Formulation and Physical–Chemical Analysis of Functional Muffin Made with Inulin, Moringa, and Cacao Adapted for Elderly People with Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Product
2.2. Physical–Chemical Analysis
2.2.1. Determination of Macronutrients
Determination of Moisture
Moisture %: 100 − Dry matter
Determination of Inorganic Matter
Determination of Energy Value
Determination of Total Nitrogen and Protein Content
- 6.25: meat, fish, eggs, legumes, and vegetable protein.
- 5.7: cereals and soy derivatives.
- 6.38: milk and dairy products.
- 5.55: jelly.
- 5.95: rice.
Determination of Carbohydrates (Carbs)
Available carbs (%): Total carbs − DF
Determination of Total Fat
2.2.2. Determination of Folate Content
2.2.3. Determination of Dietary Fiber
2.2.4. Determination of Antioxidant Capacity
Oxygen Radical Absorbance Capacity (ORAC) Technique
Ferric Reducing Antioxidant Power (FRAP) Technique
ABTS Technique
DPPH Technique
2.3. Sensory Analysis and Product Tasting
2.3.1. Colorimetry
2.3.2. Degustation of the Product (Product Tasting)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Composition of the Product and Its Benefits
3.2. Determination of Macronutrients
3.3. Determination of Dietary Fiber
3.4. Determination of Antioxidant Activity
3.5. Folate Content
3.6. Sensory Analysis
3.6.1. Colorimetry
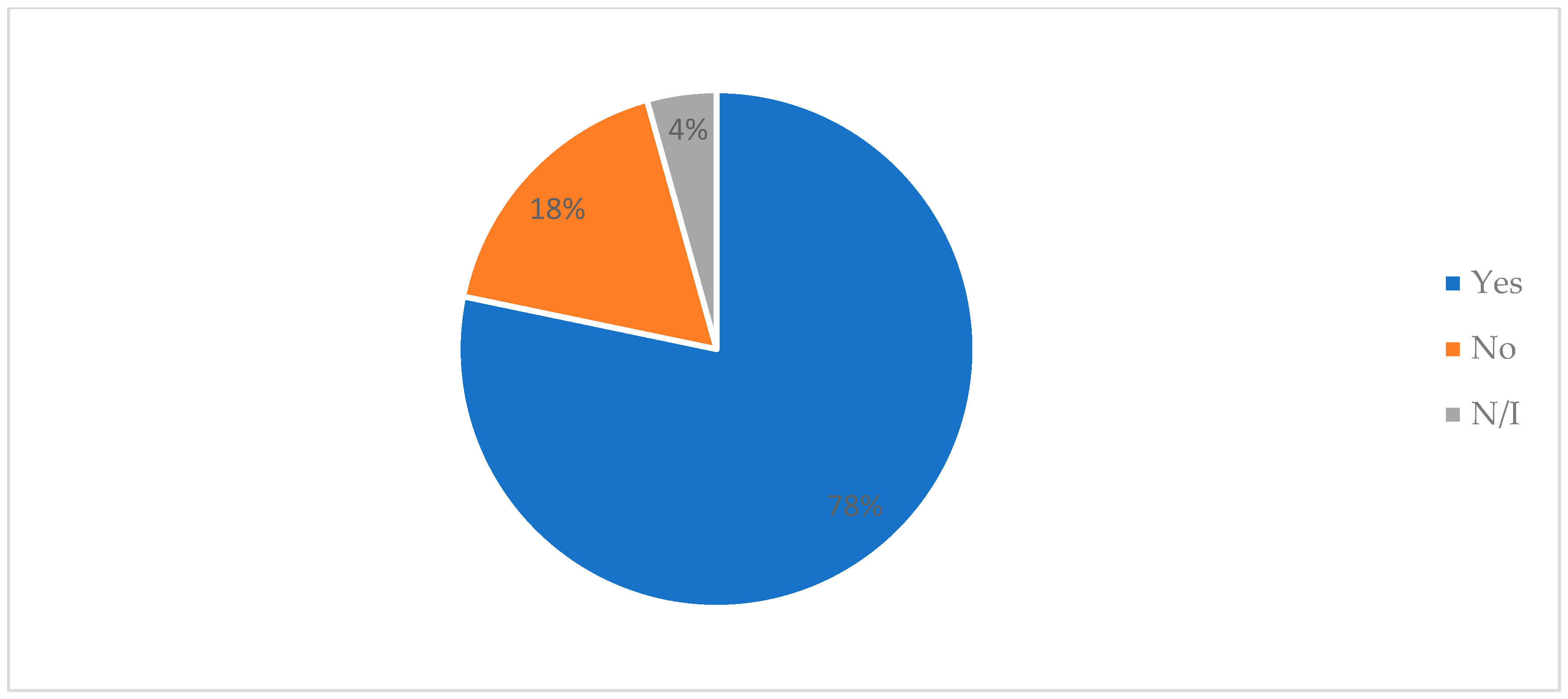
3.6.2. Product Tasting
3.6.3. The Limitations of This Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valadez-Barba, V.; Juárez-Navarro, K.; Padilla-Camberos, E.; Díaz, N.F.; Guerra-Mora, J.R.; Díaz-Martínez, N.E. Parkinson’s disease: An update on preclinical studies of induced pluripotent stem cells. Neurologia 2023, 38, 681–694. [Google Scholar] [CrossRef]
- Cófreces, P.; Ofman, S.D.; Estay, J.A.; Hermida, P.D. Enfermedad de Parkinson: Una actualización bibliográfica de los aspectos psicosociales. Rev. Fac. Cienc. Med. 2022, 79, 181. [Google Scholar] [CrossRef]
- Pérez Menéndez, A. El Número de Afectados por la Enfermedad de Parkinson se Duplicará en 20 Años y se Triplicará en 2050; Sociedad Española de Neurología: Barcelona, Spain, 2023; Available online: https://www.sen.es/saladeprensa/pdf/Link238.pdf (accessed on 19 October 2023).
- Leiva, A.M.; Martínez-Sanguinetti, M.A.; Troncoso-Pantoja, C.; Nazar, G.; Petermann-Rocha, F.; Celis-Morales, C. Chile lidera el ranking latinoamericano de prevalencia de enfermedad de Parkinson. Rev. Med. Chil. 2019, 147, 535–536. [Google Scholar] [CrossRef]
- Vial, F.; Delgado, I.; Idiaquez, J.F.; Canals, F.; Chana-Cuevas, P. Epidemiology of Parkinson’s Disease in Chile. Neuroepidemiology 2021, 55, 393–397. [Google Scholar] [CrossRef]
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jésus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar] [CrossRef]
- Parkinson’s Fundation. Síntomas Motores. Available online: https://www.parkinson.org/espanol/entendiendo-parkinson/sintomas-motores (accessed on 23 October 2023).
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Singapore, 2018; pp. 3–26. [Google Scholar]
- Parkinson’s Fundation. Síntomas No Motores. Available online: https://www.parkinson.org/espanol/entendiendo-parkinson/sintomas-no-motores (accessed on 23 October 2023).
- Berganzo, K.; Tijero, B.; González-Eizaguirre, A.; Somme, J.; Lezcano, E.; Gabilondo, I.; Fernandez, M.; Zarranz, J.J.; Gómez-Esteban, J.C. Síntomas no motores y motores en la enfermedad de Parkinson y su relación con la calidad de vida y los distintos subgrupos clínicos. Neurología 2016, 31, 585–591. [Google Scholar] [CrossRef]
- Pérez-Macho, L.; Borja-Andrés, S. Trastornos digestivos en la enfermedad de Parkinson: Atonía gástrica, malabsorción y estreñimiento. Rev. Neurol. 2010, 50 (Suppl. 2), S55–S58. [Google Scholar] [CrossRef]
- Yao, L.; Liang, W.; Chen, J.; Wang, Q.; Huang, X. Constipation in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Eur. Neurol. 2023, 86, 34–44. [Google Scholar] [CrossRef]
- Svensson, E.; Henderson, V.W.; Borghammer, P.; Horváth-Puhó, E.; Sørensen, H.T. Constipation and risk of Parkinson’s disease: A Danish population-based cohort study. Park. Relat. Disord. 2016, 28, 18–22. [Google Scholar] [CrossRef]
- Stocchi, F.; Torti, M. Constipation in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 134, 811–826. [Google Scholar]
- Quigley, E.M.M. Constipation in Parkinson’s Disease. Semin Neurol. 2023, 43, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Adams-Carr, K.L.; Bestwick, J.P.; Shribman, S.; Lees, A.; Schrag, A.; Noyce, A.J. Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Leta, V.; Urso, D.; Batzu, L.; Weintraub, D.; Titova, N.; Aarsland, D.; Martinez-Martin, P.; Borghammer, P.; Van Wamelen, D.J.; Yousaf, T.; et al. Constipation is Associated with Development of Cognitive Impairment in de novo Parkinson’s Disease: A Longitudinal Analysis of Two International Cohorts. J. Parkinson’s Dis. 2021, 11, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, P.; Hoffman, M.; Badiali, D.; Colosimo, C. Constipation: An emerging risk factor for Parkinson’s disease? Eur. J. Neurol. 2016, 23, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.; Constipation. Fox Fundation. Available online: https://www.michaeljfox.org/news/constipation (accessed on 9 November 2023).
- Fernandez Pardal, M. Neurología; Editorial Médica Panamericana: Madrid, Spain, 2019; 241p. [Google Scholar]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Diseases. 2020, 39, 403–413. [Google Scholar] [CrossRef]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Glibowski, P.; Skrzypek, M.; Ćwiklińska, M.; Drozd, M.; Kowalska, A. Chemical stability of fructans in apple beverages and their influence on chronic constipation. Food Funct. 2020, 11, 3860–3866. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Metodos Analiticos para la Determinacion de Humedad, Alcohol, Energia, Materia Grasa y Colesterol en Alimentos. Available online: https://www.fao.org/3/ah833s/Ah833s16.htm#:~:text=La%20determinaci%C3%B3n%20de%20humedad%20es,base%20tal%20como%20se%20recibi%C3%B3 (accessed on 25 October 2023).
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis of Official Analytical Chemists. Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 25 October 2023).
- Greenfield, H.; Southgate, D.A.T. Datos de Composición de Alimentos: Obtención, Gestión y Utilización; FAO—Organizacion de las Naciones Unidas para la Agricultura y la Alimentacion: Rome, Italy, 2003. [Google Scholar]
- Raymond, E. Métodos Aplicados Análisis Ley 20606 Etiquetado Nutricional. Available online: https://www.achipia.gob.cl/wp-content/uploads/2016/06/2-ISP-Salud-Ambiental-Ing.-Emilia-Raymond-.pdf (accessed on 23 October 2023).
- Peñalver, R.; Ros, G.; Nieto, G. Development of Gluten-Free Functional Bread Adapted to the Nutritional Requirements of Celiac Patients. Fermentation 2023, 9, 631. [Google Scholar] [CrossRef]
- Konings, E.J.M. A Validated Liquid Chromatographic Method for Determining Folates in Vegetables, Milk Powder, Liver, and Flour. J. AOAC Int. 1999, 82, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Rogers, L.M.; Gregory, J.F. Determination of Folate in Cereal-Grain Food Products Using Trienzyme Extraction and Combined Affinity and Reversed-Phase Liquid Chromatography. J. Agric. Food Chem. 1997, 45, 407–413. [Google Scholar] [CrossRef]
- Martín Tornero, E. Análisis de Folatos en Muestras Alimentarias Mediante Técnicas Cromatográficas de Alta Resolución. Available online: https://dehesa.unex.es/bitstream/10662/15229/1/978-84-697-4644-8_257.pdf (accessed on 29 October 2023).
- Vahteristo, L.T.; Ollilainen, V.; Koivistoinen, P.E.; Varo, P. Improvements in the Analysis of Reduced Folate Monoglutamates and Folic Acid in Food by High-Performance Liquid Chromatography. J. Agric. Food Chem. 1996, 44, 477–482. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Furda, I.; DeVries, J.W.; Schweizer, T.F.; Harland, B.F. Determination of Total Dietary Fiber in Foods and Food Products: Collaborative Study. J. AOAC Int. 1985, 68, 677–679. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC) and Phenolic and Anthocyanin Concentrations in Fruit and Leaf Tissues of Highbush Blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 15–27. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Laurri, A.; Saura-Calixto, F.; Sanchez-Moreno, C. Un procedimiento para medir la eficacia antirradical de los polifenoles. J. Cienc. Aliment. Agric. 1998, 76, 270–276. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mathias-Rettig, K.; Ah-Hen, K. El color en los alimentos un criterio de calidad medible. Agro Sur 2014, 42, 57–66. [Google Scholar] [CrossRef]
- UNE-EN ISO 8589:2010 Análisis Sensorial. Guía General Para el Diseño de una Sala de Cata [Internet]. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0045203 (accessed on 28 May 2024).
- Julia Espinoza, M. Evaluación Sensorial de los Alimentos; Torricella, R., Ed.; Universitaria: La Habana, Cuba, 2007; pp. 12–13. [Google Scholar]
- Madrigal, L.; Sangronis, E. La Inulina y Derivados Como Ingredientes Claves en Alimentos Funcionales. 2007. Available online: https://www.alanrevista.org/ediciones/2007/4/art-12/ (accessed on 16 November 2023).
- Rodriguez Garcia, J. Reformulación de Productos Horneados para Disminuir el Contenido en Grasa y Azúcar Mediante Sustitución con Inulina. Efectos Sobre la Estructura y Propiedades Físicas; Universitat Politècnica de València: Valencia, Spain, 2014. [Google Scholar]
- Gutiérrez-Luna, K.; Astiasarán, I.; Ansorena, D. Gels as fat replacers in bakery products: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3768–3781. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Ramos, S.; Alía, M.; Rúperez, P.; Goya, L.; Izquierdo-Pulido, M.; Bravo, L. Caracterización de la fibra de cacao y su efecto sobre la capacidad antioxidante en suero de animales de experimentación. Nutr. Hosp. 2006, 21, 622–628. [Google Scholar] [PubMed]
- Tafurt, G.; Suarez, O.; Lares, M.d.C.; Álvarez, C.; Liconte, N. Capacidad antioxidante de un chocolate oscuro de granos cacao orgánico sin fermentar. Rev. Digit. Postgrado 2020, 10, e280. [Google Scholar] [CrossRef]
- Beshel, J.A.; Beshel, F.N.; Nwangwa, J.N.; Okon, I.A.; Ejim, C.I.; Owu, D.U. Cardioprotective Role of Theobroma cacao against Isoproterenol-Induced Acute Myocardial Injury. Cardiovasc. Hematol. Agents Med. Chem. 2022, 20, 75–80. [Google Scholar] [CrossRef]
- Camandola, S.; Plick, N.; Mattson, M.P. Impact of Coffee and Cacao Purine Metabolites on Neuroplasticity and Neurodegenerative Disease. Neurochem. Res. 2019, 44, 214–227. [Google Scholar] [CrossRef]
- Mundkar, M.; Bijalwan, A.; Soni, D.; Kumar, P. Neuroprotective potential of Moringa oleifera mediated by NF-kB/Nrf2/HO-1 signaling pathway: A review. J. Food Biochem. 2022, 46, e14451. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xu, T.; Tian, Y.; Zhong, Y.; Ali, F.A.Z.; Yang, X.; Lu, B. Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review. Phytomedicine 2021, 93, 153771. [Google Scholar] [CrossRef]
- Cuellar-Núñez, M.L.; Gonzalez de Mejia, E.; Loarca-Piña, G. Moringa oleifera leaves alleviated inflammation through downregulation of IL-2, IL-6, and TNF-α in a colitis-associated colorectal cancer model. Food Res. Int. 2021, 144, 110318. [Google Scholar] [CrossRef]
- Peñalver, R.; Martínez-Zamora, L.; Lorenzo, J.M.; Ros, G.; Nieto, G. Nutritional and Antioxidant Properties of Moringa oleifera Leaves in Functional Foods. Foods 2022, 11, 1107. [Google Scholar] [CrossRef]
- Rodriguez, J.P. Evaluación de Inulina como Reemplazante de Grasa en Tortas de Bajo Contenido Calórico a Través de la Vida Útil; Universidad Nacional de Colombia: Bogotá, Colombia, 2016; Available online: https://repositorio.unal.edu.co/handle/unal/57928 (accessed on 28 December 2023).
- Doménech-Asensi, G.; Merola, N.; López-Fernández, A.; Ros-Berruezo, G.; Frontela-Saseta, C. Influence of the reformulation of ingredients in bakery products on healthy characteristics and acceptability of consumers. Int. J. Food Sci. Nutr. 2016, 67, 74–82. [Google Scholar] [CrossRef]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 2010, 257, 253–261. [Google Scholar] [CrossRef]
- Sanchís, G.; Mena, M.A.; Martín del Río, R.; Morales, B.; Casarejo, M.J.; de Yébenes, M.J.; Tabernero, C.; Jiménez, A.; de Yébenes, J.G. Effect of a controlled low-protein diet on the pharmacological response to levodopa and on the plasma levels of L-dopa and amino acids in patients with Parkinson’s disease. Arch. Neurobiol. 1991, 54, 296–302. [Google Scholar]
- Cai, Y.; Feng, F.; Wei, Q.; Jiang, Z.; Ou, R.; Shang, H. Sarcopenia in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 598035. [Google Scholar] [CrossRef]
- Rodríguez-García, J.; Puig, A.; Salvador, A.; Hernando, I. Optimization of a Sponge Cake Formulation with Inulin as Fat Replacer: Structure, Physicochemical, and Sensory Properties. J. Food Sci. 2012, 77, C189–C197. [Google Scholar] [CrossRef]
- Islas-Rubio, A.R.; Hernández-Zepeda, A.; Calderón de la Barca, A.M.; Ballesteros-Vásquez, M.N.; Granados-Nevárez, M.d.C.; Vásquez-Lara, F. Formulación y elaboración de pastelillos tipo brownies con más fibra y menos calorías que los convencionales. Arch. Latinoam. Nutr. 2012, 62, 185–191. [Google Scholar]
- Praznik, W.; Cieślik, E.; Filipiak-Florkiewicz, A. Soluble dietary fibres in Jerusalem artichoke powders: Composition and application in bread. Nahrung/Food 2002, 46, 151. [Google Scholar] [CrossRef]
- Bhanja, A.; Sutar, P.P.; Mishra, M. Inulin-A polysaccharide: Review on its functional and prebiotic efficacy. J. Food Biochem. 2022, 46, e14386. [Google Scholar] [CrossRef]
- de Luis, D.A.; de la Fuente, B.; Izaola, O.; Conde, R.; Gutiérrez, S.; Morillo, M. Randomized clinical trial with a inulin enriched cookie on risk cardiovascular factor in obese patients. Nutr. Hosp. 2010, 25, 53–59. [Google Scholar]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef]
- Bizeau, J.B.; Albouery, M.; Grégoire, S.; Buteau, B.; Martine, L.; Crépin, M.; Bron, A.M.; Berdeaux, O.; Acar, N.; Chassaing, B.; et al. Dietary Inulin Supplementation Affects Specific Plasmalogen Species in the Brain. Nutrients 2022, 14, 3097. [Google Scholar] [CrossRef]
- Castillo, L.C.; Tur, J.A.; Uauy, R. Fortificación de la harina de trigo con ácido fólico en Chile: Consecuencias no intencionadas. Rev. Med. Chil. 2010, 138, 832–840. [Google Scholar] [CrossRef]
- Centeno Tablante, E.; Pachón, H.; Guetterman, H.M.; Finkelstein, J.L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Organización Panamericana de la Salud (OPS). CARMEN, Observatorio de Políticas de Enfermedades No Transmisibles: Formulación de la Política de Fortificación de Harina de Trigo con Ácido Fólico. 2006. Available online: https://www3.paho.org/spanish/ad/dpc/nc/cmn-obs-pol-cor-est-caso.pdf (accessed on 23 January 2024).
- Zheng, J.; Wang, X.; Wu, B.; Qiao, L.; Zhao, J.; Pourkheirandish, M.; Wang, J.; Zheng, X. Folate (vitamin B9) content analysis in bread wheat (Triticum aestivum L.). Front. Nutr. 2022, 9, 933358. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, F. El Pan Nuestro de Cada Día: Su Historia y Fortificación—Instituto de Nutrición y Tecnología de los Alimentos—Universidad de Chile; Unidad de Nutrición Humana, INTA—Universidad de Chile: Santiago, Chile, 2022; Available online: https://inta.uchile.cl/noticias/201109/el-pan-nuestro-de-cada-dia-su-historia-y-fortificacion (accessed on 18 February 2024).
- Ministerios de Salud del Gobierno de Chile. Reglamento Sanitario de los Alimentos; Santiago, Chile, 2013. [Google Scholar]
- Wilma, F. Nutricion y Vida Avtice: Del Conocimiento a la Accion—Google Libros; Organización Panamericana de la Salud (OPS): Washington, DC, USA, 2006; pp. 99–107. [Google Scholar]
- Muller, H. Determination of the folic acid content of grain, cereal products, baked goods and legumes using high-performance liquid chromatography (HPLC). Z. Lebensm. Unters. Forsch. 1993, 197, 573–577. [Google Scholar] [PubMed]
- Cecilia, L. Modificaciones de Componentes de los Alimentos: Cambios Químicos y Bioquímicos por Procesamiento y Almacenamiento. Edulp. Buenos Aires; 2013; 158p. Available online: https://www.google.com/url?sa=i&url=https%3A%2F%2Flibros.unlp.edu.ar%2Findex.php%2Funlp%2Fcatalog%2Fdownload%2F91%2F73%2F239-1&psig=AOvVaw3oTN-vHyirDaALC3wqH_uR&ust=1708438159722000&source=images&cd=vfe&opi=89978449&ved=0CAgQrpoMahcKEwiovcrIy7eEAxUAAAAAHQAAAAAQBA (accessed on 18 February 2024).
- Russo, M.; Elichalt, M.; Vázquez, D.; Suburú, G.; Tihista, H.; Godiño, M. Fortificación de harina de trigo con ácido fólico y hierro en Uruguay; implicancias en la nutrición. Rev. Chil. Nutr. 2014, 41, 399–403. [Google Scholar] [CrossRef]
- Liu, Y.; Green, T.J.; Wong, P.; Kitts, D.D. Microencapsulation of L-5-Methyltetrahydrofolic Acid with Ascorbate Improves Stability in Baked Bread Products. J. Agric. Food Chem. 2013, 61, 247–254. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional Ingredients from Agri-Food Waste: Effect of Inclusion Thereof on Phenolic Compound Content and Bioaccessibility in Bakery Products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef]
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros, G.; Viuda-Martos, M. Valorization of citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Nieto, G.; Bañón, S.; Garrido, M.D. Incorporation of thyme leaves in the diet of pregnant and lactating ewes: Effect on the fatty acid profile of lamb. Small Rumin. Res. 2012, 105, 140–147. [Google Scholar] [CrossRef]



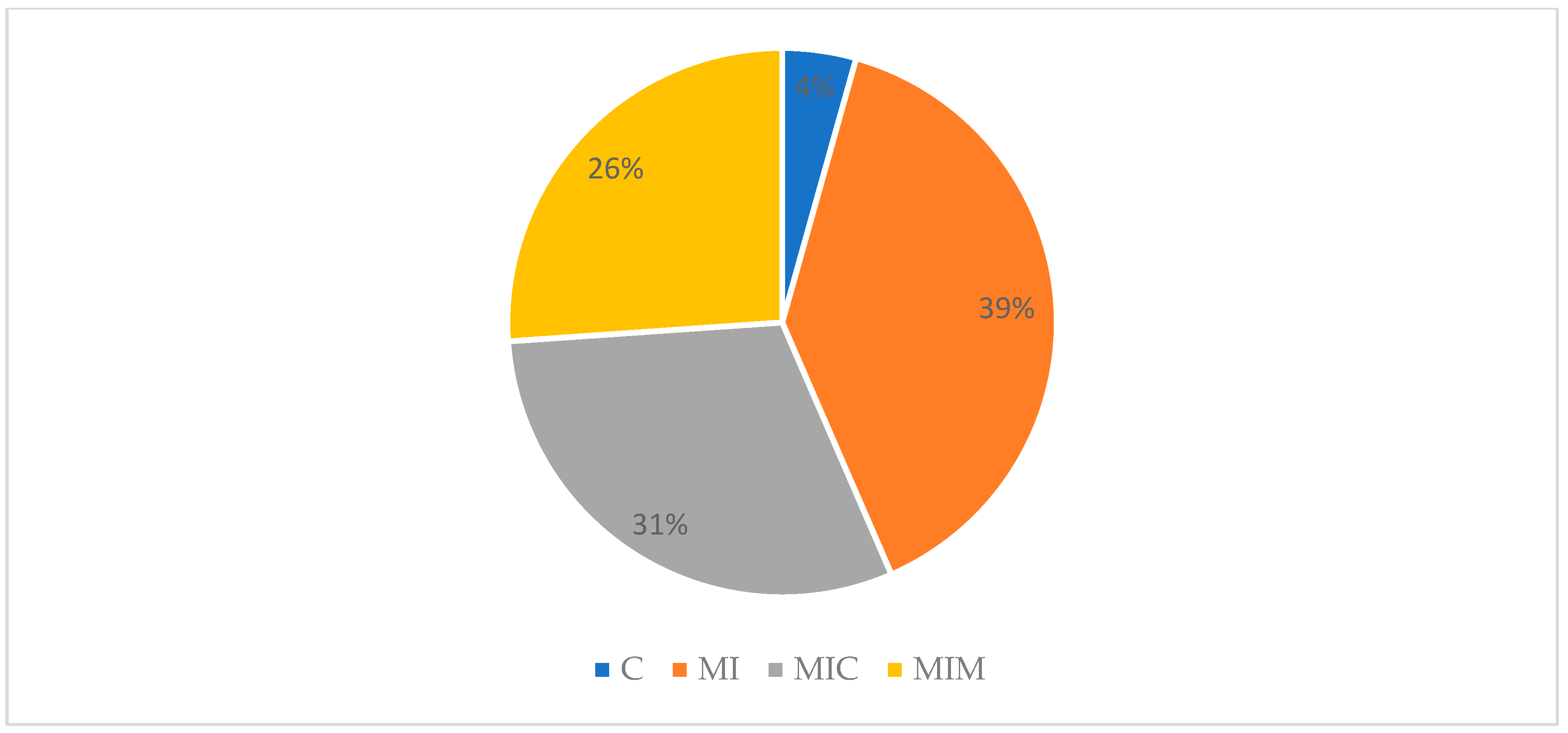
| Samples | Flour (g) | Baking Powder (g) | Sugar (g) | Eggs (units) | Semi-Skimmed Milk (g) | Olive Oil (g) | Inulin (g) | Cacao (g) | Moringa (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 150 | 5 | 100 | 1 | 115 | 50 | 0 | 0 | 0 |
| MI | 150 | 5 | 100 | 1 | 115 | 20 | 30 | 0 | 0 |
| MIC | 150 | 5 | 100 | 1 | 115 | 20 | 30 | 15 | 0 |
| MIM | 150 | 5 | 100 | 1 | 115 | 20 | 30 | 0 | 7000 |
| Parameters | Samples | ||||
|---|---|---|---|---|---|
| Control | MI | MIC | MIM | ANOVA (p Value) | |
| Moisture | 23.44 ± 0.16 | 23.55 ± 0.25 | 22.47 ± 0.31 | 24.43 ± 0.61 | p = 0.0016 |
| Energy | 367.02 ± 13.60 | 339.91 ± 4.06 | 264.42 ± 69.99 | 310.38 ± 14.56 | p = 0.1647 |
| Protein | 6.48 ± 0.19 | 7.08 ± 0.55 | 7.36 ± 0.17 | 7.41 ± 0.02 | p = 0.1054 |
| Carbohydrates | 43.23 ± 4.67 | 46.53 ± 0.53 | 43.59 ± 18.14 | 52.38 ± 0.16 | p = 0.7562 |
| Fat | 18.69 ± 3.67 | 13.94 ± 0.45 | 7.77 ± 1.80 | 7.91 ± 1.55 | p = 0.0078 |
| Ash | 0.93 ± 0.13 | 1.15 ± 0.11 | 9.09 ± 0.14.02 | 0.99 ± 0.15 | p = 0.6685 |
| Parameters | Samples | ||||
|---|---|---|---|---|---|
| Control | MI | MIC | MIM | ANOVA (p Value) | |
| Insoluble Fiber | 0.87 ± 0.41 | 0.95 ± 0.05 | 5.61 ± 0.53 | 0.57 ± 0.75 | p = 0.0015 |
| Soluble Fiber | 6.35 ± 0.68 | 6.80 ± 0.18 | 1.05 ± 0.08 | 5.97 ± 1.04 | p = 0.0023 |
| Total Fiber | 7.22 ± 1.09 | 7.75 ± 0.22 | 6.66 ± 0.60 | 6.54 ± 1.79 | p = 0.6955 |
| Assay | ||||
|---|---|---|---|---|
| Samples | DPPH (%) | ABTS (%) | ORAC (μmol TE/g) | FRAP (μmol TE/g) |
| Control | 579.92 ± 33.03 | 2446.39 ± 723.34 | 44.58 ± 6.97 | 2.16 ± 0.28 |
| MI | 661.14 ± 104.15 | 2835.81 ± 723.34 | 67.92 ± 5.84 | 3.18 ± 0.10 |
| MIC | 1844 ± 543.05 | 5515 ± 2050.84 | 99.99 ± 0.25 | 20.99 ± 4.09 |
| MIM | 548.04 ± 55.79 | 2407.16 ± 437.70 | 59.19 ± 1.43 | 4.66 ± 1.29 |
| ANOVA (p Value) | p = 0.0244 | p = 0.1419 | p = 0.0000 | p = 0.0000 |
| Row Mean-Col. Mean | ORAC (μmol TE/g) | FRAP (μmol TE/g) | ||||
|---|---|---|---|---|---|---|
| Control | MI | MIC | Control | MI | MIC | |
| MI | 23.34 ** | 1.02 | ||||
| MIC | 55.41 ** | 32.06 ** | 18.83 ** | 17.81 ** | ||
| MIM | 14.61 | −8.73 | −40.79 ** | 2.50 | 1.48 | −16.33 ** |
| Mean/Standard Deviation | ABTS (μmol TE/g) | DPPH (μmol TE/g) |
|---|---|---|
| Inulin | 262.5728 ± 34.74 | 9.092518 ± 10.43 |
| Parameters | Samples | ||||
|---|---|---|---|---|---|
| Control | MI | MIC | MIM | ANOVA (p Value) | |
| Folin (mg gallic acid/g sample) | 2.35 ± 0.03 | 3.28 ± 0.22 | 11.03 ± 0.37 | 2.71 ± 0.02 | p = 0.0000 |
| Samples (n = 4) | Folic Acid | THF | 5M-THF | 5F-THF | Total |
|---|---|---|---|---|---|
| Control | 18.98 ± 1.52 | 148.00 ± 10.15 | 70.97 ± 0.16 | 374.27 ± 2.85 | 612.22 ± 5.95 |
| MI | 34.46 ± 1.25 | 8.43 ± 1.83 | 73.16 ± 1.11 | 172.20 ± 3.87 | 288.25 ± 3.33 |
| MIC | 54.39 ± 0.98 | 135.85 ± 4.01 | 103.57 ± 1.32 | 574.97 ± 23.98 | 868.77 ± 30.29 |
| MIM | 36.19 ± 2.81 | 68.76 ± 0.27 | 86.45 ± 1.16 | 297.45 ± 7.47 | 488.85 ± 9.39 |
| ANOVA (p Value) | p = 0.0000 | p = 0.0000 | p = 0.0000 | p = 0.0000 | p = 0.0000 |
| Samples (n = 4) | Colorimetry | ||||
|---|---|---|---|---|---|
| c | l | a | b | h | |
| Control | 29.12 ± 2.35 | 61.55 ± 2.11 | 4.70 ± 0.66 | 28.72 ± 2.30 | 80.74 ± 0.61 |
| MI | 28.30 ± 1.83 | 60.66 ± 0.85 | 5.70 ± 0.50 | 27.73 ± 1.79 | 78.37 ± 0.87 |
| MIC | 12.00 ± 1.63 | 27.87 ± 1.16 | 9.03 ± 0.93 | 7.88 ± 1.43 | 40.90 ± 2.45 |
| MIM | 26.57 ± 3.22 | 56.03 ± 2.09 | 3.33 ± 0.08 | 26.36 ± 3.26 | 82.71 ± 1.14 |
| ANOVA (p Value) | p = 0.0001 | p = 0.0000 | p = 0.0000 | p = 0.0000 | p = 0.0000 |
| Samples | Appearance | Aroma | Texture | Taste | Overall Color | Purchase Intention | Overall Acceptability |
|---|---|---|---|---|---|---|---|
| Average ± SD | |||||||
| Control | 4.00 ± 0.74 | 3.74 ± 0.86 | 3.32 ± 0.99 | 4.00 ± 0.92 | 4.09 ± 0.92 | 3.36 ± 1.26 | 3.95 ± 0.722 |
| MI | 4.04 ± 1.11 | 3.13 ± 1.25 | 3.39 ± 0.98 | 3.82 ± 1.11 | 4.09 ± 0.92 | 3.22 ± 1.44 | 3.61 ± 1.03 |
| MIC | 4.13 ± 0.87 | 3.48 ± 0.95 | 3.70 ± 0.88 | 3.64 ± 0.95 | 4.14 ± 0.91 | 3.59 ± 1.37 | 3.82 ± 1.14 |
| MIM | 4.26 ± 0.915 | 3.39 ± 1.11 | 3.21 ± 0.99 | 3.77 ± 0.87 | 4.26 ± 0.75 | 3.72 ± 1.08 | 3.82 ± 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Milla, P.; Peñalver, R.; Nieto, G. Formulation and Physical–Chemical Analysis of Functional Muffin Made with Inulin, Moringa, and Cacao Adapted for Elderly People with Parkinson’s Disease. Antioxidants 2024, 13, 683. https://doi.org/10.3390/antiox13060683
García-Milla P, Peñalver R, Nieto G. Formulation and Physical–Chemical Analysis of Functional Muffin Made with Inulin, Moringa, and Cacao Adapted for Elderly People with Parkinson’s Disease. Antioxidants. 2024; 13(6):683. https://doi.org/10.3390/antiox13060683
Chicago/Turabian StyleGarcía-Milla, Paula, Rocío Peñalver, and Gema Nieto. 2024. "Formulation and Physical–Chemical Analysis of Functional Muffin Made with Inulin, Moringa, and Cacao Adapted for Elderly People with Parkinson’s Disease" Antioxidants 13, no. 6: 683. https://doi.org/10.3390/antiox13060683
APA StyleGarcía-Milla, P., Peñalver, R., & Nieto, G. (2024). Formulation and Physical–Chemical Analysis of Functional Muffin Made with Inulin, Moringa, and Cacao Adapted for Elderly People with Parkinson’s Disease. Antioxidants, 13(6), 683. https://doi.org/10.3390/antiox13060683







