Abstract
Because of its high antioxidant activity, chokeberry can be used both in the prevention and treatment of various metabolic disorders. In this study, for the first time, the synergistic effects of chokeberry juice and chokeberry fiber on selected metabolic and anthropometric parameters were assessed during a 90-day intervention including 102 people (67 women and 35 men). After 60 days of intervention with chokeberry juice, statistically significant increases in the muscle mass and antioxidant potential of the serum were observed. In turn, there were decreases in the waist circumference, systolic blood pressure, diastolic blood pressure, heart rate, glycated hemoglobin, glucose, LDL cholesterol, eGFR, and ALT level. The addition of chokeberry fiber for the next 30 days resulted in stabilizations of the diastolic blood pressure, glycated hemoglobin, glucose, and waist circumference, as well as reductions in the values of the heart rate, LDL cholesterol, insulin, and AST level. After 90 days, a significant increase in the FRAP value was also observed. This intervention indicates that chokeberry products may have a beneficial effect on metabolic health and serve as a foundation for developing functional foods.
1. Introduction
Nowadays, there is an increase in the incidence of lifestyle-related diseases, including obesity, cardiovascular diseases, liver diseases, neurodegenerative diseases, kidney diseases, and cancer [1]. Oxidative stress, which is associated with the formation of reactive oxygen species, plays an important role in the pathogenesis of these diseases [2]. Reactive oxygen species are produced naturally in the body, including through mitochondrial changes, and can also result from external factors, such as a highly processed diet, chronic stress, injuries, intense physical exercise, alcohol consumption, or cigarette smoking [3]. The disturbance of the antioxidant–oxidant balance leads to damage to bodily cells and, consequently, increases in the risks of many diseases and metabolic disorders [2,3]. The human body has natural mechanisms that protect against the negative effects of oxidative stress. These are enzymatic antioxidants (superoxide dismutase, glutathione peroxidase, and catalase) and non-enzymatic antioxidants (metal-binding proteins, glutathione, uric acid, melatonin, bilirubin, and polyamines) [4]. However, excess reactive oxygen species caused by exogenous factors significantly increase the need for antioxidants supplied in the diet. Dietary factors may significantly influence the increase in the antioxidant potential of the blood plasma. In turn, a high antioxidant potential translates to a better ability of the body to counteract and combat the effects of oxidative stress [5,6]. Natural antioxidants present in food include polyphenol compounds, antioxidant vitamins (vitamins C, E, and A), and trace elements (selenium, zinc, manganese, copper, and iron). These substances participate directly or indirectly in the fight against reactive oxygen species [7,8].
The results of many studies have shown that lifestyle modifications, including a healthy diet rich in antioxidants, are the main therapeutic strategy in the prevention and treatment of chronic diseases [9,10]. In the Polish adult population, a higher intake of dietary antioxidants was significantly associated with higher socioeconomic and health statuses [11]. It was shown that reduced total dietary antioxidants may be considered as an additional risk factor for the development of diabetes and cardiovascular diseases [12,13]. Berries, including chokeberry, are excellent sources of antioxidants. Chokeberry is distinguished by its high antioxidant potential compared to other plant products [14]. It contains many bioactive compounds, including polyphenols, vitamins (among others, vitamin C, vitamin K, and vitamin E), carotenoids, microelements (potassium, magnesium, calcium, and phosphorus), and trace elements (zinc, selenium, manganese, copper, and iron) [15]. Among the polyphenols of chokeberry fruit are flavonoids, especially anthocyanins, as well as procyanidins, flavonols, flavanols, and phenolic acids. Anthocyanins are the main antioxidants of chokeberry fruit. They include cyanidin-3-glucoside, cyanidin-3-galactoside, cyanidin-3-arabinoside, and cyanidin-3-xyloside [16]. Because of their high contents of antioxidant compounds, products made from chokeberry fruit, when regularly consumed, may increase the antioxidant potential of the blood plasma, which is confirmed by literature reports [17]. Additionally, it has been shown that the consumption of chokeberry juice may have hypotensive, lipid-lowering, and hypoglycemic effects, as well as reduce the levels of liver enzymes [18,19,20]. Studies on animals or cell cultures report chokeberry’s hepatoprotective [21,22], anti-inflammatory [23,24,25], neuroprotective [26,27], antidepressant [28], anti-aging [29], anticancer [30], antiviral [31], and antibacterial effects [32]. Because of their contents of valuable health-promoting ingredients, chokeberry products can be used both in the prevention and support of the treatment for existing metabolic disorders [18]. However, many of these issues require confirmation, and more studies involving humans are needed. Previous research has been based on assessing the impacts of the consumption of both chokeberry juice and chokeberry extracts on various health parameters; however, the impacts on some parameters are still unclear and require further investigation.
The aim of this study was to assess the impacts of a dietary intervention using organic chokeberry products (100% chokeberry juice and chokeberry fiber) on specific blood parameters and the antioxidant potential of the plasma. For the first time, the synergistic effect of these two raw materials was examined, as well as the comprehensive assessment of the synergistic effects on selected metabolic and anthropometric parameters.
2. Materials and Methods
2.1. Recruitment of Participants
We conducted this dietary intervention study among the Polish population. Volunteer participants expressed their willingness to participate in the study by completing the online application form (using Google forms). The inclusion criteria for the study were ages 30–65 and low or moderate physical activity levels.
Exclusion criteria were diabetes (all types); gastric ulcer; acute or chronic gastritis; intestinal diseases, including inflammatory bowel diseases and functional intestinal disorders; taking hypoglycemic, lipid-lowering, immunosuppressive, anticoagulant, or antihypertensive drugs; the use of steroid therapy; high physical activity levels; pregnancy; and breastfeeding.
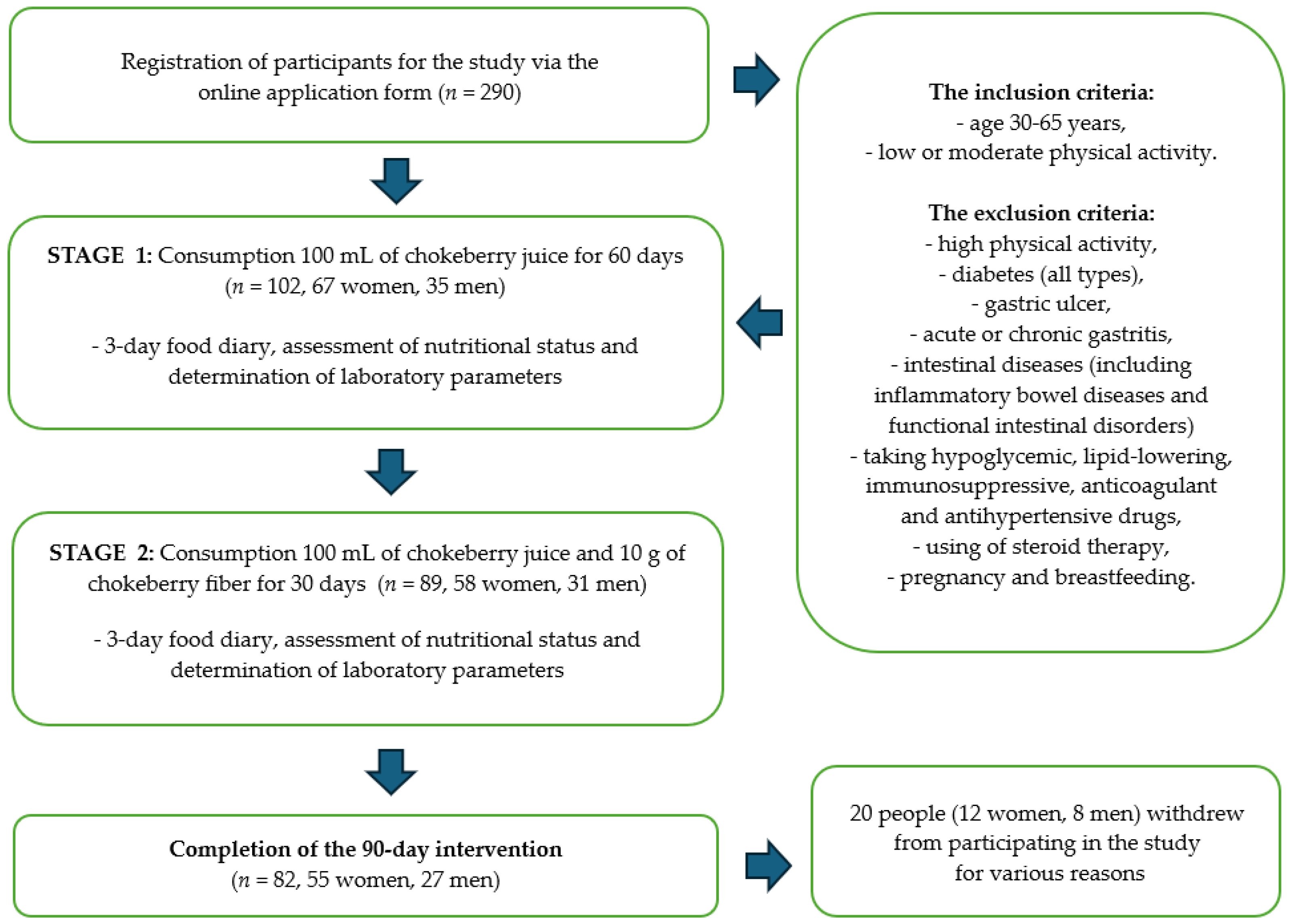
Finally, 290 people expressed their willingness to participate in the study. After we considered the data indicated in the surveys and analyzed the exclusion criteria, 102 people (67 women and 35 men) were classified to participate in the study. Twenty people withdrew from participating in the study for various reasons. One of the declared reasons for resignation was the sour taste of the chokeberry products, which was not accepted by all the study participants. Scheme of the intervention is shown on Figure 1.
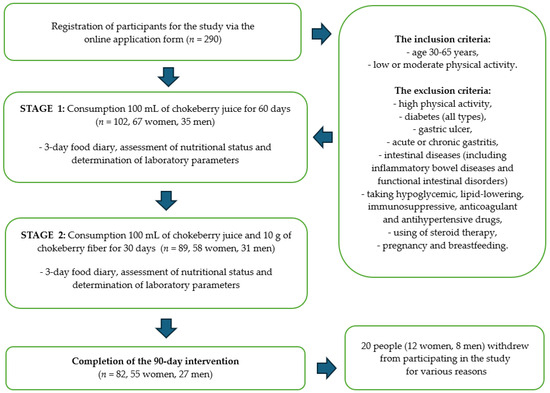
Figure 1.
Scheme of the intervention with chokeberry juice and fiber.
The dietary intervention lasted for a total of 90 days. Study participants consumed 100 mL of chokeberry juice daily for 60 days. After 2 months, the participants additionally supplemented with chokeberry fiber (10 g/day). Chokeberry juice and fiber were selected based on previous analyses confirming the quality and safety of the raw materials, which we described in detail in our previous publication [33,34]. Chokeberry juice and chokeberry fiber came from ecological cultivation (Poland). This study has the status of a clinical trial—number: NCT06435130.
Qualitative and quantitative assessments of the diet, the assessment of the nutritional status, and the determination of laboratory parameters were performed three times during the study: before the start of the dietary intervention, after 2 months of consuming chokeberry juice (60 days), and after 3 months (next 30 days), i.e., after the monthly consumption of chokeberry juice and fiber together. The characteristics of the study participants are presented in Table 1.

Table 1.
Characteristics of the study group (n = 102).
2.2. Assessment of Diet and Nutritional Status
A quantitative assessment of the diet was carried out among the study participants according to a 3-day food diary. The diary was based on 2 working days and 1 day off. The participants underwent anthropometric measurements: body height (expressed in centimeters), bodyweight (in kilograms), and waist and hip circumferences (in centimeters).
The participants were asked to keep the diet the same in each of the three stages to avoid interference from other dietary components. Dietary data (caloric intake, protein intake, fat intake, and carbohydrate intake) are presented in Table 2. Height and bodyweight were measured using a growth meter and a body composition analyzer. Body composition analysis assessed the mass and percentage of the fat tissue, the volume of the visceral fat tissue, and the mass of the muscle tissue. BMI was assessed based on these measurements. Additionally, body composition analysis was performed using the bioelectrical impedance method and an InBody 720 device (Biospace, Eonju-ro, Republic of Korea).

Table 2.
Dietary diary assessment during chokeberry intervention.
2.3. Assessment of Metabolic Disorder Parameters
Participants who qualified for the study had specific biochemical parameters tested: fasting glucose concentration, fasting insulin concentration, lipid profile (triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol), glycated hemoglobin, uric acid, creatinine, estimated glomerular filtration rate (e-GFR), C-reactive protein (CRP), creatinine, and liver enzymes (alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamylotranspeptydase (GGTP), and homocysteine). The assessment was performed by a certified external laboratory.
The study participants also had their blood pressure and heart rate measured using a blood pressure monitor (twice, at a 5 min interval, and on the non-dominant arm).
2.4. Assessment of Ferric-Ion-Reducing Antioxidant Potential (FRAP) in Blood Serum
Additionally, the antioxidant potential of the participants’ blood serum was assessed at each stage of the study using the ferric-ion-reducing antioxidant potential method (FRAP).
The ferric-ion-reducing antioxidant potential (FRAP) was determined according to Benzie and Strain’s method [35] using a Shimadzu UV spectrophotometer (Shimadzu, Kyoto, Japan). This method is based on the reduction of Fe3+ ions in the form of a complex with 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ)—TPTZ-Fe3+ to Fe2+ ions—TPTZ-Fe2+ in the presence of antioxidants in samples. The absorbance (an intense blue color) was measured, at 593 nm after 4 min of incubation at 37 °C, against a blank without a sample. The FRAP was calculated from the standard curve and expressed as millimoles per liter.
2.5. Assessment of the Compositions of Chokeberry Juice and Fiber
The compositions of the juice and fiber used for the intervention were assessed. The following parameters were determined: antioxidant potential (FRAP), total content of polyphenols (TPC), total flavonoids, total anthocyanins, vitamin C, and elements (Cu, Fe, Mg, Mn, Se, and Zn). Each parameter was determined in three repetitions.
Measurements of antioxidant properties were performed using a UV spectrophotometer (Shimadzu, Kyoto, Japan). Before that, chokeberry juice was thinned with ultrapure water, and chokeberry fiber was extracted with methanol and acetone according to the method in a study by Zujko et al. (2011) [36]. In turn, the contents of the elements were assessed by the microwave mineralization of the samples and then analyzed using the atomic absorption spectrometry (AAS) method [37]. Composition of chokeberry juice and fiber used for the dietary intervention is shown on Table 3.

Table 3.
Compositions of chokeberry juice and fiber used for the dietary intervention.
2.5.1. Assessment of the Total Anthocyanin Content
The Giusti and Wrolstad method [38] was used to assess the anthocyanin content. In this method, samples were incubated (for 15 min at room temperature); then, the absorbance was measured using buffers: chloride (pH = 1.0) and acetate buffer (pH = 4.5). The anthocyanin content was expressed as the cyanidyn-3-glucoside content (mg Cy-3-GL/kg).
2.5.2. Assessment of the Total Flavonoid Content
To assess the content of flavonoids, a method with a mixture of 2% AlCl2 and methanol was used. This method involves the creation of aluminum–flavonoid complexes [39]. Then, the samples were incubated (for 10 min at room temperature). After this time, absorbance measurements were made (wavelength: 415 nm). The total flavonoid content was presented as quercetin equivalents (mg QE/kg).
2.5.3. Assessment of the Total Polyphenol Content
The total polyphenol content was determined using the Folin–Ciocalteu reagent [40]. The yellow reagent is oxidized as a result of the reaction with polyphenol components and then reduced in the presence of sodium carbonate—a blue color is created. The resulting samples were incubated (for 30 min at room temperature), and the absorbance was measured (wavelength: 765 nm). The total polyphenol content was expressed as gallic acid equivalents (mg GAE/kg) after calculation from the standard curve.
2.5.4. Assessment of the Ferric-Ion-Reducing Antioxidant Potential (FRAP) in Chokeberry Juice and Chokeberry Fiber
The ferric-ion-reducing antioxidant potentials (FRAPs) of the chokeberry juice and fiber were determined according to Benzie and Strain’s method [35]. The method was the same as in the case of the FRAP assessment of the blood serum. The FRAP contents were expressed as millimoles per kilogram after calculation from the standard curve.
2.5.5. Assessment of the Mineral Contents
Mineralization was carried out to assess the element contents. For this purpose, the samples were weighed (accurate to 1 mg); then, spectrally pure concentrated nitric acid 69% (4.0 mL, 69% HNO3, Tracepur, Merck, Darmstadt, Germany) was added. The mineralization process was carried out in a closed-loop microwave system (Speedwave, Berghof, Eningen, Germany). Ultrapure water was used to transfer the obtained samples to the vessels. Ultrapure water was prepared using a Simplicity 185 device (Millipore, Burlington, VT, USA).
The contents of the selected elements in the samples were determined using the AAS method (Z-2000 apparatus, Hitachi, Tokyo, Japan). The mineralizates were diluted with ultrapure water, based on the ranges of the standard curves. The contents of Cu, Mn, and Se were determined by the flameless AAS technique with electrothermal atomization in a graphite cuvette. The determination of the Se content required a palladium–magnesium matrix modifier: Pd concentration—1500 mg/L and Mg concentration—900 mg/L (Merck, Darmstadt, Germany). For the measurement of the Mn, magnesium nitrate (Mg(NO3)2; concentration: 100 mg/L, Sigma-Aldrich, Merck, Darmstadt, Germany) as a modifier was used. The contents of Fe, Mg, and Zn were determined using the AAS flame technique in an acetylene–air flame with Zeeman background correction. In the case of the Mg content determination, a masking agent was used—1% lanthanum chloride (LaCl3, Sigma-Aldrich, Merck, Darmstadt, Germany). To validate this method, a certified reference material was used: tea leaves—INCT–TL–1—a Polish certified reference material for multielement trace analysis (Institute of Nuclear Chemistry and Technology, Warsaw, Poland) [41].
2.5.6. Assessment of the Vitamin C Content
The sum of the L-ascorbic acid and dehydroascorbic acid contents was determined using the high-performance liquid chromatography (HPLC) method with UV detection (wavelength: 254 nm; Perkin Elmer, Waltham, MA, USA). Dehydroascorbic acid was reduced to L-ascorbic acid using dithiothreitol (DTT). The results were expressed in milligrams per kilogram of the product [42].
2.6. Consumption of Chokeberry Juice and Fiber
Because of the sour taste of the chokeberry juice, participants could dilute the juice with spring, filtered, or boiled water. The chokeberry fiber could be divided into 2 portions a day (5 g of fiber per serving) and consumed with water or included in a meal. Participants were asked to discontinue the use of antioxidant supplements for at least 7 days prior to laboratory testing and for the duration of the dietary intervention. Additionally, they were advised not to consume larger amounts of chokeberry juice per day than recommended, other juices containing chokeberry, supplements, or other products containing chokeberry fruit.
2.7. Statistical Analysis
The obtained results were subjected to statistical analysis using Microsoft Office Excel 2019 and Statistica 13.3 (StatSoft, Tibco, Palo Alto, CA, USA).
The following descriptive statistic parameters were calculated: mean with standard deviation (SD), minimum (Min.) and maximum (Max.), median, and lower (Q1) and upper (Q3) quartiles. The normality of the data distribution was assessed using the following tests: Kolmogorov–Smirnov, Lilliefors, and Shapiro–Wilk tests.
The Wilcoxon paired-order test was used to assess differences between individual groups before and after the intervention. The assessment of the connections between the studied parameters was carried out using Spearman’s correlation. Spearman’s rank correlation coefficients were also determined. The statistically significant level was p < 0.05.
3. Results
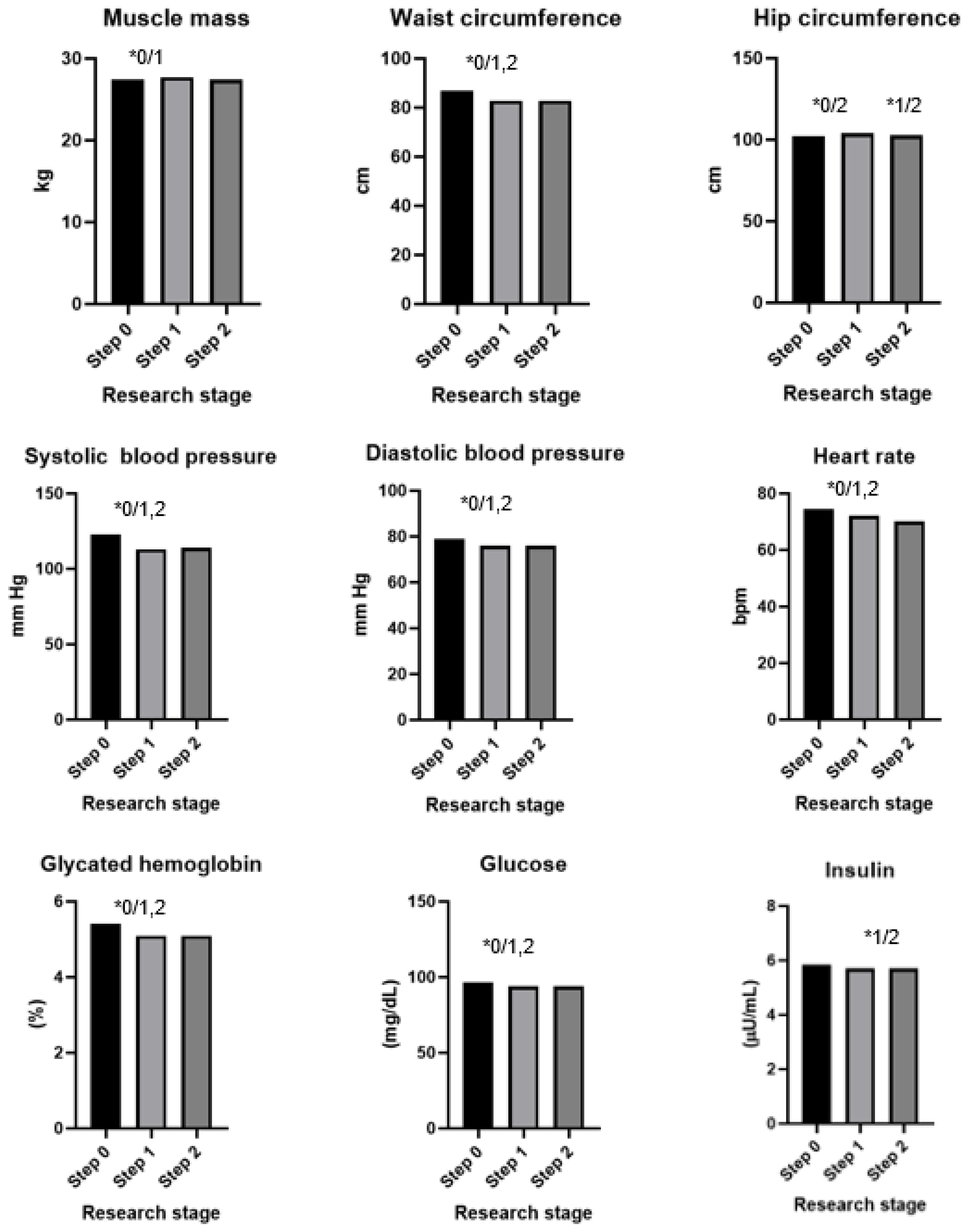
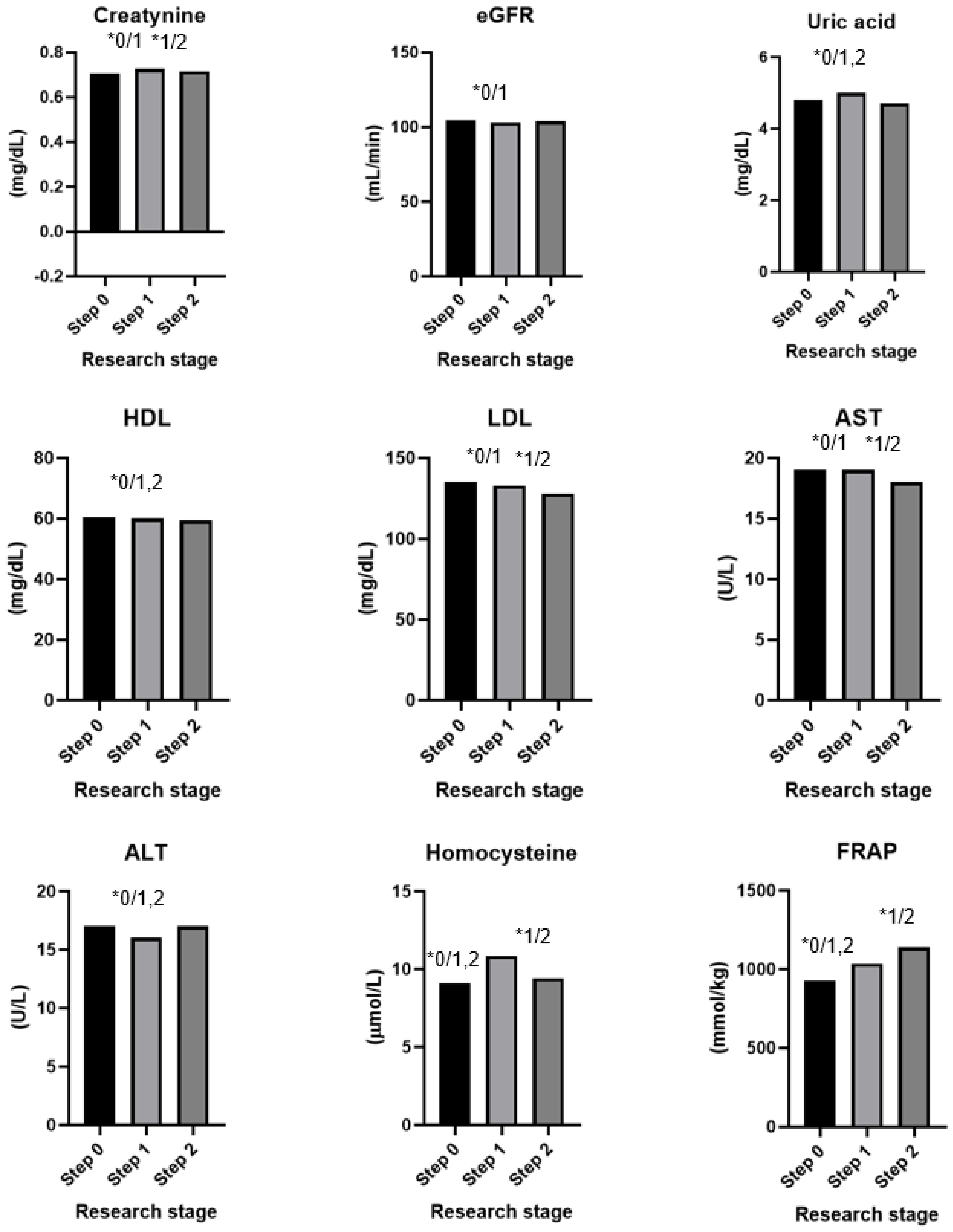
The results of the dietary intervention are presented in Table 4, Figure 2 and Figure 3. After 60 days of intervention with chokeberry juice, statistically significant increases in the muscle mass (median (Q1–Q3): 27.7 kg (24.1–36.2) vs. 27.5 kg (24.1–33.2)) and FRAP (926.02 (808.30–1010.37 mmol/L) vs. 1036.44 (914.22–1168.44 mmol/L)) were observed. The median FRAP parameter of the participants’ diets at stage 0 was 16.78 (11.81–22.99 mmol/kg). The value of this parameter, estimated on the basis of the dietary supply, did not change during the intervention period. An increase in FRAP in the serum resulted from the dietary intervention with chokeberry juice and fiber.

Table 4.
Results of an intervention study involving chokeberry juice and fiber—data without statistically significant differences (p > 0.05).
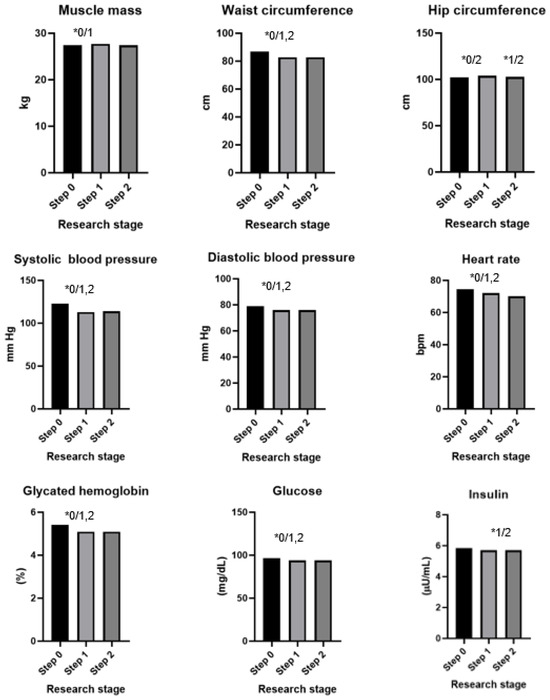

Figure 2.
Results of an intervention study involving chokeberry juice and fiber (* p < 0.05, 0—step 0, 1—step 1, 2—step 2).

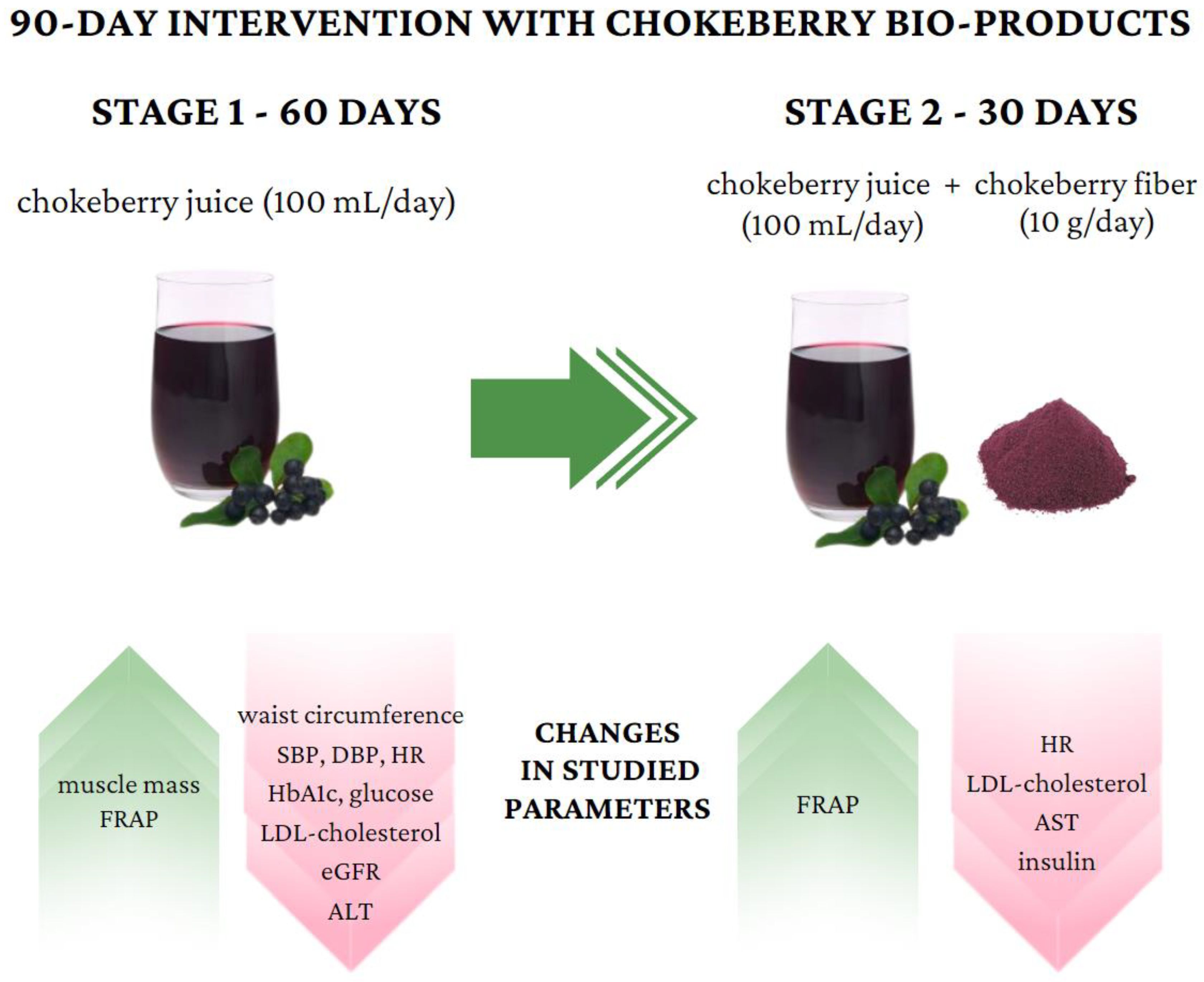
Figure 3.
Important changes in studied parameters during chokeberry intervention.
In addition, increases in the levels of creatinine (0.705, 0.630–0.790 mg/dL vs. 0.756, 0.660–0.790 mg/dL), uric acid (4.8, 3.8–5.4 mg/dL vs. 5.0, 4.2–5.6 mg/dL), and homocysteine (9.08, 7.76–10.95 µmol/L vs. 10.87, 9.46–12.60 µmol/L) were observed. A monthly dietary intervention using chokeberry fiber resulted in reductions in the values of these parameters to the following levels: 0.715, 0.660–0.780 mg/dL; 4.7, 4.0–5.5 mg/dL; 9.43, 8.12–11.16 µmol/L.
The intervention of drinking juice for 60 days resulted in decreases in the following parameters: waist circumference (86.8 cm, 80.0–96.0 vs. 82.8, 78.0–92.0 cm), systolic blood pressure (122, 112–130 vs. 113, 105–125 mm Hg), diastolic blood pressure (79, 73–87 vs. 79, 69–83 mm Hg), heart rate (75, 69–82 vs. 72, 66–77 bmp), glycated hemoglobin (5.4, 5.2–5.6 vs. 5.1, 4.9–5.4%), glucose (97, 92–101 vs. 94, 90–97 mg/dL), insulin (5.9, 4.4–7.7 vs. 5.7, 3.9–7.6 µU/mL), LDL cholesterol (136, 108–162 vs. 133, 106–158 mg/dL), ALT (17, 14–25 vs. 16, 12–22 U/L), and e-GFR (105, 98–118 vs. 103, 93–114 mL/min).
The intervention consisting of adding fiber had a bidirectional effect: In the case of some parameters, it stabilized them (waist circumference, hip circumference, diastolic blood pressure, glycated hemoglobin, glucose, and insulin); in the case of others, it additionally reduced or increased them toward favorable values (heart rate, LDL, and AST).
The strongest effect observed was an increase in the antioxidant properties of the plasma, as measured by the FRAP method. The strength of the correlation between the FRAP and the parameter values for which statistically significant differences were found was then assessed. In most cases, the strength of the correlation for the parameters assessed after the 3rd stage was higher than after the second stage. The strongest correlations were found for uric acid, 0.87 and 0.78, respectively, which may indicate a decrease in uric acid levels with prolonged use of chokeberry juice and fiber (Table S1).
4. Discussion
In the studies conducted so far, chokeberry was tested both in the form of juice and in the form of a supplement, which could be crucial in understanding its effects. Natural chokeberry products have variable compositions, despite the same forms. Differences in the contents of various nutrients will depend on the variety of the fruit used for production, the cultivation method, soil and weather conditions, the harvest time, as well as the storage and processing of the fruit at later production stages [43,44,45]. In the case of supplements, the composition may also vary, and only in studies by a few authors, supplements were standardized, i.e., they contained a specific amount of the active substance [46,47]. So far, there have been studies involving chokeberry juice, but to the best of our knowledge, the effects of chokeberry fiber have not been assessed. Dietary fiber could help maintain metabolic health. Dose–response meta-analyses suggested an inverse association between the total fiber intake and the risks of cardiovascular, all-cause, and cancer mortalities [48]. Basu et al. (2021) have noted that supplementation with 280 g of whole blueberries and 12 g of soluble fiber daily for 36 weeks may prevent excess gestational weight gain, improve glycemic control, and reduce inflammation factors (CRP protein) in women with obesity. However, no effect on the lipid profile was observed [49]. In another study from 2023, it was observed that dietary fiber supplementation (24 g of dietary fiber powder/day) during pregnancy may prevent gestational diabetes mellitus and preterm birth in women before 20 weeks of gestation [50].
Our intervention assessed various anthropometric parameters. However, the influences of chokeberry fruit on the selected parameters are not clear. The mechanisms include decreasing lipogenesis and adipogenesis [51] and affecting cellular metabolism by modulating signaling pathways, including 5’-AMP-activated protein kinase [52]. Adipose tissue, especially visceral fat, plays an important role in the pathogenesis of metabolic disorders. Its excess, it contributes to the development of chronic low-grade systemic inflammation and, consequently, insulin resistance [53]. The body mass index (BMI) takes into account both the height and weight. A BMI score of 25–29.9 is overweight, while a score of 30 and above is obesity [54]. It is worth noting that BMI is not reliable because people with a high muscle mass can have a high BMI. In turn, skeletal muscles play an important role in maintaining metabolic health. This is mainly related to their participation in glucose uptake and increasing the sensitivity of cells to insulin [55]. In our study, no changes were observed in the bodyweight, BMI, fat tissue mass (kg), fat tissue percentage (%), or visceral fat (cm2) after 90 days of intervention. However, after 60 days of consuming only chokeberry juice, an increase in the muscle mass and a decrease in the waist circumference were observed. Then, after 30 days and adding chokeberry fiber—a decrease in the hip circumference was observed. In studies by other authors, no significant impacts of chokeberry juice consumption on anthropometric parameters (body mass, BMI, and waist circumference) were observed [56,57,58,59,60,61]. Only in a study by Kardum et al. (2014), there were observed decreases in the BMI and waist circumference in postmenopausal women with abdominal obesity after 4 weeks of chokeberry juice consumption [62]. Chokeberry juice and fiber do not seem to have significant impacts on anthropometric parameters. The overall diet and physical activity likely played a greater role, but chokeberry products may possibly support metabolic changes.
The next assessed parameters were the blood pressure and heart rate. Hypertension could be diagnosed when a person’s systolic blood pressure (SBP), in the office or clinic, is ≥140 mm Hg and/or their diastolic blood pressure (DBP) is ≥90 mm Hg, following repeated examinations [63]. Among the risk factors for hypertension, we can mention cardiovascular diseases, dyslipidemia, diabetes, a highly processed diet, smoking, alcohol consumption, and genetic predispositions, like a family history of hypertension [64]. The influence of chokeberry fruit on lowering blood pressure has already been documented. Hawkins et al. (2021), in a meta-analysis, have confirmed that chokeberry supplementation (both in the forms of juice and extract) can reduce systolic blood pressure [19]. Potential mechanisms include decreasing the oxidative stress and damage of the endothelium and reducing the activities of some factors, like endoothelin-1 and angiotensin-converting enzyme-1 [65,66]. Our study has also confirmed the beneficial effects of chokeberry juice consumption on the blood pressure—both systolic and diastolic. However, adding chokeberry fiber for 4 weeks did not enhance the antihypertensive effect. Other studies have shown positive effects for consuming juice in the amounts of 100–300 mL per day. The beneficial effects of the chokeberry juice on the blood pressure could be observed after just 4 weeks [59,62,67,68]. The exception was a study by Milutinović et al. (2019), in which no effects were observed [69]. In the case of the heart rate—initially, after 8 weeks, there was a decrease in this value and then the heart rate returned to the value at the beginning of the study. Comparing these results with those of other authors, Kardum et al. (2015) did not observe any impacts of chokeberry juice consumption on the 24 h pulse blood pressure or asleep pulse blood pressure, but they noticed a decrease in the awake pulse blood pressure [68]. Another study evaluated the heart rate using 30 mL of a standardized chokeberry extract, with the authors not reporting any observed changes [46].
Chokeberry may have a positive effect on carbohydrate metabolism, and anthocyanins probably could play the main role [70]. Several possible mechanisms are taken into account—including the protection of pancreatic ß-cells against oxidative stress [71,72], the effect on the increase in adiponectin, and the decrease in visfatin levels, which play roles in insulin sensitivity [73], increasing the degradation of glucose—alpha-amylase and alpha-glucosidase [74], impacting the activities of hepatic glucose metabolism enzymes [75], and inhibiting the activity of dipeptidyl peptidase IV (DPP IV) [74]. In a study from 2019 on mice with diabetes, a reduction in glucose levels was observed when the mice were given chokeberry juice. Chokeberry juice inhibited the activity of alpha-glucosidase in the upper part of the small intestine and the activity of DPP IV [76]. In another study from 2020, chokeberry extract given to rats with type 2 diabetes resulted in lower blood glucose and insulin levels. The mechanism responsible for this was the effects on liver enzymes involved in glucose metabolism—pyruvate kinase, phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, and glucokinase [75]. The impact of chokeberry consumption by people on carbohydrate metabolism has been previously studied by other authors [59,62,67,68,69,77]. Changes in fasting glucose levels were noticed with the consumption of 150 mL of juice in studies by Gancheva et al. (2021) and Milutinović et al. (2019) for 12 weeks and with the consumption of 250 mL for 12 weeks in a study by Skoczyńska et al. (2007) [67,69]. Moreover, Gancheva et al. (2021) and Milutinović et al. (2019) observed decreasing HbA1c levels by drinking chokeberry juice [69,77]. In our study, there were reductions in the levels of glucose and glycated hemoglobin using smaller amounts of chokeberry juice, and the duration of the intervention was shorter. Importantly, extending this time to 12 weeks and adding fiber at the same time did not result in further reductions in glucose levels. In an earlier review [18], the authors hypothesized that a longer intervention time may be important, but this study does not confirm that conclusion. So far, insulin levels have not been tested in the case of chokeberry interventions involving people. High insulin levels may indicate developing insulin resistance in cells and an increase in hyperinsulinemia [78]. In studies assessing the effects of anthocyanins, it was shown that supplementation with purified anthocyanin (80 mg/capsule) could have beneficial effects on the homeostatic model assessment of insulin resistance (HOMA-IR) and adiponectin levels [79]. Our study did not show any important changes in insulin levels.
The positive impact of chokeberry consumption on the lipid metabolism may be based on several mechanisms, including reducing oxidative stress and pro-inflammatory factors, like TNF-alpha, or inhibiting the expression of specific genes, which are responsible for de novo lipogenesis, like fatty acid synthase [80,81,82]. In a study on intestinal CaCo2 cells, it was shown that the administration of chokeberry extract reduced the expression of genes involved in lipid metabolism, including sterol regulatory element-binding protein 1c, fatty acid synthase, and acyl-CoA oxidase 1 [82]. In other study, supplementation with anthocyanins derived from chokeberry improved blood lipid levels, had a beneficial effect on the intestinal microbiome, and regulated the AMP-activated protein kinase signaling pathway [83]. It has been shown that the activation of the AMPK pathway inhibits the enzyme 3-hydroxy-3-methylglutaryl coenzyme-A reductase, which reduces cholesterol biosynthesis [84]. Elevated levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) are directly associated with increased cardiovascular risk and mortality [85]. In 2021, a meta-analysis was conducted and showed that daily supplementation with chokeberry for 6–8 weeks lowers total cholesterol levels. However, in this study, both chokeberry juice and chokeberry extract were taken into account. This impact was the most visible in people over 50 years of age [19]. In our study, the consumption of chokeberry juice also contributed to a decrease in TC, and the addition of fiber enhanced this effect. In studies by other authors, the effects of chokeberry juice on TC levels varied. Skoczyńska et al. (2007) noticed a decrease in the TC level after 12 weeks of juice consumption (250 mL) in people with mild hypocholesterolemia. Milutinović et al. (2019) also observed reductions in TC levels after 12 weeks in people with DM2 and on oral antidiabetic drug therapy for at least 6 months (150 mL of juice). The remaining authors did not notice any effect of the juice on the TC level. The duration of the intervention was 4–12 weeks, and the amount of juice was 100–300 mL [56,59,62,68,77]. The LDL-C level did not change significantly after 8 weeks of juice intervention, but there was a statistically significant decrease after the addition of the fiber. Similar conclusions were drawn by other researchers for consuming 100–200 mL of chokeberry juice over a period of 4–12 weeks [56,61,62,68,77]—LDL-C levels did not change. In turn, there are studies in which reductions in LDL-C levels have been observed—after consuming 150–200 mL of juice/day for 12 weeks [66,69]. It seems that despite the health-promoting properties of chokeberry juice, the overall diet is important in the case of LDL-C levels. Taking into account the impacts of this intervention on HDL-C levels, after 8 and 12 weeks of the intervention, the levels were lower than before its initiation. Other authors have demonstrated varied effects of chokeberry juice on HDL-C levels [56,59,62,69,77]. Kardum et al. (2014) and Milutinović et al. (2019) showed decreases in HDL-C levels after consuming 100–150 mL of chokeberry juice for 4–12 weeks [62,69]. Loo et al. (2016) and Gancheva et al. (2021), in contrast, did not observe any changes in HDL-C levels [59,77]—participants consumed 150–300 mL of juice for 8–12 weeks. Interestingly, in a study by Pokimica et al. (2019), the consumption of 100 mL of standardized chokeberry juice with a low polyphenol content reduced HDL-C levels, while juice with a high polyphenol content had no effect [56]. In the case of the triglyceride level, in our study, a downward trend could be observed after 8 weeks of consuming only chokeberry juice. Adding chokeberry fiber resulted in a return to preintervention values. Most studies conducted so far have not observed changes in TG levels [56,59,62,77]. In two studies involving juice, decreases in TG levels were noticed [67,68]. The homocysteine level did not change after 12 weeks of our intervention. In a study by Skoczyńska et al. (2007), a decrease in homocysteine was observed after a 12-week study using 250 mL of chokeberry juice [67]. Homocysteine is an amino acid that could be a risk factor for the development of cardiovascular diseases; therefore, its reduction may play an important role [86].
The liver has numerous necessary functions in the body, among which are the metabolism of proteins, lipids, and carbohydrates; detoxification processes; and the production of some substances, like bile [87]. Increasing liver enzymes, like alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGTP), could be among the symptoms of liver diseases [88]. It is known that dietary antioxidants, including polyphenols, can improve liver function and reduce inflammation [89]. Polyphenols contained in chokeberry may improve liver functions by influencing the expressions of genes responsible for de novo lipogenesis in the liver (sterol regulatory element-binding protein, acetyl-CoA carboxylase, and fatty acid synthase); inhibiting proinflammatory factors, like TNF-alpha; and reducing oxidative stress [22,90,91]. In our study, the levels of AST and GGTP did not change after 8 weeks of juice consumption, but a decrease in the level of ALT was observed. Adding fiber after 8 weeks led to decreases in AST levels. The effects of juice consumption on liver functions have been assessed so far in three studies. Kardum et al. (2015) and Gancheva et al. (2021) did not observe any impacts of juice consumption on the levels of AST and ALT [68,77]. For the GGTP enzyme, Gancheva et al. (2021) reported a decrease, in contrast to Loo et al. (2016)—who observed no changes [59,77].
Uric acid is a product of the enzymatic breakdown of purine nucleosides and free nitrogen bases [92]. Elevated levels of uric acid are observed, among other things, in obesity, hypertriglyceridemia, hypertension, kidney diseases, and diabetes. There is also a relationship between hyperuricemia and the components of the metabolic syndrome [93]. However, uric acid could have antioxidant and pro-oxidative potentials [94]. The level of creatinine, in turn, can be a sign of the condition of the kidneys. However, creatinine values may fluctuate depending on the diet and protein intake, muscle mass, or some medications. Therefore, it is not the best diagnostic indicator because of its variability [95]. The glomerular filtration rate determines the amount of glomerular filtration in the kidneys. The GFR is usually estimated using equations based on concentrations of endogenous serum filtration markers, mainly creatinine. An e-GFR of ≥90 mL/min/1.73 m2 is considered as normal [96]. In our study, after 8 weeks of chokeberry juice consumption, there were increases in creatinine and uric acid and a decrease in the e-GFR index. The addition of fiber enhanced the decrease in creatinine. The impacts of chokeberry products on kidney functions have not been well researched, and so far, other authors have not confirmed the important role of chokeberry [67,68,69].
Inflammation is a part of the body’s defense mechanism. It is the process by which the immune system recognizes and removes harmful and foreign stimuli and begins the healing process. Inflammation can be either acute or chronic. Long-term inflammation is a pathological condition and increases the risk for developing many diseases and disorders, such as cardiovascular diseases, diabetes, cancer, and liver diseases [97]. We can assess inflammation in the body using various markers, including acute-phase proteins, TNF-alpha, and specific cytokines [98]. Because of its high antioxidant activity, chokeberry fruit may reduce inflammation [59]. The antioxidant and anti-inflammatory effects have been confirmed by studies on animals and cell lines [23,24,99,100]. In our study, there were no effects of the consumptions of chokeberry juice and fiber on CRP protein. Other authors also did not observe any changes in CRP levels [67,68,69]. The CRP level decreased in a study on overweight women after 12 weeks of consuming 150 mL of juice [77]. Not all studies confirm the beneficial effects of chokeberry on inflammatory markers. However, the effects of chokeberry seem to be promising because of its high antioxidant content, which is very important in the prevention and the treatment of many disorders.
The antioxidant potential of the plasma is our body’s ability to fight reactive oxygen species. One method for measuring this potential is the FRAP method [101]. The value of this potential is influenced by both endogenous antioxidants, such as antioxidant enzymes and uric acid, and exogenous antioxidants—supplied with the diet, including vitamins—vitamins C, E, and β-carotene; elements—magnesium, selenium, copper, manganese, zinc, and iron; and polyphenols, such as anthocyanins [7,102]. Chokeberry fruits have a high antioxidant potential compared to those of other fruits, which may translate to an increase in the body’s defense capabilities [103]. A meta-analysis from 2019 found that following a diet high in total antioxidants was associated with reduced risks of all-cause death, cardiovascular disease, and cancer [104]. In our study, there was a statistically significant increase in the antioxidant potential of the plasma after 8 and 12 weeks of intervention. Other studies did not assess the antioxidant potential of the FRAP, but the authors noted increases in antioxidant enzymes after the intervention with juice—there were increases in glutathione peroxidase [62] and superoxide dismutase [77]. However, the effect on the superoxide dismutase activity is not clear—in the study by Kardum et al. (2014) [62], this effect was not confirmed. The activity of the catalase did not change [62,77]. The consumption of chokeberry products may have a beneficial effect on the antioxidant capacity and, thus, increase the body’s defense mechanisms in the fight against excessive oxidative stress.
5. Conclusions
Our intervention has confirmed the positive impacts of the combination of chokeberry juice and chokeberry fiber consumptions on reductions in the systolic and diastolic blood pressures, heart rate, aspartate transaminase level, LDL cholesterol level, glycated hemoglobin level, glucose level, and waist circumference. After 90 days, increases in the antioxidant potentials of the serum were also observed. The impacts of this dietary intervention on other parameters seem to be ambiguous. This study’s findings suggest that these products may support metabolic health, but this hypothesis requires further research in the future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antiox13060699/s1: Table S1: Correlations between the FRAP and values of selected parameters, divided into research stages.
Author Contributions
Conceptualization, M.E.Z., A.P.-J., and E.O.; methodology, M.E.Z., A.P.-J., E.O., K.S., and C.P.; software, E.O. and A.P.-J.; validation, E.O., A.P.-J., and M.E.Z.; formal analysis, A.P.-J. and M.E.Z.; investigation, E.O.; resources, E.O., A.P.-J., and M.E.Z.; data curation, E.O. and M.E.Z.; writing—original draft preparation, E.O.; writing—review and editing, A.P.-J., M.E.Z., K.S., and C.P.; visualization, E.O. and A.P.-J.; supervision, M.E.Z.; project administration, M.E.Z.; funding acquisition, M.E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Białystok: SUB/3/DN/22/003/3317, B.SUB.23.180, and B.SUB.24.149.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the Medical University of Białystok, Poland (approval number: APK.002.475.2021; date of approval: 18 November 2021). The study has the status of a clinical trial—number: NCT06435130.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
All the data are available on request from the corresponding author.
Acknowledgments
The authors thank all the study participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Puertollano, M.A.; Puertollano, E.; de Cienfuegos, G.Á.; de Pablo, M.A. Dietary antioxidants: Immunity and host defense. Curr. Top. Med. Chem. 2011, 11, 1752–1766. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Dietary Antioxidants and Chronic Diseases. Antioxidants 2023, 12, 362. [Google Scholar] [CrossRef]
- Zujko, M.E.; Rożniata, M.; Zujko, K. Individual Diet Modification Reduces the Metabolic Syndrome in Patients Before Pharmacological Treatment. Nutrients 2021, 13, 2102. [Google Scholar] [CrossRef]
- Zujko, M.E.; Waśkiewicz, A.; Drygas, W.; Cicha-Mikołajczyk, A.; Zujko, K.; Szcześniewska, D.; Kozakiewicz, K.; Witkowska, A.M. Dietary habits and dietary antioxidant intake are related to socioeconomic status in Polish adults: A nationwide study. Nutrients 2020, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Waśkiewicz, A.; Witkowska, A.M.; Cicha-Mikołajczyk, A.; Zujko, K.; Drygas, W. Dietary total antioxidant capacity—A new indicator of healthy diet quality in cardiovascular diseases: A polish cross-sectional study. Nutrients 2022, 14, 3219. [Google Scholar] [CrossRef] [PubMed]
- Cyuńczyk, M.; Zujko, M.E.; Jamiołkowski, J.; Zujko, K.; Łapińska, M.; Zalewska, M.; Kondraciuk, M.; Witkowska, A.M.; Kamiński, K.A. Dietary total antioxidant capacity is inversely associated with prediabetes and insulin resistance in Bialystok PLUS population. Antioxidants 2022, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia Melanocarpa L.—A qualitative composition, phenolic profile and antioxidant potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef] [PubMed]
- Kardum, N.; Konić-Ristić, A.; Šavikin, K.; Spasić, S.; Stefanović, A.; Ivanišević, J.; Miljković, M. Effects of polyphenol-rich chokeberry juice on antioxidant/pro-oxidant status in healthy subjects. J. Med. Food 2014, 17, 869–874. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E. Chokeberry (A. melanocarpa (Michx.) Elliott)—A Natural Product for Metabolic Disorders? Nutrients 2022, 14, 2688. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily supplementation with Aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: A meta analysis of controlled clinical trials. J. Diet. Suppl. 2021, 18, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Clark, C.; Varkaneh, H.K.; Lakiang, T.; Vasanthan, L.T.; Onyeche, V.; Mousavi, S.M.; Zhang, Y. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Kempisty, H.; Nowicki, M.; Jodynis-Liebert, J.; Kurpik, M.; Ewertowska, M.; Adamska, T.; Oszmiański, J.; Kujawska, M. Assessment of hepatoprotective effect of chokeberry juice in rats treated chronically with carbon tetrachloride. Molecules 2020, 25, 1268. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yan, T.; Tong, Y.; Deng, H.; Tan, C.; Wan, M.; Wang, M.; Meng, X.; Wang, Y. Gut microbiota modulation by polyphenols from Aronia melanocarpa of lps-induced liver diseases in rats. J. Agric. Food Chem. 2021, 69, 3312–3325. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Zheng, Y.; Liu, W.; Ding, C. Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota. Sci. Rep. 2021, 11, 20558. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, J.; Martin, D.A.; Valdez, J.C.; Jeffety, J.; Barrett-Wilt, G.A.; Liu, Z.; Bolling, B.W. Aronia berry supplementation mitigates inflammation in t cell transfer-induced colitis by decreasing oxidative stress. Nutrients 2019, 11, 1316. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Kim, M.-B.; Park, Y.-K.; Bae, M.; Kang, H.; Hu, S.; Pham, T.X.; Carpenter, R.; Lee, J.; Lee, O.-H.; et al. Anthocyanin-rich aronia berry extract mitigates high-fat and high-sucrose diet-induced adipose tissue inflammation by inhibiting nuclear factor-κb activation. J. Med. Food 2021, 24, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Cui, H.; Tian, H.; Zhang, X.; Ma, L.; Ramassamy, C.; Li, J. Isolation of Neuroprotective Anthocyanins from Black Chokeberry (Aronia melanocarpa) against Amyloid-β-Induced Cognitive Impairment. Foods 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Daskalova, E.; Delchev, S.; Topolov, M.; Dimitrova, S.; Uzunova, Y.; Valcheva-Kuzmanova, S.; Kratchanova, M.; Vladimirova-Kitova, L.; Denev, P. Aronia melanocarpa (Michx.) Elliot fruit juice reveals neuroprotective effect and improves cognitive and locomotor functions of aged rats. Food Chem. Toxicol. 2019, 132, 110674. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Ignjatović, Đ.; Tovilović-Kovačević, G.; Krstić-Milošević, D.; Ranković, S.; Popović, T.; Glibetić, M. Reduction of anxiety-like and depression-like behaviors in rats after one month of drinking Aronia melanocarpa berry juice. Food Funct. 2016, 7, 3111–3120. [Google Scholar] [CrossRef]
- Lee, H.R.; Ryu, H.G.; Lee, Y.; Park, J.A.; Kim, S.; Lee, C.E.; Jung, S.; Lee, K.H. Effect of Aronia extract on collagen synthesis in human skin cell and dermal equivalent. Oxid. Med. Cell Longev. 2022, 2022, 4392256. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Alhosin, M.; Auger, C.; Minker, C.; Kim, J.H.; Etienne-Selloum, N.; Bories, P.; Gronemeyer, H.; Lobstein, A.; Bronner, C.; et al. Aronia melanocarpa juice induces a redox-sensitive p73-related caspase 3-dependent apoptosis in human leukemia cells. PLoS ONE 2012, 7, e32526. [Google Scholar] [CrossRef]
- Ochnik, M.; Franz, D.; Sobczyński, M.; Naporowski, P.; Banach, M.; Orzechowska, B.; Sochocka, M. Inhibition of human respiratory influenza a virus and human betacoronavirus-1 by the blend of double-standardized extracts of Aronia melanocarpa (Michx.) elliot and Sambucus nigra l. Pharmaceuticals 2022, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Kong, Y.; Zhu, J.; Jiao, X.; Tong, Y.; Wan, M.; Zhao, Y.; Lin, S.; Ma, Y.; Meng, X. Proteomic analyses revealed the antibacterial mechanism of Aronia melanocarpa isolated anthocyanins against Escherichia coli O157: H7. Curr. Res. Food Sci. 2022, 5, 1559–1569. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K.; Cyuńczyk, M.; Zujko, M.E. Antioxidant properties of chokeberry products-assessment of the composition of juices and fibers. Foods 2023, 12, 4029. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K.; Zujko, M.E. Are Chokeberry Products Safe for Health? Evaluation of the Content of Contaminants and Health Risk. Foods 2023, 12, 3271. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of selected food. Int. J. Food Prop. 2011, 14, 300–308. [Google Scholar] [CrossRef]
- Żmudzińska, A.; Puścion-Jakubik, A.; Soroczyńska, J.; Socha, K. Evaluation of Selected Antioxidant Parameters in Ready-to-Eat Food for Infants and Young Children. Nutrients 2023, 15, 3160. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–179. [Google Scholar]
- Polish Certified Reference Material for Multielement Trace Analysis; Tea Leaves (INCT-TL-1); Department of Analytical Chemistry, Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2002.
- Klimczak, I.; Gliszczynska-Swigło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kobus, Z.; Nadulski, R.; Wilczyński, K.; Kozak, M.; Guz, T.; Rydzak, L. Effect of the black chokeberry (Aronia melanocarpa (Michx.) Elliott) juice acquisition method on the content of polyphenols and antioxidant activity. PLoS ONE 2019, 14, e0219585. [Google Scholar] [CrossRef]
- Schmid, V.; Steck, J.; Mayer-Miebach, E.; Behsnilian, D.; Briviba, K.; Bunzel, M.; Karbstein, H.P.; Emin, M.A. Impact of defined thermomechanical treatment on the structure and content of dietary fiber and the stability and bioaccessibility of polyphenols of chokeberry (Aronia melanocarpa) pomace. Food Res. Int. 2020, 134, 109232. [Google Scholar] [CrossRef]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Grupcheva, C.; Galunska, B. Comparative phytochemical analysis of Aronia melanocarpa L. fruit juices on Bulgarian market. Plants 2022, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: A prospective controlled trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, I.; Jakovljevic, V.; Petrovic, D.; Draginic, N.; Jeremic, J.; Mitrovic, M.; Zivkovic, V.; Srejovic, I.; Stojic, V.; Bolevich, S.; et al. Standardized Aronia melanocarpa extract regulates redox status in patients receiving hemodialysis with anemia. Mol. Cell. Biochem. 2021, 476, 4167–4175. [Google Scholar] [CrossRef] [PubMed]
- Mirrafiei, A.; Jayedi, A.; Shab-Bidar, S. Total and different dietary fiber subtypes and the risk of all-cause, cardiovascular, and cancer mortality: A dose-response meta-analysis of prospective cohort studies. Food Funct. 2023, 14, 10667–10680. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary blueberry and soluble fiber supplementation reduces risk of gestational diabetes in women with obesity in a randomized controlled trial. J. Nutr. 2021, 15, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sheng, J.; Zhang, D.; Chen, L.; Jiang, Y.; Cheng, D.; Su, Y.; Yu, Y.; Jia, H.; He, P.; et al. The role of dietary fiber on preventing gestational diabetes mellitus in an at-risk group of high triglyceride-glucose index women: A randomized controlled trial. Endocrine 2023, 82, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-Y.; Thomas, S.S.; Hwang, D.-I.; Lee, J.-H.; Kim, K.-A.; Cha, Y.-S. Anti-Obesity Effects of Morus alba L. and Aronia melanocarpa in a High-Fat Diet-Induced Obese C57BL/6J Mouse Model. Foods 2021, 10, 1914. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Pokimica, B.; García-Conesa, M.-T.; Zec, M.; Debeljak-Martacic, J.; Ranković, S.; Vidović, N.; Petrović-Oggiano, G.; Konić-Ristić, A.; Glibetić, M. Chokeberry Juice Containing Polyphenols Does Not Affect Cholesterol or Blood Pressure but Modifies the Composition of Plasma Phospholipids Fatty Acids in Individuals at Cardiovascular Risk. Nutrients 2019, 11, 850. [Google Scholar] [CrossRef]
- Sikora, J.; Broncel, M.; Markowicz, M.; Chałubi’nski, M.; Wojdan, K.; Mikiciuk-Olasik, E. Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. Eur. J. Nutr. 2012, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Broncel, M.; Mikiciuk-Olasik, E. Aronia melanocarpa Elliot reduces the activity of Angiotensin I-converting enzyme—In vitro and ex vivo studies. Oxidative Med. Cell. Longev. 2014, 2014, 739721. [Google Scholar] [CrossRef] [PubMed]
- Loo, B.-M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of chokeberry (Aronia mitschurinii) products modestly lowered blood pressure and reduced low-grade inflammation in patients with mildly elevated blood pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, CR28–CR34. [Google Scholar] [PubMed]
- Naruszewicz, M.; Łaniewska, I.; Millo, B.; Dłuzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef]
- Kardum, N.; Petrović-Oggiano, G.; Takic, M.; Glibetić, N.; Zec, M.; Debeljak-Martacic, J.; Konić-Ristić, A. Effects of Glucomannan-Enriched, Aronia Juice-Based Supplement on Cellular Antioxidant Enzymes and Membrane Lipid Status in Subjects with Abdominal Obesity. Sci. World J. 2014, 2014, 869250. [Google Scholar] [CrossRef] [PubMed]
- European Society of Hypertension. 2023 Guidelines for the Management of Arterial Hypertension. Available online: https://www.eshonline.org/guidelines/2023-guidelines/ (accessed on 10 April 2024).
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary polyphenol intake, blood pressure, and hypertension: A systematic review and meta-analysis of observational studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Potential Factors Influencing the Effects of Anthocyanins on Blood Pressure Regulation in Humans: A Review. Nutrients 2019, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Skoczynska, A.; Jedrychowska, I.; Porêba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial Effects of Polyphenol-Rich Chokeberry Juice Consumption on Blood Pressure Level and Lipid Status in Hypertensive Subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Milutinović, M.; Radovanović, R.V.; Šavikin, K.; Radenković, S.; Arvandi, M.; Pešić, M.; Kostić, M.; Miladinović, B.; Branković, S.; Kitić, D. Chokeberry juice supplementation in type 2 diabetic patients—Impact on health status. J. Appl. Biomed. 2019, 17, 218–224. [Google Scholar] [CrossRef]
- Putta, S.; Yarla, N.S.; Kumar, E.K.; Lakkappa, D.B.; Kamal, M.A.; Scotti, L.; Scotti, M.T.; Ashraf, G.; Rao, B.S.B.; Kumari, S.; et al. Preventive and Therapeutic Potentials of Anthocyanins in Diabetes and Associated Complications. Curr. Med. Chem. 2019, 25, 5347–5371. [Google Scholar] [CrossRef] [PubMed]
- Jebur, A.B.; Mokhamer, M.H.; El-Demerdash, F.M. A Review on Oxidative Stress and Role of Antioxidants in Diabetes Mellitus. Austin Endocrinol. Diabetes Case Rep. 2016, 1, 1006. [Google Scholar]
- Rugină, D.; Diaconeasa, Z.; Coman, C.; Bunea, A.; Socaciu, C.; Pintea, A. Chokeberry Anthocyanin Extract as Pancreatic β-Cell Protectors in Two Models of Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2015, 429075. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ling, W.; Yang, Y.; Chen, Y.; Tian, Z.; Du, Z.; Chen, J.; Xie, Y.; Liu, Z.; Yang, L. Role of Purified Anthocyanins in Improving Cardiometabolic Risk Factors in Chinese Men and Women with Prediabetes or Early Untreated Diabetes—A Randomized Controlled Trial. Nutrients 2017, 9, 1104. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, Anthocyanins and Procyanidins from Aronia melanocarpa as Radical Scavengers and Enzyme Inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of blood glucose levels and obesity in mice given aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef]
- Gancheva, S.; Ivanova, I.; Atanassova, A.; Gancheva-Tomova, D.; Eftimov, M.; Moneva, K.; Zhelyazkova-Savova, M.; Valcheva Kuzmanova, S. Effects of Aronia melanocarpa fruit juice on oxidative stress, energy homeostasis, and liver function in overweight and healthy-weight individuals. Scr. Sci. Med. 2021, 53, 39–46. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Lorrain, B.; Dangles, O.; Genot, C.; Dufour, C. Chemical Modeling of Heme-Induced Lipid Oxidation in Gastric Conditions and Inhibition by Dietary Polyphenols. J. Agric. Food Chem. 2010, 58, 676–683. [Google Scholar] [CrossRef]
- Lorrain, B.; Dangles, O.; Loonis, M.; Armand, M.; Dufour, C. Dietary Iron-Initiated Lipid Oxidation and Its Inhibition by Polyphenols in Gastric Conditions. J. Agric. Food Chem. 2012, 60, 9074–9081. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, Y.; Wegner, C.J.; Bolling, B.W.; Lee, J. Polyphenol-rich black chokeberry (Aronia melanocarpa) extract regulates the expression of genes critical for intestinal cholesterol flux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1564–1570. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Liu, S.; Zhang, M.; Wang, C.; Xia, X.; Lou, Y.; Xu, H. The effect of lipid metabolism regulator anthocyanins from Aronia melanocarpa on 3T3-L1 preadipocytes and C57BL/6 mice via activating AMPK signaling and gut microbiota. Food Funct. 2021, 12, 6254–6270. [Google Scholar] [CrossRef]
- Mahdavi, A.; Bagherniya, M.; Fakheran, O.; Reiner, Ž.; Xu, S.; Sahebkar, A. Medicinal plants and bioactive natural compounds as inhibitors of HMG-CoA reductase: A literature review. BioFactors 2020, 46, 906–926. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Beltran, D.; Gil-Guillen, V.F.; Redon, J.; Martin-Moreno, J.M.; Pallares-Carratala, V.; Navarro-Perez, J.; Valls-Roca, F.; Sanchis-Domenech, C.; Fernandez-Gimenez, A.; Perez-Navarro, A.; et al. Correction: Lipid profile, cardiovascular disease and mortality in a Mediterranean high-risk population: The ESCARVAL-RISK study. PLoS ONE 2018, 13, e0205047. [Google Scholar] [CrossRef]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The Contribution of homocysteine metabolism disruption to endothelial dysfunction: State-of-the-art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef]
- Arias, I.M.; Alter, H.J.; Boyer, J.L.; Cohen, D.E.; Shafritz, D.A.; Thorgeirsson, S.S.; Wolkoff, A.W. The Liver: Biology and Pathobiology, 6th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Tamber, S.S.; Bansal, P.; Sharma, S.; Singh, R.B.; Sharma, R. Biomarkers of liver diseases. Mol. Biol. Rep. 2023, 50, 7815–7823. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Park, H.; Liu, Y.; Kim, H.-S.; Shin, J.-H. Chokeberry attenuates the expression of genes related to de novo lipogenesis in the hepatocytes of mice with nonalcoholic fatty liver disease. Nutr. Res. 2016, 36, 57–64. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. berries prevents cadmium-induced oxidative stress in the liver: A study in a rat model of low-level and moderate lifetime human exposure to this toxic metal. Nutrients 2019, 11, 21. [Google Scholar] [CrossRef]
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not So Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Titan, S. Measurement and Estimation of GFR for Use in Clinical Practice: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 78, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.R.; Shipkowski, K.A.; Frawley, R.P.; Evans, E. Markers of Inflammation. In Immunotoxicity Testing. Methods in Molecular Biology; DeWitt, J., Rockwell, C., Bowman, C., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1803, pp. 57–79. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, A.; Kuzmanova, V.; Tzaneva, M. Aronia melanocarpa fruit juice ameliorates the symptoms of inflammatory bowel disease in TNBS-induced colitis in rats. Food Chem. Toxicol. 2018, 113, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-κB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.J.; Mehta, R.G.; Kinghorn, A.D.; Pezzuto, J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High. Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).