Melatonin Interaction with Other Phytohormones in the Regulation of Abiotic Stresses in Horticultural Plants
Abstract
:1. Introduction
2. Yield Losses in Horticultural Crops: A Threat to Food Security and Nutrition
3. Multifunctional Role of MT in Horticultural Plants
3.1. Stress Mitigation in Horticultural Crops: Impacts of MT
3.2. MT and Salinity Stress
3.3. MT and Drought Stress
3.4. MT and Cold Stress
3.5. MT and Heat Stress
3.6. MT and HMs Stress
4. Multifunctional Role of Phytohormone in Horticultural Plants
| Phytohormones | Stress | Vegetables | Key Findings | References |
|---|---|---|---|---|
| JA | Drought | Potato | Reduced relative leaf water potential was the result of StJAZ1 overexpression in plants. MDA was improved. JA is useful for lowering MDA production. and lipid peroxidation. | [129] |
| SA | Heat stress | Potato | An amount of 2, 3, 8, 14, and 21 dpi was applied on Chicago and Gala cultivars, resulting in improved physiological and biochemical processes | [130] |
| ABA | Salinity | Lettuce | A concentration of 0, 100, 200, 300, and 400 mg L−1 increased lettuce output and performance. | [46] |
| MT | Drought | Cucumber | A concentration of 100 μM suggestively enriched growth, yield, and defense mechanism. | [51] |
| SA | Drought | Tomato | A concentration of 10–5 M improved growth under 10 days and water holding capacity. | [131] |
| SA | Chilling injury | Sweet potato | By increasing plant antioxidant capacity, SA treatment reduced the likelihood of chilling harm. | [132] |
| Osmoprotectants | Drought | Tomato | A total of 50–57% of the field capacity also increased the level of salts in plants. Germination was very poor in seeds. | [46] |
| SA | UV-radiation | Pea | A concentration of 0.4 mM enhances growth by improving the defense system. | [133] |
| Polyamines (PAs) | Salinity | Cucumber | The application of PAs such as spermidine can be controlled when harmful effects from 50 mM NaCl arise. | [47] |
| SA | HMs | Melon | The amount of Cd adsorption, excessive ROS production, the level of proline, the amount of protein, and the level of oxidative enzymes all decreased at the required 0.1 mM concentration. | [134] |
| SA | Chilling injury | Sponge gourd | A concentration of 1.5 mM L−1 was found to be more effective for the reduction of chilling injury under storage conditions. | [135] |
| SA | Heat stress | Cucumber | The plant defense system was strengthened by exogenous spray (1 mM) by lowering MDA, H2O2, and ROS. SA thereby boosted the amount of photosynthesis that was disturbed due to temperature extremities. | [128] |
| BRs | Salinity | Peppermint | Antioxidant enzyme system maintained cellular membrane integrity, and secondary metabolites generation. | [136] |
| Epibrassinolide | Cold stress | Cucumber | Better seedling health index, increased chlorophyll content, antioxidant enzyme activity, upregulated gene expression, and reduced oxidative stress level. | [137] |
| BRs | Salinity | Lettuce | Lessened the adverse effect of salinity by reduced oxidative injury and improved antioxidant enzyme action. | [138] |
| BRs | Cadmium | Radish | Improved seed germination level, proline meditation, antioxidant enzymes, and fresh seedling weight of radish. | [139] |
| Brassinolide | Lead | Radish | Reduced oxidative stress in plants growing under leaf toxicity. Moreover, antioxidant enzymes were also activated. | [140] |
| SA | Heat | Strawberry | Improved plant growth under stressful conditions. Better crop performance under heat stress. Activation of plant defense system. | [141] |
| SA | Boron toxicity | Watermelon | Better melon plant performance under boron toxicity. Regulation in the photosynthetic system of plants. Lessening of oxidative harms. | [142] |
| SA | Chromium | Tomato | Regulation of photosynthetic pigment under chromium toxicity. Improved plant growth by regulation of oxidative damage. | [143] |
| MT | Drought | Pea | Reduced oxidative stress in plants growing under water stress. Antioxidant enzymes were also activated. | [144] |
4.1. MT Interacts with Other Phytohormones in Horticultural Plants
4.2. MT and SA
4.3. MT and JAs
4.4. MT and BRs
4.5. MT and GABA
4.6. MT and PAs
5. MT Interact with PGRs in Horticultural Plants
5.1. MT and AUXs
5.2. MT and GA
5.3. MT and CKs
6. Conclusions and Future Concerns
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric acid-mediated abiotic stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Nguyen, H.M.; Seki, M. Advances in chemical priming to enhance abiotic stress tolerance in plants. Plant Cell Physiol. 2020, 61, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic stress in crop species: Improving tolerance by applying plant metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Cui, X.; Wang, K.; Wang, Y.; He, Y. Phytohormones regulate the abiotic stress: An overview of physiological, biochemical, and molecular responses in horticultural crops. Front. Plant Sci. 2023, 13, 095363. [Google Scholar] [CrossRef]
- Wang, L.; Tanveer, M.; Wang, H.; Arnao, M.B. Melatonin as a key regulator in seed germination under abiotic stress. J. Pineal Res. 2024, 76, e12937. [Google Scholar] [CrossRef]
- Huang, L.; Fu, W.; Zhang, Y.; Liu, X.; Wang, Q.; Wang, L.; Tanveer, M. The role of melatonin in regulating horticultural crop production under various abiotic stresses. Sci. Hortic. 2024, 323, 112508. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Lal, S.K.; Pawar, J.; Narayan, O.P. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene 2021, 25, 100264. [Google Scholar]
- Altaf, M.A.; Sharma, N.; Singh, J.; Samota, M.K.; Sankhyan, P.; Singh, B.; Kumar, A.; Naz, S.; Lal, M.K.; Tiwari, R.K.; et al. Mechanistic insights on melatonin-mediated plant growth regulation and hormonal cross-talk process in Solanaceous vegetables. Sci. Hortic. 2023, 308, 111570. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a regulator of plant ionic homeostasis: Implications for abiotic stress tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Bajguz, A.; Alam, P.; Hayat, S. Silicon mediated abiotic stress tolerance in plants using physio-biochemical, omic approach and cross-talk with phytohormones. Plant Physiol. Biochem. 2021, 166, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Basit, A.; Ahmad, I.; Mohamed, H.I.; Wasila, H. Effect of Salicylic Acid and Salinity Stress on the Performance of Tomato Plants. Gesunde Pflanz. 2020, 72, 393–402. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; Shu, H.; Mumtaz, M.A.; Cheng, S.; Alyemeni, M.N.; Ahmad, P.; Wang, Z. Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Kuppusamy, S.; Cho, K.M.; Kim, P.J.; Kwack, Y.B.; Lee, Y.B. Influence of cold stress on contents of soluble sugars, vitamin C and free amino acids including gamma-aminobutyric acid (GABA) in spinach (Spinacia oleracea). Food Chem. 2017, 215, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, G.; Yuan, M.; Guo, S.; Wang, Y.; Sun, J. CsbZIP2-miR9748-CsNPF4. 4 module mediates high temperature tolerance of cucumber through jasmonic acid pathway. Front. Plant Sci. 2022, 13, 883876. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, X.; Li, L.; Sun, Q.; Wang, Q.; Huang, H.; Tong, Z.; Zhang, J. Melatonin-mediated development and abiotic stress tolerance in plants. Front. Plant Sci. 2023, 14, 1100827. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin affects the photosynthetic performance of pepper (Capsicum annuum L.) seedlings under cold stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: Current status and future perspectives. Sustainability 2020, 13, 294. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Erland, L.A.; Murch, S.J.; Reiter, R.J.; Saxena, P.K. A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant Signal. Behav. 2015, 10, e1096469. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M. Melatonin and gibberellic acid promote growth and chlorophyll biosynthesis by regulating antioxidant and methylglyoxal detoxification system in tomato seedlings under salinity. J. Plant Growth Regul. 2020, 39, 1488–1502. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Exploring the synergistic effects of melatonin and salicylic acid in enhancing drought stress tolerance in tomato plants through fine-tuning oxidative-nitrosative processes and methylglyoxal metabolism. Sci. Hortic. 2023, 321, 112368. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin Suppression of Heat-Induced Leaf Senescence Involves Changes in Abscisic Acid and Cytokinin Biosynthesis and Signaling Pathways in Perennial Ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Shi, S.; Dou, F.; Song, Y.; Ma, F. Exogenous melatonin alleviates alkaline stress in Malus hupehensis Rehd. by regulating the biosynthesis of polyamines. Molecules 2017, 22, 1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Lin, W.; Jahan, M.S.; Wang, J.; Sun, J.; Jiang, J.; Gu, W.; Zou, J.; Shu, S.; et al. Foliar application of a mixture of putrescine, melatonin, proline, and potassium fulvic acid alleviates high temperature stress of cucumber plants grown in the greenhouse. Technol. Hortic. 2022, 2, 6. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.M.D.; Anjum, M.A.; Ahmad, R.; Ercisli, S. Foliar Application of Moringa and Mint Leaf Extracts Enhances Carnation Growth and Flower Yield Under Saline Conditions by Improving Plant Defense Mechanism. J. Plant Growth Regul. 2023, 1–11. [Google Scholar] [CrossRef]
- Sardar, H.; Bashir, M.M.; Naz, S.; Nawaz, A.; Ahmad, R.; Ejaz, S.; Ali, S.; Alhag, S.K.; Al-Shuraym, L.A. Melatonin Mitigates Adverse Effects of Salinity in Stevia Through Physiological and Biochemical Adjustments. Sci. Hortic. 2023, 322, 112390. [Google Scholar] [CrossRef]
- Kang, G.; Wang, C.; Sun, G.; Wang, Z. Salicylic Acid Changes Activities of H2O2-Metabolizing Enzymes and Increases the Chilling Tolerance of Banana Seedlings. Environ. Exp. Bot. 2003, 50, 9–15. [Google Scholar] [CrossRef]
- Ahmad, R.; Naz, S.; Muhammad, H.M.D.; Ahmad, I.; Tiwari, R.K.; Lal, M.K.; Altaf, M.A. Scenarios for Improvement of Jujube Tolerance against Drought with Molecular Tools. Erwerbs-Obstbau 2023, 65, 2615–2622. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Hajilou, J.; Tabatabaei, S.J. Photosynthetic and Growth Responses of Olive to Proline and Salicylic Acid Under Salinity Condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 579–585. [Google Scholar] [CrossRef]
- Sardar, H.; Nisar, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Ali, S.; Javed, M.S.; Ahmad, R. Foliar Spray of Moringa Leaf Extract Improves Growth and Concentration of Pigment, Minerals and Stevioside in Stevia (Stevia rebaudiana Bertoni). Ind. Crops Prod. 2021, 166, 113485. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Lian, X.; Zhao, X.; Zhao, Q.; Wang, G.; Li, Y.; Hao, Y. MdDREB2A in Apple Is Involved in the Regulation of Multiple Abiotic Stress Responses. Hortic. Plant J. 2021, 7, 197–208. [Google Scholar] [CrossRef]
- Jangid, K.K.; Dwivedi, P. Physiological Responses of Drought Stress in Tomato: A Review. Int. J. Agric. Environ. Biotechnol. 2016, 9, 53. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous Spermidine Affects Polyamine Metabolism in Salinity-Stressed Cucumis sativus Roots and Enhances Short-Term Salinity Tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Jakubowska, D.; Janicka, M. The Role of Brassinosteroids in the Regulation of the Plasma Membrane H+-ATPase and NADPH Oxidase under Cadmium Stress. Plant Sci. 2017, 264, 37–47. [Google Scholar] [PubMed]
- Wang, R.K.; Li, L.L.; Cao, Z.H.; Zhao, Q.; Li, M.; Zhang, L.Y.; Hao, Y.J. Molecular Cloning and Functional Characterization of a Novel Apple MdCIPK6L Gene Reveals Its Involvement in Multiple Abiotic Stress Tolerance in Transgenic Plants. Plant Mol. Biol. 2012, 79, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Z.; Cui, J.; Ding, J.; Xia, X.; Liu, D.; Yu, J. Alleviation of Chilling-Induced Oxidative Damage by Salicylic Acid Pretreatment and Related Gene Expression in Eggplant Seedlings. Plant Growth Regul. 2011, 65, 101–108. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin Promotes Water-Stress Tolerance, Lateral Root Formation, and Seed Germination in Cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biol. Plant. 2017, 61, 571–578. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of Heat Stress on Peroxidase Activity and Total Protein Content in Strawberry Plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 180101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, Y.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 441357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–522. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Yu, Y.; Lv, Y.; Shi, Y.; Li, T.; Chen, Y.; Zhao, D.; Zhao, Z. The role of phyto-melatonin and related metabolites in response to stress. Molecules 2018, 23, 1887. [Google Scholar] [CrossRef]
- Pardo-Hernández, M.; López-Delacalle, M.; Rivero, R.M. ROS and NO regulation by melatonin under abiotic stress in plants. Antioxidants 2020, 9, 1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.A.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yakupoğlu, G. Effects of Magnetic Field and Ultrasound Applications on Endogenous Melatonin Content and Drought Stress Tolerance of Pepper Seedlings. Horticulturae 2023, 9, 704. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Scott, E.R.; Liu, J.W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef]
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M.; Khanizadeh, S. Exogenous melatonin alleviates heat-induced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) Plant. J. Plant Growth Regul. 2022, 41, 52–64. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, S.; Wang, M.; Zeb, S.; Khan, M.N.; Chen, Y.; Zhu, G.; Zhu, Z. Enhancement of sweetpotato tolerance to chromium stress through melatonin and glutathione: Insights into photosynthetic efficiency, oxidative defense, and growth parameters. Plant Physiol. Biochem. 2024, 208, 108509. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Chourasia, K.N.; More, S.J.; Kumar, A.; Kumar, D.; Singh, B.; Bhardwaj, V.; Kumar, A.; Das, S.K.; Singh, R.K.; Zinta, G.; et al. Salinity responses and tolerance mechanisms in underground vegetable crops: An integrative review. Planta 2022, 255, 68. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Khan, L.U.; Shahid, S.; Jahan, M.S. Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci. Hortic. 2021, 285, 110145. [Google Scholar] [CrossRef]
- Brengi, S.H.; Abd Allah, E.M.; Abouelsaad, I.A. Effect of melatonin or cobalt on growth, yield and physiological responses of cucumber (Cucumis sativus L.) plants under salt stress. J. Saudi Soc. Agric. Sci. 2022, 21, 51–60. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Efimova, M.V.; Danilova, E.D.; Zlobin, I.E.; Kolomeichuk, L.V.; Murgan, O.K.; Boyko, E.V.; Kuznetsov, V.V. Priming potato plants with melatonin protects stolon formation under delayed salt stress by maintaining the photochemical function of photosystem II, ionic homeostasis and activating the antioxidant system. Int. J. Mol. Sci. 2023, 24, 6134. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Abadía, J.; Marjani, M. Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Guo, D.; Wang, T.; Tian, Y.; Ma, C.; Liu, X.; Wang, C.; Zheng, X. Melatonin enhances KCl salinity tolerance by maintaining K+ homeostasis in Malus hupehensis. Plant Biotechnol. J. 2023, 21, 2273–2290. [Google Scholar] [CrossRef]
- Mohamadi Esboei, M.; Ebrahimi, A.; Amerian, M.R.; Alipour, H. Melatonin confers fenugreek tolerance to salinity stress by stimulating the biosynthesis processes of enzymatic, non-enzymatic antioxidants, and diosgenin content. Front. Plant Sci. 2022, 13, 890613. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought stress memory at the plant cycle level: A review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Z.; Amiri, H.; Ismaili, A. Improving drought stress tolerance in fenugreek (Trigonella foenum-graecum) by exogenous melatonin. Plant Biosyst. Int. J. Deal. All Asp. 2020, 154, 643–655. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in micro-tom tomato: Improved drought tolerance via the regulation of the photosynthetic apparatus, membrane stability, osmoprotectants, and root system. Life 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Ardıç, Ş.K.; Szafrańska, K.; Havan, A.; Karaca, A.; Aslan, M.Ö.; Sözeri, E.; Yakupoğlu, G.; Korkmaz, A. Endogenous melatonin content confers drought stress tolerance in pepper. Environ. Exp. Bot. 2023, 216, 105536. [Google Scholar] [CrossRef]
- El-Bauome, H.A.; Abdeldaym, E.A.; Abd El-Hady, M.A.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; et al. Exogenous proline, methionine, and melatonin stimulate growth, quality, and drought tolerance in cauliflower plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- El-Yazied, A.A.; Ibrahim, M.F.; Ibrahim, M.A.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M.; et al. Melatonin mitigates drought induced oxidative stress in potato plants through modulation of osmolytes, sugar metabolism, ABA homeostasis and antioxidant enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Q.N.; SEVGİN, N.; Rizwana, H.; Arif, N. Exogenous melatonin mitigates the adverse effects of drought stress in strawberry by upregulating the antioxidant defense system. S. Afr. J. Bot. 2023, 162, 658–666. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Ren, L.; Xie, F.; Wang, M.; Zhang, S. Jasmonate and melatonin act synergistically to potentiate cold tolerance in tomato plants. Front. Plant Sci. 2022, 12, 763284. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Değer, Ö.; Szafrańska, K.; Köklü, Ş.; Karaca, A.; Yakupoğlu, G.; Kocacinar, F. Melatonin effects in enhancing chilling stress tolerance of pepper. Sci. Hortic. 2021, 289, 110434. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin mitigates chilling-induced oxidative stress and photosynthesis inhibition in tomato plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef]
- He, Q.L.; Zhang, H.; Wang, Z.W.; Wan, L.Q.; Hai, M.R. Effects of exogenous melatonin on antioxidant system of potato seedlings under low temperature stress. Acta Agric. Boreali-Sin. 2022, 37, 103–111. [Google Scholar]
- Zhang, Y.P.; Xu, S.; Yang, S.J.; Chen, Y.Y. Melatonin alleviates cold-induced oxidative damage by regulation of ascorbate–glutathione and proline metabolism in melon seedlings (Cucumis melo L.). J. Hortic. Sci. Biotechnol. 2017, 92, 313–324. [Google Scholar] [CrossRef]
- Hayat, F.; Sun, Z.; Ni, Z.; Iqbal, S.; Xu, W.; Gao, Z.; Qiao, Y.; Tufail, M.A.; Jahan, M.S.; Khan, U.; et al. Exogenous melatonin improves cold tolerance of strawberry (Fragaria × ananassa Duch.) through modulation of DREB/CBF-COR pathway and antioxidant defense system. Horticulturae 2022, 8, 194. [Google Scholar] [CrossRef]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Arif, M.S.; Ahmad, R.; Hasanuzzaman, M.; Ali, B.; Hussain, A. Approaches in enhancing thermotolerance in plants: An updated review. J. Plant Growth Regul. 2020, 39, 456–480. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Zhao, C.; Nawaz, G.; Cao, Q.; Xu, T. Melatonin is a potential target for improving horticultural crop resistance to abiotic stress. Sci. Hortic. 2022, 291, 110560. [Google Scholar] [CrossRef]
- Colombage, R.; Singh, M.B.; Bhalla, P.L. Melatonin and abiotic stress tolerance in crop plants. Int. J. Mol. Sci. 2023, 24, 7447. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.; Ibrahim, M.F.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Ping, X.; Khan, R.A.A.; Li, Y.; Yang, Y.; Xie, B.; Mao, Z.; Ling, J. Draft genome resource of Fusarium oxysporum f. sp. capsici, the infectious agent of pepper Fusarium wilt. Mol. Plant-Microb. Int. 2021, 34, 715–717. [Google Scholar]
- Li, M.; Zhou, J.; Du, J.; Li, X.; Sun, Y.; Wang, Z.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; et al. Comparative physiological and transcriptomic analyses of improved heat stress tolerance in celery (Apium graveolens L.) caused by exogenous melatonin. Int. J. Mol. Sci. 2022, 23, 11382. [Google Scholar] [CrossRef]
- He, Q.; Wu, X.; Liu, Y.; Zhang, W.; Liu, J.; Munir, S.; Hai, M. Elucidating the molecular mechanisms of exogenous melatonin for improving heat tolerance in Solanum tuberosum L. seedlings. Sci. Hortic. 2023, 322, 112423. [Google Scholar]
- Xing, X.; Ding, Y.; Jin, J.; Song, A.; Chen, S.; Chen, F.; Fang, W.; Jiang, J. Physiological and transcripts analyses reveal the mechanism by which melatonin alleviates heat stress in chrysanthemum seedlings. Front. Plant Sci. 2021, 12, 673236. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; Rehman, K.U.; Islam, R.U.; Wahab, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2021, 321, 114455. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Pei, Z.Q.; Xu, T.T.; Dong, C.Y.; Bai, X.; Ma, C.; Zhu, Q.; Chai, C.H.; Wang, J.; Zheng, S.; et al. Exogenous melatonin enhanced cadmium stress tolerance of cucumber seedlings (Cucumis sativus L.). Biologia 2024, 1–18. [Google Scholar] [CrossRef]
- Saqib, M.; Khalofah, A.; Rehman, A.U.; Altaf, M.A. Alleviating effects of exogenous melatonin on nickel toxicity in two pepper genotypes. Sci. Hortic. 2024, 325, 112635. [Google Scholar] [CrossRef]
- Rizwan, M.; Nawaz, A.; Irshad, S.; Manoharadas, S. Exogenously applied melatonin enhanced chromium tolerance in pepper by up-regulating the photosynthetic apparatus and antioxidant machinery. Sci. Hortic. 2024, 323, 112468. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Ali Shah, A.; Ahmed, S.; Yasin, N.A. Cadmium stress consolation in melatonin supplemented Cucumis sativus through modulation of antioxidative defense system. Iranian J. Plant Physiol. 2020, 10, 3135–3154. [Google Scholar]
- Moussa, H.R.; Algamal, S.M.A. Does exogenous application of melatonin ameliorate boron toxicity in spinach plants? Int. J. Veg. Sci. 2017, 23, 233–245. [Google Scholar] [CrossRef]
- Altaf, M.M.; Awan, Z.A.; Ashraf, S.; Altaf, M.A.; Zhu, Z.; Alsahli, A.A.; Ahmad, P. Melatonin induced reversibility of vanadium toxicity in muskmelon by regulating antioxidant defense and glyoxalase systems. J. Hazard. Mater. 2024, 473, 134452. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Saqib, M.; Shahzad, U.; Zulfiqar, F.; Tiwari, R.K.; Lal, M.K.; Naz, S.; Jahan, M.S.; Awan, Z.A.; El-Sheikh, M.A.; Altaf, M.A. Exogenous melatonin alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in strawberry. S. Afr. J. Bot. 2023, 157, 10–18. [Google Scholar] [CrossRef]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Ahmad, J.; Hayat, F.; Khan, U.; Ahmed, N.; Li, J.; Ercisli, S.; Iqbal, S.; Javed, H.U.; Alyas, T.; Tu, P.; et al. Melatonin: A promising approach to enhance abiotic stress tolerance in horticultural plants. S. Afr. J. Bot. 2024, 164, 66–76. [Google Scholar] [CrossRef]
- Bartha, K.S.; Bujdosó, G.; Stefanovits-Bányai, É. Biochemical Methods for The Characterisation of Walnut Genotypes with Different Levels of Frost Tolerance. Erwerbs-Obstbau 2023, 65, 293–298. [Google Scholar] [CrossRef]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of Salinity Stress on Color Parameters, Leaf Pigmentation, Polyphenol and Flavonoid Contents, And Antioxidant Activity of Amaranthus lividus Leafy Vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of Auxin in Growth and Stress Response of Cucumber. Vegetos 2022, 2, 13. [Google Scholar] [CrossRef]
- Taskin, S.; Ertan, E. Exogenous Applications of Kaolin and Glycine Betaine Increased the Yield and Quality of Olive Fruit and Olive Oil. Erwerbs-Obstbau 2023, 65, 337–346. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, And Antioxidative Defense Mechanism in Plants Under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Shi, Y.; Zhang, Z.; Zou, Z.; Zhang, H.; Zhao, J. Effect of Exogenous Spermidine on Polyamine Content and Metabolism in Tomato Exposed to Salinity-Alkalinity Mixed Stress. Plant Physiol. Biochem. 2012, 57, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.T.; Oates, R.P.; Subbiah, S.; Payton, P.R.; Singh, K.P. Carbon Nanotubes Affect Early Growth, Flowering Time and Phytohormones in Tomato. Chemosphere 2020, 256, 127042. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of Different Treatments of Salicylic Acid on Heat Tolerance, Chlorophyll Fluorescence, and Antioxidant Enzyme Activity in Seedlings of Cucumis sativus L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Jing, S.; Begum, S.; Yu, L.; Kawochar, M.A.; Wang, E.; Chen, Y.; Zhao, J.; Song, B. Stjaz1-Like Mediated Root Architecture Plays Critical Roles in Drought Susceptibility in Potato. Environ. Exp. Bot. 2022, 202, 105008. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection with Potato Virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Fariduddin, Q.; Ahmad, A. Growth of Tomato (Lycopersicon esculentum) in Response to Salicylic Acid under Water Stress. J. Plant Interact. 2008, 3, 297–304. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Huang, C.; Lu, G.; Huang, J. Effects of Different Treatments on Chilling Injury and Antioxidative Metabolism in Sweet Potato. J. Nucl. Agric. Sci. 2014, 28, 1407–1412. [Google Scholar]
- Martel, A.B.; Qaderi, M.M. Does Salicylic Acid Mitigate the Adverse Effects of Temperature and Ultraviolet-B Radiation on Pea (Pisum sativum) Plants? Environ. Exp. Bot. 2016, 122, 39–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Yang, S.; Chen, Y. Salicylic Acid Alleviates Cadmium-Induced Inhibition of Growth and Photosynthesis through Upregulating Antioxidant Defense System in Two Melon Cultivars (Cucumis melo L.). Protoplasma 2015, 252, 911–924. [Google Scholar] [CrossRef]
- Cong, H.A.N.; Zuo, J.H.; Qing, W.; Dong, H.Z.; Gao, L.P. Salicylic Acid Alleviates Postharvest Chilling Injury of Sponge Gourd (Luffa cylindrica). J. Integr. Agric. 2017, 16, 735–741. [Google Scholar]
- Çoban, Ö.; Baydar, N.G. Brassinosteroid Effects on Some Physical and Biochemical Properties and Secondary Metabolite Accumulation in Peppermint (Mentha piperita L.) Under Salt Stress. Indust. Crops Prod. 2016, 86, 251–258. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide Ameliorates Endogenous Hormone Levels to Enhance Low-Temperature Stress Tolerance in Cucumber Seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef]
- Serna, M.; Coll, Y.; Zapata, P.J.; Botella, M.Á.; Pretel, M.T.; Amorós, A. A Brassinosteroid Analogue Prevented the Effect of Salt Stress on Ethylene Synthesis and Polyamines in Lettuce Plants. Sci. Hortic. 2015, 185, 105–112. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S.E.R. Amelioration of Lead Toxicity in Radish (Raphanus sativus L.) plants by brassinolide. J. Appl. Biol. Sci. 2011, 5, 43–48. [Google Scholar]
- Anuradha, S.; Rao, S.S.R. The Effect of Brassinosteroids on Radish (Raphanus sativus L.) Seedlings Growing Under Cadmium Stress. Plant Soil Environ. 2007, 53, 465. [Google Scholar] [CrossRef]
- Gupta, S.; Seth, C.S. Salicylic Acid Alleviates Chromium (VI) Toxicity by Restricting its Uptake, Improving Photosynthesis and Augmenting Antioxidant Defense in Solanum lycopersicum L. Physiol. Mol. Biol. Plants 2021, 27, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Karlidag, H.; Yildirim, E.; Turan, M. Salicylic Acid Ameliorates the Adverse Effect of Salt Stress on Strawberry. Sci. Agric. 2009, 66, 180–187. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Ullah, N.; Alam, S.S.; Ali, A.; Naz, I.; Ahmad, B.; Ahmad, M.; Ullah, I. Management of Ralstonia solanacearum (Smith) Wilt in Tomato Using Green Manure of the Medicinal Plant Adhatoda vasica (L.) Nees. Gesunde Pflanz. 2020, 72, 129. [Google Scholar] [CrossRef]
- Ahmad, R.; Alsahli, A.A.; Alansi, S.; Altaf, M.A. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic efficiency and antioxidant defense system of pea (Pisum sativum L.). Sci. Hortic. 2023, 322, 112431. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A Growth-Stimulating Compound Present in Lupin Tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; An, B.; Wei, Y.; Reiter, R.J.; Shi, H.; Luo, H.; He, C. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 2016, 60, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef]
- Tan, X.; Long, W.; Zeng, L.; Ding, X.; Cheng, Y.; Zhang, X.; Zou, X. Melatonin-induced transcriptome variation of rapeseed seedlings under salt stress. Int. J. Mol. Sci. 2019, 20, 5355. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, Z.; Xu, W.; Vetukuri, R.R.; Xu, X. Exogenous melatonin-stimulated transcriptomic alterations of Davidia involucrata seedlings under drought stress. Trees 2021, 35, 1025–1038. [Google Scholar] [CrossRef]
- Wang, J.; Lv, P.; Yan, D.; Zhang, Z.; Xu, X.; Wang, T.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Exogenous melatonin improves seed germination of wheat (Triticum aestivum L.) under salt stress. Int. J. Mol. Sci. 2022, 23, 8436. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Imran, M.; Yaseen, M.; ul Haq, T.; Jamshaid, M.U.; Rukh, S.; Ikram, R.M.; Ali, M.; Ali, A.; Maqbool, M.; et al. Role of salicylic acid in regulating ethylene and physiological characteristics for alleviating salinity stress on germination, growth and yield of sweet pepper. PeerJ 2020, 8, e8475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously Applied Melatonin Stimulates Root Growth and Raises Endogenous Indoleacetic Acid in Roots of Etiolated Seedlings of Brassica Juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Checker, V.G.; Khurana, P. Molecular and Functional Characterization of Mulberry EST Encoding Remorin (Mirem) Involved in Abiotic Stress. Plant Cell Rep. 2013, 32, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in Plant Growth, Development and Senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.F.; Wang, Q.C. Exogenous Application of Melatonin Improves Plant Resistance to Virus Infection. Plant Pathol. 2019, 68, 1287–1295. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin Induces Disease Resistance to Botrytis cinerea in Tomato Fruit by Activating Jasmonic Acid Signaling Pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Muhammad, H.M.D.; Naz, S.; Lal, M.K.; Tiwari, R.K.; Ahmad, R.; Nawaz, M.A.; Das, R.; Altaf, M.A. Melatonin in Business with Abiotic Stresses in Vegetable Crops. Sci. Hortic. 2014, 324, 112594. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl Jasmonate-Induced Alteration in Lipid Peroxidation, Antioxidative Defence System and Yield in Soybean under Drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A.; Nick, P. Exploring Jasmonates in the Hormonal Network of Drought and Salinity Responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Alam, S.S.; Najeeb, S.; Ali, A.; Ahmad, A.; Shakoor, A.; Tong, L. Mitigating Cd and bacterial wilt stress in tomato plants through trico-synthesized silicon nanoparticles and Trichoderma metabolites. Environ. Pollut. 2023, 333, 122041. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wan, C.; Wu, W.; Zhang, Y.; Pan, Y.; Chen, X.; Li, C.; Pi, J.; Wang, Z.; Ye, Y.; et al. Methyl Jasmonate (MeJA) Enhances Salt Tolerance of Okra (Abelmoschus esculentus L.) plants by Regulating ABA Signaling, Osmotic Adjustment Substances, Photosynthesis and ROS Metabolism. Sci. Hortic. 2023, 319, 112145. [Google Scholar] [CrossRef]
- Ayub, Q.; Khan, S.M.; Mehmood, A.; Haq, N.U.; Ali, S.; Ahmad, T.; Ayub, M.U.; Hassan, M.; Hayat, U.; Shoukat, M.F. Enhancement of Physiological and Biochemical Attributes of Okra by Application of Salicylic Acid under Drought Stress. J. Hortic. Sci. Technol. 2020, 3, 113–119. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; An, X.; Lv, Y.; Khan, R.A.A.; Xue, M.; Chen, J.; Liu, T. Trichoderma asperellum GD040 upregulates defense-related genes and reduces lesion size in Coffea canephora leaves inoculated with Colletotrichum cairnsense. Biol. Control 2023, 181, 105213. [Google Scholar] [CrossRef]
- Qin, C.; Lian, H.; Alqahtani, F.M.; Ahanger, M.A. Chromium mediated damaging effects on growth, nitrogen metabolism and chlorophyll synthesis in tomato can be alleviated by foliar application of melatonin and jasmonic acid priming. Sci. Hortic. 2024, 323, 112494. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids Make Plant Life Easier under Abiotic Stresses Mainly by Modulating Major Components of Antioxidant Defense System. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Ahmad, I.; Ahmad, A. Brassinosteroids and Their Role in Response of Plants to Abiotic Stresses. Biol. Plant. 2014, 58, 9–17. [Google Scholar] [CrossRef]
- Jiroutova, P.; Oklestkova, J.; Strnad, M. Crosstalk between Brassinosteroids and Ethylene during Plant Growth and under Abiotic Stress Conditions. Int. J. Mol. Sci. 2018, 19, 3283. [Google Scholar] [CrossRef]
- Kaur, N.; Pati, P.K. Harnessing the Potential of Brassinosteroids in Abiotic Stress Tolerance in Plants. In Brassinosteroids: Plant Growth and Development; Springer: Singapore, 2019; pp. 407–423. [Google Scholar]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and Physiological Responses in Plant Growth and Abiotic Stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Suhel, M.; Husain, T.; Prasad, S.M.; Singh, V.P. GABA Requires Nitric Oxide for Alleviating Arsenate Stress in Tomato and Brinjal Seedlings. J. Plant Growth Regul. 2023, 42, 670–683. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide Application in Plants: An Implication for Improving Drought Stress Tolerance in Plants. Plant Physiol. Biochem. 2019, 135, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G. The Effects of GABA in Plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against Abiotic Stress Tolerance in Legumes: A Review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, R.; Hou, J.; Khan, R.A.A.; Li, X.; Wei, H.; Chen, J.; Wang, R.; Zhang, J.; Liu, T. The unique sugar conversion and complex CAZyme system of Trichoderma brev T069 during solid-state fermentation of cassava peel. Ind. Crops Prod. 2023, 193, 116263. [Google Scholar] [CrossRef]
- Suhel, M.; Husain, T.; Pandey, A.; Singh, S.; Dubey, N.K.; Prasad, S.M.; Singh, V.P. An Appraisal of Ancient Molecule GABA in Abiotic Stress Tolerance in Plants and Its Crosstalk with Other Signaling Molecules. J. Plant Growth Regul. 2023, 42, 614–629. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Jisha, K.C.; Puthur, J.T. GABA/BABA Priming: A Means for Enhancing Abiotic Stress Tolerance Potential of Plants with Less Energy Investments on Defense Cache. Acta Physiol. Plant. 2016, 38, 230. [Google Scholar] [CrossRef]
- Groppa, M.D.; Benavides, M.P. Polyamines and Abiotic Stress: Recent Advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and Abiotic Stress in Plants: A Complex Relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Polyamines and Abiotic Stress Tolerance in Plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bitrián, M.; Zarza, X.; Altabella, T.; Tiburcio, A.F.; Alcázar, R. Polyamines under Abiotic Stress: Metabolic Crossroads and Hormonal Crosstalks in Plants. Metabolites 2012, 2, 516–528. [Google Scholar] [CrossRef]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of Polyamines in Plant Response to Abiotic Stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Zhu, R.Y.; Zhang, G.Y.; Dai, Y.R. Attenuation of Cold-Induced Apoptosis by Exogenous Melatonin in Carrot Suspension Cells: The Possible Involvement of Polyamines. J. Pineal Res. 2004, 36, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Kumar, R.; Lal, M.K.; Kumar, A.; Altaf, M.A.; Devi, R.; Mangal, V.; Naz, S.; Altaf, M.M.; Dey, A.; et al. Melatonin-Polyamine Interplay in the Regulation of Stress Responses in Plants. J. Plant Growth Regul. 2023, 42, 4834–4850. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Zhu, L.; Ma, Z.; Wang, J.; Zhu, J. Exogenous Melatonin Improves Plant Iron Deficiency Tolerance via Increased Accumulation of Polyamine-Mediated Nitric Oxide. Int. J. Mol. Sci. 2016, 17, 1777. [Google Scholar] [CrossRef]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Melatonin influences stomatal behavior, root morphology, cell viability, photosynthetic responses, fruit yield, and fruit quality of tomato plants exposed to salt stress. J. Plant Growth Regul. 2023, 42, 2408–2432. [Google Scholar] [CrossRef]
- Tanveer, M.; Shabala, S. Neurotransmitters in signalling and adaptation to salinity stress in plants. In Neurotransmitters in Plant Signaling and Communication; Springer: Cham, Switzerland, 2020; pp. 49–73. [Google Scholar]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-Seq Approach to Discriminate Gene Expression Profiles in Response to Melatonin on Cucumber Lateral Root Formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Pre sowing Seed Treatment with Melatonin Protects Red Cabbage Seedlings against Toxic Copper Ion Concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Shi, K.; Shan, D.; Zhu, Y.; Wang, C.; Bai, Y.; Yan, T.; Zheng, X.; Kong, J. Apple tree flowering is mediated by low level of melatonin under the regulation of seasonal light signal. J. Pineal Res. 2019, 66, e12551. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Niu, C.; Li, K.; Chen, S.; Tahir, M.M.; Han, M.; Zhang, D. Melatonin Promotes Adventitious Root Formation in Apple by Promoting the Function of Mdwox11. BMC Plant Biol. 2020, 20, 536. [Google Scholar] [CrossRef]
- Chen, A.; Najeeb, S.; Wang, Y.; Khan, R.A.A.; Ping, X.; Ling, J. First report of Colletotrichum circinans causing anthracnose in Allium fistulosum var. giganteum in Gansu Province, China. Plant Dis. 2022, 106, 1985. [Google Scholar] [CrossRef] [PubMed]
- Tijero, V.; Munoz, P.; Munné-Bosch, S. Melatonin as An Inhibitor of Sweet Cherries Ripening in Orchard Trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Zheng, J.; Dong, Y.; Liu, Q.; Yang, X.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X. Local Melatonin Application Induces Cold Tolerance in Distant Organs of Citrullus lanatus L. via Long Distance Transport. Sci. Rep. 2017, 7, 40858. [Google Scholar] [CrossRef]
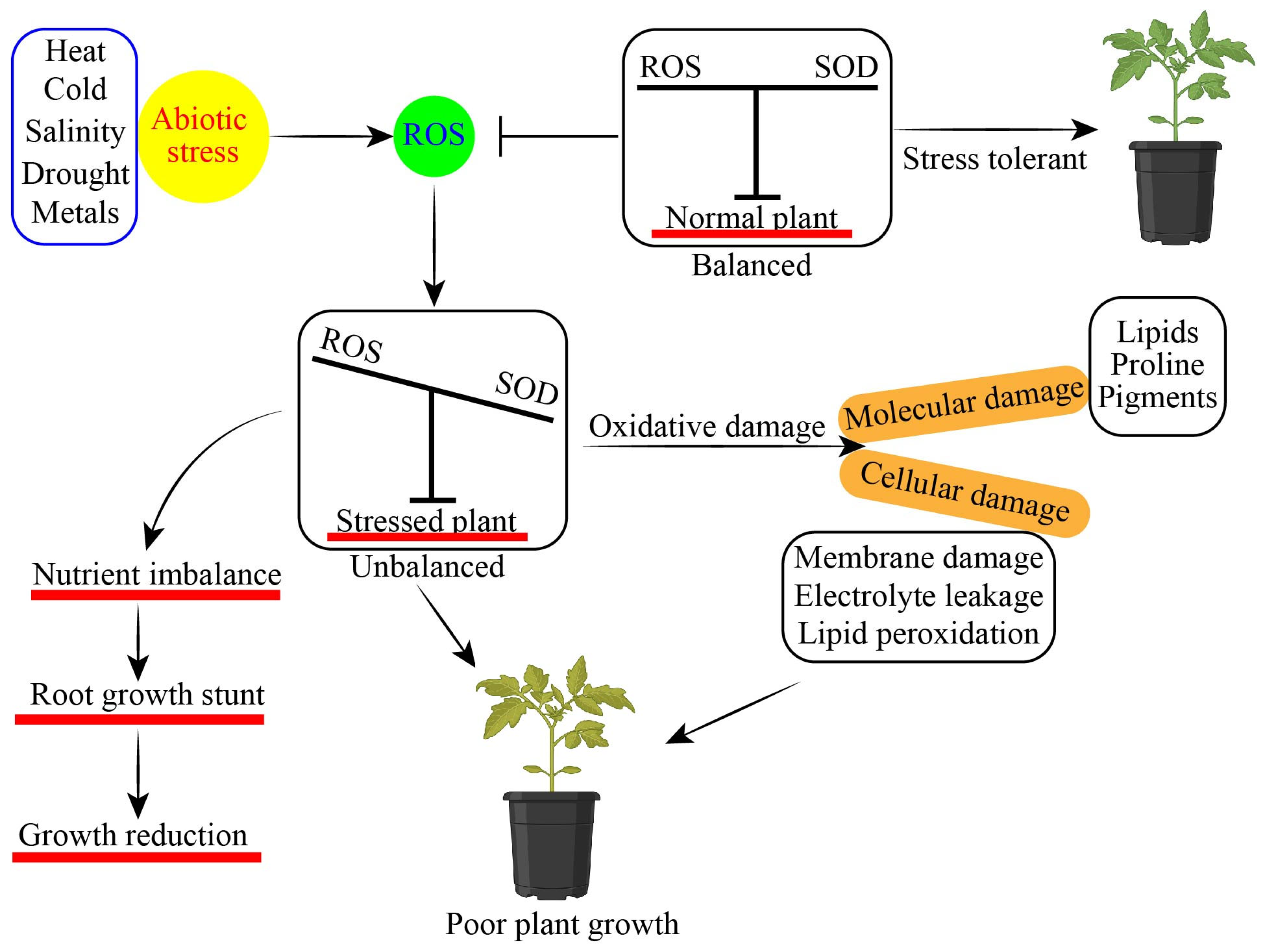


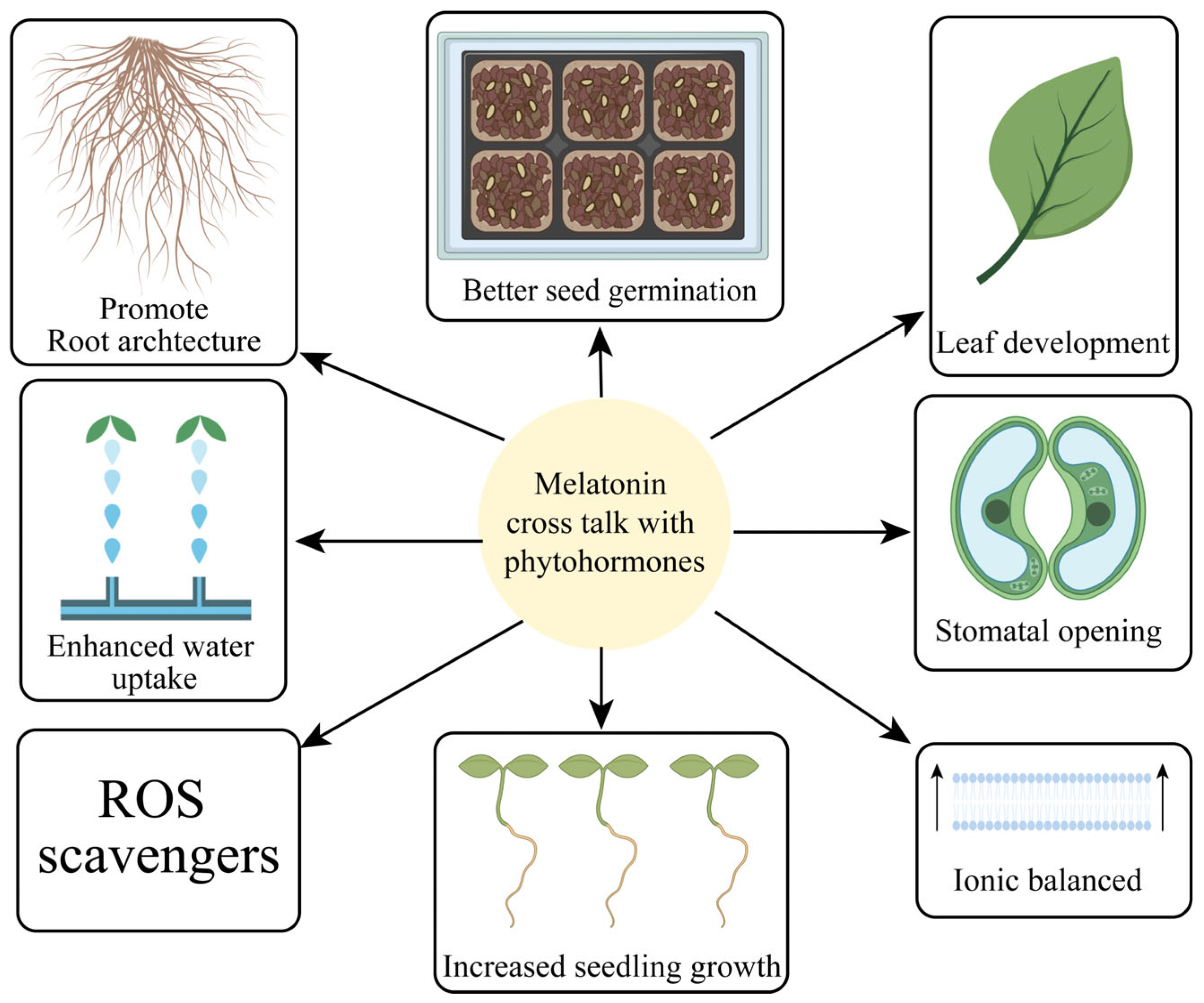
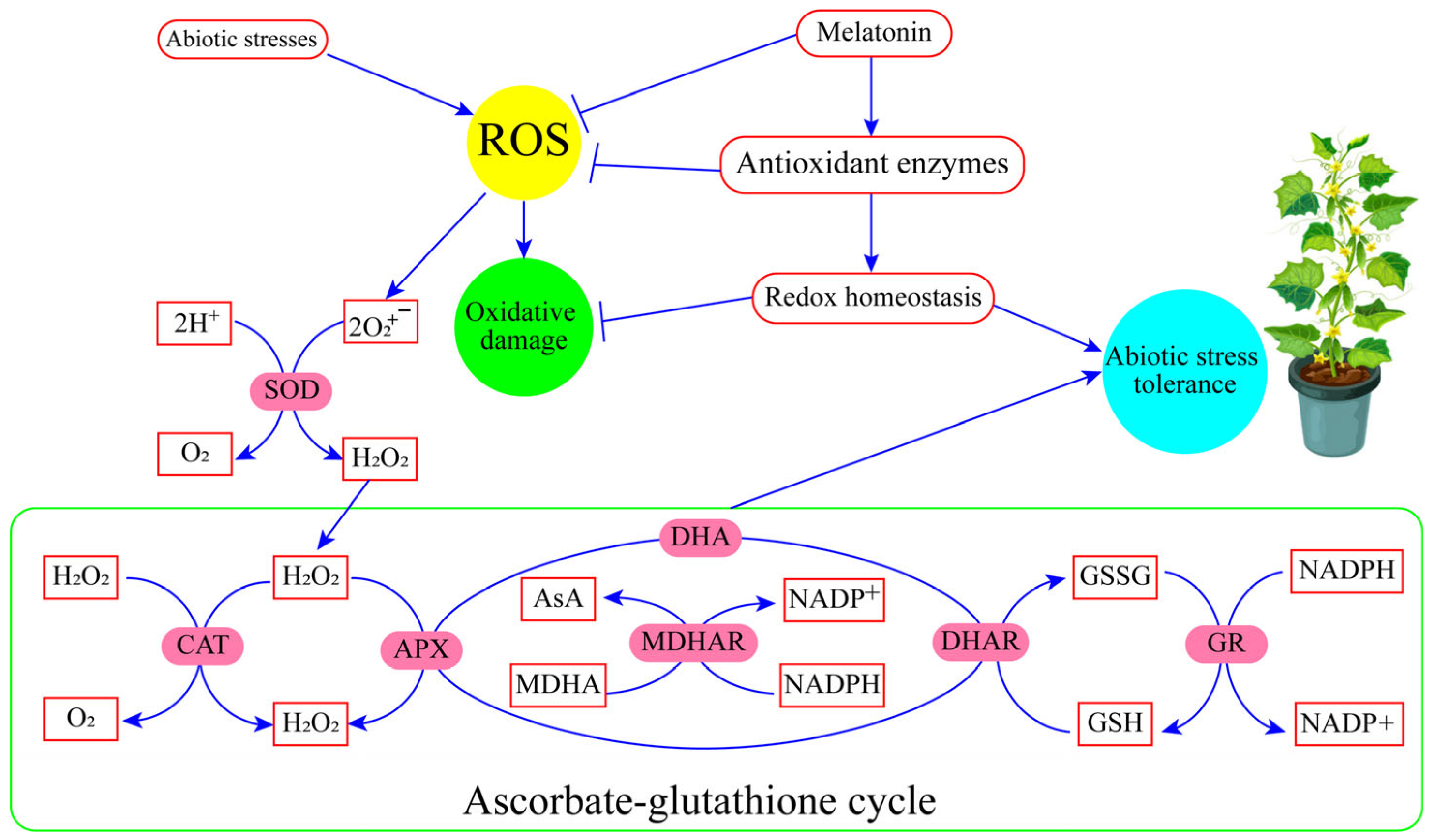
| Stress Type | Crops | Key Finding | Reference |
|---|---|---|---|
| Drought and salt | Apple | Stress tolerance improved via transcriptional factor MdDREB2A. | [45] |
| Drought | Tomato | About 70–75% of field capacity also increased the level of salt accumulation in the root zone of vegetables | [46] |
| Salinity | Cucumber | The application of 50 mM NaCl, which may be controlled by spraying various phytohormones on vegetable crops, had a negative effect. | [47] |
| Salinity | Lettuce | Lettuce performance was decreased because salinity resulted in the decrease of plant productivity. | [46] |
| HMs | Tomato | Overexpression of numerous genes like StJAZ1 might reduce the relative leaf water capability in the vegetables. | [48] |
| Salinity | Apple | A salt-induced MdCIPK6L gene was isolated from an apple. Its expression was positively induced by abiotic stresses, stress-related hormones | [49] |
| Drought | Cucumber | The development, yield, and defense-related activities of the cucumber plants were expressly promoted by osmotic damage as well as oxidative stress. | [50] |
| Heat | Peppers | Temperature extremities are the major cause of proteins denaturing contributing in proper growth and yield. | [50] |
| Drought | Potato | Lipid peroxidation, hydrogen peroxide (H2O2), ROS, and malondialdehyde (MDA) were increased. Phytohormones can effectively lower lipid peroxidation and MDA generation. | [51] |
| Cold | Melon | The cooling stress conditions had a very negative impact on seed germination. Chiller injuries were lessened in part through CBF-responsive pathways. | [52] |
| Heat | Strawberry | Physiological, biochemical, and molecular basis were disturbed due to abiotic stressors. | [53] |
| Functions | Reference |
|---|---|
| Regulated seed germination | [55] |
| Modulated Antioxidant defense mechanism | [22] |
| Maintained redox homeostasis | [56] |
| Reduced oxidative damage and membrane damage | [57] |
| Lowered electrolyte leakage and lipid peroxidation production | [36] |
| Enhanced photosynthetic machinery | [27] |
| Modified root system architecture | [13] |
| Boosted growth and development | [31] |
| Regulated lateral root development | [58] |
| Decreased HMs accumulation | [59] |
| Regulated fruit maturation | [56] |
| Upregulated secondary metabolites accumulation | [60] |
| Increased photosynthetic pigments content | [24] |
| Development of leaf size and plant height | [22] |
| Development of flowering | [61] |
| Vegetative growth promotion | [27] |
| Regulated production of phytohormones | [36] |
| Crop Type | MT Spray | Major Effects | References |
|---|---|---|---|
| Tomato fruits | 50 μM | It can induce stress-related responses, including stomatal closure, root growth inhibition, leaf senescence, and the activation of stress-responsive genes. MT treatment has been shown to downregulate the expression of ethylene biosynthesis genes, such as ACC synthase and ACC oxidase, leading to reduced ethylene production. Ethylene receptor genes were expressed. | [126] |
| Tomato plants | 0.1 mM | Increase in the potential of ascorbic acid and lycopene contents. Elevated quality and yield of tomato fruits. | [158] |
| Cassava roots | 100 μM | Downregulation of ethylene biosynthesis genes by MT may contribute to the mitigation of stress-induced ethylene responses in plants. Reduction in the post-harvest losses. Increase in the shelf life of tuberous. Improved plant defense system against biotic and abiotic stress. | [125] |
| Peach fruits | 0.1 mM | Reduction in post-harvest senescence. Increase in fruit firmness and flavor. Reduction in physiological weight losses. | [159] |
| Strawberry fruits | 100 μM | Reduction in the post-harvest senescence. Increase in the ATP, antioxidants, and shelf life. Improved potential of defense system against pathogens. | [53] |
| Crop Type | MT Spray | Major Effects | References |
|---|---|---|---|
| Tomato | 12.5–100 μM | IAA increased up to two-fold. AUX carrier proteins were significantly activated. Increase of IAA signaling molecules. Drought tolerance improved in the plants. | [126] |
| Tomato overexpressing of serotonin N-acetyltransferase | 0.2 μM | Abiotic stress tolerance was proved by modifications in plant physiological mechanisms. Improvement in the IAA up to 7-fold. | [126] |
| Banana | 0.01–0.5 μM | IAA up to 2-fold was found to be induced. Root morphology was improved. | [40] |
| Pepper seedling | 5 μM | MT enhanced seedling growth; Reduced oxidative damage and upregulated the antioxidant enzyme system | [20] |
| Pepper seedlings | 5 μM | MT protected leaf photosynthetic efficiency, enhanced mineral nutrient accumulation, and decreased heavy metal uptake | [20] |
| Cucumber seedlings | 1 μM | GA biosynthesis was improved. Tolerance against salinity was enhanced by modulation of physiological and biochemical processes. An increase in germination rate was recorded. | [123] |
| Crop Type | MT Spray | Major Effects | References |
|---|---|---|---|
| Watermelon plants | 1.5–150 μM | MT and CK can coordinately modulate the expression of stress-related genes, including those involved in antioxidant defense, osmotic regulation, and stress signaling pathways. The activation of cold-responsive genes was recorded in plants. Plant tolerance was improved against cold. Activation of IAA, GAs, ethylene, and JA-related genes. ABA receptor PYL8 gene expression. | [198] |
| Apple leaves | 10–500 μM | ABA endogenous level was restricted. Reduced ABA biosynthesis genes. Increase in ABA catabolism genes. | [148] |
| Cucumber seedlings | 1 μM | The ABA endogenous level was suppressed. Reduced ABA biosynthesis occurs. Increase in the ABA catabolism. | [192] |
| Ryegrass | 20 μM | Photosynthesis potential was improved. Cell membrane stability was regulated. CKs endogenous level enhanced. Expression of CKs biosynthesis genes. Activation of CKs signaling transcription factors. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Jin, S. Melatonin Interaction with Other Phytohormones in the Regulation of Abiotic Stresses in Horticultural Plants. Antioxidants 2024, 13, 663. https://doi.org/10.3390/antiox13060663
Huang S, Jin S. Melatonin Interaction with Other Phytohormones in the Regulation of Abiotic Stresses in Horticultural Plants. Antioxidants. 2024; 13(6):663. https://doi.org/10.3390/antiox13060663
Chicago/Turabian StyleHuang, Shanxia, and Songheng Jin. 2024. "Melatonin Interaction with Other Phytohormones in the Regulation of Abiotic Stresses in Horticultural Plants" Antioxidants 13, no. 6: 663. https://doi.org/10.3390/antiox13060663





