Senegenin Attenuates Pulmonary Fibrosis by Inhibiting Oxidative-Stress-Induced Epithelial Cell Senescence through Activation of the Sirt1/Pgc-1α Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiment
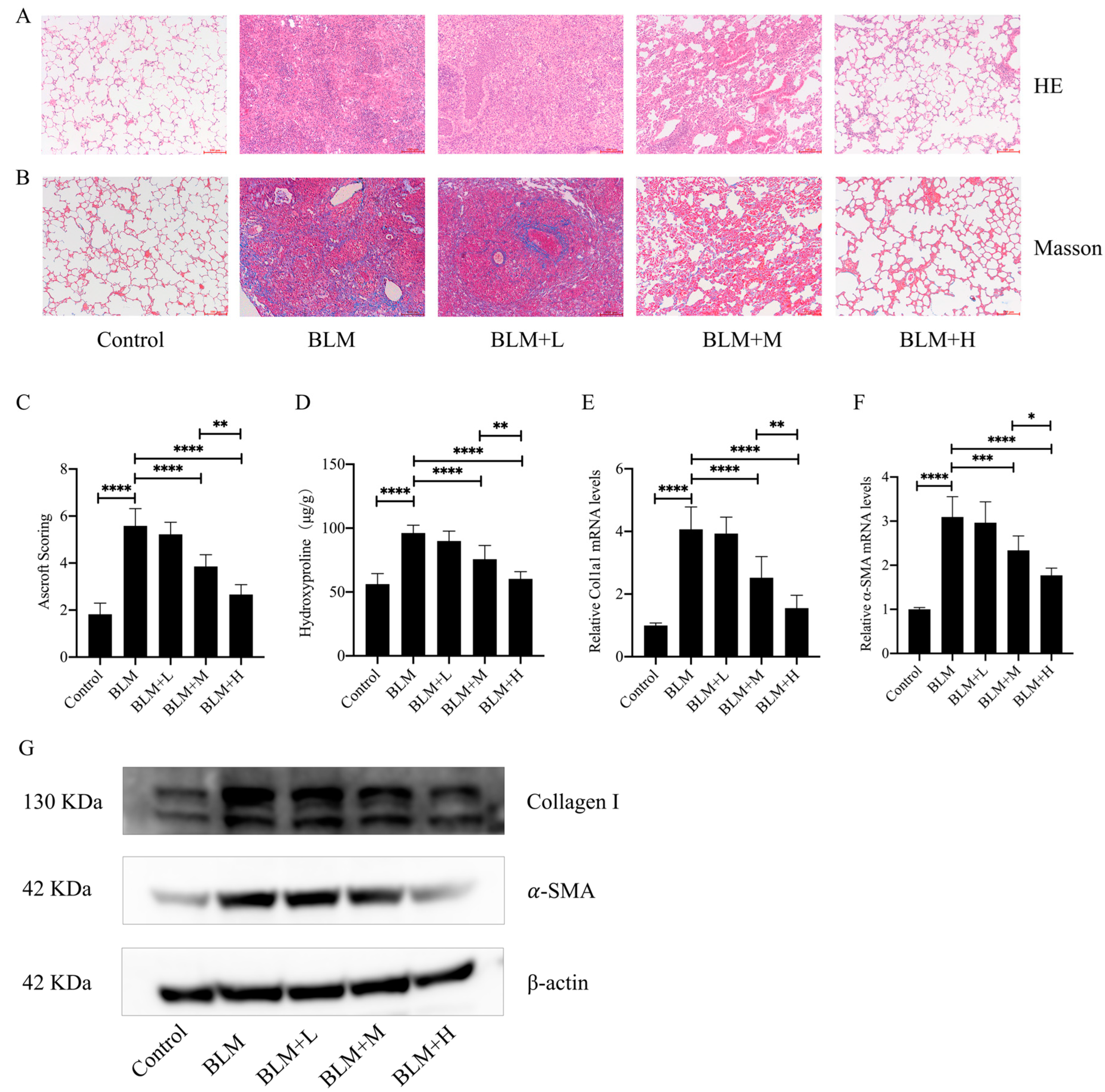
2.2. Hematoxylin–Eosin (HE) Staining, Masson Staining, and Ashcroft Score
2.3. Determination of Hydroxyproline in Lung Tissue
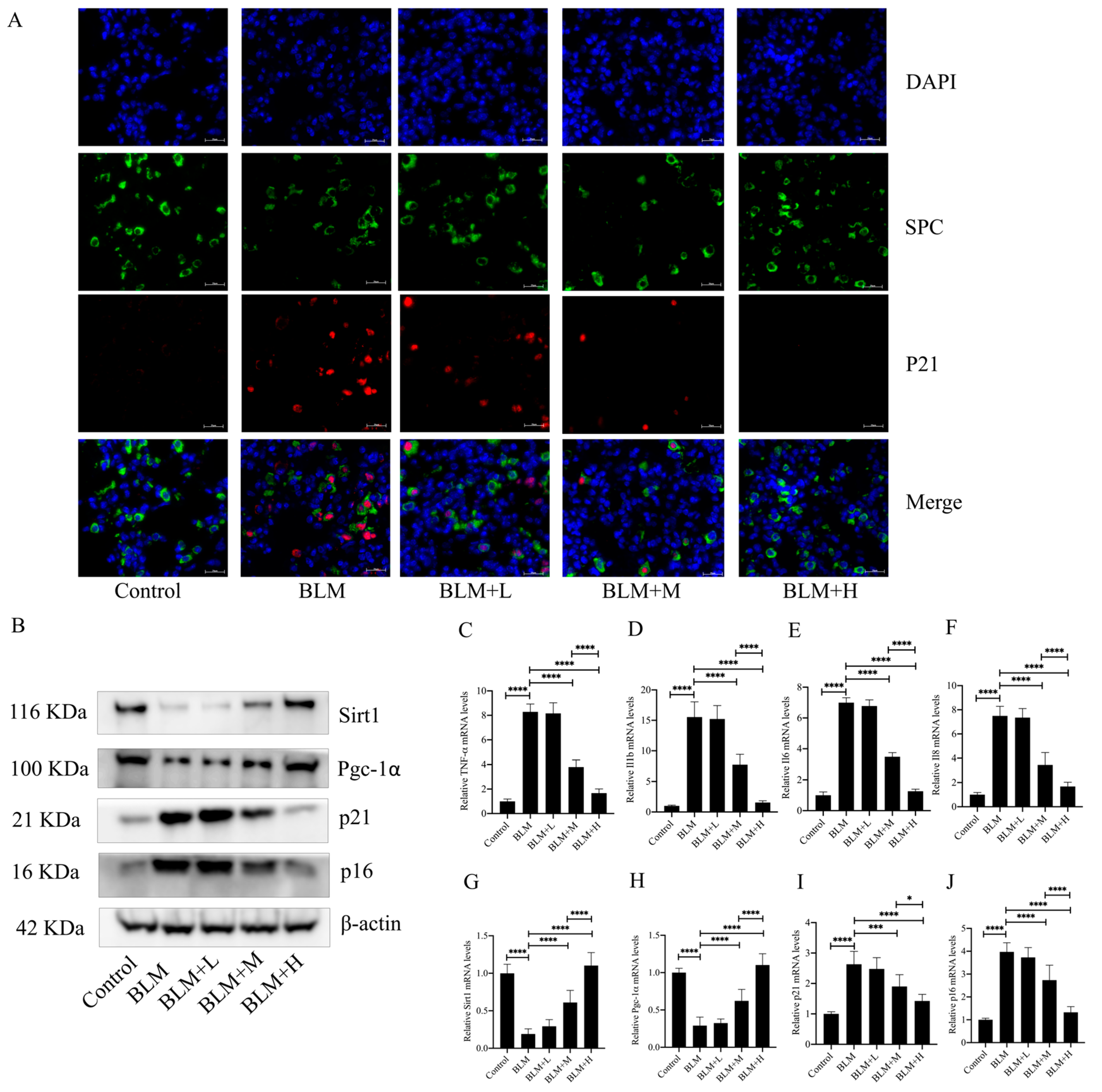
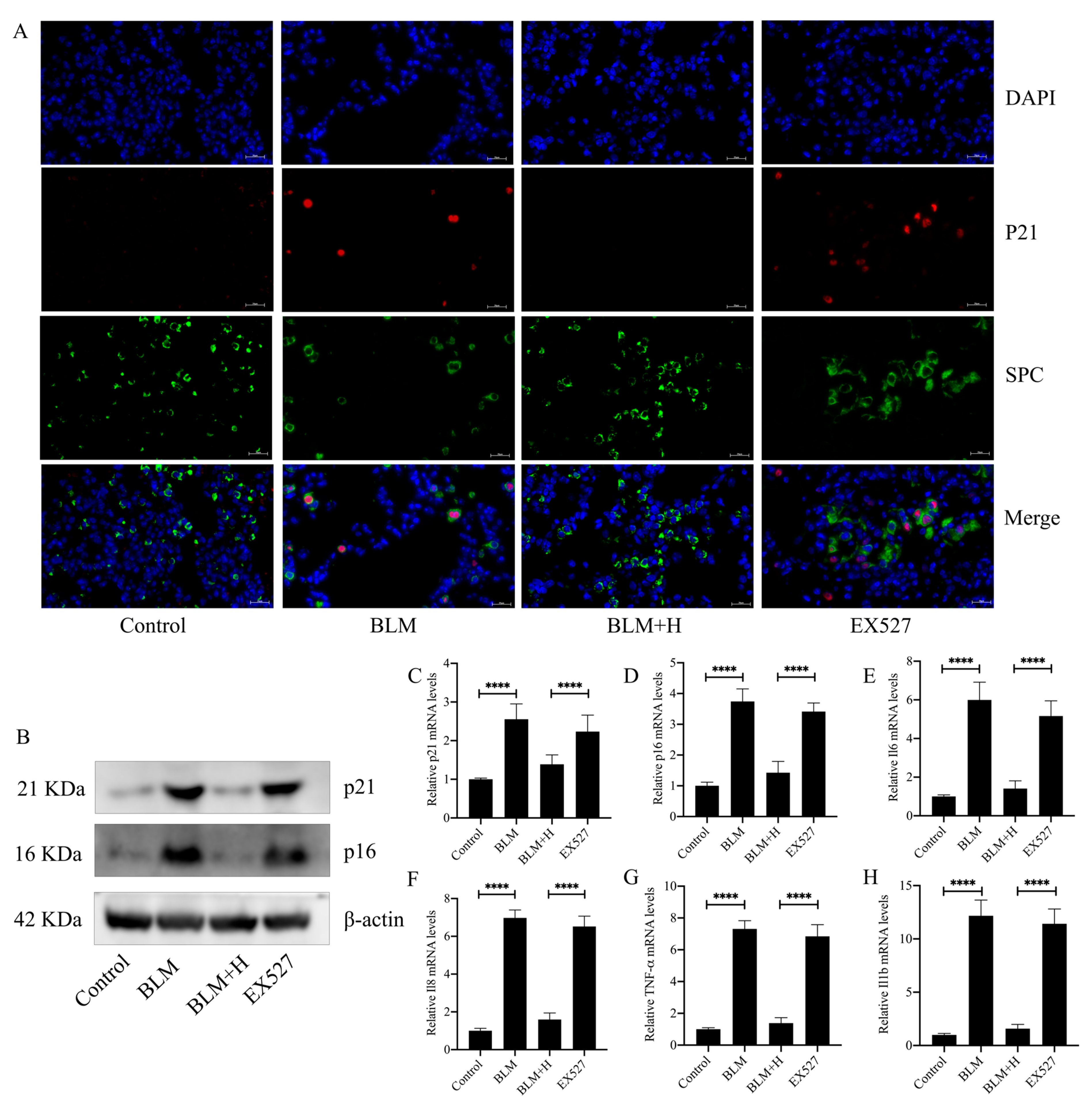
2.4. Immunofluorescence Staining
2.5. ROS Staining
2.6. Determination of Malondialdehyde (MDA) and Glutathione (GSH) Content and Total Superoxide Dismutase (SOD) Activity in Lung Tissue
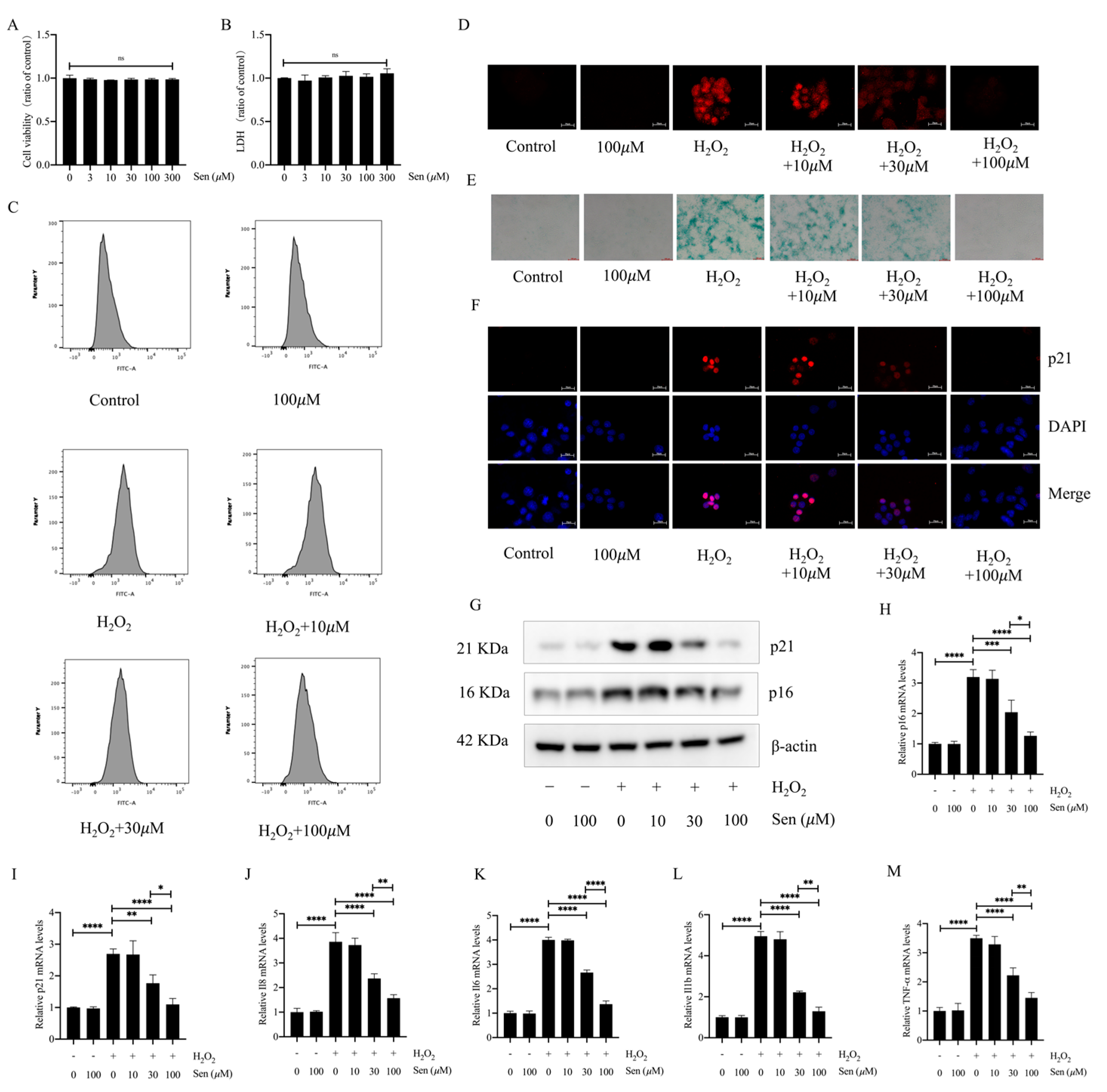
2.7. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.8. Western Blotting
2.9. Cell Culture and Hydrogen–Peroxide–Induced Epithelial Cell Senescence
2.10. Cell Viability Assay
2.11. SiRNA–Specific Gene Silencing
2.12. Statistical Analysis
3. Results
3.1. Senegenin Inhibited Bleomycin-Induced Pulmonary Fibrosis in Mice
3.2. Senegenin Reduced Oxidative Stress Levels in the Lung Tissues of Pulmonary Fibrosis Mice
3.3. Senegenin Inhibited Epithelial Cell Senescence in Lung Tissues of Pulmonary Fibrosis Mice
3.4. Senegenin Inhibited Hydrogen-Peroxide-Induced Epithelial Cell Senescence
3.5. The Sirt1/Pgc-1α Pathway Mediated the Antisenescent Effect of Senegenin
3.6. Sirt1 Mediated the In Vivo Antifibrotic Effects of Senegenin
3.7. Sirt1 Mediated the In Vivo Antioxidative Effect of Senegenin
3.8. Sirt1 Mediated the Antisenescent Effect of Senegenin In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, Y.; Li, H.; Qiu, T.; Dai, J.; Zhang, Y.; Chen, J.; Cai, H. Loss of PTEN induces lung fibrosis via alveolar epithelial cell senescence depending on NF-κB activation. Aging Cell 2019, 18, e12858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarker, R.S.J.; Conlon, T.M.; Morrone, C.; Srivastava, B.; Konyalilar, N.; Verleden, S.E.; Bayram, H.; Fehrenbach, H.; Yildirim, A. CARM1 regulates senescence during airway epithelial cell injury in COPD pathogenesis. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L602–L614. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Y.; Wang, X.; Xie, H.Y.; Yang, N.H.; Li, J.Y.; Sun, X.A.; Guo, H.J.; Zhou, L.; Zhang, W.; Liu, J.; et al. Deubiquitinating enzyme USP11 promotes renal tubular cell senescence and fibrosis via inhibiting the ubiquitin degradation of TGF-β receptor II. Acta Pharmacol. Sin. 2023, 44, 584–595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, S.; Ji, C.; Wei, K. Cellular Senescence and Regulated Cell Death of Tubular Epithelial Cells in Diabetic Kidney Disease. Front. Endocrinol. 2022, 13, 924299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, C.; Guan, X.; Carraro, G.; Parimon, T.; Liu, X.; Huang, G.; Mulay, A.; Soukiasian, H.J.; David, G.; Weigt, S.S.; et al. Senescence of Alveolar Type 2 Cells Drives Progressive Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2021, 203, 707–717, Erratum in Am. J. Respir. Crit. Care Med. 2021, 204, 113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, J.; Huang, G.; Liu, X.; Taghavifar, F.; Liu, N.; Wang, Y.; Deng, N.; Yao, C.; Xie, T.; Kulur, V.; et al. The ZIP8/SIRT1 axis regulates alveolar progenitor cell renewal in aging and idiopathic pulmonary fibrosis. J. Clin. Investig. 2022, 132, e157338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheresh, P.; Kim, S.J.; Jablonski, R.; Watanabe, S.; Lu, Z.; Chi, M.; Helmin, K.A.; Gius, D.; Budinger, G.R.S.; Kamp, D.W. SIRT3 Overexpression Ameliorates Asbestos-Induced Pulmonary Fibrosis, mt-DNA Damage, and Lung Fibrogenic Monocyte Recruitment. Int. J. Mol. Sci. 2021, 22, 6856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minagawa, S.; Araya, J.; Numata, T.; Nojiri, S.; Hara, H.; Yumino, Y.; Kawaishi, M.; Odaka, M.; Morikawa, T.; Nishimura, S.L.; et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 300, L391–L401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Yang, Y.; Han, Y.; Wang, X. Neuroprotective Effects and Mechanisms of Senegenin, an Effective Compound Originated from the Roots of Polygala Tenuifolia. Front. Pharmacol. 2022, 13, 937333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.H.; Zhang, W.D.; Wang, J.J.; Feng, S.D. Senegenin Ameliorate Acute Lung Injury Through Reduction of Oxidative Stress and Inhibition of Inflammation in Cecal Ligation and Puncture-Induced Sepsis Rats. Inflammation 2016, 39, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, Y.; Gu, X.; Zheng, Y.; Sun, Y.E.; Liang, Y.; Bo, J.; Ma, Z. Senegenin attenuates hepatic ischemia-reperfusion induced cognitive dysfunction by increasing hippocampal NR2B expression in rats. PLoS ONE 2012, 7, e45575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.Q.; Wu, M.F.; Gu, R.; Liu, J.B.; Li, Y.; Jiang, J.L. Senegenin inhibits neuronal apoptosis after spinal cord contusion injury. Neural Regen. Res. 2016, 11, 657–663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashcroft, T.; Simpson, J.M.; Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988, 41, 467–470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Zhou, W.; Li, J.; Qiu, Z.; Wang, X.; Xu, H.; Wang, H.; Lu, D.; Qi, R. Senegenin Rescues PC12 Cells with Oxidative Damage Through Inhibition of Ferroptosis. Mol. Neurobiol. 2022, 59, 6983–6992. [Google Scholar] [CrossRef] [PubMed]
- Otoupalova, E.; Smith, S.; Cheng, G.; Thannickal, V.J. Oxidative Stress in Pulmonary Fibrosis. Compr. Physiol. 2020, 10, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Jin, W.; Zhao, Y.; Wang, H.; He, S.; Zhang, W.; Zhou, J. Sulfated Fucogalactan from Laminaria Japonica Ameliorates β-Cell Failure by Attenuating Mitochondrial Dysfunction via SIRT1–PGC1-α Signaling Pathway Activation. Front. Endocrinol. 2022, 13, 881256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papaconstantinou, J. The Role of Signaling Pathways of Inflammation and Oxidative Stress in Development of Senescence and Aging Phenotypes in Cardiovascular Disease. Cells 2019, 8, 1383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, J.H.; Baskaran, R.; Wang, M.F.; Yang, H.S.; Lo, Y.H.; Mohammedsaleh, Z.M.; Lin, W.T. Bioactive Peptides and Exercise Modulate the AMPK/SIRT1/PGC-1α/FOXO3 Pathway as a Therapeutic Approach for Hypertensive Rats. Pharmaceuticals 2022, 15, 819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, S.; Lv, Y.; Yang, D.; Li, J.; Yang, Q.; Wu, P.; Lv, Z.; Zhang, Z. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019, 10, 5555–5565. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Di, W.; Zhang, Z.; Li, N.; Qiu, Z.; Shi, W.; Lei, W.; Tang, J.; Yang, Y.; Xu, B.; et al. Activation of ITLN-1 attenuates oxidative stress injury via activating SIRT1/PGC1-α signaling in neuroblastoma cells. J. Cell. Physiol. 2024, 239, e31144. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, L.; Zhou, S.; Ma, Y.R.; Xiao, X.; Jiang, Q.; Kang, Z.H.; Liu, M.L.; Liu, T.X. Erythropoietin promotes energy metabolism to improve LPS-induced injury in HK-2 cells via SIRT1/PGC1-α pathway. Mol. Cell. Biochem. 2023, 478, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. Cellular senescence: Molecular mechanisms, in vivo significance, and redox considerations. Antioxid. Redox Signal. 2009, 11, 59–98. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Kennedy, B.K. SIRT6, oxidative stress, and aging. Cell Res. 2016, 26, 143–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, Z.; Liang, Z.; Yang, H.; Pan, Y.; Zheng, Y.; Wang, X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J. Neuroinflamm. 2017, 14, 256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, W.; Zheng, Z.; Wang, W.; Yuan, Y.; Hong, Q.; Lin, J.; Li, X.; Meng, Y. Cigarette smoke-inactivated SIRT1 promotes autophagy-dependent senescence of alveolar epithelial type 2 cells to induce pulmonary fibrosis. Free. Radic. Biol. Med. 2021, 166, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, D.; Ran, Y.; Yu, R.; Liu, G.; Ran, D.; Liu, Z. SIRT1 regulates osteoblast senescence through SOD2 acetylation and mitochondrial dysfunction in the progression of Osteoporosis caused by Cadmium exposure. Chem.-Biol. Interact. 2023, 382, 110632. [Google Scholar] [CrossRef] [PubMed]
- Karnewar, S.; Pulipaka, S.; Katta, S.; Panuganti, D.; Neeli, P.K.; Thennati, R.; Jerald, M.K.; Kotamraju, S. Mitochondria-targeted esculetin mitigates atherosclerosis in the setting of aging via the modulation of SIRT1-mediated vascular cell senescence and mitochondrial function in Apoe−/− mice. Atherosclerosis 2022, 356, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiong, R.; Li, G.; Wang, B.; Geng, Q. PM2.5 contributed to pulmonary epithelial senescence and ferroptosis by regulating USP3-SIRT3-P53 axis. Free Radic. Biol. Med. 2023, 205, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1α-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Front. Cell Dev. Biol. 2022, 10, 871357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huss, J.M.; Kelly, D.P. Mitochondrial energy metabolism in heart failure: A question of balance. J. Clin. Investig. 2005, 115, 547–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggarwal, R.; Potel, K.N.; McFalls, E.O.; Butterick, T.A.; Kelly, R.F. Novel Therapeutic Approaches Enhance PGC1-alpha to Reduce Oxidant Stress-Inflammatory Signaling and Improve Functional Recovery in Hibernating Myocardium. Antioxidants 2022, 11, 2155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.J.; Ng, S.C.; Hsu, J.Y.; Liu, H.; Chen, C.J.; Huang, C.Y.; Kuo, W.W. Galangin Reverses H2O2-Induced Dermal Fibroblast Senescence via SIRT1-PGC-1α/Nrf2 Signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, N.; Xu, M.H.; Li, Y. Bioactive Oligopeptides from Ginseng (Panax ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, D.; Zhao, X.Y.; Meng, Q.P.; Teng, D.; Deng, K.; Lin, N. Resveratrol activates the SIRT1/PGC-1 pathway in mice to improve synaptic-related cognitive impairment after TBI. Brain Res. 2022, 1796, 148109. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Liu, P.; Zhu, X.; Chen, M.; Wang, H.; Lu, D.; Qi, R. Senegenin Inhibits Hypoxia/Reoxygenation-Induced Neuronal Apoptosis by Upregulating RhoGDIα. Mol. Neurobiol. 2015, 52, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Qiao, J.; Shen, Y.; Li, L. Protective effects of tenuigenin on Staphylococcus aureus-induced pneumonia in mice. Microb. Pathog. 2017, 110, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Y.; Gai, W.; Yu, B. Protective role of tenuigenin on sepsis-induced acute kidney injury in mice. Exp. Ther. Med. 2017, 14, 5051–5056. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Gene Name | Forward [5′–3′] | Reverse [5′–3′] |
|---|---|---|
| Mouse collagen I | GAGCGGAGAGTACTGGATCG | GCTTCTTTTCCTTGGGGTTC |
| Mouse α-SMA | TGGCTATTCAGGCTGTGCTGTC | CAATCTCACGCTCGGCAGTAGT |
| Mouse β-Actin | GTGCTATGTTGCTCTAGACTTCG | ATGCCACAGGATTCCATACC |
| Mouse p21 | CCTGGTGATGTCCGACCTGTTC | CGAAGTCAAAGTTCCACCGTTCTC |
| Mouse p16 | AAGAGCGGGGACATCAAGACATC | AAAGACCACCCAGCGGAACAC |
| Mouse Pgc-1α | TTCGCTGCTCTTGAGAATGGATATAC | TCGTCTGAGTTGGTATCTAGGTCTG |
| Mouse Sirt1 | GTGGCAGTAACAGTGACAGTGG | TCCAGATCCTCCAGCACATTCG |
| Mouse TNF-α | GTGCCTATGTCTCAGCCTCTTCTC | TGGTTTGTGAGTGTGAGGGTCTG |
| Mouse IL-1β | CTCGCAGCAGCACATCAACAAG | CCACGGGAAAGACACAGGTAGC |
| Mouse IL-6 | TTCTTGGGACTGATGCTGGTGAC | GTGGTATCCTCTGTGAAGTCTCCTC |
| Mouse IL-8 | CTCCTGCTGGCTGTCCTTAACC | TGGGACTGCTATCACTTCCTTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Luo, Y.; Sang, X.; Liao, M.; Wen, B.; Hu, Z.; Sun, M.; Luo, Z.; Huang, X.; Liu, W.; et al. Senegenin Attenuates Pulmonary Fibrosis by Inhibiting Oxidative-Stress-Induced Epithelial Cell Senescence through Activation of the Sirt1/Pgc-1α Signaling Pathway. Antioxidants 2024, 13, 675. https://doi.org/10.3390/antiox13060675
Zeng Q, Luo Y, Sang X, Liao M, Wen B, Hu Z, Sun M, Luo Z, Huang X, Liu W, et al. Senegenin Attenuates Pulmonary Fibrosis by Inhibiting Oxidative-Stress-Induced Epithelial Cell Senescence through Activation of the Sirt1/Pgc-1α Signaling Pathway. Antioxidants. 2024; 13(6):675. https://doi.org/10.3390/antiox13060675
Chicago/Turabian StyleZeng, Qian, Yuyang Luo, Xiaoxue Sang, Minlin Liao, Binbin Wen, Zhengang Hu, Mei Sun, Ziqiang Luo, Xiaoting Huang, Wei Liu, and et al. 2024. "Senegenin Attenuates Pulmonary Fibrosis by Inhibiting Oxidative-Stress-Induced Epithelial Cell Senescence through Activation of the Sirt1/Pgc-1α Signaling Pathway" Antioxidants 13, no. 6: 675. https://doi.org/10.3390/antiox13060675
APA StyleZeng, Q., Luo, Y., Sang, X., Liao, M., Wen, B., Hu, Z., Sun, M., Luo, Z., Huang, X., Liu, W., & Tang, S. (2024). Senegenin Attenuates Pulmonary Fibrosis by Inhibiting Oxidative-Stress-Induced Epithelial Cell Senescence through Activation of the Sirt1/Pgc-1α Signaling Pathway. Antioxidants, 13(6), 675. https://doi.org/10.3390/antiox13060675






