.; Zembron-Lacny, A.; Golab-Janowska, M.; Nowacki, P.; Szczuko, M. The Association of Free Fatty Acids and Eicosanoids with the Severity of Depressive Symptoms in Stroke Patients. Int. J. Mol. Sci. 2020, 21, 5220. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Marcheselli, V.L.; Khoutorova, L.; Rodriguez de Turco, E.B.; Busto, R.; Ginsberg, M.D.; Bazan, N.G. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke 2005, 36, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Khoutorova, L.; Atkins, K.D.; Bazan, N.G. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke 2009, 40, 3121–3126. [Google Scholar] [CrossRef] [PubMed]
- Huun, M.U.; Garberg, H.T.; Escobar, J.; Chafer, C.; Vento, M.; Holme, I.M.; Saugstad, O.D.; Solberg, R. DHA reduces oxidative stress following hypoxia-ischemia in newborn piglets: A study of lipid peroxidation products in urine and plasma. J. Perinat. Med. 2018, 46, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, S.; Hu, M.; Sun, X.; Qiu, W.; Zheng, S.; Hu, X.; Lu, Z. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res. 2018, 9, 669–680. [Google Scholar] [CrossRef]
- Quartu, M.; Serra, M.P.; Boi, M.; Pillolla, G.; Melis, T.; Poddighe, L.; Del Fiacco, M.; Falconieri, D.; Carta, G.; Murru, E.; et al. Effect of acute administration of Pistacia lentiscus L. essential oil on rat cerebral cortex following transient bilateral common carotid artery occlusion. Lipids Health Dis. 2012, 11, 8. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Zhan, L.; Shu, A. Effects and mechanisms of docosahexaenoic acid on the generation of angiopoietin-2 by rat brain microvascular endothelial cells under an oxygen- and glucose-deprivation environment. Springerplus 2016, 5, 1518. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, W.J.; Choi, Y.K.; Song, H.S.; Son, M.J.; Gelman, I.H.; Kim, Y.J.; Kim, K.W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 2003, 9, 900–906. [Google Scholar] [CrossRef]
- Yu, Y.; Fang, H.; Qiu, Z.; Xia, Z.; Zhou, B. DHA Attenuates Hypoxia/Reoxygenation Injury by Activating SSeCKS in Human Cerebrovascular Pericytes. Neurochem. Res. 2020, 45, 310–321. [Google Scholar] [CrossRef]
- Okabe, N.; Nakamura, T.; Toyoshima, T.; Miyamoto, O.; Lu, F.; Itano, T. Eicosapentaenoic acid prevents memory impairment after ischemia by inhibiting inflammatory response and oxidative damage. J. Stroke Cerebrovasc. Dis. 2011, 20, 188–195. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G.; Gudkov, S.V.; Plotnikov, E.Y. The Protective Mechanism of Deuterated Linoleic Acid Involves the Activation of the Ca2+ Signaling System of Astrocytes in Ischemia In Vitro. Int. J. Mol. Sci. 2021, 22, 13216. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N. The nutraceutical potential of omega-3 alpha-linolenic acid in reducing the consequences of stroke. Biochimie 2016, 120, 49–55. [Google Scholar] [CrossRef]
- Blondeau, N.; Nguemeni, C.; Debruyne, D.N.; Piens, M.; Wu, X.; Pan, H.; Hu, X.; Gandin, C.; Lipsky, R.H.; Plumier, J.C.; et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: A versatile potential therapy for stroke. Neuropsychopharmacology 2009, 34, 2548–2559. [Google Scholar] [CrossRef]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G.; et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [CrossRef]
- Nguemeni, C.; Delplanque, B.; Rovère, C.; Simon-Rousseau, N.; Gandin, C.; Agnani, G.; Nahon, J.L.; Heurteaux, C.; Blondeau, N. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol. Res. 2010, 61, 226–233. [Google Scholar] [CrossRef]
- Bourourou, M.; Heurteaux, C.; Blondeau, N. Alpha-linolenic acid given as enteral or parenteral nutritional intervention against sensorimotor and cognitive deficits in a mouse model of ischemic stroke. Neuropharmacology 2016, 108, 60–72. [Google Scholar] [CrossRef]
- Miao, Z.; Schultzberg, M.; Wang, X.; Zhao, Y. Role of polyunsaturated fatty acids in ischemic stroke—A perspective of specialized pro-resolving mediators. Clin. Nutr. 2021, 40, 2974–2987. [Google Scholar] [CrossRef]
- Marcheselli, V.L.; Hong, S.; Lukiw, W.J.; Tian, X.H.; Gronert, K.; Musto, A.; Hardy, M.; Gimenez, J.M.; Chiang, N.; Serhan, C.N.; et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003, 278, 43807–43817. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.M.; Kautzmann, M.I.; Andrew, G.; Obenaus, A.; Mukherjee, P.K.; Khoutorova, L.; Ji, J.X.; Roque, C.R.; Oria, R.B.; Habeb, B.F.; et al. NPD1 Plus RvD1 Mediated Ischemic Stroke Penumbra Protection Increases Expression of Pro-homeostatic Microglial and Astrocyte Genes. Cell. Mol. Neurobiol. 2023, 43, 3555–3573. [Google Scholar] [CrossRef]
- Zuo, G.; Zhang, D.; Mu, R.; Shen, H.; Li, X.; Wang, Z.; Li, H.; Chen, G. Resolvin D2 protects against cerebral ischemia/reperfusion injury in rats. Mol. Brain 2018, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Tułowiecka, N.; Kotlęga, D.; Prowans, P.; Szczuko, M. The Role of Resolvins: EPA and DHA Derivatives Can Be Useful in the Prevention and Treatment of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7628. [Google Scholar] [CrossRef] [PubMed]
- Sobrado, M.; Pereira, M.P.; Ballesteros, I.; Hurtado, O.; Fernández-López, D.; Pradillo, J.M.; Caso, J.R.; Vivancos, J.; Nombela, F.; Serena, J.; et al. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J. Neurosci. 2009, 29, 3875–3884. [Google Scholar] [CrossRef] [PubMed]
- Tułowiecka, N.; Kotlęga, D.; Bohatyrewicz, A.; Szczuko, M. Could Lipoxins Represent a New Standard in Ischemic Stroke Treatment? Int. J. Mol. Sci. 2021, 22, 4207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Fan, M.; Jin, W. Lipoxins in the Nervous System: Brighter Prospects for Neuroprotection. Front. Pharmacol. 2022, 13, 781889. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Wu, Y.; Xiong, W.; Li, L.; Li, T.; Pan, S.; Song, L.; Hu, L.; Pei, L.; Yao, S.; et al. The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem. Biophys. Res. Commun. 2016, 472, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Pillon, C.; Moliere, P.; Lagarde, M.; Lecerf, J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. 1994, 267 Pt 2, R1273–R1279. [Google Scholar] [CrossRef]
- Bernoud, N.; Fenart, L.; Molière, P.; Dehouck, M.P.; Lagarde, M.; Cecchelli, R.; Lecerf, J. Preferential transfer of 2-docosahexaenoyl-1-lysophosphatidylcholine through an in vitro blood-brain barrier over unesterified docosahexaenoic acid. J. Neurochem. 1999, 72, 338–345. [Google Scholar] [CrossRef]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thiès, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204; discussion 215–221. [Google Scholar] [CrossRef]
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Mechanisms of DHA transport to the brain and potential therapy to neurodegenerative diseases. Biochimie 2016, 130, 163–167. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Wong, B.H.; Silver, D.L. Mfsd2a: A Physiologically Important Lysolipid Transporter in the Brain and Eye. Adv. Exp. Med. Biol. 2020, 1276, 223–234. [Google Scholar] [CrossRef]
- Cater, R.J.; Chua, G.L.; Erramilli, S.K.; Keener, J.E.; Choy, B.C.; Tokarz, P.; Chin, C.F.; Quek, D.Q.Y.; Kloss, B.; Pepe, J.G.; et al. Structural basis of omega-3 fatty acid transport across the blood-brain barrier. Nature 2021, 595, 315–319. [Google Scholar] [CrossRef]
- Lagarde, M.; Hachem, M.; Bernoud-Hubac, N.; Picq, M.; Véricel, E.; Guichardant, M. Biological properties of a DHA-containing structured phospholipid (AceDoPC) to target the brain. Prostaglandins Leukot. Essent. Fat. Acids. 2015, 92, 63–65. [Google Scholar] [CrossRef]
- Hachem, M.; Géloën, A.; Van, A.L.; Foumaux, B.; Fenart, L.; Gosselet, F.; Da Silva, P.; Breton, G.; Lagarde, M.; Picq, M.; et al. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2016, 53, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC. Nutrients 2020, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Lo Van, A.; Bernoud-Hubac, N.; Lagarde, M. Esterification of Docosahexaenoic Acid Enhances Its Transport to the Brain and Its Potential Therapeutic Use in Brain Diseases. Nutrients 2022, 14, 4550. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Cho, T.H.; Perez, M.; Guichardant, M.; Riou, A.; Aguettaz, P.; Picq, M.; Lagarde, M.; Berthezène, Y.; Nighoghossian, N.; et al. Brain-targeting form of docosahexaenoic acid for experimental stroke treatment: MRI evaluation and anti-oxidant impact. Curr. Neurovasc. Res. 2011, 8, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Fourmaux, B.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Targeting the Brain with a Neuroprotective Omega-3 Fatty Acid to Enhance Neurogenesis in Hypoxic Condition in Culture. Mol. Neurobiol. 2019, 56, 986–999. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y.; et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef]
- Williams, J.J.; Mayurasakorn, K.; Vannucci, S.J.; Mastropietro, C.; Bazan, N.G.; Ten, V.S.; Deckelbaum, R.J. N-3 fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice. PLoS ONE 2013, 8, e56233. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Jiang, X.; Hu, X.; Xia, J.; Hong, D.; Zhang, W.; Gao, Y.; Chen, J.; Shi, Y. Delayed Docosahexaenoic Acid Treatment Combined with Dietary Supplementation of Omega-3 Fatty Acids Promotes Long-Term Neurovascular Restoration After Ischemic Stroke. Transl. Stroke Res. 2016, 7, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Sabry, S.A.; Abd El Razek, A.M.; Nabil, M.; Khedr, S.M.; El-Nahas, H.M.; Eissa, N.G. Brain-targeted delivery of Valsartan using solid lipid nanoparticles labeled with Rhodamine B; a promising technique for mitigating the negative effects of stroke. Drug Deliv. 2023, 30, 2179127. [Google Scholar] [CrossRef]
- Nong, J.; Glassman, P.M.; Shuvaev, V.V.; Reyes-Esteves, S.; Descamps, H.C.; Kiseleva, R.Y.; Papp, T.E.; Alameh, M.G.; Tam, Y.K.; Mui, B.L.; et al. Targeting lipid nanoparticles to the blood-brain barrier to ameliorate acute ischemic stroke. Mol. Ther. 2024, 32, 1344–1358. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Jiménez Garavito, M.C.; Desor, F.; Huguet, M.; Soligot-Hognon, C.; Linder, M.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Use of Active Salmon-Lecithin Nanoliposomes to Increase Polyunsaturated Fatty Acid Bioavailability in Cortical Neurons and Mice. Int. J. Mol. Sci. 2021, 22, 11859. [Google Scholar] [CrossRef]
- Passeri, E.; Bun, P.; Elkhoury, K.; Linder, M.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons. Pharmaceutics 2022, 14, 2172. [Google Scholar] [CrossRef]
- Andone, S.; Farczádi, L.; Imre, S.; Bălașa, R. Fatty Acids and Lipid Paradox-Neuroprotective Biomarkers in Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 10810. [Google Scholar] [CrossRef]
- Kotlęga, D.; Peda, B.; Palma, J.; Zembroń-Łacny, A.; Gołąb-Janowska, M.; Masztalewicz, M.; Nowacki, P.; Szczuko, M. Free Fatty Acids Are Associated with the Cognitive Functions in Stroke Survivors. Int. J. Environ. Res. Public Health 2021, 18, 6500. [Google Scholar] [CrossRef]
- Kurth, T.; Everett, B.M.; Buring, J.E.; Kase, C.S.; Ridker, P.M.; Gaziano, J.M. Lipid levels and the risk of ischemic stroke in women. Neurology 2007, 68, 556–562. [Google Scholar] [CrossRef]
- Glasser, S.P.; Mosher, A.; Howard, G.; Banach, M. What is the association of lipid levels and incident stroke? Int. J. Cardiol. 2016, 220, 890–894. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Chen, S.; Yang, X.; Liu, F.; Li, Y.; Li, J.; Cao, J.; Liu, X.; Chen, J.; et al. Association of Lipids with Ischemic and Hemorrhagic Stroke: A Prospective Cohort Study Among 267 500 Chinese. Stroke 2019, 50, 3376–3384. [Google Scholar] [CrossRef]
- Shahar, E.; Chambless, L.E.; Rosamond, W.D.; Boland, L.L.; Ballantyne, C.M.; McGovern, P.G.; Sharrett, A.R. Plasma lipid profile and incident ischemic stroke: The atherosclerosis risk in communities (ARIC) study. Stroke 2003, 34, 623–631. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. ApoB and Non-HDL Cholesterol Versus LDL Cholesterol for Ischemic Stroke Risk. Ann. Neurol. 2022, 92, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Çoban, E.K. Can TG/HDL Ratio be an Accurate Predictor in the Determination of the Risk of Cerebrovascular Events in Youngsters? Sisli Etfal Hastan Tip Bul. 2018, 52, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kwon, H.M.; Jeong, H.Y.; Park, J.H.; Kwon, H.; Jeong, S.M. High triglyceride/HDL cholesterol ratio is associated with silent brain infarcts in a healthy population. BMC Neurol. 2019, 19, 147. [Google Scholar] [CrossRef]
- Jové, M.; Mauri-Capdevila, G.; Suárez, I.; Cambray, S.; Sanahuja, J.; Quílez, A.; Farré, J.; Benabdelhak, I.; Pamplona, R.; Portero-Otín, M.; et al. Metabolomics predicts stroke recurrence after transient ischemic attack. Neurology 2015, 84, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xu, S.; Zhou, M.; Luo, J.; Zha, F.; Shan, L.; Yang, Q.; Zhou, B.; Wang, Y. Lysophosphatidylcholines and phosphatidylcholines as biomarkers for stroke recovery. Front. Neurol. 2022, 13, 1047101. [Google Scholar] [CrossRef]
- Jensen, M.; Liu, S.; Stepula, E.; Martella, D.; Birjandi, A.A.; Farrell-Dillon, K.; Chan, K.L.A.; Parsons, M.; Chiappini, C.; Chapple, S.J.; et al. Opto-Lipidomics of Tissues. Adv. Sci. 2024, 11, e2302962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernoud-Hubac, N.; Lo Van, A.; Lazar, A.-N.; Lagarde, M. Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies. Antioxidants 2024, 13, 634. https://doi.org/10.3390/antiox13060634
Bernoud-Hubac N, Lo Van A, Lazar A-N, Lagarde M. Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies. Antioxidants. 2024; 13(6):634. https://doi.org/10.3390/antiox13060634
Chicago/Turabian StyleBernoud-Hubac, Nathalie, Amanda Lo Van, Adina-Nicoleta Lazar, and Michel Lagarde. 2024. "Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies" Antioxidants 13, no. 6: 634. https://doi.org/10.3390/antiox13060634
APA StyleBernoud-Hubac, N., Lo Van, A., Lazar, A.-N., & Lagarde, M. (2024). Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies. Antioxidants, 13(6), 634. https://doi.org/10.3390/antiox13060634





