Food and Food Waste Antioxidants: Could They Be a Potent Defence against Parkinson’s Disease?
Abstract
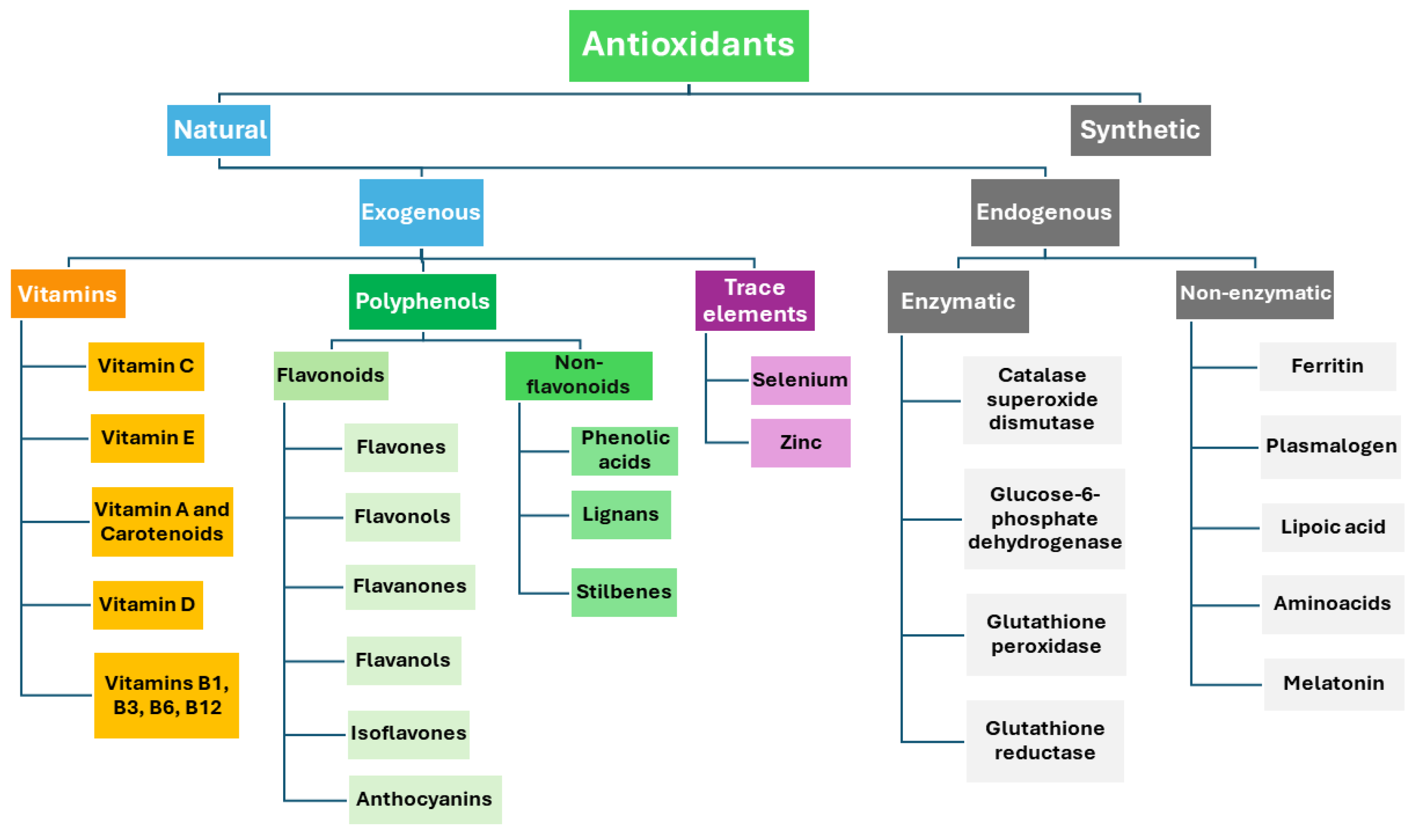
:1. Introduction
2. Material and Methods
3. Vitamins
3.1. Ascorbic Acid
3.2. Vitamin E
3.3. Vitamin A and Carotenoids
3.4. Vitamin D
3.5. Vitamins B
3.5.1. Vitamin B1 (Thiamine)
3.5.2. Vitamin B3
3.5.3. Vitamin B6
3.5.4. Vitamin B12
4. Polyphenols
5. Trace Elements
5.1. Selenium
5.2. Zinc
6. Other Antioxidants
6.1. Linalool
6.2. Caffeine
6.3. Zingerone
7. Discussions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Srivastava, A.K.; Arnold, W.D.; Singh, M.P.; Zhang, Y. Neurodegeneration: Etiologies and New Therapies. BioMed Res. Int. 2015, 2015, 272630. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in Neurodegenerative Diseases: Pathogenesis and Therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Troulinaki, K.; Tavernarakis, N. Necrotic Cell Death and Neurodegeneration: The Involvement of Endocytosis and Intracellular Trafficking. Worm 2012, 1, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Miura, M. Programmed Cell Death in Neurodevelopment. Dev. Cell 2015, 32, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Prata, C.; Freschi, M.; Barbalace, M.; Hrelia, S. Mechanisms Underlying Neurodegenerative Disorders and Potential Neuroprotective Activity of Agrifood By-Products. Antioxidants 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Gatti, M.; Prata, C.; Hrelia, S.; Maraldi, T. Role of Mesenchymal Stem Cells in Counteracting Oxidative Stress—Related Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3299. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Ferreira, M.E.S.; De Vasconcelos, A.S.; Da Costa Vilhena, T.; Da Silva, T.L.; Da Silva Barbosa, A.; Gomes, A.R.Q.; Dolabela, M.F.; Percário, S. Oxidative Stress in Alzheimer’s Disease: Should We Keep Trying Antioxidant Therapies? Cell Mol. Neurobiol. 2015, 35, 595–614. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-S.; Geng, W.-S.; Jia, J.-J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 175909141877743. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Maresova, P.; Klimova, B.; Novotny, M.; Kuca, K. Alzheimer’s and Parkinson’s Diseases: Expected Economic Impact on Europe—A Call for a Uniform European Strategy. J. Alzheimer’s Dis. 2016, 54, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Rajaee, S.M.; Johnston, T.P.; Sahebkar, A. Neuroprotective Effects of Antioxidants in the Management of Neurodegenerative Disorders: A Literature Review. J. Cell. Biochem. 2019, 120, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.; Chircov, C.; Grumezescu, A.; Volceanov, A.; Teleanu, D. Antioxidant Therapies for Neuroprotection—A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Marinelli, L.; Cacciatore, I.; Stefano, A.D. Role of Dietary Supplements in the Management of Parkinson’s Disease. Biomolecules 2019, 9, 271. [Google Scholar] [CrossRef]
- Hughes, K.C.; Gao, X.; Kim, I.Y.; Rimm, E.B.; Wang, M.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A. Intake of Antioxidant Vitamins and Risk of Parkinson’s Disease: Antioxidant Vitamin Intake and PD. Mov. Disord. 2016, 31, 1909–1914. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Gao, R.; Li, J. Ascorbic Acid Protects against Colistin Sulfate-Induced Neurotoxicity in PC12 Cells. Toxicol. Mech. Methods 2013, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Chapter Nine—Ascorbic Acid as Antioxidant. In Antioxidants; Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 121, pp. 247–270. [Google Scholar]
- Hantikainen, E.; Trolle Lagerros, Y.; Ye, W.; Serafini, M.; Adami, H.-O.; Bellocco, R.; Bonn, S. Dietary Antioxidants and the Risk of Parkinson Disease: The Swedish National March Cohort. Neurology 2021, 96, E895–E903. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef] [PubMed]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of Phenolic Compounds and Vitamin C and Antioxidant Activity in Wasted Parts of Sudanese Citrus Fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Dhankhar, S.; Chauhan, S.; Garg, N.; Bhattacharya, T.; Ali, M.; Chaudhary, A.A.; Rudayni, H.; Al-Zharani, M.; Ahmad, W.; et al. A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson’s Disease. Pharmaceuticals 2023, 16, 908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef]
- Yap, H.-M.; Lye, K.-L. An Insight of Vitamin E as Neuroprotective Agents. Prog. Microbes Mol. Biol. 2020, 3, a0000071. [Google Scholar] [CrossRef]
- Matsura, T. Protective Effect of Tocotrienol on In Vitro and In Vivo Models of Parkinson’s Disease. J. Nutr. Sci. Vitaminol. 2019, 65, S51–S53. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Sokolova, T.V.; Avrova, N.F. α-Tocopherol Prevents ERK1/2 Activation in PC12 Cells. Bull. Exp. Biol. Med. 2013, 155, 44–47. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Akšić, M.F.; Lazarević, K.; Šegan, S.; Natić, M.; Tosti, T.; Ćirić, I.; Meland, M. Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus Domestica Borkh.) Grown in Norway. Foods 2021, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements. Vitamin A and Carotenoids. Available online: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/ (accessed on 23 April 2024).
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-de-Mesquita, B.; Gallo, V. Vitamin A and Carotenoids and the Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2014, 42, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Otto, L.R.; Clemens, V.; Üsekes, B.; Cosma, N.C.; Regen, F.; Hellmann-Regen, J. Retinoid Homeostasis in Major Depressive Disorder. Transl. Psychiatry 2023, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Marie, A.; Darricau, M.; Touyarot, K.; Parr-Brownlie, L.C.; Bosch-Bouju, C. Role and Mechanism of Vitamin A Metabolism in the Pathophysiology of Parkinson’s Disease. J. Park. Dis. 2021, 11, 949–970. [Google Scholar] [CrossRef] [PubMed]
- Marie, A.; Leroy, J.; Darricau, M.; Alfos, S.; De Smedt-Peyrusse, V.; Richard, E.; Vancassel, S.; Bosch-Bouju, C. Preventive Vitamin A Supplementation Improves Striatal Function in 6-Hydroxydopamine Hemiparkinsonian Rats. Front. Nutr. 2022, 9, 811843. [Google Scholar] [CrossRef] [PubMed]
- Enenebeaku, C.K.; Enenebeaku, U.E.; Ezejiofor, T.I.N. Evaluation of Selected Agricultural Wastes as Viable Sources of Vitamin Supplements in Poultry Feeds. World News Nat. Sci. 2018, 20, 103–120. [Google Scholar]
- Pinheiro, K.H.; Watanabe, L.S.; Nixdorf, S.L.; Barão, C.E.; Pimentel, T.C.; Matioli, G.; De Moraes, F.F. Cassava Bagasse as a Substrate to Produce Cyclodextrins. Starch Stärke 2018, 70, 1800073. [Google Scholar] [CrossRef]
- de Souza, C.B.; Roeselers, G.; Troost, F.; Jonkers, D.; Koenen, M.E.; Venema, K. Prebiotic Effects of Cassava Bagasse in TNO’s in Vitro Model of the Colon in Lean versus Obese Microbiota. J. Funct. Foods 2014, 11, 210–220. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Chaves, N.S.G.; Janner, D.E.; Poetini, M.R.; Fernandes, E.J.; De Almeida, F.P.; Musachio, E.A.S.; Reginaldo, J.C.; Dahleh, M.M.M.; De Carvalho, A.S.; Leimann, F.V.; et al. β-Carotene-Loaded Nanoparticles Protect against Neuromotor Damage, Oxidative Stress, and Dopamine Deficits in a Model of Parkinson’s Disease in Drosophila Melanogaster. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 268, 109615. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Gutiérrez, M.T.; Serrano-García, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chang, C.; Lai, G. Reactive Oxygen Species Scavenging Activities in a Chemiluminescence Model and Neuroprotection in Rat Pheochromocytoma Cells by Astaxanthin, Beta-carotene, and Canthaxanthin. Kaohsiung J. Med. Scie 2013, 29, 412–421. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Liu, G.; Kang, J.; Wang, B.; Wang, J.; Li, J.; Wang, H. Lutein Attenuated Methylglyoxal-induced Oxidative Damage and Apoptosis in PC12 Cells via the PI3K/Akt Signaling Pathway. J. Food Biochem. 2022, 46, e14382. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dilxat, T.; Shi, Q.; Qiu, T.; Lin, J. The Combination of Nicotinamide Mononucleotide and Lycopene Prevents Cognitive Impairment and Attenuates Oxidative Damage in D-Galactose Induced Aging Models via Keap1-Nrf2 Signaling. Gene 2022, 822, 146348. [Google Scholar] [CrossRef] [PubMed]
- Kulaberoglu, Y.; Gundogdu, R.; Hergovich, A. The Role of P53/P21/P16 in DNA-Damage Signaling and DNA Repair. In Genome Stability; Elsevier: Amsterdam, The Netherlands, 2016; pp. 243–256. ISBN 978-0-12-803309-8. [Google Scholar]
- Dutta, D.; Nayak, A.; Dutta, D. Reconnoitring the Usage of Agroindustrial Waste in Carotenoid Production for Food Fortification: A Sustainable Approach to Tackle Vitamin A Deficiency. Food Bioprocess. Technol. 2023, 16, 467–491. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y.; Chen, N.; Guo, J.; Kanaya, S.; Lange, K.M.; Li, S. Diet and Medical Foods in Parkinson’s Disease. Food Sci. Hum. Wellness 2019, 8, 83–95. [Google Scholar] [CrossRef]
- Barichella, M.; Garrì, F.; Caronni, S.; Bolliri, C.; Zocchi, L.; Macchione, M.C.; Ferri, V.; Calandrella, D.; Pezzoli, G. Vitamin D Status and Parkinson’s Disease. Brain Sci. 2022, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.; Yu, Y.; Shao, G.; Wang, Q. Vitamin D and Sunlight Exposure in Newly-Diagnosed Parkinson’s Disease. Nutrients 2016, 8, 142. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B. Serum 25-Hydroxyvitamin D Predicts Severity in Parkinson’s Disease Patients. Neurol. Sci. 2014, 35, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Sirajo, M.U.; Oyem, J.C.; Badamasi, M.I. Supplementation with Vitamins D3 and a Mitigates Parkinsonism in a Haloperidol Mice Model. J. Chem. Neuroanat. 2024, 135, 102366. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Arora, A.; Singla, R.K.; Sehgal, A.; Makeen, H.A.; Albratty, M.; Meraya, A.M.; Najmi, A.; Bungau, S.G. Understanding the Role of “Sunshine Vitamin D” in Parkinson’s Disease: A Review. Front. Pharmacol. 2022, 13, 993033. [Google Scholar] [CrossRef] [PubMed]
- Claro Da Silva, T.; Hiller, C.; Gai, Z.; Kullak-Ublick, G.A. Vitamin D 3 Transactivates the Zinc and Manganese Transporter SLC30A10 via the Vitamin D Receptor. J. Steroid Biochem. Mol. Biol. 2016, 163, 77–87. [Google Scholar] [CrossRef] [PubMed]
- FAO (Ed.) Contributing to Food Security and Nutrition for All; The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2016; ISBN 978-92-5-109185-2. [Google Scholar]
- Lithgow, D.; De La Lanza, G.; Silva, R. Ecosystem-Based Management Strategies to Improve Aquaculture in Developing Countries: Case Study of Marismas Nacionales. Ecol. Eng. 2019, 130, 296–305. [Google Scholar] [CrossRef]
- Wong, M.-H.; Mo, W.-Y.; Choi, W.-M.; Cheng, Z.; Man, Y.-B. Recycle Food Wastes into High Quality Fish Feeds for Safe and Quality Fish Production. Environ. Pollut. 2016, 219, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef] [PubMed]
- Håglin, L.; Johansson, I.; Forsgren, L.; Bäckman, L. Intake of Vitamin B before Onset of Parkinson’s Disease and Atypical Parkinsonism and Olfactory Function at the Time of Diagnosis. Eur. J. Clin. Nutr. 2017, 71, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Sandeep; Sahu, M.; Rani, L.; Kharat, A.; Mondal, A. Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease? Brain Sci. 2023, 13, 272. [Google Scholar] [CrossRef]
- Costantini, A.; Pala, M.I.; Compagnoni, L.; Colangeli, M. High-Dose Thiamine as Initial Treatment for Parkinson’s Disease. Case Rep. 2013, 2013, bcr2013009289. [Google Scholar] [CrossRef]
- Liu, D.; Ke, Z.; Luo, J. Thiamine Deficiency and Neurodegeneration: The Interplay Among Oxidative Stress, Endoplasmic Reticulum Stress, and Autophagy. Mol. Neurobiol. 2017, 54, 5440–5448. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tang, L.; Wei, W.; Hong, Y.; Chen, H.; Ying, W.; Chen, S. Nicotinamide Mononucleotide Improves Energy Activity and Survival Rate in an in Vitro Model of Parkinson’s Disease. Exp. Ther. Med. 2014, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Sadik, N.A.H.; Hamed, M.A.; Ali, S.A.; Khalil, W.K.B.; Ahmed, Y.R. Potential Therapeutic Effects of Antagonizing Adenosine A2A Receptor, Curcumin and Niacin in Rotenone-Induced Parkinson’s Disease Mice Model. Mol. Cell Biochem. 2020, 465, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Sebastián, A.; González-Robles, C.; García De Yébenes, J. Vitamin B6 Deficiency in Patients With Parkinson Disease Treated With Levodopa/Carbidopa. Clin. Neuropharm. 2020, 43, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.J.M.; Khemiri, S.; Simões, S.; Prista, C.; Sousa, I.; Raymundo, A. The Importance, Prevalence and Determination of Vitamins B6 and B12 in Food Matrices: A Review. Food Chem. 2023, 426, 136606. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Associations between B Vitamins and Parkinson’s Disease. Nutrients 2015, 7, 7197–7208. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Said-Al Ahl, H.A.H.; Bratovcic, A.; Tkachenko, K.G.; Sharifi-Rad, J.; Kačániová, M.; Elhourri, M.; Atanassova, M. Banana Peels: A Waste Treasure for Human Being. Evid.-Based Complement. Altern. Med. 2022, 2022, 7616452. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, Y.; Wei, W.; Zhao, W.; Lu, F.; Liu, F. Vitamin B12 Inhibits α-Synuclein Fibrillogenesis and Protects against Amyloid-Induced Cytotoxicity. Food Funct. 2019, 10, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Temova Rakuša, Ž.; Roškar, R.; Hickey, N.; Geremia, S. Vitamin B12 in Foods, Food Supplements, and Medicines—A Review of Its Role and Properties with a Focus on Its Stability. Molecules 2022, 28, 240. [Google Scholar] [CrossRef]
- McCarter, S.J.; Stang, C.; Turcano, P.; Mielke, M.M.; Ali, F.; Bower, J.H.; Savica, R. Higher Vitamin B12 Level at Parkinson’s Disease Diagnosis Is Associated with Lower Risk of Future Dementia. Park. Relat. Disord. 2020, 73, 19–22. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Yang, N.; Xin, C.; Li, Z.; Xu, J.; Ma, B.; Lim, K.-L.; Li, L.; Wu, Q.; et al. Vitamin B12 Ameliorates the Pathological Phenotypes of Multiple Parkinson’s Disease Models by Alleviating Oxidative Stress. Antioxidants 2023, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Langston, J.W. The MPTP Story. J. Park. Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.F.; De Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Tavan, M.; Hanachi, P.; De La Luz Cádiz-Gurrea, M.; Segura Carretero, A.; Mirjalili, M.H. Natural Phenolic Compounds with Neuroprotective Effects. Neurochem. Res. 2024, 49, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Kim, S.R. Beneficial Effects of Flavonoids Against Parkinson’s Disease. J. Med. Food 2018, 21, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, M.; Takemoto, H. Synthesis of Theaflavins and Their Functions. Molecules 2018, 23, 918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Suen, C.L.-C.; Yang, C.; Quek, S.Y. Antioxidant Capacity and Major Polyphenol Composition of Teas as Affected by Geographical Location, Plantation Elevation and Leaf Grade. Food Chem. 2018, 244, 109–119. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Moon, G.-H.; Shim, D.; Kim, J.C.; Lee, K.-J.; Chung, K.-H.; An, J.H. Neuroprotective Effects of Fermented Tea in MPTP-Induced Parkinson’s Disease Mouse Model via MAPK Signaling-Mediated Regulation of Inflammation and Antioxidant Activity. Food Res. Int. 2023, 164, 112133. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Liu, Y.; Xue, N.-J.; Zheng, R.; Yan, Y.-Q.; Wang, Z.-X.; Li, Y.-L.; Ying, C.-Z.; Song, Z.; Tian, J.; et al. Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson’s Disease by Inhibiting Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 7769355. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Li, H.; Liu, J.; Zhang, P.; Cheng, Y.; Qin, X.; Hu, Y.; Wei, Y. The Neuroprotective Effects of Isoquercitrin Purified from Apple Pomace by High-Speed Countercurrent Chromatography in the MPTP Acute Mouse Model of Parkinson’s Disease. Food Funct. 2021, 12, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Long, T.; Wu, Q.; Wei, J.; Tang, Y.; He, Y.-N.; He, C.-L.; Chen, X.; Yu, L.; Yu, C.-L.; Law, B.Y.-K.; et al. Ferulic Acid Exerts Neuroprotective Effects via Autophagy Induction in C. Elegans and Cellular Models of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. New By-products Rich in Bioactive Substances from the Olive Oil Mill Processing. J. Sci. Food Agric. 2018, 98, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Du, Z.-R.; Liu, X.; Wang, X.; Li, C.; Zhou, S.-N.; Liu, J.-R.; Xu, P.-Y.; Ye, J.-L.; Zhao, Q.; et al. Low and High Doses of Oral Maslinic Acid Protect against Parkinson’s Disease via Distinct Gut Microbiota-Related Mechanisms. Biomed. Pharmacother. 2023, 165, 115100. [Google Scholar] [CrossRef] [PubMed]
- Lofrumento, D.D.; Nicolardi, G.; Cianciulli, A.; Nuccio, F.D.; Pesa, V.L.; Carofiglio, V.; Dragone, T.; Calvello, R.; Panaro, M.A. Neuroprotective Effects of Resveratrol in an MPTP Mouse Model of Parkinson’s-like Disease: Possible Role of SOCS-1 in Reducing pro-Inflammatory Responses. Innate Immun. 2014, 20, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory Effects of Resveratrol on Endoplasmic Reticulum Stress-Associated Apoptosis and Oxido-Inflammatory Markers in a Rat Model of Rotenone-Induced Parkinson’s Disease. Chem.-Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef]
- Ramazani, E.; Ebrahimpour, F.; Emami, S.A.; Shakeri, A.; Javadi, B.; Sahebkar, A.; Tayarani-Najaran, Z. Neuroprotective Effects of Sesamum Indicum, Sesamin and Sesamolin against 6-OHDA-Induced Apoptosis in PC12 Cells. Recent Patents Food Nutr. Agric. 2023, 14, 126–133. [Google Scholar] [CrossRef]
- Ben Youssef, S.; Brisson, G.; Doucet-Beaupré, H.; Castonguay, A.-M.; Gora, C.; Amri, M.; Lévesque, M. Neuroprotective Benefits of Grape Seed and Skin Extract in a Mouse Model of Parkinson’s Disease. Nutr. Neurosci. 2021, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A Fascinating Antioxidant of Protective Properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, F.; Liang, W.; Huang, K.; Jia, C.; Zhang, J.; Li, X.; Wei, W.; Gong, R.; Chen, J. Integrated Insight into the Molecular Mechanisms of Selenium-Modulated, MPP+-Induced Cytotoxicity in a Parkinson’s Disease Model. J. Trace Elem. Med. Biol. 2023, 79, 127208. [Google Scholar] [CrossRef] [PubMed]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in Soils, Water and Food Crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: An Antioxidant and Anti-Inflammatory Agent: Role of Zinc in Degenerative Disorders of Aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef]
- Mbiydzenyuy, N.E.; Ninsiima, H.I.; Valladares, M.B.; Pieme, C.A. Zinc and Linoleic Acid Pre-Treatment Attenuates Biochemical and Histological Changes in the Midbrain of Rats with Rotenone-Induced Parkinsonism. BMC Neurosci. 2018, 19, 29. [Google Scholar] [CrossRef]
- Dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; Do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent Updates on Bioactive Properties of Linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef]
- Park, H.; Seol, G.H.; Ryu, S.; Choi, I.-Y. Neuroprotective Effects of (−)-Linalool against Oxygen-Glucose Deprivation-Induced Neuronal Injury. Arch. Pharm. Res. 2016, 39, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Migheli, R.; Lostia, G.; Galleri, G.; Rocchitta, G.; Serra, P.A.; Bassareo, V.; Acquas, E.; Peana, A.T. Neuroprotective Effect of (R)-(-)-Linalool on Oxidative Stress in PC12 Cells. Phytomed. Plus 2021, 1, 100073. [Google Scholar] [CrossRef]
- Geraci, A.; Di Stefano, V.; Di Martino, E.; Schillaci, D.; Schicchi, R. Essential Oil Components of Orange Peels and Antimicrobial Activity. Nat. Prod. Res. 2017, 31, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Caffeine: An Overview of Its Beneficial Effects in Experimental Models and Clinical Trials of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 4766. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Di Luca, D.G.; Orrú, M.; Xu, Y.; Chen, J.-F.; Schwarzschild, M.A. Neuroprotection by Caffeine in the MPTP Model of Parkinson’s Disease and Its Dependence on Adenosine A2A Receptors. Neuroscience 2016, 322, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Paudel, Y.N.; Papageorgiou, S.G.; Piperi, C. Elucidating the Beneficial Effects of Ginger (Zingiber officinale Roscoe) in Parkinson’s Disease. ACS Pharmacol. Transl. Sci. 2022, 5, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Bae, W.; Park, C.; Jeong, J. Zingerone Activates VMAT2 during MPP+-induced Cell Death. Phytother. Res. 2015, 29, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Pifl, C.; Rajput, A.; Reither, H.; Blesa, J.; Cavada, C.; Obeso, J.A.; Rajput, A.H.; Hornykiewicz, O. Is Parkinson’s Disease a Vesicular Dopamine Storage Disorder? Evidence from a Study in Isolated Synaptic Vesicles of Human and Nonhuman Primate Striatum. J. Neurosci. 2014, 34, 8210–8218. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Muñoz-Garcia, M.; Godos, J.; Martinez-Gonzalez, M.A. Dietary Patterns and Cognitive Decline: Key Features for Prevention. Curr. Pharm. Des. 2019, 25, 2428–2442. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of Mediterranean Diet with Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef]
- Szczuko, M.; Migrała, R.; Drozd, A.; Banaszczak, M.; Maciejewska, D.; Chlubek, D.; Stachowska, E. Role of Water Soluble Vitamins in the Reduction Diet of an Amateur Sportsman. Open Life Sci. 2018, 13, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Pinto, A.; Acuña, A.; Beltrán, F.; Torres-Díaz, L.; Castro, M. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef] [PubMed]
- Gvozdjáková, A.; Kucharská, J.; Rausová, Z.; Lopéz-Lluch, G.; Navas, P.; Palacka, P.; Bartolčičová, B.; Sumbalová, Z. Effect of Vaccination on Platelet Mitochondrial Bioenergy Function of Patients with Post-Acute COVID-19. Viruses 2023, 15, 1085. [Google Scholar] [CrossRef] [PubMed]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, Tocopherol, and Retinol Concentrations in Elderly Human Brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar] [PubMed]
- Choi, Y.-J.; Kwon, J.-W.; Jee, D. The Relationship between Blood Vitamin A Levels and Diabetic Retinopathy: A Population-Based Study. Sci. Rep. 2024, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D Deficiency 2.0: An Update on the Current Status Worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Spector, R. Niacin and niacinamide transport in the central nervous system. in vivo studies. J. Neurochem. 1979, 33, 895–904. [Google Scholar] [CrossRef]
- Hsu, J.M.; Kawin, B.; Minor, P.; Mitchell, J.A. Vitamin B12 Concentrations in Human Tissues. Nature 1966, 210, 1264–1265. [Google Scholar] [CrossRef]
- Torabian, S.; Haddad, E.; Rajaram, S.; Banta, J.; Sabaté, J. Acute Effect of Nut Consumption on Plasma Total Polyphenols, Antioxidant Capacity and Lipid Peroxidation. J. Hum. Nutr. Diet. 2009, 22, 64–71. [Google Scholar] [CrossRef]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef]
- Smith, L.D.; Garg, U. Chapter 17—Disorders of Trace Metals. In Biomarkers in Inborn Errors of Metabolism; Clinical Aspects and Laboratory Determination; Garg, U., Smith, L.D., Eds.; Elsevier: San Diego, CA, USA, 2017; pp. 399–426. ISBN 978-0-12-802896-4. [Google Scholar]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Anatomical Regional Differences in Selenium Levels in the Human Brain. Biol. Trace Elem. Res. 2015, 163, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Buxaderas, S.C.; Farré-Rovira, R. Whole Blood and Serum Zinc Levels in Relation to Sex and Age. Rev. Esp. Fisiol. 1985, 41, 463–470. [Google Scholar] [PubMed]
- Wang, B.; Fang, T.; Chen, H. Zinc and Central Nervous System Disorders. Nutrients 2023, 15, 2140. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean Diet Adherence Is Related to Reduced Probability of Prodromal Parkinson’s Disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Elhefian, E.A.; Sulayman, L.A.; Emkebish, A.S.; Alfalah, N.M. Estimation of Vitamin C in Selected Fruits and Vegetables Commonly Consumed in Sabratha, Northwestern Libya. Int. J. Mod. Sci. Technol. 2019, 4, 148–151. [Google Scholar]
- Chabi, I.B.; Zannou, O.; Dedehou, E.S.C.A.; Ayegnon, B.P.; Oscar Odouaro, O.B.; Maqsood, S.; Galanakis, C.M.; Pierre Polycarpe Kayodé, A. Tomato Pomace as a Source of Valuable Functional Ingredients for Improving Physicochemical and Sensory Properties and Extending the Shelf Life of Foods: A Review. Heliyon 2024, 10, e25261. [Google Scholar] [CrossRef] [PubMed]
- Chitchumroonchokchai, C.; Diretto, G.; Parisi, B.; Giuliano, G.; Failla, M.L. Potential of Golden Potatoes to Improve Vitamin A and Vitamin E Status in Developing Countries. PLoS ONE 2017, 12, e0187102. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Lācis, G.; Mišina, I.; Ikase, L. Tocopherols in Cultivated Apple Malus Sp. Seeds: Composition, Variability and Specificity. Plants 2023, 12, 1169. [Google Scholar] [CrossRef]
- Álvarez, R.; Araya, H.; Navarro-Lisboa, R.; de Dicastillo, C.L. Evaluation of Polyphenols and Antioxidant Capacity of Fruits and Vegetables Using a Modified Enzymatic Extraction Method. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Venkateswarlu, K.; Megharaj, M. Examining the Polyphenol Content, Antioxidant Activity and Fatty Acid Composition of Twenty-One Different Wastes of Fruits, Vegetables, Oilseeds and Beverages. SN Appl. Sci. 2020, 2, 673. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Brennan, A.; Browne, S. Food Waste and Nutrition Quality in the Context of Public Health: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 5379. [Google Scholar] [CrossRef] [PubMed]
- Majerska, J.; Michalska, A.; Figiel, A. A Review of New Directions in Managing Fruit and Vegetable Processing By-Products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
| Antioxidants | Exogenous Antioxidants’ Levels in Blood | Exogenous Antioxidants’ Levels in Brain | Ref. |
|---|---|---|---|
| Vitamin C | 0.977 ± 0.086 ng/μL | 1–10 mM | [115,116] |
| Vitamin E | (α-tocopherol) 34.9 ± 2.84 µmol/L | (Tocopherols isomers) 0.11–17.9 nmol/g | [117,118] |
| Vitamin A | 0.3–0.6 mg/L | 87.8–163.3 pmol/g | [118,119] |
| Carotenoids | Β-carotene 0.327 ± 0.054 | 1.8–23.0 pmol/g | [117,118] |
| Vitamin D | 50 nmol/L (20 ng/mL) | Not found | [120] |
| Thiamine (B1) | 679.3 ± 188.7 ng/μL | Not found | [115] |
| Niacin (B3) | 0.260 ± 0.053 ng/μL | 0.5 mmol/kg | [115,121] |
| Pyridoxine (B6) | 0.368 ± 0.201 ng/μL | Not found | [115] |
| Vitamin B12 | 0.405 ± 0.291 ng/μL | 0.027 ± 0.013 pg/g wet tissues | [115,122] |
| Polyphenols | 171.6 ± 3.1–208.4 ± 2.45 (mg L−1) GAE | 1 nmol/g of brain tissue | [123,124] |
| Selenium | 60–150 ng/mL | 552–1435 ng/g | [125,126] |
| Zinc | 585.2–607.0 µg/100 mL | 150 µmol/L | [127,128] |
| Antioxidant | Food | Antioxidant Content in Food | Food Waste | Antioxidant Content in Food Waste | Ref. |
|---|---|---|---|---|---|
| Ascorbic acid (mg/100g) | Orange | 82.34 | Peel | 110.4 | [25,131,132] |
| Lemon | 58.12 | Peel | 58.59 | ||
| Tomato | 5.71–101.29 | Peel | 110.00 | ||
| Seeds | 9.50 | ||||
| Vitamin A | Potato | 91 μg/g dry weight | Peel | 0.24 ± 0.03 mg/L | [39,133] |
| Tocopherols | Apple | 4.54–18.56 mg/100 g dry weight | Seeds | 1.391–1.811 µg/g | [134] |
| Vitamin B3/Niacin | Potato | 1035–1573 μg/100 g | Peel | 3.25 ± 0.20 mg/L | [39,61] |
| Vitamin B6 | Potato | 0.16 mg/100 g | Peel | 0.55 ± 0.21 mg/L | [39,69] |
| Polyphenols | Strawberry | 422 ± 15 mg/100 mg of fresh mass | Pomace | 24.4 mg gallic acid/g dry sample | [135,136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannas, C.; Lostia, G.; Serra, P.A.; Peana, A.T.; Migheli, R. Food and Food Waste Antioxidants: Could They Be a Potent Defence against Parkinson’s Disease? Antioxidants 2024, 13, 645. https://doi.org/10.3390/antiox13060645
Cannas C, Lostia G, Serra PA, Peana AT, Migheli R. Food and Food Waste Antioxidants: Could They Be a Potent Defence against Parkinson’s Disease? Antioxidants. 2024; 13(6):645. https://doi.org/10.3390/antiox13060645
Chicago/Turabian StyleCannas, Claudia, Giada Lostia, Pier Andrea Serra, Alessandra Tiziana Peana, and Rossana Migheli. 2024. "Food and Food Waste Antioxidants: Could They Be a Potent Defence against Parkinson’s Disease?" Antioxidants 13, no. 6: 645. https://doi.org/10.3390/antiox13060645






