Omega-3-Rich Tuna Oil Derived from By-Products of the Canned Tuna Industry Enhances Memory in an Ovariectomized Rat Model of Menopause
Abstract
:1. Introduction
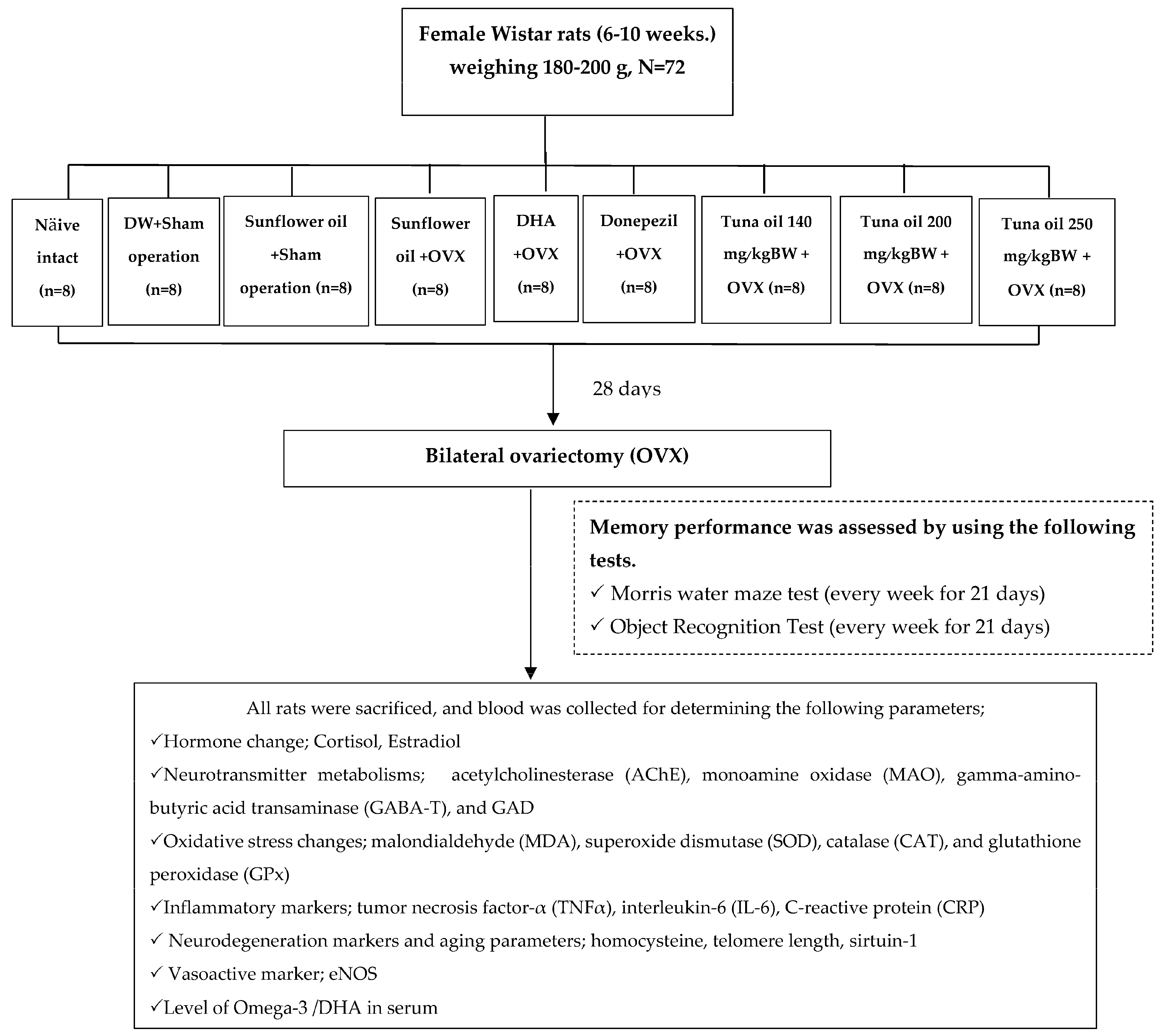
2. Materials and Methods
2.1. Tuna Oil
2.2. Experimental Animals and Treatments
- Group I.
- Naïve intact control group.
- Group II.
- DW (distilled water) + sham operation group: Rats in this group were orally administered DW with an intragastric needle for 28 days to induce the same feeding stress as that induced in the other treatment groups and were subjected to a sham operation procedure, that is, the same processes as that in bilateral ovariectomy but without actual ovariectomy being carried out. Next, they were continually administered DW with an intragastric needle once daily and provided normal diet and water for 21 days after the operation. The purpose of this group was to demonstrate the effect of a sham operation along with stress feeding, because distilled water is known to produce no effect on memory, a primary outcome in this study.
- Group III.
- Vehicle (sunflower oil) + sham operation group: All rats were subjected to a sham operation as described in Group II, but DW administration was replaced with vehicle administration. This group could provide information about the effect of a vehicle (in this case, sunflower oil) which was used as a delivery system for tuna oil.
- Group IV.
- Vehicle (sunflower oil) + OVX-treated group: Rats in this group were orally administered sunflower oil, a vehicle used in this study, for 28 days before the bilateral ovariectomy (OVX) and for 21 days continually after the operation. The purpose of this group was to emphasize the effect of bilateral ovariectomy (OVX).
- Group V.
- DHA + OVX-treated group: The experimental rats were treated with DHA at a dose of 360 mg/kg of BW. This dose was selected based on a previous study that showed memory enhancement [25]. The treatment was performed for 28 days before the bilateral ovariectomy (OVX) and for 21 days continually after the operation. Based on the memory-enhancing effect of DHA mentioned earlier, we also administered DHA to OVX rats, and it also served as a positive control.
- Group VI.
- Donepezil + OVX-treated group: Rats in this group were orally administered donepezil for 28 days before the bilateral ovariectomy (OVX) and for 21 days continually after the operation. In this group, donepezil, a standard drug used for improving memory in humans, was administered to OVX rats, and it served as a positive control group. We used this comparison with an available standard drug to find out whether it is worth moving on to clinical research, which is expensive.
- Group VII.
- Group IX tuna oil + OVX-treated groups (doses of 140, 200, and 250 mg/kg of BW were applied): All animals were subjected to the treatment as mentioned in Group IV and V except that the experimental rats were orally fed tuna oil at the doses of 140, 200, and 250 mg/kg of BW for 28 days before the bilateral ovariectomy (OVX) and for 21 days continually after an operation. These groups were experimental groups.
2.3. Ovariectomy
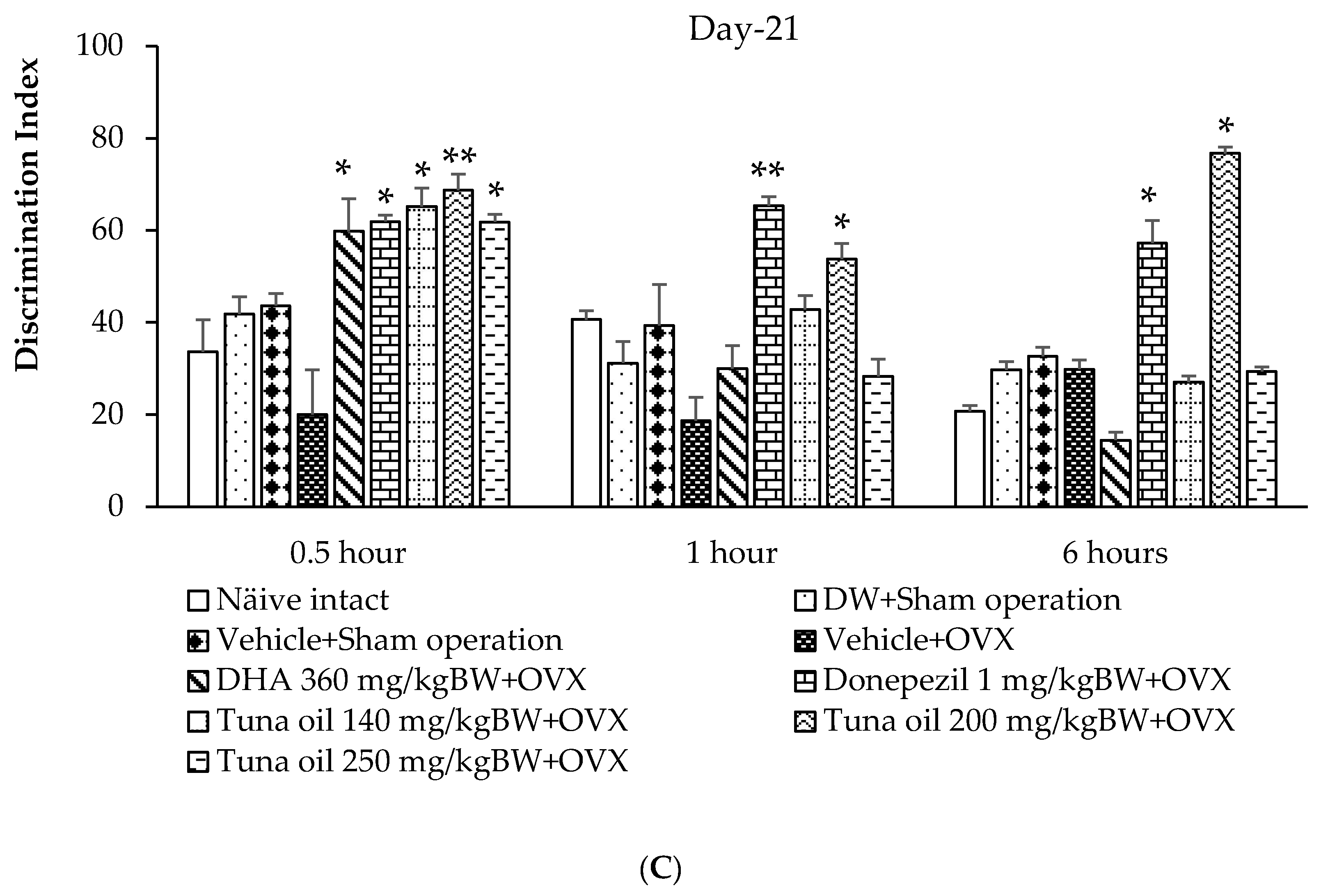
2.4. Memory Assessment
Exploration Time) × 100
2.5. Biochemical Assessment
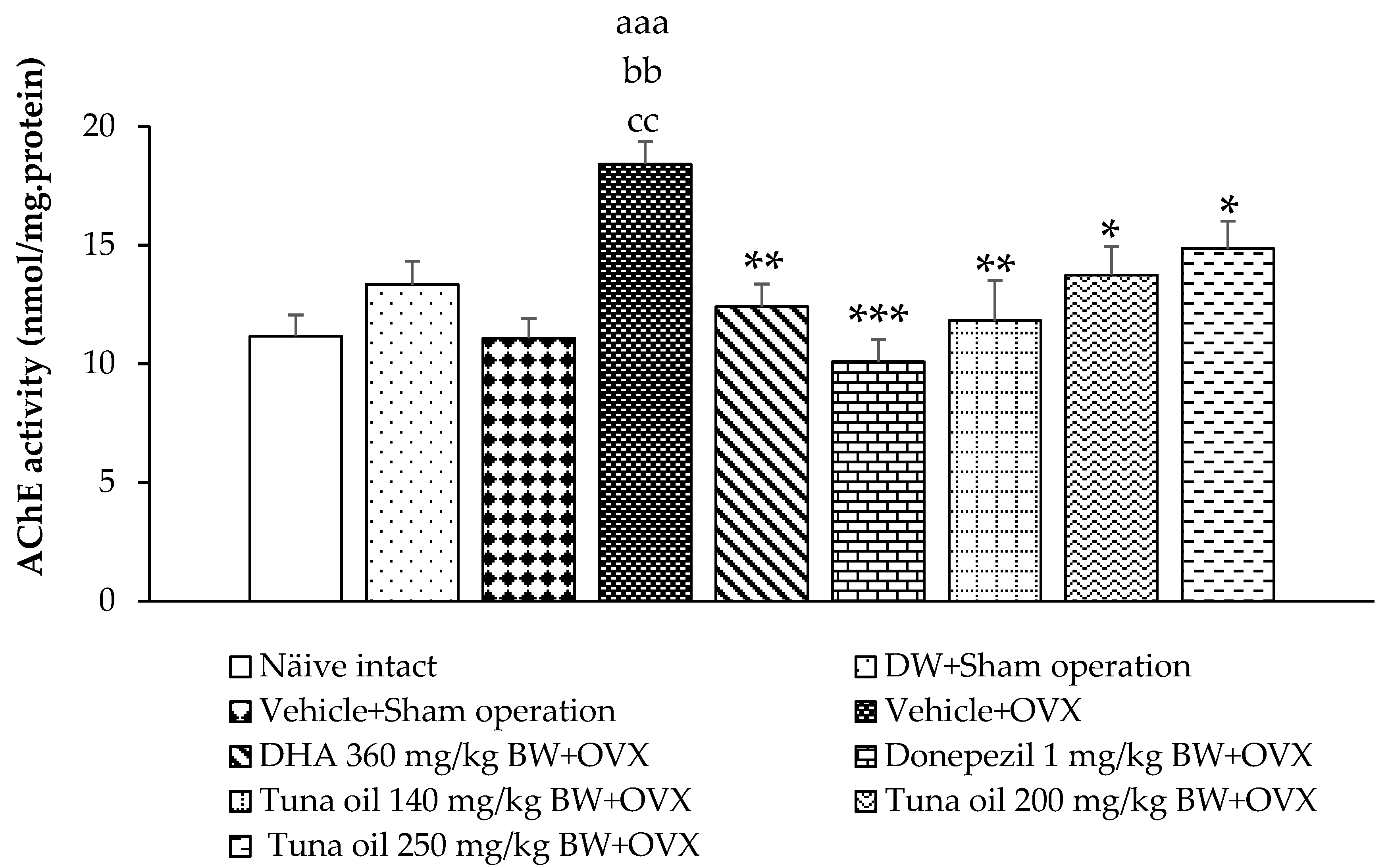
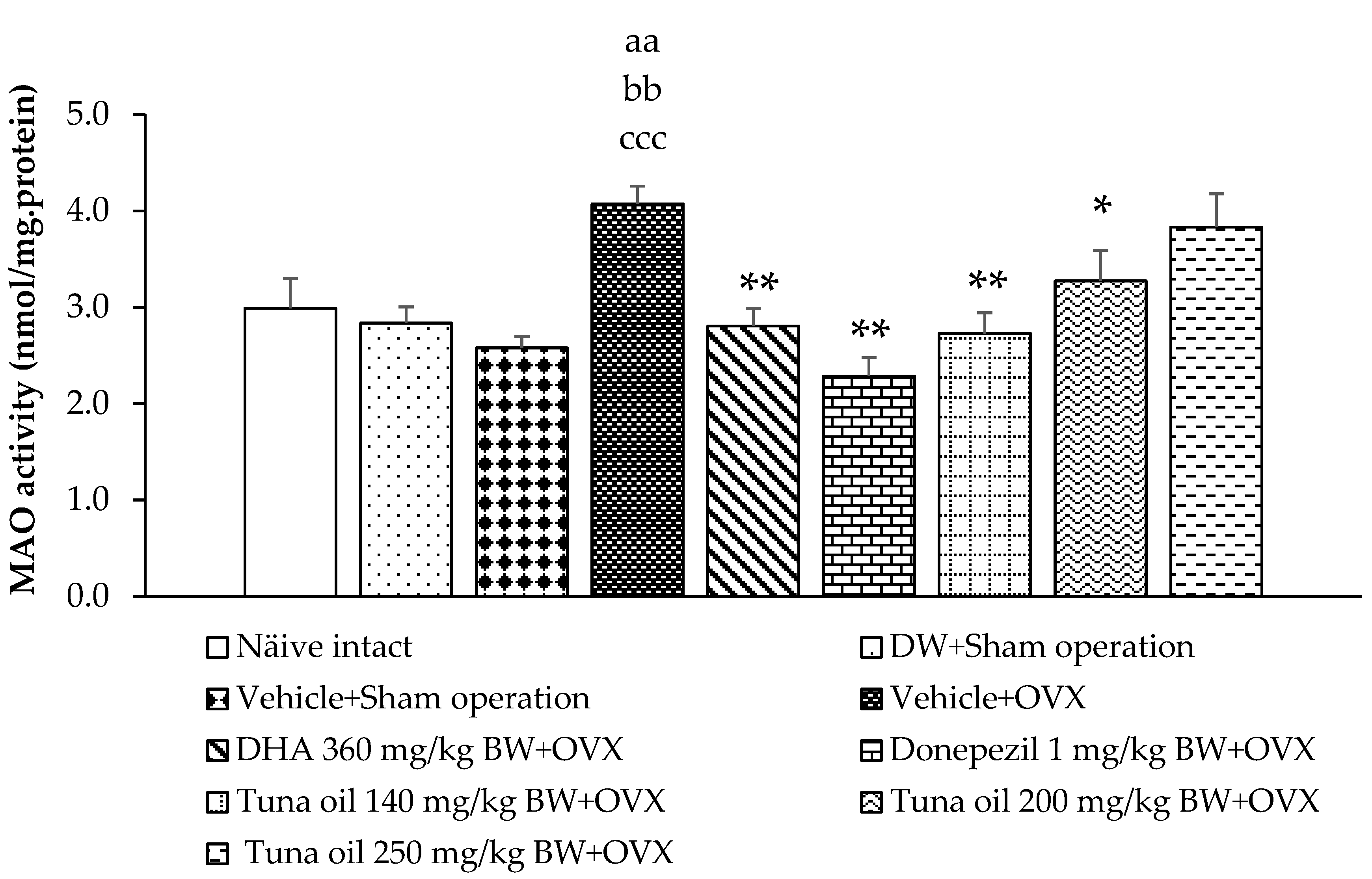
2.5.1. Assessment of Neurotransmitter Changes
2.5.2. Oxidative Stress Markers
2.5.3. Inflammatory Marker Assessment
2.5.4. Assessment of Endothelial Nitric Oxide Synthase (eNOS) Activity
2.5.5. Measurement of Telomere Length
2.5.6. Sirtuin-1 Assessment
2.5.7. Assessment of Homocysteine, Cortisol, and Estradiol
2.6. Measurement of Serum Levels of Docosahexaenoic Acid (DHA)
2.7. Statistical Analysis
3. Results
3.1. Effect of Omega-3-Rich Tuna Oil on Spatial Memory
3.2. Effect of Omega-3-Rich Tuna Oil on Non-Spatial Memory
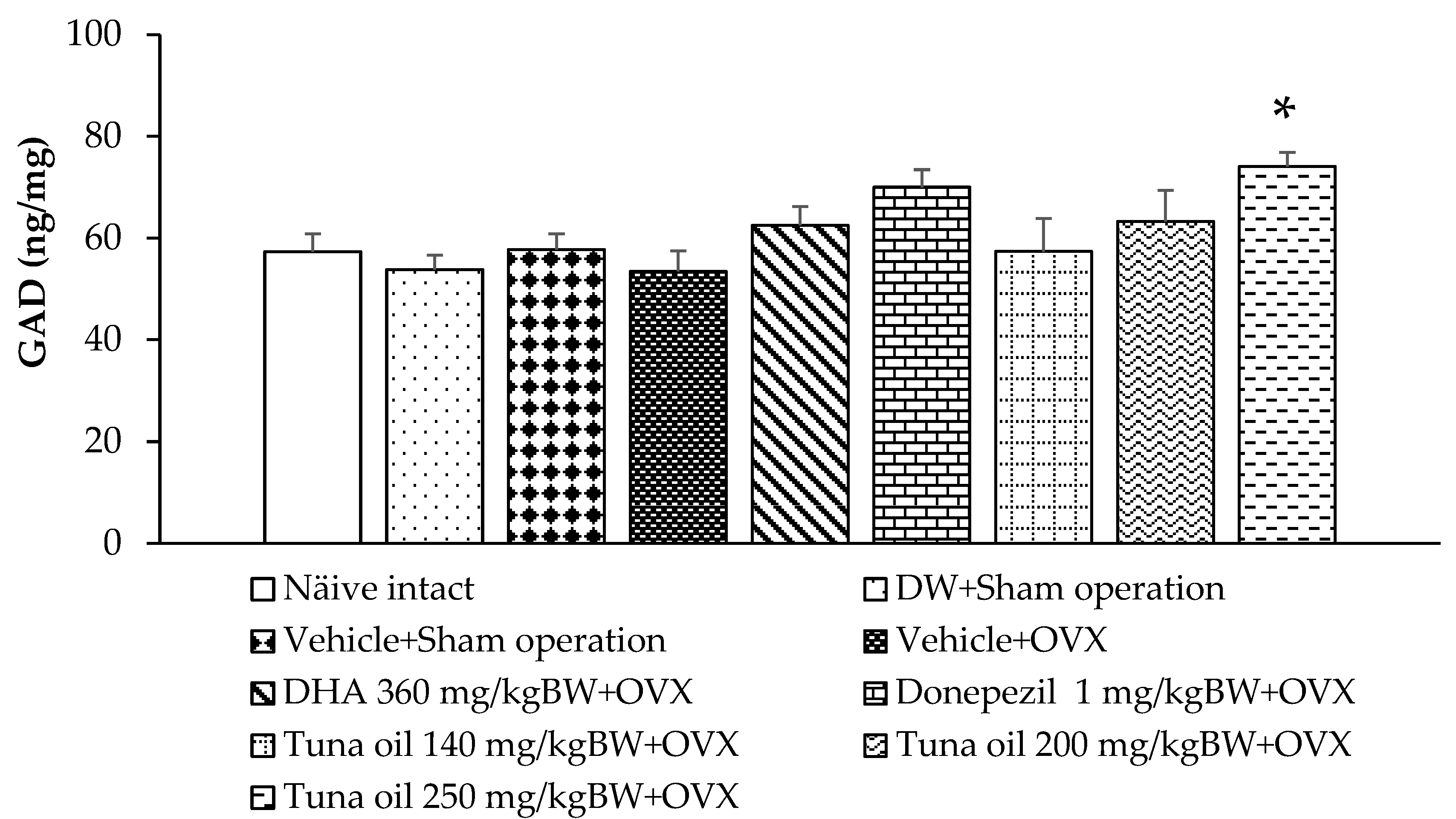
3.3. Neurotransmitter Changes
3.4. Changes in Oxidative Stress Markers
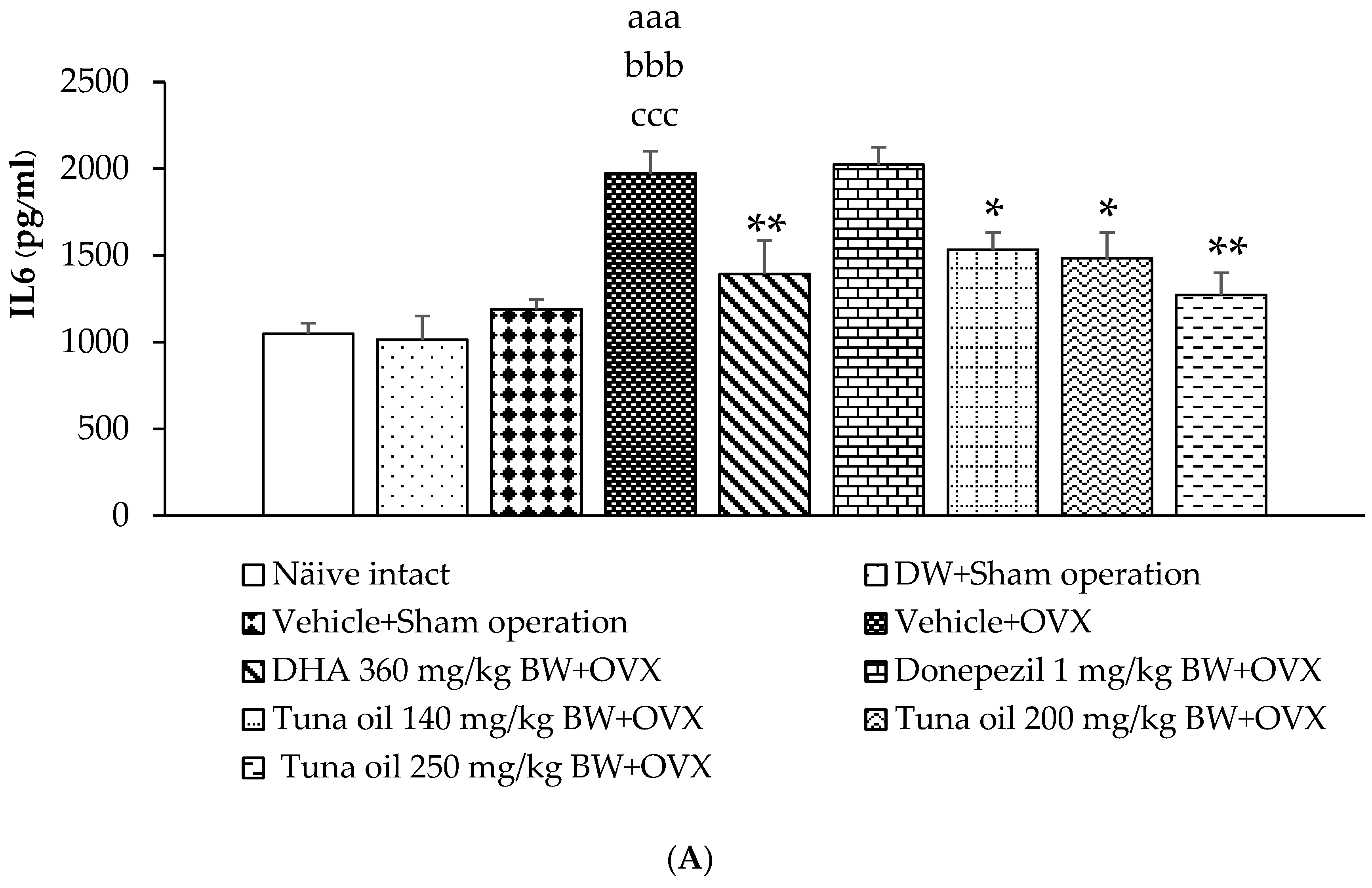
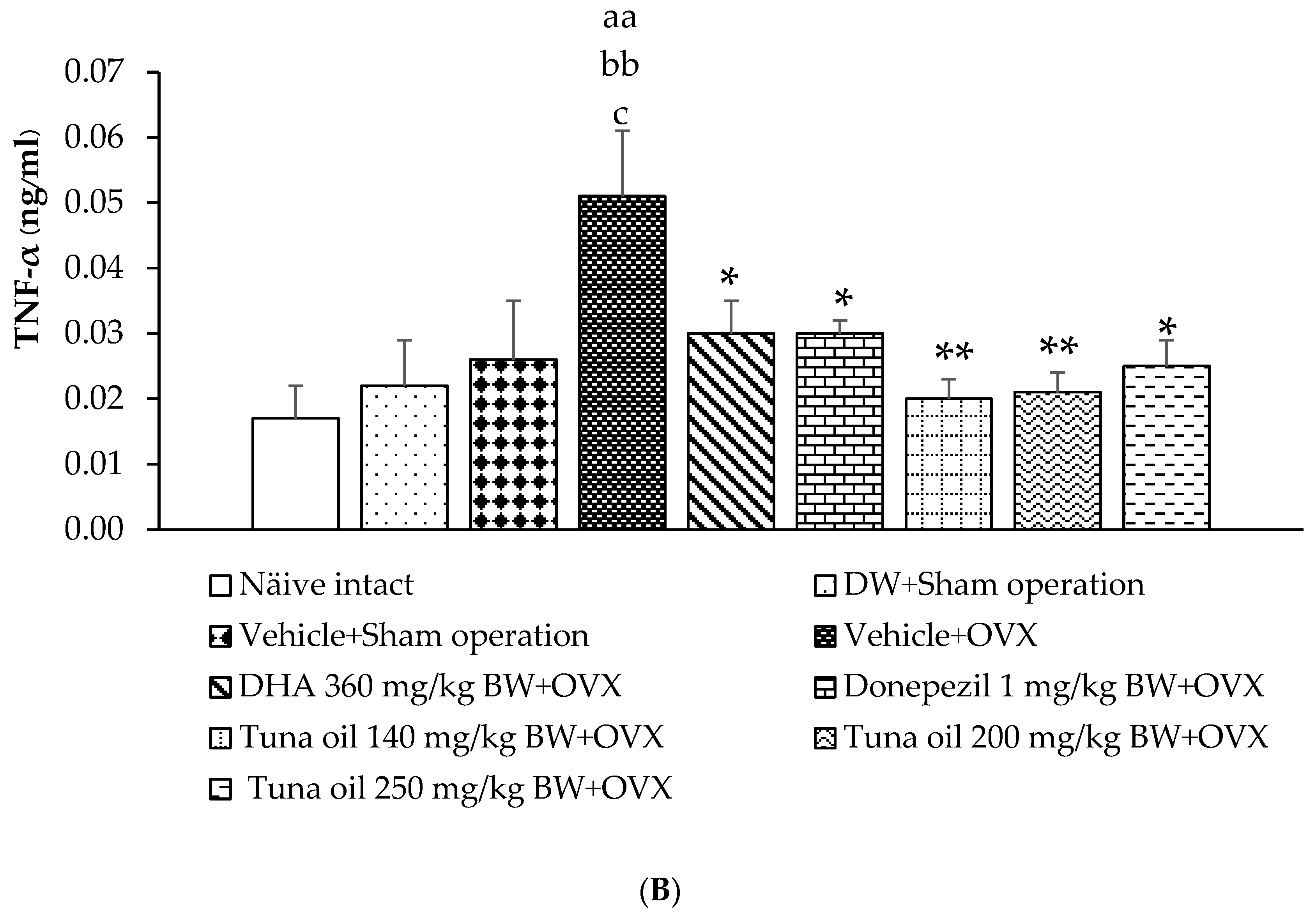
3.5. Inflammatory Marker Changes
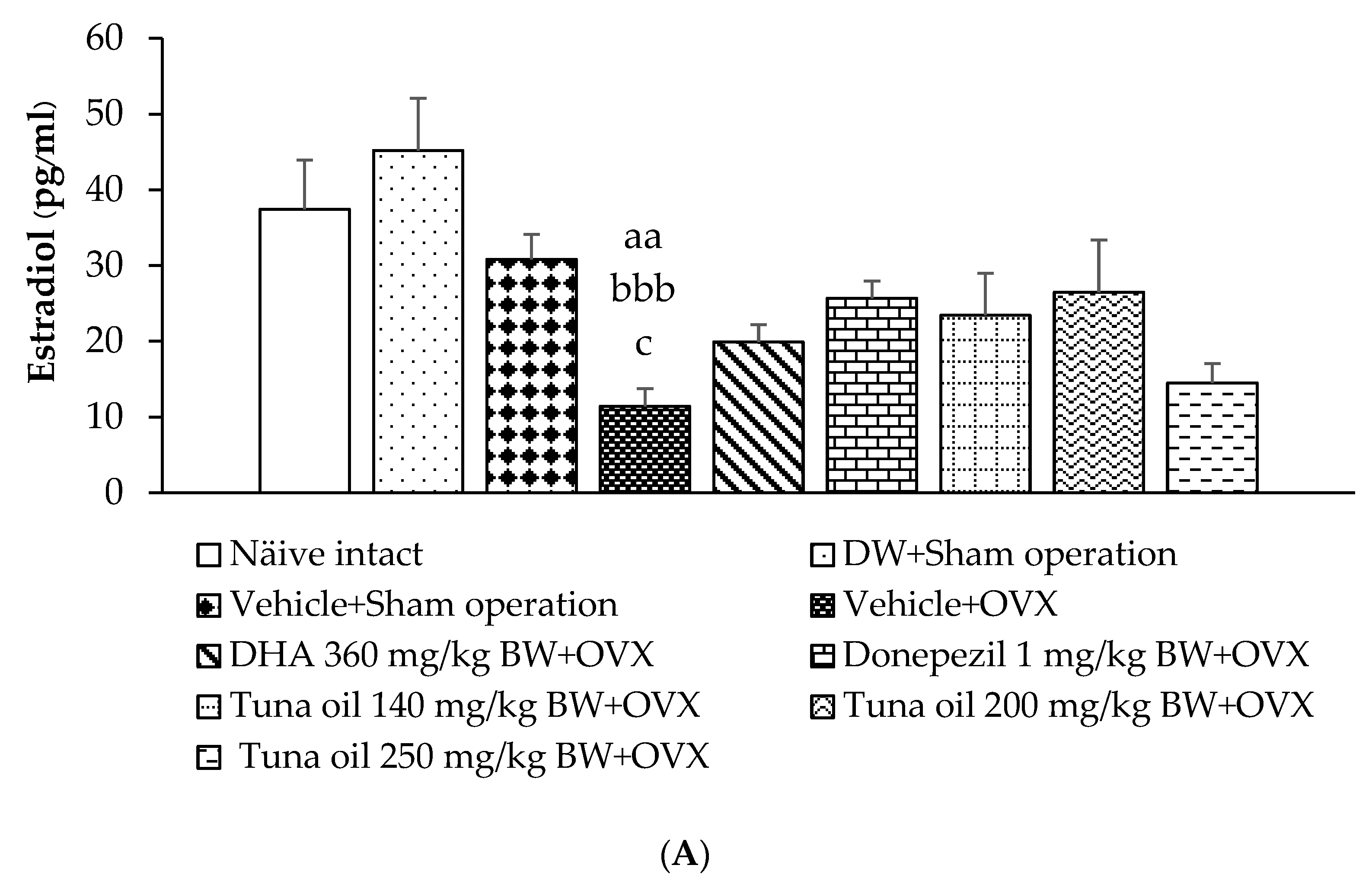
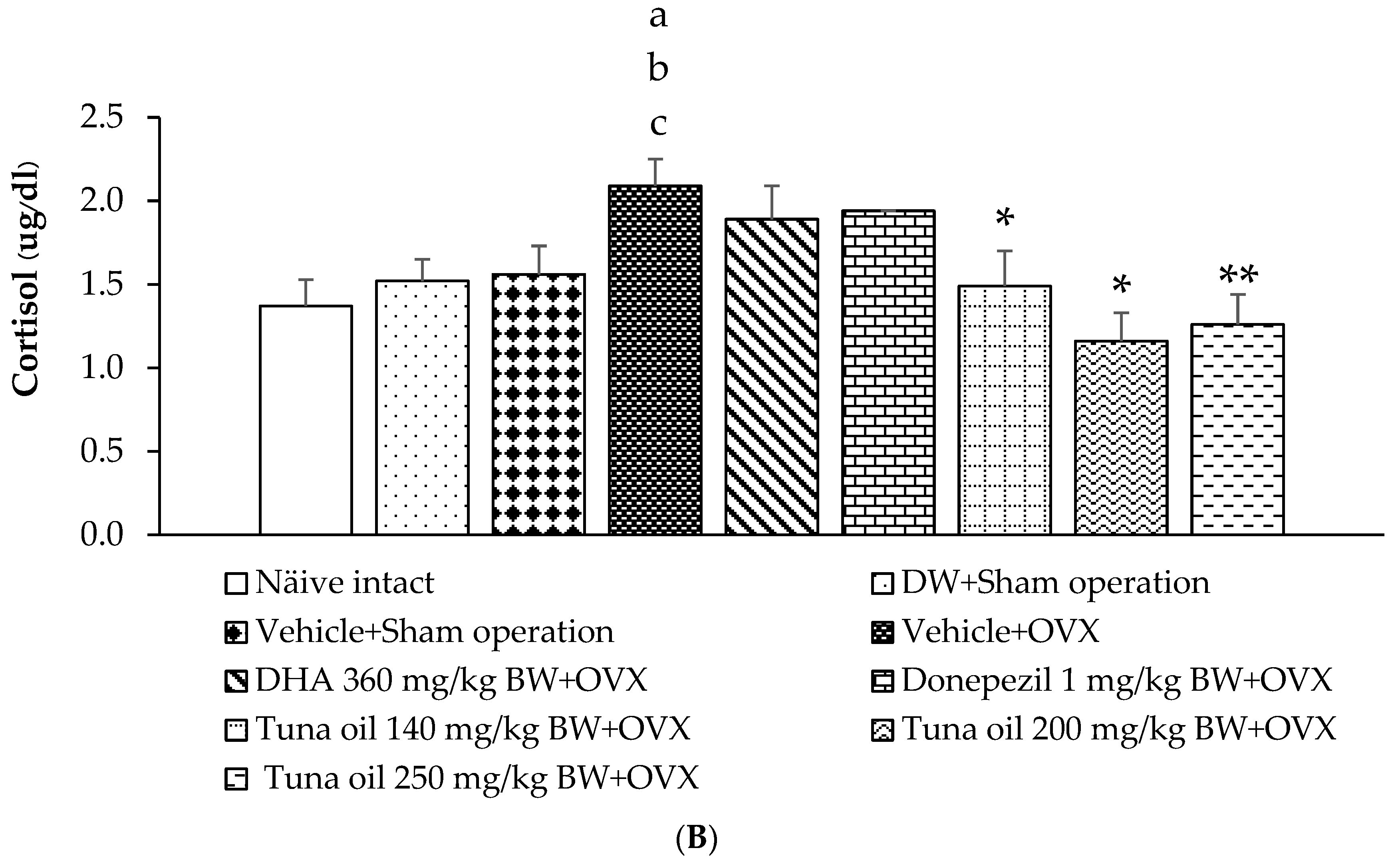
3.6. Hormonal Changes
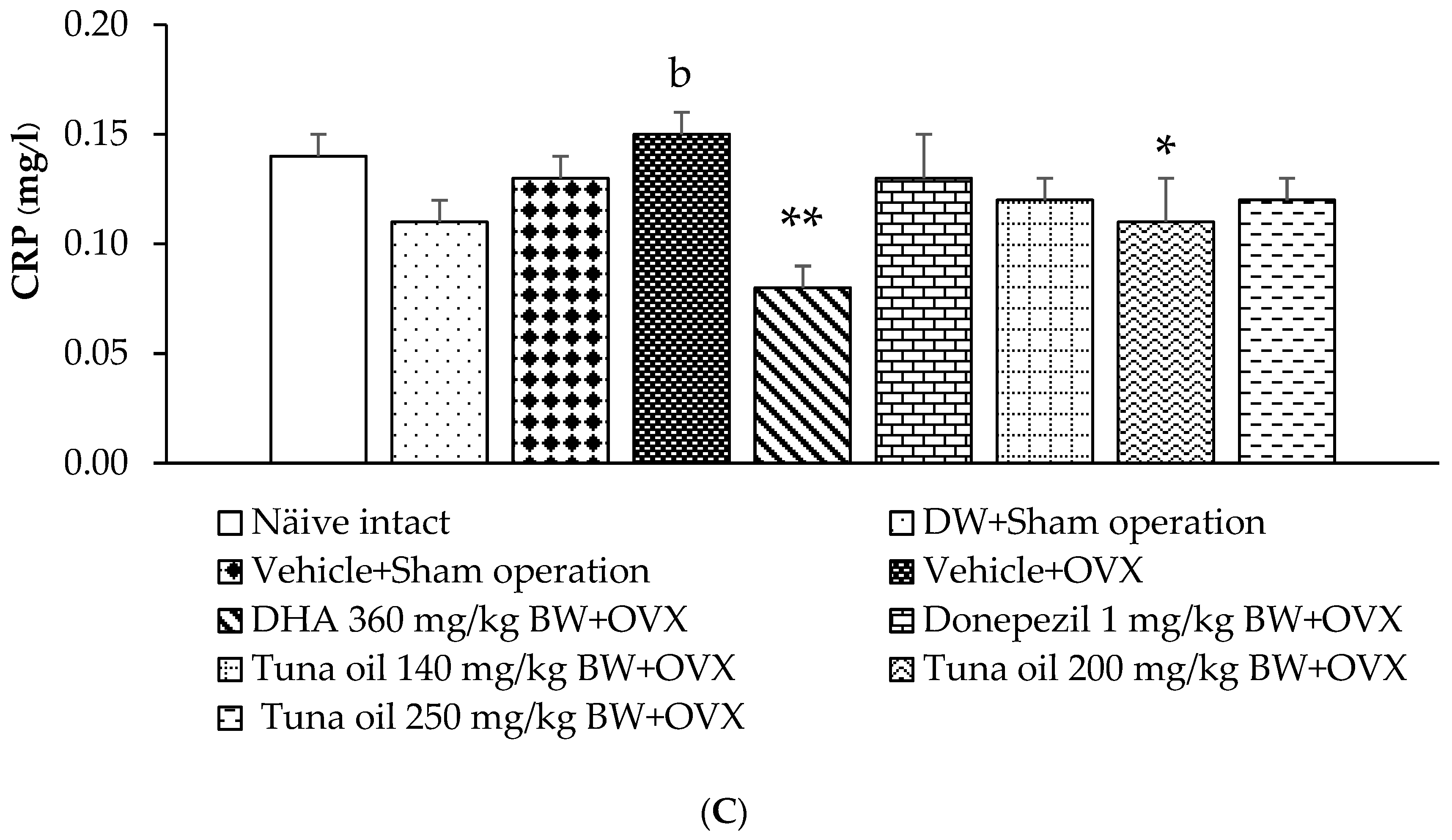
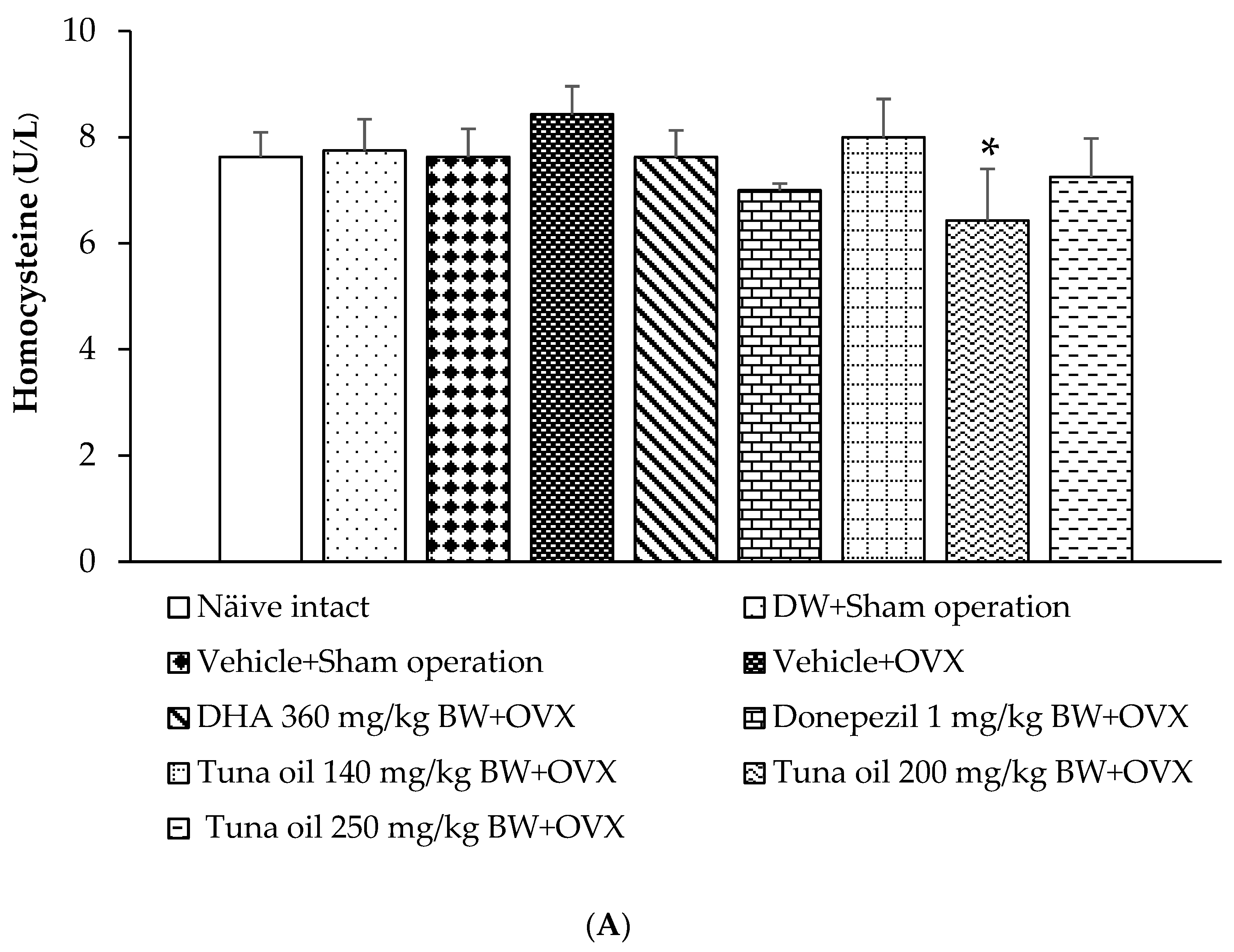
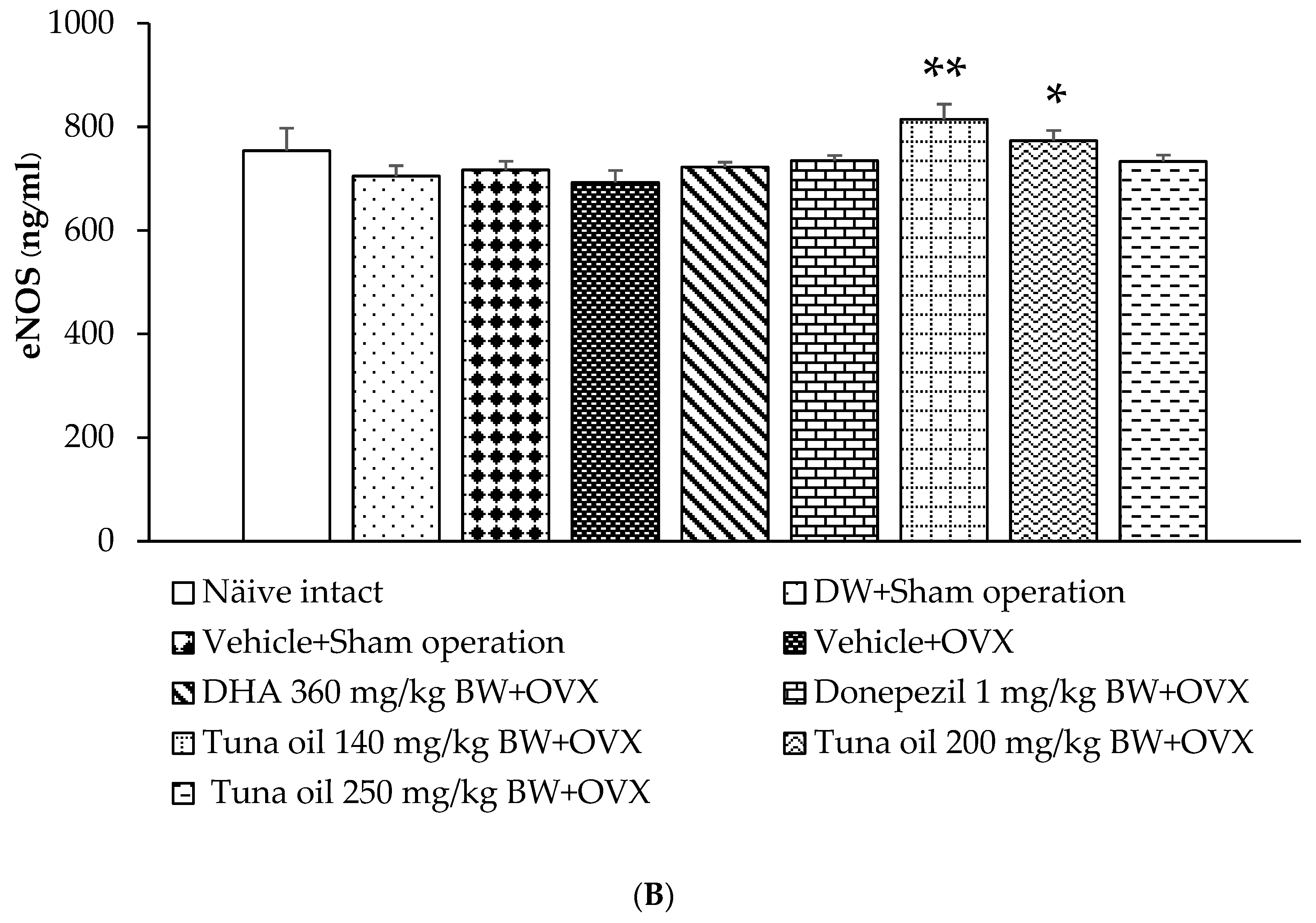
3.7. Changes in Various Surrogate Markers
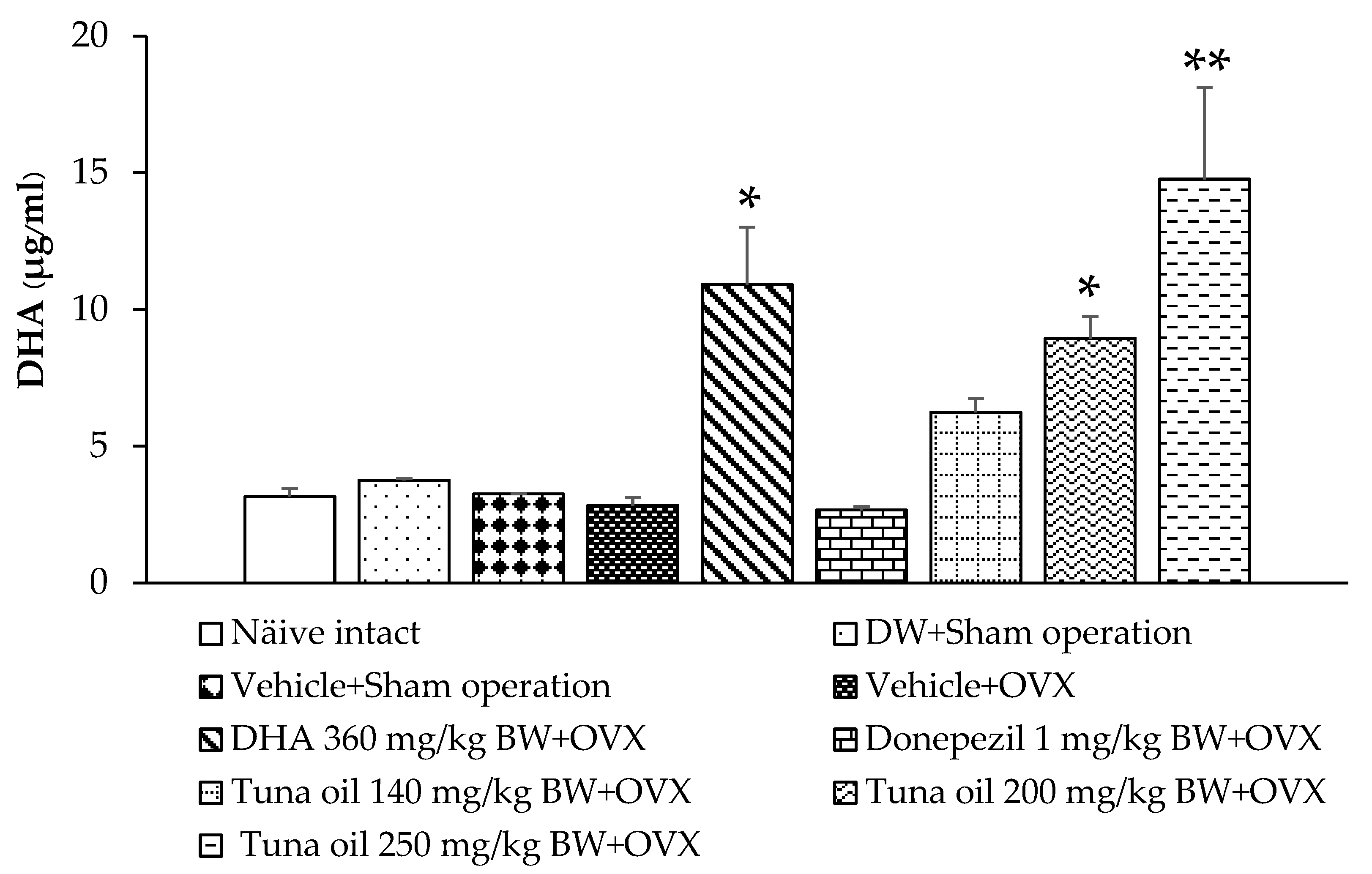
3.8. Serum DHA Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.G.; Dudley, E.C.; Robertson, D.M.; Dennerstein, L. Hormonal changes in the menopause transition. Recent Prog. Horm. Res. 2002, 57, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Conde, D.M.; Verdade, R.C.; Valadares, A.L.R.; Mella, L.F.B.; Pedro, A.O.; Costa-Paiva, L. Menopause and cognitive impairment: A narrative review of current knowledge. World J. Psychiatry 2021, 11, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Tamaria, A.; Bharti, R.; Sharma, M.; Dewan, R.; Kapoor, G.; Aggarwal, A.; Batra, A.; Batra, A. Risk assessment for psychological disorders in postmenopausal, women. J. Clin. Diagn. Res. 2013, 7, 2885–2888. [Google Scholar] [PubMed]
- Sliwinski, J.R.; Johnson, A.K.; Elkins, G.R. Memory Decline in Peri- and Post-menopausal Women: The Potential of Mind-Body Medicine to Improve Cognitive Performance. Integr. Med. Insights 2014, 9, 17–23. [Google Scholar] [CrossRef]
- Hayashi, K.; Ideno, Y.; Nagai, K.; Lee, J.S.; Yasui, T.; Kurabayashi, T.; Takamatsu, K. Complaints of reduced cognitive functioning during perimenopause: A cross-sectional analysis of the Japan Nurses’ Health Study. Womens Midlife Health 2022, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Delrobaei, F.; Fatemi, I.; Shamsizadeh, A.; Allahtavakoli, M. Ascorbic acid attenuates cognitive impairment and brain oxidative stress in ovariectomized mice. Pharmacol. Rep. 2019, 71, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.B.; Agarwal, A. The role of oxidative stress in menopause. J. Midlife Health 2013, 4, 140–146. [Google Scholar] [PubMed]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflamm. 2020, 17, 317. [Google Scholar] [CrossRef]
- Newhouse, P.; Dumas, J. Estrogen-cholinergic interactions: Implications for cognitive aging. Horm. Behav. 2015, 74, 173–185. [Google Scholar] [CrossRef]
- Long, T.; Yao, J.K.; Li, J.; Kirshner, Z.Z.; Nelson, D.; Dougherty, G.G.; Gibbs, R.B. Comparison of transitional vs. surgical menopause on monoamine and amino acid levels in the rat brain. Mol. Cell. Endocrinol. 2018, 476, 139–147. [Google Scholar] [CrossRef]
- Gilfarb, R.A.; Leuner, B. GABA System Modifications During Periods of Hormonal Flux Across the Female Lifespan. Front. Behav. Neurosci. 2022, 16, 802530. [Google Scholar] [CrossRef]
- Raszewski, G.; Loroch, M.; Owoc, A.; Łukawski, K.; Filip, R.; Bojar, I. Homocysteine and cognitive disorders of postmenopausal women measured by a battery of computer tests—Central nervous system vital signs. Arch. Womens Ment. Health 2015, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; He, P.; Imam, M.U.; Qi, J.; Tang, S.; Song, C.; Ismail, M. Edible Bird’s Nest Prevents Menopause-Related Memory and Cognitive Decline in Rats via Increased Hippocampal Sirtuin-1 Expression. Oxid. Med. Cell. Longev. 2017, 2017, 7205082. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Mattei, P.; Sudano, I.; Bernini, G.; Pinto, S.; Salvetti, A. Menopause is associated with endothelial dysfunction in women. Hypertension 1996, 28, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Seals, D.R.; Silver, A.E.; Moreau, K.L. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J. Clin. Endocrinol. Metab. 2009, 94, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Nevzati, E.; Shafighi, M.; Bakhtian, K.D.; Treiber, H.; Fandino, J.; Fathi, A.R. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir. Suppl. 2015, 120, 141–145. [Google Scholar] [PubMed]
- Arikawe, A.P.; Olusanya, A.W.; Udenze, I.K.; Oyedeji, K.S.; Nwaiwu, O.; Atobiloye, A.; Akinibosun, O.A.; Ogunsola, A.O. L-Arginine Administration Improves Cognition and Oxidative Stress Parameters in the Hippocampus and Frontal Lobe of 4-Vinylcyclohexene Diepoxide Perimenopausal Female Rats. Afr. J. Biomed. Res. 2019, 22, 287–293. [Google Scholar]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Thailand Now World’s 2nd Largest Exporter of Canned/Processed Fish. Available online: https://www.nationthailand.com/thailand/general/40029429 (accessed on 19 February 2024).
- Garofalo, S.F.; Cavallini, N.; Demichelis, F.; Savorani, F.; Mancini, G.; Fino, D.; Tommasi, T. From tuna viscera to added-value products: A circular approach for fish-waste recovery by green enzymatic hydrolysis. Food Bioprod. Process. 2023, 137, 155–167. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, Z.I.; Norulaini, N.; Oliverira, A.; Yunnus, K.; Chowdury, A.J.; Akanda, J.; Omar, M. Quality of Tuna Fish Oils Extracted from Processing the By-Products of Three Species of Neritic Tuna Using Supercritical Carbon Dioxide. J. Food Process. Preserv. 2015, 39, 432–441. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D.; Pagani, M.; Caporali, P.; Gulbusera, A.; Laricchiuta, D.; Foti, F.; Neri, C.; Spalletta, G.; Caltagirone, C.; Petrosini, L.; et al. Effects of Omega-3 Fatty Acid Supplementation on Cognitive Functions and Neural Substrates: A Voxel-Based Morphometry Study in Aged Mice. Front. Aging Neurosci. 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Lee, Y.J.; Chung, C.K. Bone mineral density changes after ovariectomy in rats as an osteopenic model: Stepwise description of double dorso-lateral approach. J. Korean Neurosurg. Soc. 2010, 48, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Nakyam, T.; Wattanathorn, J.; Thukham-Mee, W.; Muchimapura, S. The Polyherbal Functional Ingredient Containing Ginger, Chinese Date, and Wood Ear Mushroom Protects against Dementia following Metabolic Syndrome. Biomed. Res. Int. 2023, 2023, 9911397. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.; Sharman, D.F.; Baker, G.B.; Palcic, M.M. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal. Biochem. 1997, 244, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Matsumoto, T.; Motomura, E.; Shiroyama, T. The sleep-enhancing effect of valerian inhalation and sleep-shortening effect of lemon inhalation. Chem. Senses 2006, 31, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Srichomphu, P.; Wattanathorn, J.; Thukham-Mee, W.; Muchimapura, S. Anxiety, Insomnia, and Memory Impairment in Metabolic Syndrome Rats Are Alleviated by the Novel Functional Ingredients from Anacardium occidentale. Antioxidants 2022, 11, 2203. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef]
- Mumtaz, S.; Ali, S.; Qureshi, M.Z.; Muhammad, A.; Manan, A.; Akbar Mughal, T. Antioxidant and anti-aging role of silk sericin in D-galactose induced mice model. Saudi J. Biol. Sci. 2023, 30, 103872. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.V.; Satoor, S.N.; Wong, W.K.; Cheng, F.; Ma, R.C.; Hardikar, A.A. An optimised step-by-step protocol for measuring relative telomere length. Methods Protoc. 2020, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.J.; Fenech, M. A quantitative PCR method for measuring absolute telomere length. Biol. Proced. Online 2011, 13, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Savage, S.A.; Alsaggaf, R.; Aubert, G.; Dagnall, C.L.; Spellman, S.R.; Lee, S.J.; Hicks, B.; Jones, K.; Katki, H.A.; et al. Telomere Length Calibration from qPCR Measurement: Limitations of Current Method. Cells 2018, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewsk, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef] [PubMed]
- Serafim, V.; Tiugan, D.A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC-MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24, 360. [Google Scholar] [CrossRef]
- Morris, R.G.M.; Doyle, J. Successive incompatible tasks: Evidence for separate subsystems for storage of spatial knowledge. In Electrical Activity of the Archicortex; Buzsaki, G., Vanderwolf, C.H., Eds.; Akademiai Kiado: Budapest, Hungary, 1985. [Google Scholar]
- Zhou, G.; Xiong, W.; Zhang, X.; Ge, S. Retrieval of Consolidated Spatial Memory in the Water Maze Is Correlated with Expression of pCREB and Egr1 in the Hippocampus of Aged Mice. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; McIntosh, A.R.; Cabeza, R.; Habib, R.; Houle, S.; Tulving, E. General and specific brain regions involved in encoding and retrieval of events: What, where, and when. Proc. Natl. Acad. Sci. USA 1996, 93, 11280–11285. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Gaskin, S.; Squire, L.R.; Clark, R.E. Object recognition memory and the rodent hippocampus. Learn. Mem. 2009, 17, 5–11. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Khodabandehloo, F.; Hosseini, M.; Rajaei, Z.; Soukhtanloo, M.; Farrokhi, E.; Rezaeipour, M. Brain tissue oxidative damage as a possible mechanism for the deleterious effect of a chronic high dose of estradiol on learning and memory in ovariectomized rats. Arq. Neuropsiquiatr. 2013, 71, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Turek, J.; Gąsior, Ł. Estrogen fluctuations during the menopausal transition are a risk factor for depressive disorders. Pharmacol. Rep. 2023, 75, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.B.; Mazzanti, C.M.; França, R.T.; Pagnoncelli, M.; Costa, M.M.; de Souza, E.M.; Gonçalves, J.; Spanevello, R.; Schmatz, R.; da Costa, P.; et al. 17-β estradiol in the acetylcholinesterase activity and lipid peroxidation in the brain and blood of ovariectomized adult and middle-aged rats. Life Sci. 2012, 90, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Puri, V.; Puri, S. Effects of estrogen on the serotonergic system and calcitonin gene-related peptide in trigeminal ganglia of rats. Ann. Neurosci. 2012, 19, 151–157. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; la Marca, A.; Talluri, B.; D’Antona, D.; Morgante, G. Hypothalamo-pituitary-adrenal axis and adrenal function before and after ovariectomy in premenopausal women. Eur. J. Endocrinol. 1998, 138, 430–435. [Google Scholar] [CrossRef]
- Hedberg, Y.; Dalin, A.M.; Forsberg, M.; Lundeheim, N.; Sandh, G.; Hoffmann, B.; Ludwig, C.; Kindahl, H. Effect of ACTH (tetracosactide) on steroid hormone levels in the mare. Part B: Effect in ovariectomized mares (including estrous behavior). Anim. Reprod. Sci. 2007, 100, 92–106. [Google Scholar] [CrossRef]
- Isaac, A.; Ibrahim, Y.; Andrew, A.; Edward, D.; Solomon, A. The Cortisol Steroid Levels as a Determinant of Health Status in Animals. J. Proteom. Bioinform. 2017, 10, 277–283. [Google Scholar]
- Cay, M.; Ucar, C.; Senol, D.; Cevirgen, F.; Ozbag, D.; Altay, Z.; Yildiz, S. Effect of increase in cortisol level due to stress in healthy young individuals on dynamic and static balance scores. North Clin. Istanb. 2018, 5, 295–301. [Google Scholar]
- Bowles, N.P.; Thosar, S.S.; Butler, M.P.; Clemons, N.A.; Robinson, L.D.; Ordaz, O.H.; Herzig, M.X.; McHill, A.W.; Rice, S.P.M.; Emens, J.; et al. The circadian system modulates the cortisol awakening response in humans. Front. Neurosci. 2022, 16, 995452. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Parle, M.; Dhingra, D. Ascorbic Acid: A promising memory-enhancer in mice. J. Pharmacol. Sci. 2003, 93, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Zeng, P.; Qu, N.; Ning, L.N.; Chu, J.; Zhang, T.; Zhou, X.W.; Tian, Q. Evidence of altered depression and dementia-related proteins in the brains of young rats after ovariectomy. J. Neurochem. 2018, 146, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Rachoń, D.; Myśliwska, J.; Suchecka-Rachoń, K.; Wieckiewicz, J.; Myśliwski, A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J. Endocrinol. 2002, 172, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Omega-3 fatty acids modulate cyclophosphamide induced markers of immunosuppression and oxidative stress in pigs. Sci. Rep. 2019, 9, 2684. [Google Scholar] [CrossRef] [PubMed]
- Coghill, A.E.; Schenk, J.M.; Mahkoul, Z.; Orem, J.; Phipps, W.; Casper, C. Omega-3 decreases IL-6 levels in HIV and human herpesvirus-8 coinfected patients in Uganda. AIDS 2018, 32, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zugno, A.I.; Chipindo, H.; Canever, L.; Budni, J.; Alves de Castro, A.; Bittencourt de Oliveira, M.; Heylmann, A.S.; Gomes Wessler, P.; da Rosa Silveira, F.; Damázio, L.S.; et al. Omega-3 fatty acids prevent the ketamine-induced increase in acetylcholinesterase activity in an animal model of schizophrenia. Life Sci. 2015, 121, 65–69. [Google Scholar] [CrossRef]
- Chalon, S.; Delion-Vancassel, S.; Belzung, C.; Guilloteau, D.; Leguisquet, A.M.; Besnard, J.C.; Durand, G. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J. Nutr. 1998, 128, 2512–2519. [Google Scholar] [CrossRef]
- Okuda, Y.; Kawashima, K.; Sawada, T.; Tsurumaru, K.; Asano, M.; Suzuki, S.; Soma, M.; Nakajima, T.; Yamashita, K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem. Biophys. Res. Commun. 1997, 232, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Omura, M.; Kobayashi, S.; Mizukami, Y.; Mogami, K.; Todoroki-Ikeda, N.; Miyake, T.; Matsuzaki, M. Eicosapentaenoic acid (EPA) induces Ca2+-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001, 487, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Felau, S.M.; Sales, L.P.; Solis, M.Y.; Hayashi, A.P.; Roschel, H.; Sá-Pinto, A.L.; Andrade, D.C.O.; Katayama, K.Y.; Irigoyen, M.C.; Consolim-Colombo, F.; et al. Omega-3 Fatty Acid Supplementation Improves Endothelial Function in Primary Antiphospholipid Syndrome: A Small-Scale Randomized Double-Blind Placebo-Controlled Trial. Front. Immunol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Lee, S.M.; Lee, M.H.; Son, Y.K.; Kim, S.E.; An, W.S. Omega-3 Fatty Acids Upregulate SIRT1/3, Activate PGC-1α via Deacetylation, and Induce Nrf1 Production in 5/6 Nephrectomy Rat. Model. Mar. Drugs 2021, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.K.; Al-Daihan, S.K.; El-Gezeery, A.R. On the protective effect of omega-3 against propionic acid-induced neurotoxicity in rat pups. Lipids Health Dis. 2011, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Sohouli, M.H.; Roshan, M.M.; Olusola, O.F.; Fatahi, S.; Omidi, H.R.; Sharifi, P.; Hekmatdoost, A.; Kutbi, E.; Abu-Zaid, A. Impact of Omega-3 supplementation on homocysteine levels in humans: A systematic review and meta-regression analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Perrig, W.J.; Perrig, P.; Stähelin, H.B. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997, 45, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, F.; Scarfò, G.; Guidotti, S.; Fusi, J.; Asomov, M.; Pruneti, C. Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 729757. [Google Scholar] [CrossRef] [PubMed]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Cools, R.; Arnsten, A.F.T. Neuromodulation of prefrontal cortex cognitive function in primates: The powerful roles of monoamines and acetylcholine. Neuropsychopharmacology 2022, 47, 309–328. [Google Scholar] [CrossRef]
- Calvo-Flores Guzmán, B.; Vinnakota, C.; Govindpani, K.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The GABAergic system as a therapeutic target for Alzheimer’s disease. J. Neurochem. 2018, 146, 649–669. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Shen, Y.; Chopp, M.; Zacharek, A.; Venkat, P.; Chen, Z.; Li, W.; Qian, Y.; Landschoot-Ward, J.; Chen, J. Deficiency of Endothelial Nitric Oxide Synthase (eNOS) Exacerbates Brain Damage and Cognitive Deficit in A Mouse Model of Vascular Dementia. Aging Dis. 2021, 12, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Fagerli, E.; Escobar, I.; Ferrier, F.J.; Jackson, C.W.; Perez-Lao, E.J.; Perez-Pinzon, M.A. Sirtuins and cognition: Implications for learning and memory in neurological disorders. Front. Physiol. 2022, 13, 908689. [Google Scholar] [CrossRef] [PubMed]
- de Souza-Talarico, J.N.; Marin, M.F.; Sindi, S.; Lupien, S.J. Effects of stress hormones on the brain and cognition: Evidence from normal to pathological aging. Dement. Neuropsychol. 2011, 5, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.I.; Mayer, L.; Talboom, J.S.; Tsang, C.W.; Smith, C.J.; Enders, C.K.; Bimonte-Nelson, H.A. Transitional versus surgical menopause in a rodent model: Etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology 2009, 150, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094. [Google Scholar] [CrossRef]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]





| Groups | Malondialdehyde Level (ng/mg protein) | Superoxide Dismutase Activity (U/mg protein) | Catalase Activity (U/mg protein) | Glutathione Peroxidase Activity (U/mg protein) |
| Naïve intact | 0.055 ± 0.004 | 23.481 ± 1.848 | 4.356 ± 0.216 | 2.223 ± 0.571 |
| DW + sham operation | 0.063 ± 0.003 | 24.541 ± 1.561 | 4.357 ± 0.311 | 2.255 ± 0.420 |
| Vehicle + sham operation | 0.064 ± 0.004 | 24.835 ± 1.660 | 3.724 ± 0.265 | 1.792 ± 0.623 |
| Vehicle + OVX | 0.080 ± 0.009 aa,b,c | 19.172 ± 1.713 | 4.162 ± 0.787 | 0.767 ± 0.180 |
| DHA 360 mg/kg of BW + OVX | 0.057 ± 0.004 ** | 25.683 ± 0.733 * | 3.742 ± 0.308 | 2.968 ± 0.814 |
| Donepezil 1 mg/kg of BW + OVX | 0.071 ± 0.004 | 25.088 ± 0.884 * | 3.931 ± 0.239 | 3.766 ± 0.806 * |
| Tuna oil 140 mg/kg of BW + OVX | 0.063 ± 0.005 * | 29.807 ± 3.856 ** | 4.888 ± 0.785 | 3.508 ± 0.618 * |
| Tuna oil 200 mg/kg of BW + OVX | 0.063 ± 0.002 * | 28.299 ± 2.360 ** | 4.948 ± 0.527 | 3.407 ± 0.798 * |
| Tuna oil 250 mg/kg of BW + OVX | 0.061 ± 0.005 ** | 29.023 ± 2.537 ** | 5.037 ± 0.433 | 2.934 ± 0.884 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanathorn, J.; Thukham-Mee, W. Omega-3-Rich Tuna Oil Derived from By-Products of the Canned Tuna Industry Enhances Memory in an Ovariectomized Rat Model of Menopause. Antioxidants 2024, 13, 637. https://doi.org/10.3390/antiox13060637
Wattanathorn J, Thukham-Mee W. Omega-3-Rich Tuna Oil Derived from By-Products of the Canned Tuna Industry Enhances Memory in an Ovariectomized Rat Model of Menopause. Antioxidants. 2024; 13(6):637. https://doi.org/10.3390/antiox13060637
Chicago/Turabian StyleWattanathorn, Jintanaporn, and Wipawee Thukham-Mee. 2024. "Omega-3-Rich Tuna Oil Derived from By-Products of the Canned Tuna Industry Enhances Memory in an Ovariectomized Rat Model of Menopause" Antioxidants 13, no. 6: 637. https://doi.org/10.3390/antiox13060637






