Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity
Abstract
1. Introduction
2. Materials and Methods
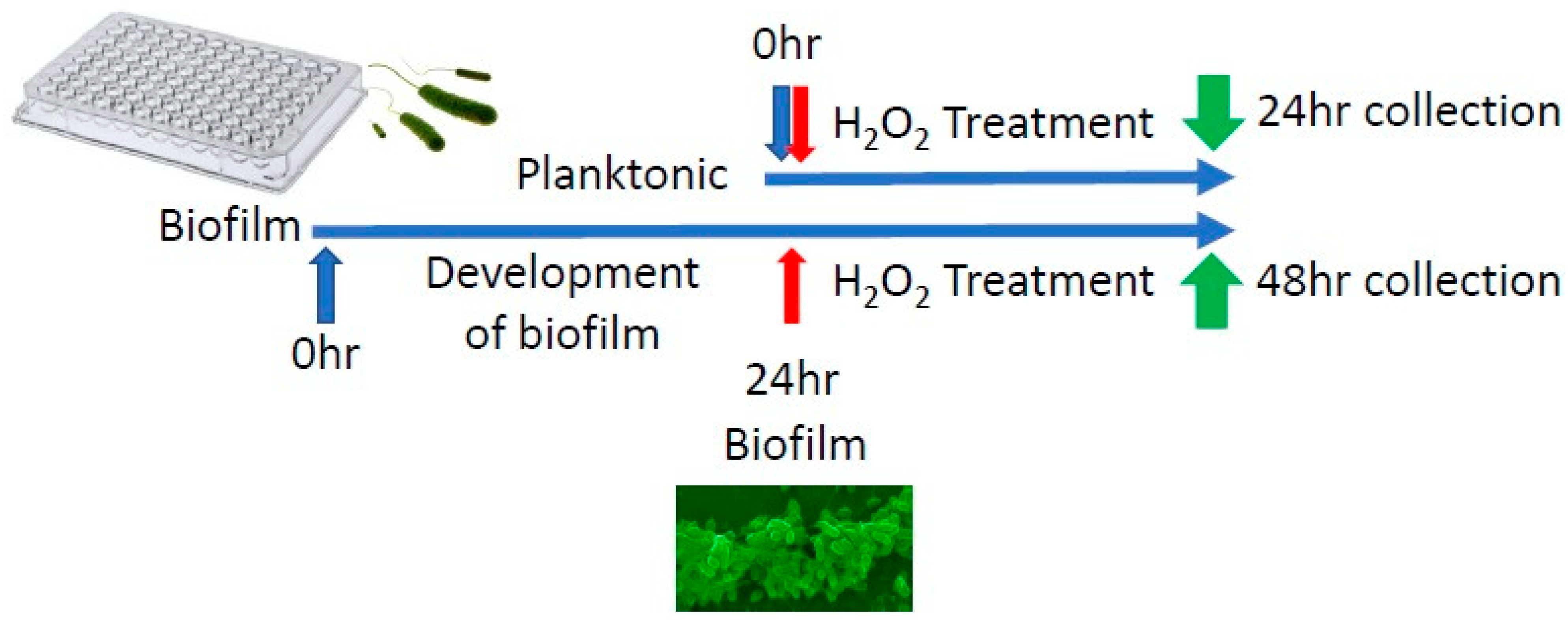
2.1. Pseudomonas aeruginosa’s Isolation, Growth and Treatment In Vitro
2.2. Chronic Wounds Caused by Infection with RPA
2.3. RNA Collection from Mouse Wounds
2.4. RNA Extraction, DNase1 Treatment and RNA Cleanup
2.5. Reverse Transcription and Quantitative Polymerase Chain Reaction
2.6. RNA Sequencing
2.7. Sequencing Genomic DNA and Annotations
2.8. Bioinformatic Analysis
3. Results
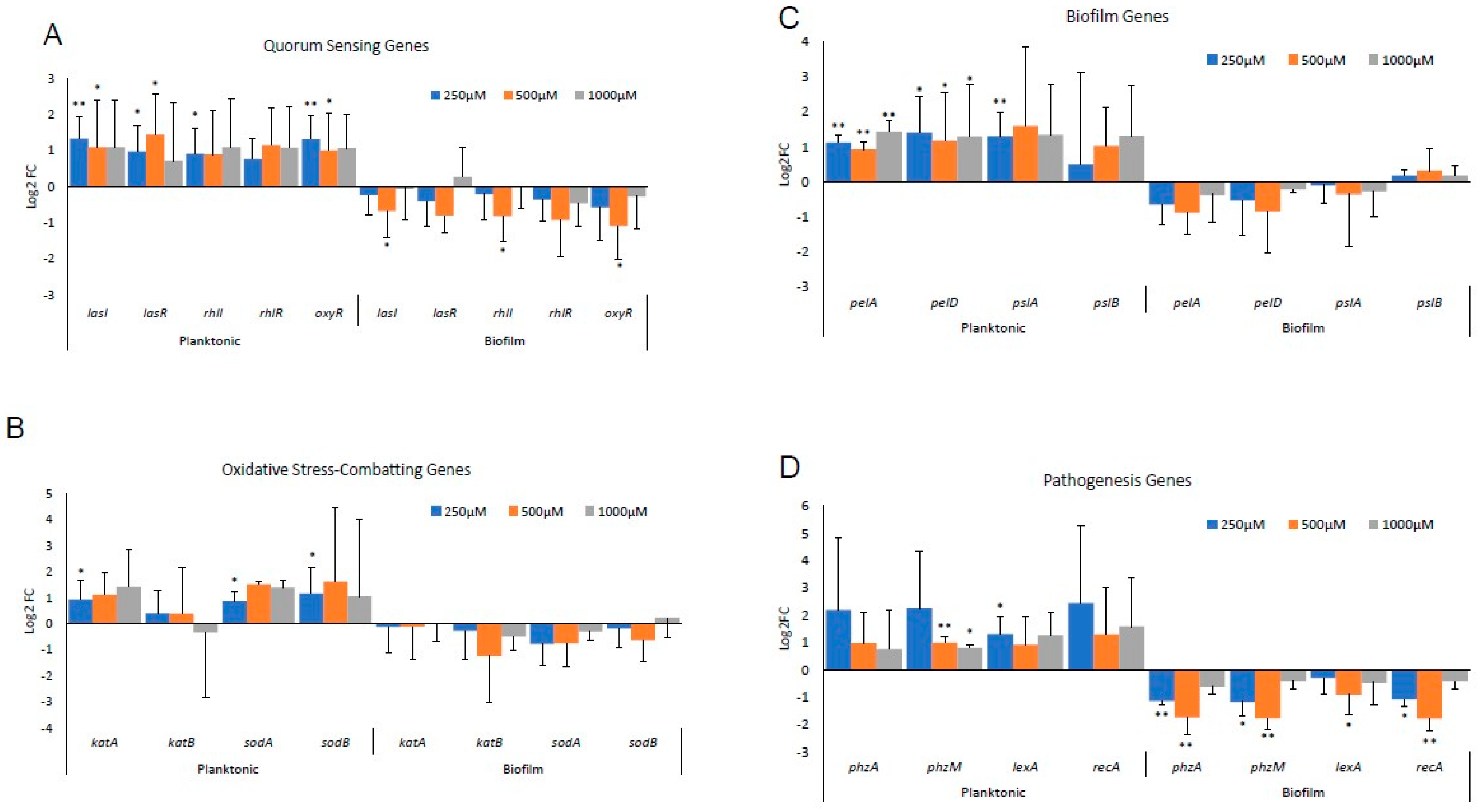
3.1. Differential Gene Expression In Vitro between Biofilm-Forming PA and PA Already in a Biofilm
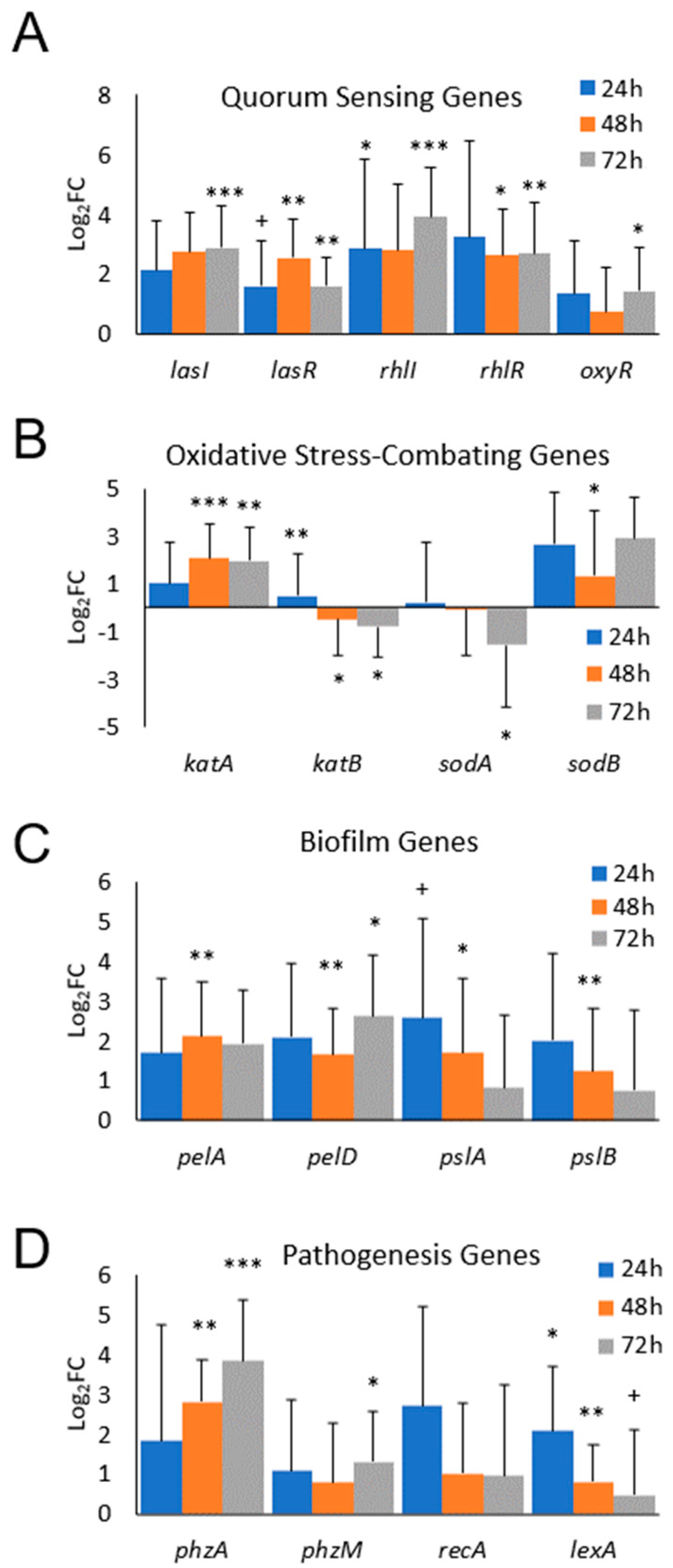
3.2. Differential Gene Expression of Biofilm-Forming PA In Vivo
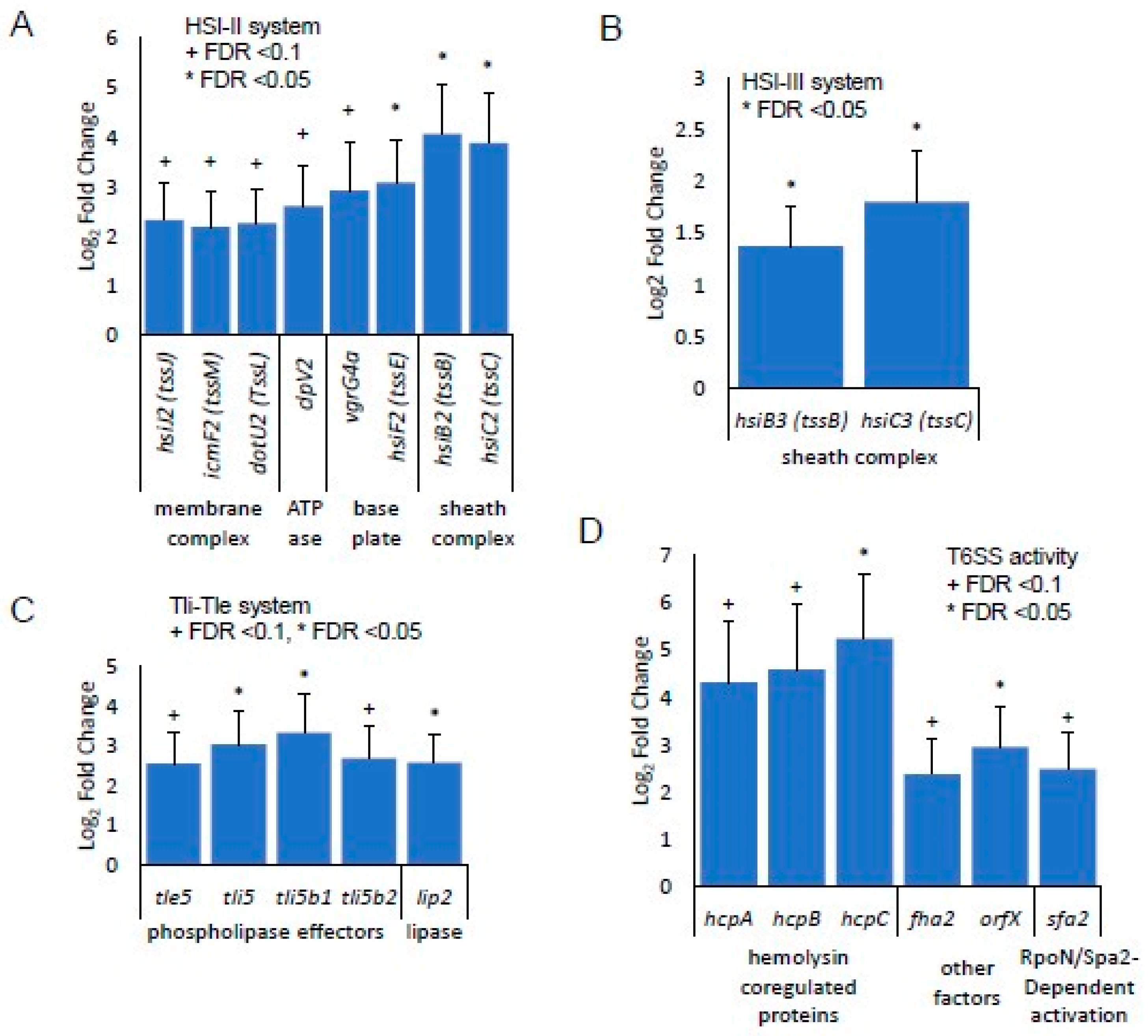
3.3. Identification of Gene Expression in RPA-Induced Biofilm In Vivo by RNA-seq Detection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.H.H.; Ahamed, A.; Chen, K.; Lebig, E.G.G.; Petros, B.; Saeed, S.; Martins-Green, M. Skin microbiota and its role in health and disease with an emphasis on wound healing and chronic wound development. In Microbiome, Immunity, Digestive Health and Nutrition: Epidemiology, Pathophysiology, Prevention and Treatment; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Loomis, K.H.; Wu, S.K.; Ernlund, A.; Zudock, K.; Reno, A.; Blount, K.; Karig, D.K. A mixed community of skin microbiome representatives influences cutaneous processes more than individual members. Microbiome 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Biswas, S.; Shang, Y.; Collard, E.; Azad, A.; Kauh, C.; Bhasker, V.; Gordillo, G.M.; Sen, C.K.; Roy, S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 2010, 5, e9539. [Google Scholar] [CrossRef] [PubMed]
- Flowers, L.; Grice, E.A. The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 2020, 28, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Egozi, E.I.; Ferreira, A.M.; Burns, A.L.; Gamelli, R.L.; Dipietro, L.A. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair Regen. 2003, 11, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Peus, D.; Achterberg, V.; Meyer-Ingold, W.; Scharffetter-Kochanek, K. Protease inhibitors protect growth factor activity in chronic wounds. Br. J. Dermatol. 1997, 137, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The role of chemokines in wound healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.M.; Werner, S.; SCHAFER, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E.; Zhao, G.; Lippman, S.I.; James, G.A.; et al. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity–monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to chronic wound infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Phalak, P.; Chen, J.; Carlson, R.P.; Henson, M.A. Metabolic modeling of a chronic wound biofilm consortium predicts spatial partitioning of bacterial species. BMC Syst. Biol. 2016, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.S.; Franklin, M.J.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, M.; Steinberg, D.; Meshner, S. Microbial biofilms and the human skin microbiome. npj Biofilms Microbiomes 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. 2010, 18, 467–477. [Google Scholar] [CrossRef]
- Nagoba, B.S.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic Environment and Wound Healing: A Review. Wounds 2015, 27, 5–11. Available online: http://www.woundsresearch.com/article/acidic-environment-and-wound-healing-review (accessed on 15 September 2016).
- Harrington, N.E.; Sweeney, E.; Harrison, F. Building a better biofilm–Formation of in vivo-like biofilm structures by Pseudomonas aeruginosa in a porcine model of cystic fibrosis lung infection. Biofilm 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Withycombe, C.; Purdy, K.J.; Maddocks, S.E. Micro-management: Curbing chronic wound infection. Mol. Oral Microbiol. 2017, 32, 263–274. [Google Scholar] [CrossRef] [PubMed]
- El-Khashaab, T.H.; Erfan, D.M.; Kamal, A. Pseudomonas aeruginosa Biofilm Formation and Quorum Sensing lasR Gene in Patients with Wound Infection. Egypt. J. Med. Microbiol. 2016, 25, 101–108. [Google Scholar] [CrossRef]
- Olejnickova, K.; Hola, V.; Ruzicka, F. Virulence factors of Pseudomonas aeruginosa strains isolated from catheterized patients. Clin. Microbiol. Infect. 2010, 16, S569. Available online: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L70196521%5Cnhttp://dx.doi.org/10.1111/j.1469-0691.2010.03239.x%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=1198743X&id=doi:10.1111/j.1469-0691.2010.03239.x&atitle=Virulence+ (accessed on 28 October 2019).
- Caldwell, C.C.; Chen, Y.; Goetzmann, H.S.; Hao, Y.; Borchers, M.T.; Hassett, D.J.; Young, L.R.; Mavrodi, D.; Thomashow, L.; Lau, G.W. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am. J. Pathol. 2009, 175, 2473–2488. [Google Scholar] [CrossRef] [PubMed]
- Stedfeld, R. How To Build a Better Bus. Mater. Eng. 1978, 88, 37–41. [Google Scholar]
- Arai, H. Regulation and Function of Versatile Aerobic and Anaerobic Respiratory Metabolism in Pseudomonas aeruginosa. Front. Microbiol. 2011, 2, 103. [Google Scholar] [CrossRef] [PubMed]
- Spero, M.A.; Newman, D.K. Chlorate Specifically Targets Oxidant-Starved, Antibiotic-Tolerant Populations of Pseudomonas aeruginosa Biofilms. mBio 2018, 9, e01400-18. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvarajah, S.; Mathioudakis, N.; Sherman, R.L.; Hines, K.F.; Black, J.H.; Abularrage, C.J. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs. Ann. Vasc. Surg. 2016, 33, 149–158. [Google Scholar] [CrossRef] [PubMed]
- MacWilliams, M.P.; Liao, M. Luria Broth (LB) and Luria Agar (LA) Media and Their Uses Protocol; ASM: Washington, DC, USA, 2006; Available online: https://asm.org/getattachment/5d82aa34-b514-4d85-8af3-aeabe6402874/LB-Luria-Agar-protocol-3031.pdf (accessed on 3 April 2024).
- Kim, J.H.; Spero, M.; Lebig, E.G.; Lonergan, Z.R.; Trindade, I.B.; Newman, D.K.; Martins-Green, M. Targeting Anaerobic Respiration in Pseudomonas aeruginosa with Chlorate Improves Healing of Chronic Wounds. Adv. Wound Care 2023, 13, 53–69. [Google Scholar] [CrossRef]
- Kim, J.H.; Ruegger, P.R.; Lebig, E.G.; VanSchalkwyk, S.; Jeske, D.R.; Hsiao, A.; Borneman, J.; Martins-Green, M. High Levels of Oxidative Stress Create a Microenvironment That Significantly Decreases the Diversity of the Microbiota in Diabetic Chronic Wounds and Promotes Biofilm Formation. Front. Cell. Infect. Microbiol. 2020, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Martins-Green, M. Protocol to Create Chronic Wounds in Diabetic Mice. J. Vis. Exp. 2019, 2019, e57656. [Google Scholar] [CrossRef]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [PubMed]
- Savli, H.; Karadenizli, A.; Kolayli, F.; Gundes, S.; Ozbek, U.; Vahaboglu, H. Expression stability of six housekeeping genes: A proposal for resistance gene quantification studies of Pseudomonas aeruginosa by real-time quantitative RT-PCR. J. Med. Microbiol. 2003, 52, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. metaSPAdes: A new versatile metagenomic assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef] [PubMed]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Siguier, P. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. In Gene Prediction: Methods and Protocols; Humana: New York, NY, USA, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Edgar, R.C. PILER-CR: Fast and accurate identification of CRISPR repeats. BMC Bioinform. 2007, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Casadio, R. DeepSig: Deep learning improves signal peptide detection in proteins. Bioinformatics 2018, 34, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Nawrocki, E.P.; Eddy, S.R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 2013, 29, 2933–2935. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic Analysis by Maximum Likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.R.; Abresch, H.E.; Ulrich, N.J.; Sano, E.B.; Demaree, A.H.; Oman, A.R.; Garber, A.I. Bacterial adaptation by a transposition burst of an invading IS element. Genome Biol. Evol. 2021, 13, evab245. [Google Scholar] [CrossRef] [PubMed]
- Garber, A. BagOfTricks. GitHub Repos. 2021. Available online: https://github.com/Arkadiy-Garber/BagOfTricks (accessed on 18 February 2023).
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Krueger, F. Trim Galore. A Wrapper Tool Around Cutadapt and FastQC to Consistently Apply Quality and Adapter Trimming to FastQ Files; Babraham Institute: Cambridge, UK, 2015. [Google Scholar]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef] [PubMed]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Stover, C.K.; Pham, X.Q.; Erwin, A.L.; Mizoguchi, S.D.; Warrener, P.; Hickey, M.J.; Brinkman, F.S.L.; Hufnagle, W.O.; Kowalik, D.J.; Lagrou, M.; et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000, 406, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, G.B.; Marmont, L.S.; Ostaszewski, A.; Rich, J.D.; Whitney, J.C.; Parsek, M.R.; Harrison, J.J.; Howell, P.L. Pel Polysaccharide Biosynthesis Requires an Inner Membrane Complex Comprised of PelD, PelE, PelF, and PelG. J. Bacteriol. 2020, 202, e00684-19. [Google Scholar] [CrossRef]
- Yeoh-Ellerton, S.; Stacey, M.C. Iron and 8-Isoprostane Levels in Acute and Chronic Wounds. J. Investig. Dermatol. 2003, 121, 918–925. [Google Scholar] [CrossRef]
- Wright, J.A.; Richards, T.; Srai, S.K.S. The role of iron in the skin and cutaneous wound healing. Front. Pharmacol. 2014, 5, 156. [Google Scholar] [CrossRef]
- Wilderman, P.J.; Sowa, N.A.; FitzGerald, D.J.; FitzGerald, P.C.; Gottesman, S.; Ochsner, U.A.; Vasil, M.L. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 9792–9797. [Google Scholar] [CrossRef]
- Cavinato, L.; Genise, E.; Luly, F.R.; Di Domenico, E.G.; Del Porto, P.; Ascenzioni, F. Escaping the Phagocytic Oxidative Burst: The Role of SODB in the Survival of Pseudomonas aeruginosa Within Macrophages. Front. Microbiol. 2020, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Nizer, W.S.; Inkovskiy, V.; Versey, Z.; Strempel, N.; Cassol, E.; Overhage, J. Oxidative Stress Response in Pseudomonas aeruginosa. Pathogens 2021, 10, 1187. [Google Scholar] [CrossRef]
- Hassett, D.J.; Ma, J.-F.; Elkins, J.G.; McDermott, T.R.; Ochsner, U.A.; West, S.E.H.; Huang, C.-T.; Fredericks, J.; Burnett, S.; Stewart, P.S.; et al. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 1999, 34, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Panmanee, W.; Wilson, J.J.; Mahtani, H.K.; Li, Q.; VanderWielen, B.D.; Makris, T.M.; Rogers, M.; McDaniel, C.; Lipscomb, J.D.; et al. Catalase (KatA) Plays a Role in Protection against Anaerobic Nitric Oxide in Pseudomonas aeruginosa. PLoS ONE 2014, 9, e91813. [Google Scholar] [CrossRef]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef]
- O’Malley, Y.Q.; Reszka, K.J.; Rasmussen, G.T.; Abdalla, M.Y.; Denning, G.M.; Britigan, B.E. The Pseudomonas secretory product pyocyanin inhibits catalase activity in human lung epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L1077–L1086. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, Y.Q.; Reszka, K.J.; Spitz, D.R.; Denning, G.M.; Britigan, B.E. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2004, 287, L94–L103. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Phillips, G.; Thomashow, L.S. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [CrossRef] [PubMed]
- Filloux, A.; Hachani, A.; Bleves, S. The bacterial type VI secretion machine: Yet another player for protein transport across membranes. Microbiology 2008, 154, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yam, J.K.H.; Cai, Z.; Ding, Y.; Zhang, L.-H.; Deng, Y.; Yang, L. Population dynamics and transcriptomic responses of Pseudomonas aeruginosa in a complex laboratory microbial community. npj Biofilms Microbiomes 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Potvin, E.; Lehoux, D.E.; Kukavica-Ibrulj, I.; Richard, K.L.; Sanschagrin, F.; Lau, G.W.; Levesque, R.C. In vivo functional genomics of Pseudomonas aeruginosa for high-throughput screening of new virulence factors and antibacterial targets. Environ. Microbiol. 2003, 5, 1294–1308. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.G.; Hachani, A.; Bucior, I.; Soscia, C.; Garvis, S.; Termine, E.; Engel, J.; Filloux, A.; Bleves, S. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and fur and modulates internalization in epithelial cells. J. Biol. Chem. 2012, 287, 27095–27105. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer | Reverse Primer | Gene Accession Number |
|---|---|---|---|
| sodA | CAACCACTCGCTGTTCTGGA | CTTGGTGAACGCATCCTTGAAC | PA4468 |
| sodB | AACACCTACGTGGTGAACCTGA | TGACGATCTCTTCGAGGCTCTT | PA4366 |
| katA | GAACAGCTTCAACCAGTGGCAG | CTCGTCGGTGAACAGATGGAAC | PA4236 |
| katB | CGCTTCGATTTCTTCTCCCACG | CTTGTAGGCATGCACGCTGTTG | PA4613 |
| lasI | GCCCCTACATGCTGAAGAACAC | CCTCCAGCGTACAGTCGGAAAA | PA1432 |
| lasR | ATGGCCTTGGTTGACGGTTTTC | CCTAAGGACAGCCAGGACTACG | PA1430 |
| rhlR | CCTCGGAAATGGTGGTCTGGAG | GGAAAGCACGCTGAGCAAATTG | PA3477 |
| rhlI | CGACCAGGAATTCGACCAGTTC | GTTTCGCTGCACAGGTAGGC | PA3476 |
| pelA | AGTACTACGCGCCGATCATCAA | AAGTGGTAGTACAGGTGCAGGC | PA3064 |
| pelD | TGCCTGTATGCCTTCGAGTTGA | GAAGTCAGCGGCAACAACACC | PA3061 |
| pslA | GCTACAACAACCGGCTGATCTG | GATGCTGGTCTTGCGGATGAAG | PA2231 |
| pslB | CCTCAACACCAACGAATCCACC | CGTAGATGTCGTTGAAGCGGAC | PA2232 |
| recA | TCACCGGCAATATCAAGAACGC | GACCGAGGCGTAGAACTTCAGT | PA3617 |
| lexA | GCGAGGAGGTCACGGTGAAA | GCCTTCGATGATCAGTTCCTGC | PA3007 |
| proC | CAGGCCGGGCAGTTGCTGTC | GGTCAGGCGCGAGGCTGTCT | PA0393 |
| oxyR | CCGCTGTACATCGAGGAGAACT | ACATAGAAGGGCTCGTCGAACA | PA5344 |
| phzA1&2 | GACCGAGGATCCGAACCACTTC | CGTTTTATCCGGCCGTTCTCG | PA4210, PA1899 |
| phzM | GTGGCCTTCGAGATCTTCCAGG | GGAACTCCTCGCCGTAGAACAG | PA4209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Dong, J.; Le, B.H.; Lonergan, Z.R.; Gu, W.; Girke, T.; Zhang, W.; Newman, D.K.; Martins-Green, M. Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity. Antioxidants 2024, 13, 655. https://doi.org/10.3390/antiox13060655
Kim JH, Dong J, Le BH, Lonergan ZR, Gu W, Girke T, Zhang W, Newman DK, Martins-Green M. Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity. Antioxidants. 2024; 13(6):655. https://doi.org/10.3390/antiox13060655
Chicago/Turabian StyleKim, Jane H., Julianna Dong, Brandon H. Le, Zachery R. Lonergan, Weifeng Gu, Thomas Girke, Wei Zhang, Dianne K. Newman, and Manuela Martins-Green. 2024. "Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity" Antioxidants 13, no. 6: 655. https://doi.org/10.3390/antiox13060655
APA StyleKim, J. H., Dong, J., Le, B. H., Lonergan, Z. R., Gu, W., Girke, T., Zhang, W., Newman, D. K., & Martins-Green, M. (2024). Pseudomonas aeruginosa Activates Quorum Sensing, Antioxidant Enzymes and Type VI Secretion in Response to Oxidative Stress to Initiate Biofilm Formation and Wound Chronicity. Antioxidants, 13(6), 655. https://doi.org/10.3390/antiox13060655







