Empagliflozin Alleviates Carfilzomib-Induced Cardiotoxicity in Mice by Modulating Oxidative Stress, Inflammatory Response, Endoplasmic Reticulum Stress, and Autophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Cardiotoxicity Markers
2.5. Histological Examination
2.6. Determination of Catalase (CAT), Reduced Glutathione (GSH), and SOD Activity
2.7. Inflammatory Cytokine Determination
2.8. Determination of ER Stress Markers
2.9. qRT-PCR Determination of Autophagy Markers, LC3B and Beclin-1
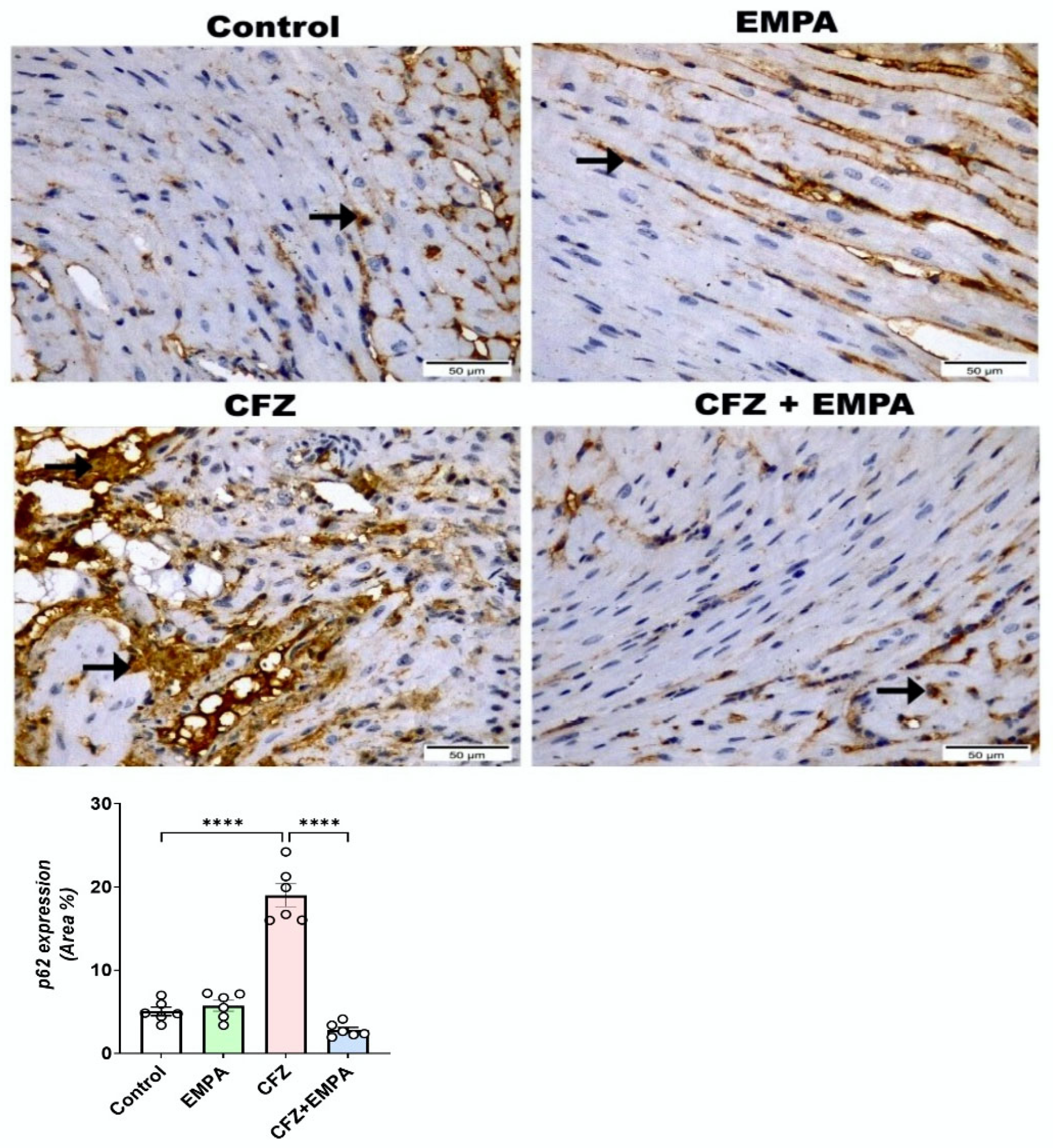
2.10. Immunohistochemical Determination of Active Caspase-3 and p62
2.11. Cytotoxicity Assay
2.11.1. Cell Culture
2.11.2. Cell Viability Assay
2.12. Protein Determination
2.13. Statistical Analysis
3. Results
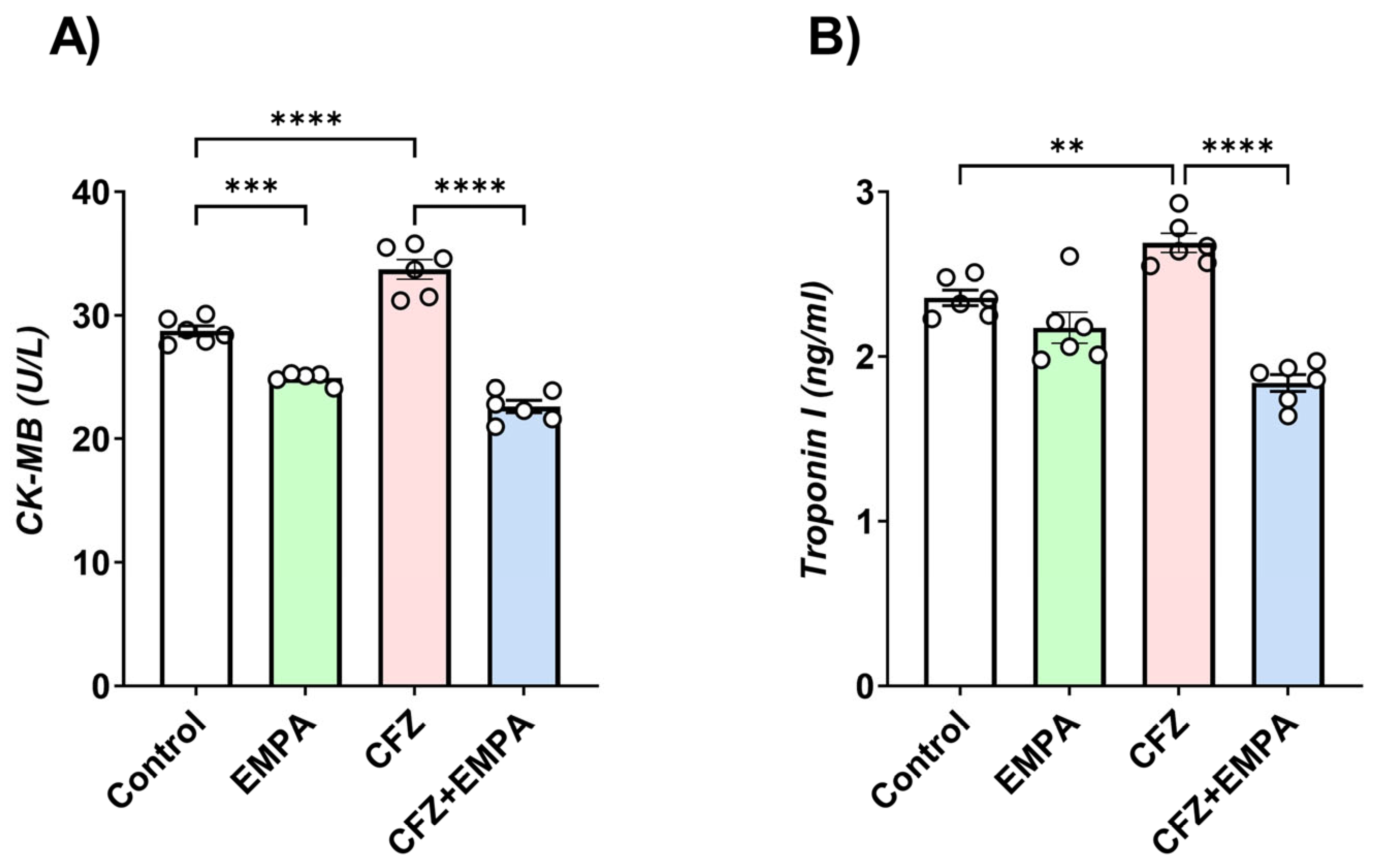
3.1. Effect of EMPA and CFZ on Cardiotoxicity Markers
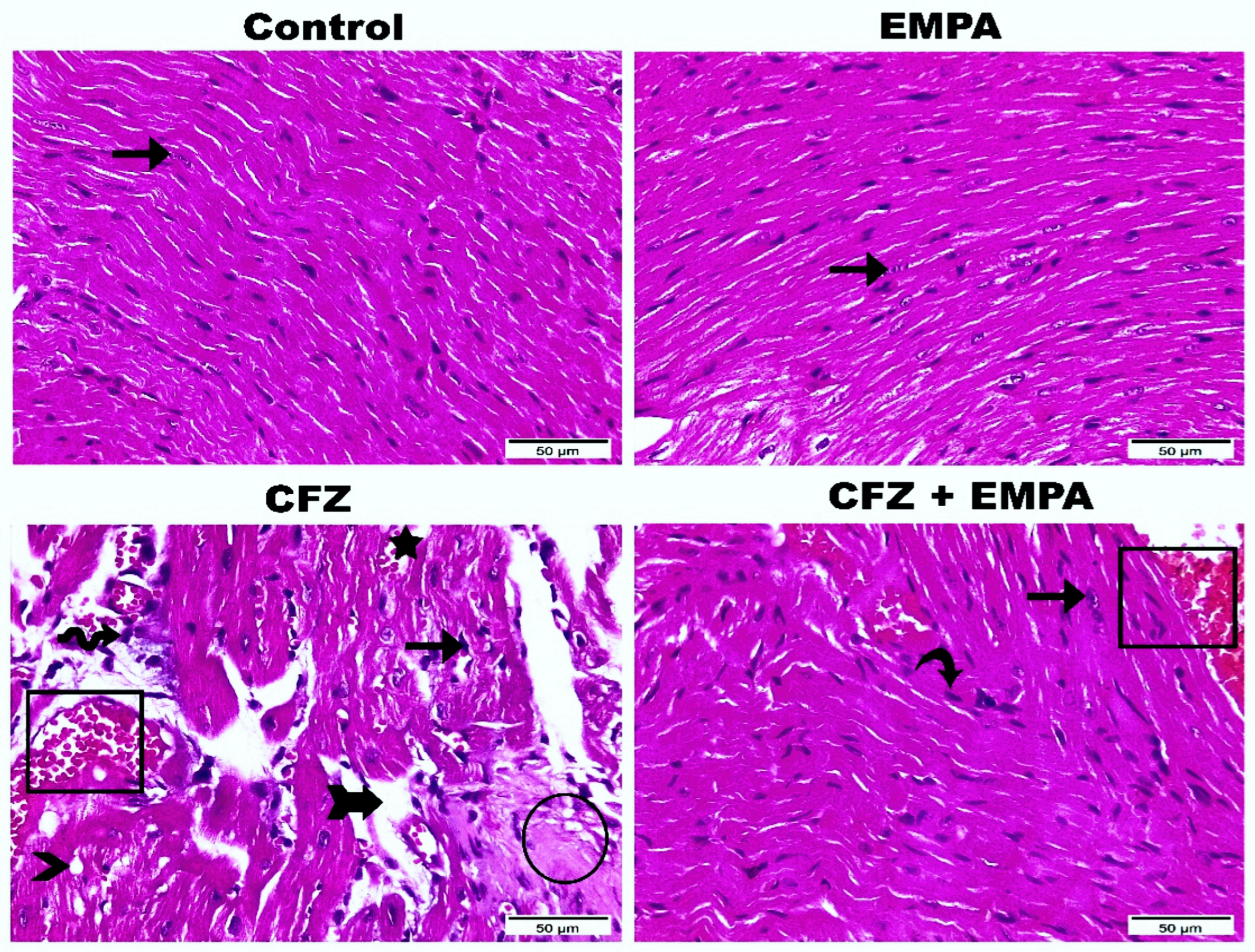
3.2. Histological Examination
3.3. EMPA Mitigates CFZ-Induced Oxidative Stress in Mice
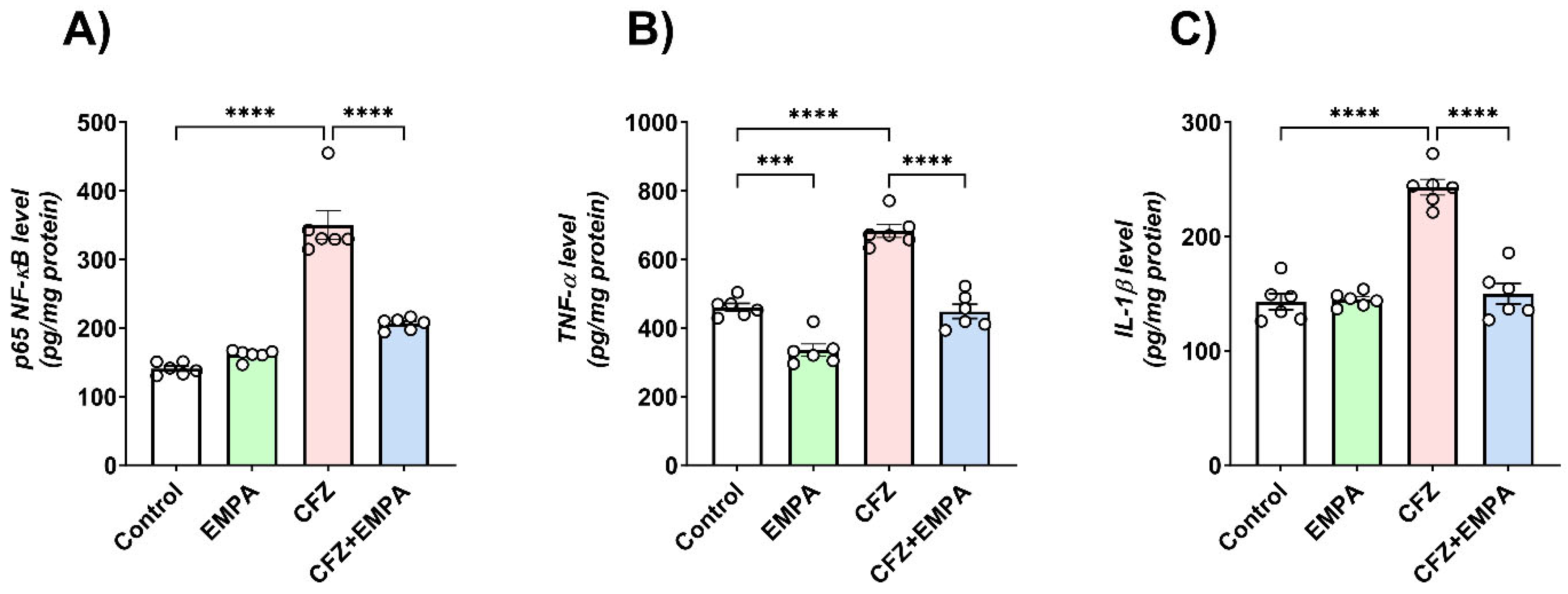
3.4. Effect of EMPA on the Inflammatory Response Induced by CFZ
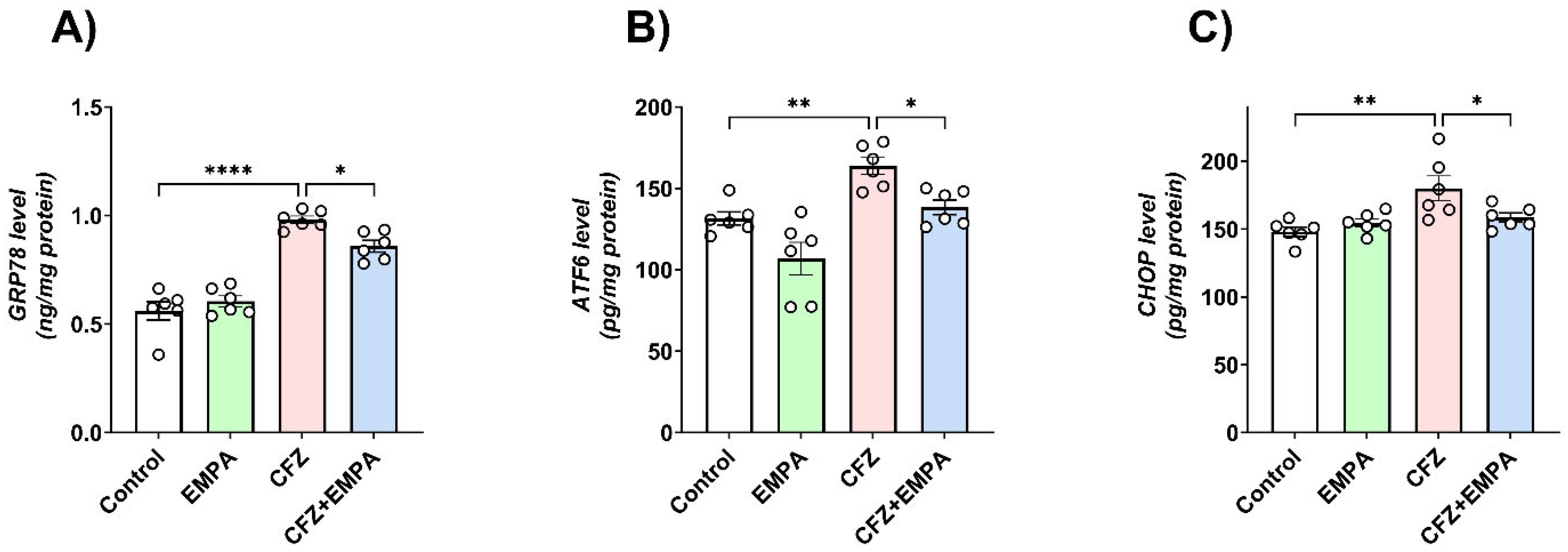
3.5. ER Stress Signaling Changes Associated with CFZ-Induced Cardiotoxicity
3.6. Effect of EMPA on Autophagy Markers LC3B, Beclin-1, and p62 Altered by CFZ
3.7. EMPA Downregulates Caspase-3 Immunohistochemical Induced by CFZ
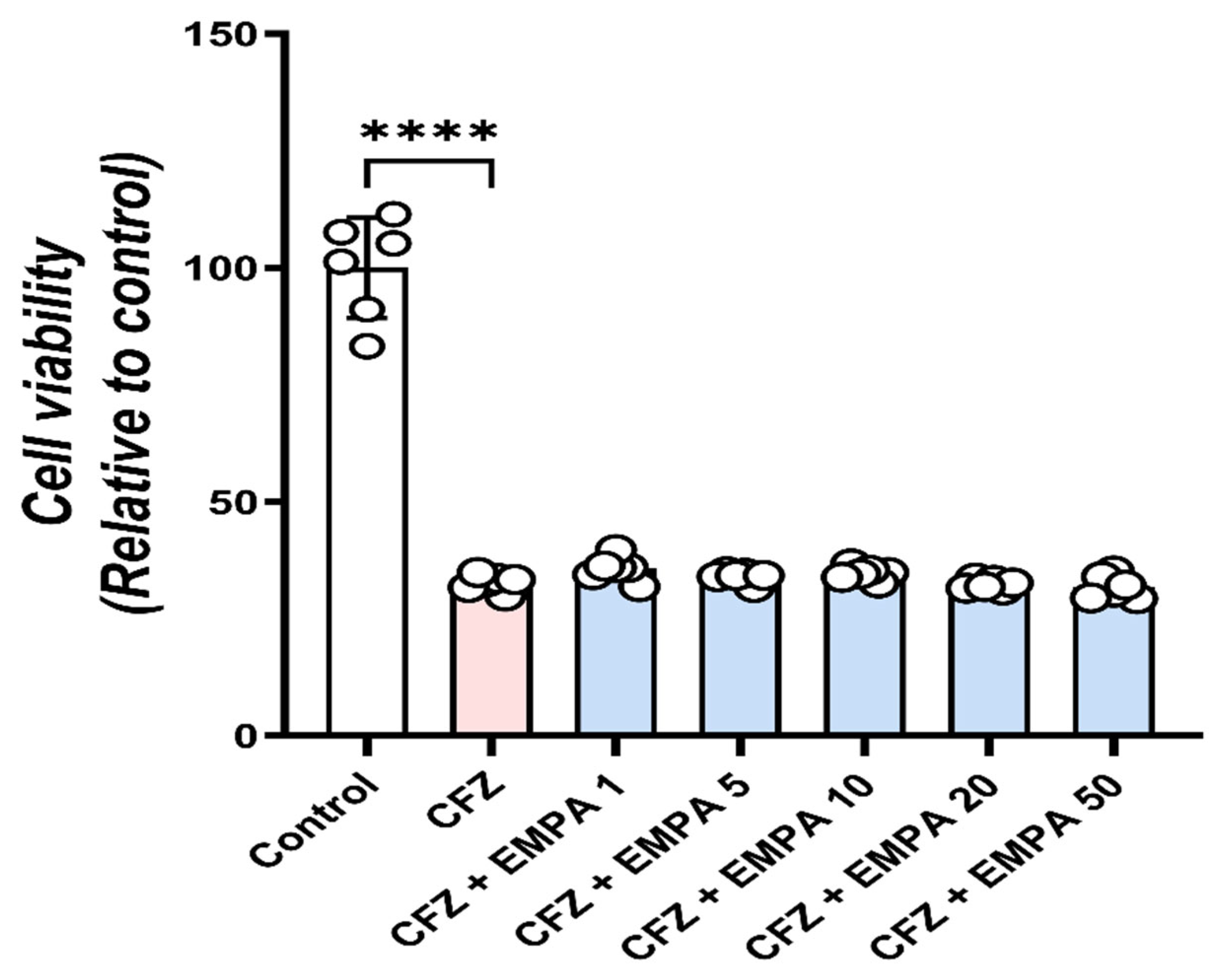
3.8. EMPA Does Not Interfere with the Anticancer Activity of CFZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.; Denlinger, N.; Yang, Y. Recent Advances and Challenges in Cancer Immunotherapy. Cancers 2022, 14, 3972. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Pokorna, Z.; Jirkovsky, E.; Hlavackova, M.; Jansova, H.; Jirkovska, A.; Lencova-Popelova, O.; Brazdova, P.; Kubes, J.; Sotakova-Kasparova, D.; Mazurova, Y.; et al. In vitro and in vivo investigation of cardiotoxicity associated with anticancer proteasome inhibitors and their combination with anthracycline. Clin. Sci. Lond. Engl. 1979 2019, 133, 1827–1844. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, R.J.; Berger, J.; Sebag, I.A. Cardiotoxicity of cancer therapeutics: Current issues in screening, prevention, and therapy. Front. Pharmacol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Berenson, J.; Hilger, J.; Yellin, O.; Dichmann, R.; Patel-Donnelly, D.; Boccia, R.V.; Bessudo, A.; Stampleman, L.; Gravenor, D.; Eshaghian, S.; et al. Replacement of bortezomib with carfilzomib for multiple myeloma patients progressing from bortezomib combination therapy. Leukemia 2014, 28, 1529–1536. [Google Scholar] [CrossRef]
- VVij, R.; Siegel, D.S.; Jagannath, S.; Jakubowiak, A.J.; Stewart, A.K.; McDonagh, K.; Bahlis, N.; Belch, A.; Kunkel, L.A.; Wear, S.; et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br. J. Haematol. 2012, 158, 739–748. [Google Scholar] [CrossRef]
- Waxman, A.J.; Clasen, S.; Hwang, W.T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbia, M.M.; Korashy, H.M.; Ansari, M.A.; Sayed-Ahmed, M.M.; Nagi, M.N.; Iqbal, M.; Khalid Answer, M.; Kazmi, I.; et al. Rutin Attenuates Carfilzomib-Induced Cardiotoxicity Through Inhibition of NF-κB, Hypertrophic Gene Expression and Oxidative Stress. Cardiovasc. Toxicol. 2017, 17, 58–66. [Google Scholar] [CrossRef]
- Efentakis, P.; Kremastiotis, G.; Varela, A.; Nikolaou, P.E.; Papanagnou, E.D.; Davos, C.H.; Tsoumani, M.; Agrogiannis, G.; Konstantinidou, A.; Kastritis, E.; et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood 2019, 133, 710–723. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, D.; Inzucchi, S.E.; Cannon, C.P.; McGuire, D.K.; Scirica, B.M.; Johansen, O.E.; Sambevski, S.; Kaspers, S.; Pfarr, E.; George, J.T.; et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019, 139, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Fitchett, D.; Inzucchi, S.E.; Pfarr, E.; George, J.T.; Zinman, B. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: Subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia 2018, 61, 1712–1723. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Koyani, C.N.; Plastira, I.; Sourij, H.; Hallström, S.; Schmidt, A.; Rainer, P.P.; Bugger, H.; Frank, S.; Malle, E.; von Lewinski, D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol. Res. 2020, 158, 104870. [Google Scholar] [CrossRef] [PubMed]
- Barış, V.Ö.; Dinçsoy, A.B.; Gedikli, E.; Zırh, S.; Müftüoğlu, S.; Erdem, A. Empagliflozin Significantly Prevents the Doxorubicin-induced Acute Cardiotoxicity via Non-antioxidant Pathways. Cardiovasc. Toxicol. 2021, 21, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Z.; Ergun, D.D.; Durmus, S.; Sahin, H.; Senturk, G.E.; Gelisgen, R.; Senyigit, A.; Uzun, H. Empagliflozin and sacubitril/valsartan reverse methotrexate cardiotoxicity by repressing oxidative stress and hypoxia in heart embryonic H9c2 cardiomyocytes—The role of morphology of mitochondria observed on electron microscopy. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3979–3992. [Google Scholar] [CrossRef]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovascular. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK, 2016; Volume 120, p. 131. [Google Scholar]
- Aebi, H. Catalase invitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, M.B. Pharmacologic effect of selenium and antioxidants on liver and kidney of cadmium intoxicated rats. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhong, X.; Gao, Z.; Jiang, W.; Peng, L.; Cao, Y.; Zhou, Z.; Huang, L. Dynamic changes in Beclin-1, LC3B and p62 at various time points in mice with temporary middle cerebral artery occlusion and reperfusion (tMCAO). Brain Res. Bull. 2021, 173, 124–131. [Google Scholar] [CrossRef] [PubMed]
- George, M.Y.; El-Derany, M.O.; Ahmed, Y.; Zaher, M.; Ibrahim, C.; Waleed, H.; Khaled, H.; Khaled, G.; Saleh, A.; Alshafei, H.; et al. Design and evaluation of chrysin-loaded nanoemulsion against lithium/pilocarpine-induced status epilepticus in rats; emphasis on formulation, neuronal excitotoxicity, oxidative stress, microglia polarization, and AMPK/SIRT-1/PGC-1α pathway. Expert Opin. Drug Deliv. 2023, 20, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Oren, O.; Gertz, M.A.; Yang, E.H. Proteasome Inhibitor-Related Cardiotoxicity: Mechanisms, Diagnosis, and Management. Curr. Oncol. Rep. 2020, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Dabour, M.S.; George, M.Y.; Daniel, M.R.; Blaes, A.H.; Zordoky, B.N. The Cardioprotective and Anticancer Effects of SGLT2 Inhibitors: JACC: CardioOncology State-of-the-Art Review. JACC Cardiooncol. 2024, 6, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, P.E.; Mylonas, N.; Makridakis, M.; Makrecka-Kuka, M.; Iliou, A.; Zerikiotis, S.; Efentakis, P.; Kampoukos, S.; Kostomitsopoulos, N.; Vilskersts, R.; et al. Cardioprotection by selective SGLT-2 inhibitors in a non-diabetic mouse model of myocardial ischemia/reperfusion injury: A class or a drug effect? Basic. Res. Cardiol. 2022, 117, 27. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Repetto, A.; Bello, B.D.; Pasotti, M.; Agozzino, M.; Viganò, M.; Klersy, C.; Tavazzi, L.; Arbustini, E. Coronary atherosclerosis in endstage idiopathic dilated cardiomyopathy: An innocent bystander? Eur. Heart J. 2005, 26, 1519–1527. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbi, M.M.; Ansari, M.A.; Almutairi, M.M.; Alshammari, M.; Almukhlafi, T.S.; Ansari, M.N.; Aljerian, K.; Ahmad, S.F. Apremilast reversed carfilzomib-induced cardiotoxicity through inhibition of oxidative stress, NF-κB and MAPK signaling in rats. Toxicol. Mech. Methods 2016, 26, 700–708. [Google Scholar] [CrossRef]
- Al-Harbi, N.O. Carfilzomib-induced cardiotoxicity mitigated by dexrazoxane through inhibition of hypertrophic gene expression and oxidative stress in rats. Toxicol. Mech. Methods 2016, 26, 189–195. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, Y.; Qian, X.Q.; Zhao, H.; Wang, D.; Zuo, G.X.; Wang, K. Empagliflozin alleviates myocardial I/R injury and cardiomyocyte apoptosis via inhibiting ER stress-induced autophagy and the PERK/ATF4/Beclin1 pathway. J. Drug Target. 2022, 30, 858–872. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Liu, H.; Chen, Y.; Li, P.; Liu, L.; Li, J.; Ren, Y.; Huang, J.; Xiong, E.; et al. Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function. Oxidative Med. Cell. Longev. 2022, 2022, 1122494. [Google Scholar] [CrossRef]
- Mohammed, N.N.; Tadros, M.G.; George, M.Y. Empagliflozin repurposing in Parkinson’s disease; modulation of oxidative stress, neuroinflammation, AMPK/SIRT-1/PGC-1α, and wnt/β-catenin pathways. Inflammopharmacology 2024, 32, 777–794. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Habib, C.N.; Ali, A.E.; Anber, N.H.; George, M.Y. Lactoferrin ameliorates carfilzomib-induced renal and pulmonary deficits: Insights to the inflammasome NLRP3/NF-κB and PI3K/Akt/GSK-3β/MAPK axes. Life Sci. 2023, 15, 122245. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Al-Shabanah, O.A.; Alotaibi, M.R.; As Sobeai, H.M.; Afzal, M.; Kazmi, I.; Al Rikabi, A.C. Rutin inhibits carfilzomib-induced oxidative stress and inflammation via the NOS-mediated NF-κB signaling pathway. Inflammopharmacology 2019, 27, 817–827. [Google Scholar] [CrossRef]
- Maamoun, H.; Benameur, T.; Pintus, G.; Munusamy, S.; Agouni, A. Crosstalk Between Oxidative Stress and Endoplasmic Reticulum (ER) Stress in Endothelial Dysfunction and Aberrant Angiogenesis Associated With Diabetes: A Focus on the Protective Roles of Heme Oxygenase (HO)-1. Front. Physiol. 2019, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Desouky, M.A.; George, M.Y.; Michel, H.E.; Elsherbiny, D.A. Roflumilast escalates α-synuclein aggregate degradation in rotenone-induced Parkinson’s disease in rats: Modulation of the ubiquitin-proteasome system and endoplasmic reticulum stress. Chem. Biol. Interact. 2023, 1, 110491. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Erratum: Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 1088, Erratum in Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef]
- Wolfson, J.J.; May, K.L.; Thorpe, C.M.; Jandhyala, D.M.; Paton, J.C.; Paton, A.W. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell Microbiol. 2008, 10, 1775–1786. [Google Scholar] [CrossRef]
- Awad, H.H.; Desouky, M.A.; Zidan, A.; Bassem, M.; Qasem, A.; Farouk, M.; AlDeab, H.; Fouad, M.; Hany, C.; Basem, N.; et al. Neuromodulatory effect of vardenafil on aluminium chloride/D-galactose induced Alzheimer’s disease in rats: Emphasis on amyloid-beta, p-tau, PI3K/Akt/p53 pathway, endoplasmic reticulum stress, and cellular senescence. Inflammopharmacology 2023, 31, 2653–2673. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Ansari, N.; Nikolopoulou, C.; Papoutsi, K.; Kyrou, I.; Mantzoros, C.S.; Kyriakopoulos, G.; Chatzigeorgiou, A.; Kalotychou, V.; Randeva, M.S.; Chatha, K.; et al. Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE(−/−) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int. J. Mol. Sci. 2021, 22, 818. [Google Scholar] [CrossRef]
- Murrow, L.; Debnath, J. Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu. Rev. Pathol. 2013, 8, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Bono, E.; Ghigo, A. The Interplay Between Autophagy and Senescence in Anthracycline Cardiotoxicity. Curr. Heart Fail. Rep. 2021, 18, 180–190. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Efentakis, P.; Doerschmann, H.; Witzler, C.; Siemer, S.; Nikolaou, P.E.; Kastritis, E.; Stauber, R.; Dimopoulos, M.A.; Wenzel, P.; Andreadou, I.; et al. Investigating the Vascular Toxicity Outcomes of the Irreversible Proteasome Inhibitor Carfilzomib. Int. J. Mol. Sci. 2020, 21, 5185. [Google Scholar] [CrossRef]
- Efentakis, P.; Psarakou, G.; Varela, A.; Papanagnou, E.D.; Chatzistefanou, M.; Nikolaou, P.E.; Davos, C.H.; Gavriatopoulou, M.; Trougakos, I.P.; Dimopoulos, M.A.; et al. Elucidating Carfilzomib’s Induced Cardiotoxicity in an In Vivo Model of Aging: Prophylactic Potential of Metformin. Int. J. Mol. Sci. 2021, 22, 10956. [Google Scholar] [CrossRef]
- Cai, C.; Guo, Z.; Chang, X.; Li, Z.; Wu, F.; He, J.; Cao, T.; Wang, K.; Shi, N.; Zhou, H.; et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion through activating the AMPKα1/ULK1/FUNDC1/mitophagy pathway. Redox Biol. 2022, 52, 102288. [Google Scholar] [CrossRef]
- Dabour, M.S.; Abdelgawad, I.Y.; Grant, M.K.O.; El-Sawaf, E.S.; Zordoky, B.N. Canagliflozin mitigates carfilzomib-induced endothelial apoptosis via an AMPK-dependent pathway. Biomed. Pharmacother. 2023, 164, 114907. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Q.X.; Li, G.Z.; Wang, T.; Zhou, H. Empagliflozin ameliorates diabetic cardiomyopathy probably via activating AMPK/PGC-1α and inhibiting the RhoA/ROCK pathway. World J. Diabetes 2023, 14, 1862–1876. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.T.K.; Ng, L.; Wong, J.W.H.; Loong, H.H.F.; Chan, W.W.L.; Lee, C.H.; Wong, C.K.H. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment—A Review. Rev. Endocr. Metab. Disord. 2021, 22, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, F.; Lin, L.; Duan, S.; Liu, X.; Li, X.; Li, T.; Xue, M.; Cheng, Y.; Ren, H.; et al. An SGLT2 inhibitor modulates SHH expression by activating AMPK to inhibit the migration and induce the apoptosis of cervical carcinoma cells. Cancer Lett. 2020, 495, 200–210. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, M.Y.; Dabour, M.S.; Rashad, E.; Zordoky, B.N. Empagliflozin Alleviates Carfilzomib-Induced Cardiotoxicity in Mice by Modulating Oxidative Stress, Inflammatory Response, Endoplasmic Reticulum Stress, and Autophagy. Antioxidants 2024, 13, 671. https://doi.org/10.3390/antiox13060671
George MY, Dabour MS, Rashad E, Zordoky BN. Empagliflozin Alleviates Carfilzomib-Induced Cardiotoxicity in Mice by Modulating Oxidative Stress, Inflammatory Response, Endoplasmic Reticulum Stress, and Autophagy. Antioxidants. 2024; 13(6):671. https://doi.org/10.3390/antiox13060671
Chicago/Turabian StyleGeorge, Mina Y., Mohamed S. Dabour, Eman Rashad, and Beshay N. Zordoky. 2024. "Empagliflozin Alleviates Carfilzomib-Induced Cardiotoxicity in Mice by Modulating Oxidative Stress, Inflammatory Response, Endoplasmic Reticulum Stress, and Autophagy" Antioxidants 13, no. 6: 671. https://doi.org/10.3390/antiox13060671






