Supplementation of Mangiferin to a High-Starch Diet Alleviates Hepatic Injury and Lipid Accumulation Potentially through Modulating Cholesterol Metabolism in Channel Catfish (Ictalurus punctatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish and Feeding Trial
2.3. Sample Collection
2.4. Analysis of the Chemical Composition of the Experimental Diets and Fish Whole Body
2.5. H&E Staining and Oil Red O Staining
2.6. Primary Hepatocyte Isolation and Culture
2.7. Cell Line Source and Culture Conditions
2.8. Cell Viability Assay
2.9. Plasma, Liver, and Cell Biochemical Analyses
2.10. Quantitative Real-Time PCR (qPCR)
2.11. Immunofluorescence Assay
2.12. Molecular Docking
2.13. Statistics
3. Results
3.1. MGF Improves the Growth Performance of Channel Catfish
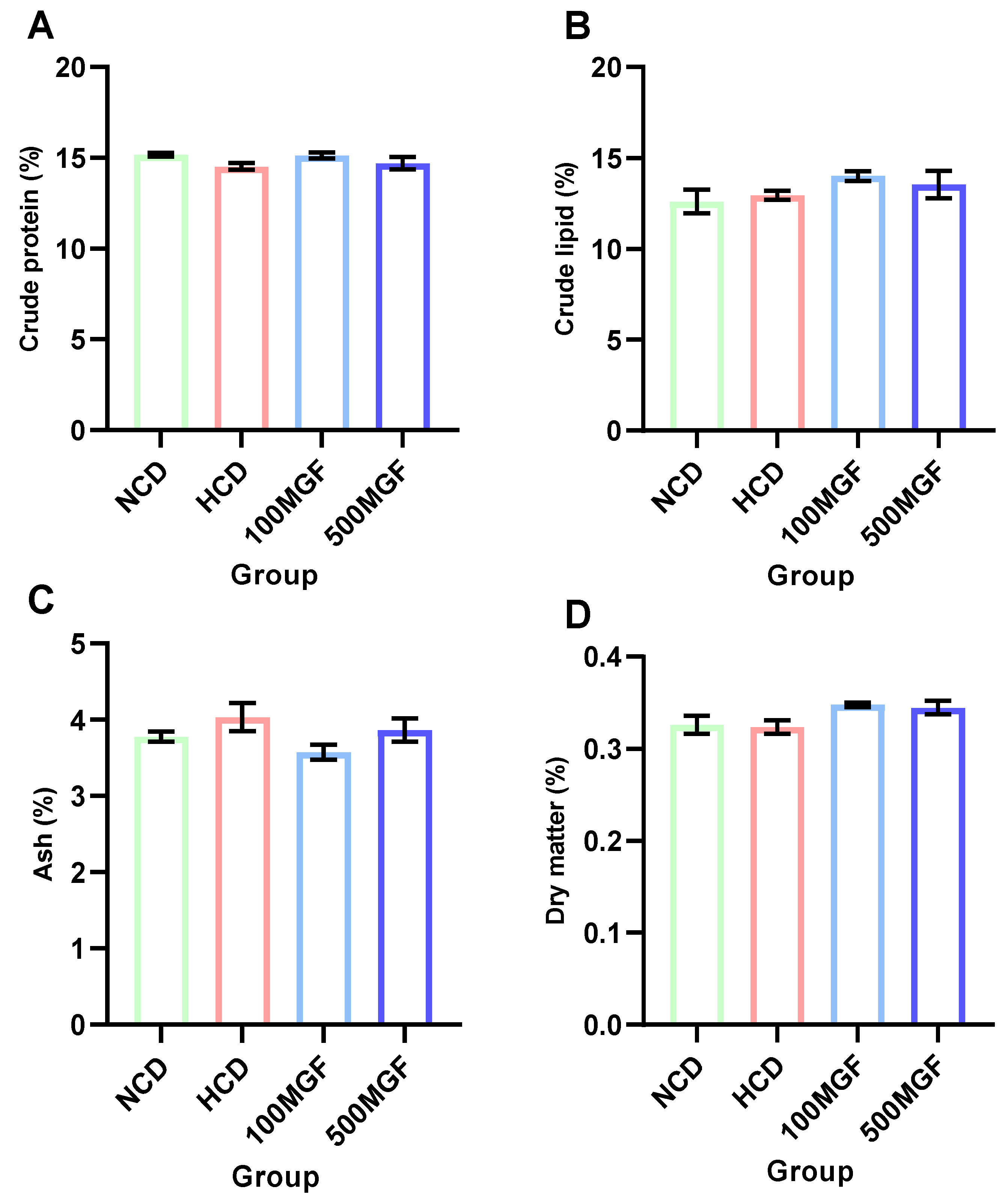
3.2. Proximate Body Composition
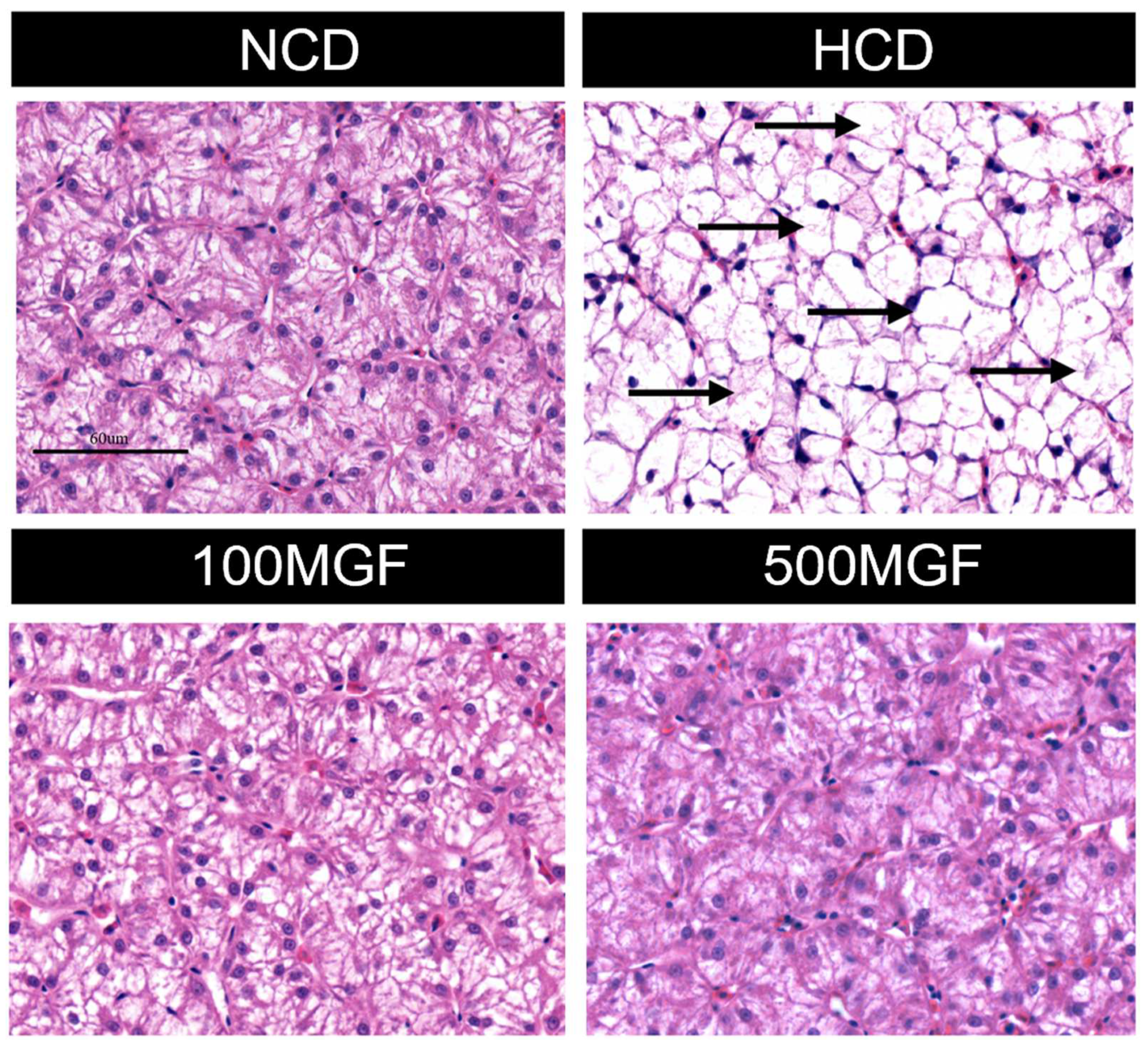
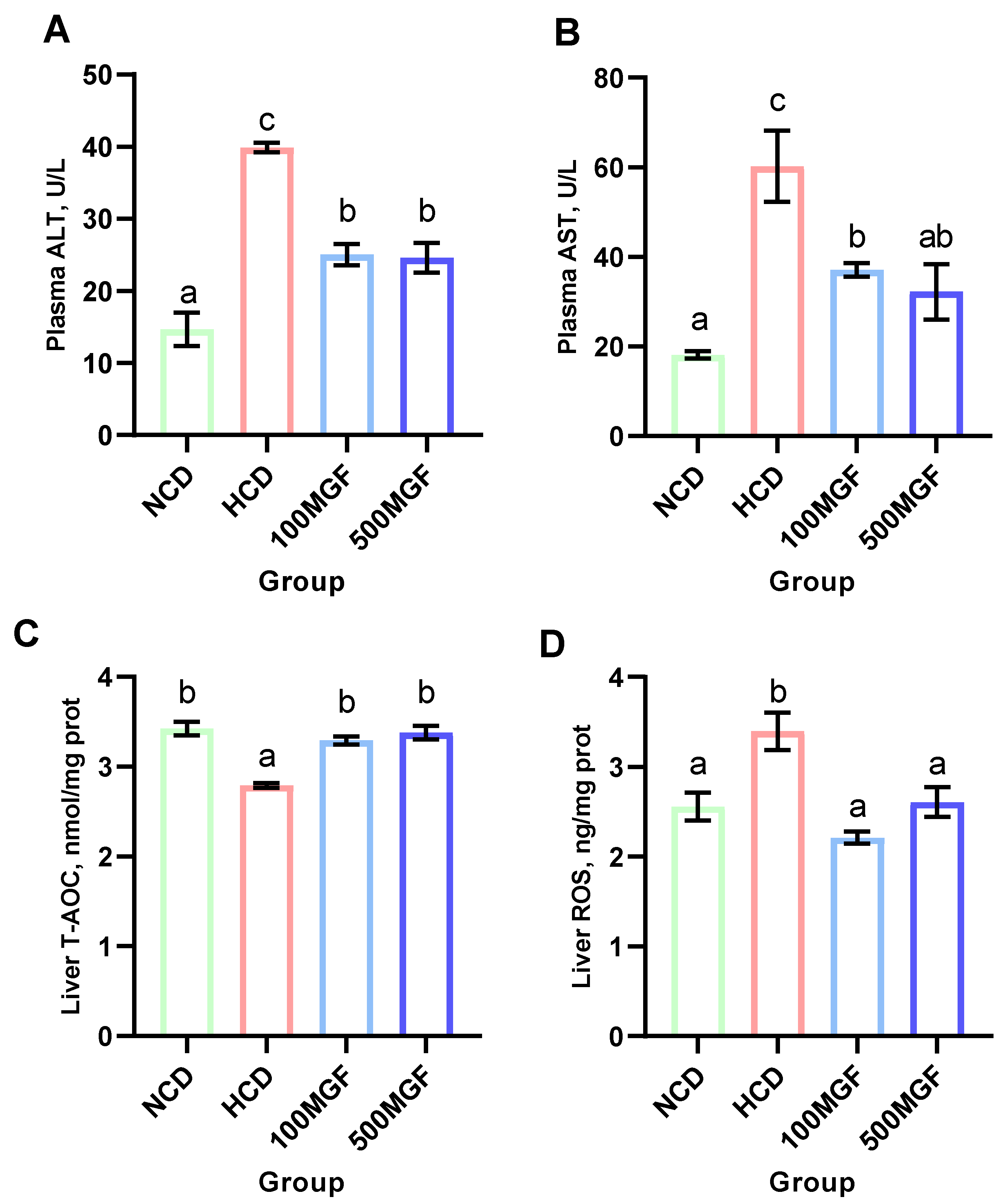
3.3. MGF Effects on HCD-Induced Hepatic Damage
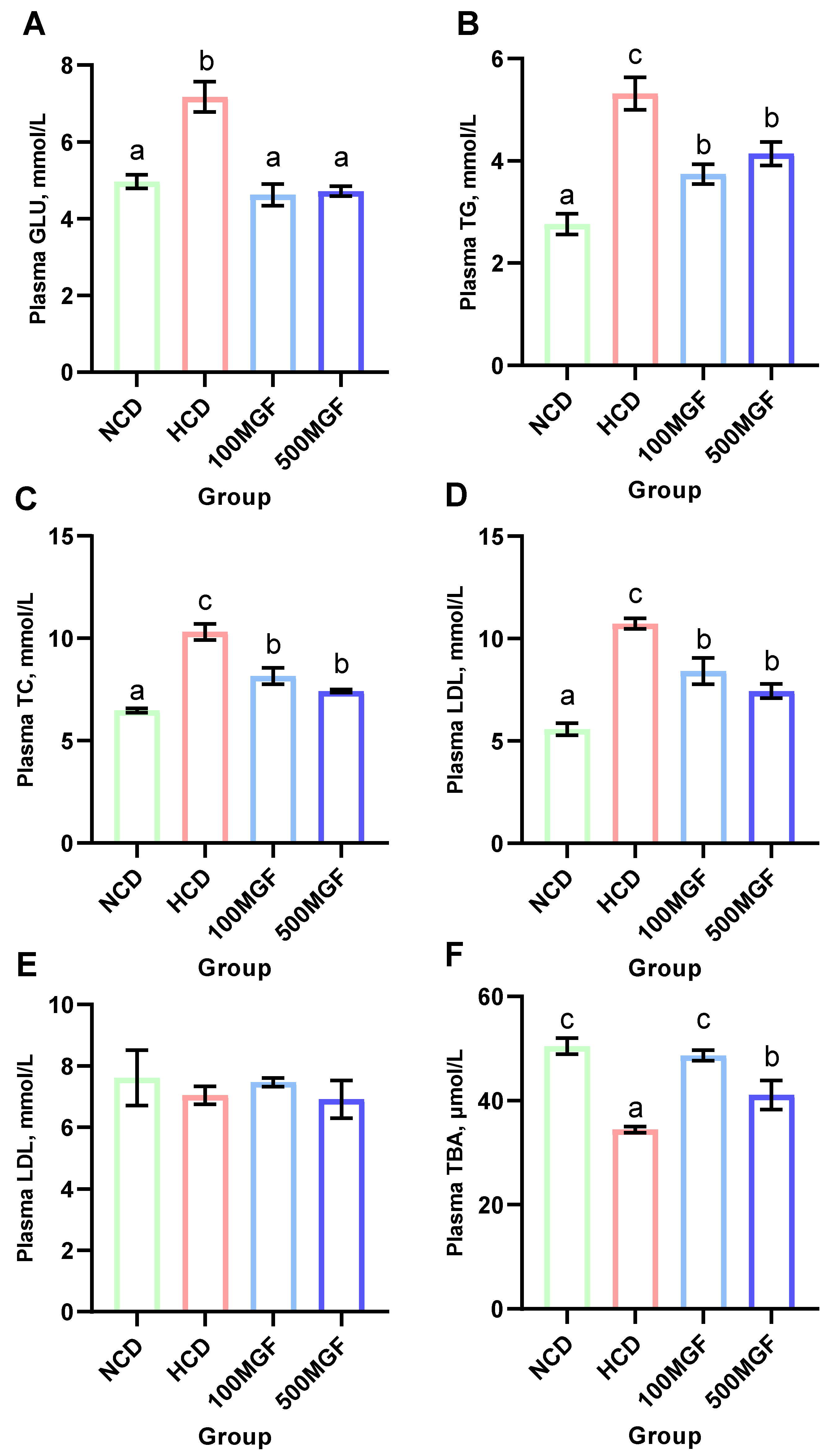
3.4. MGF Reduces the High Blood Glucose and Lipid Levels Caused by an HCD Diet
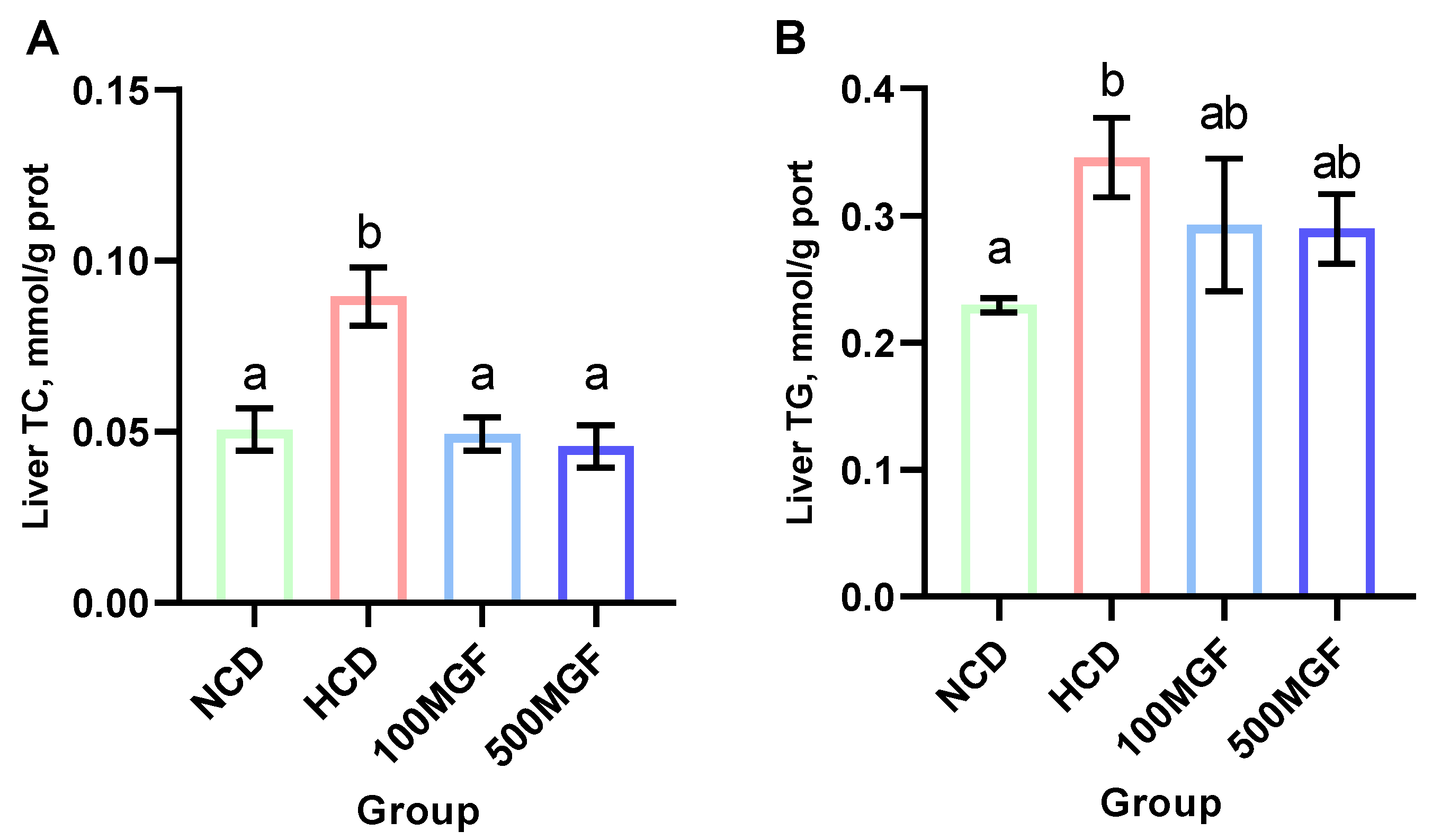
3.5. MGF Alleviates HCD-Induced Hepatic Lipid Accumulation
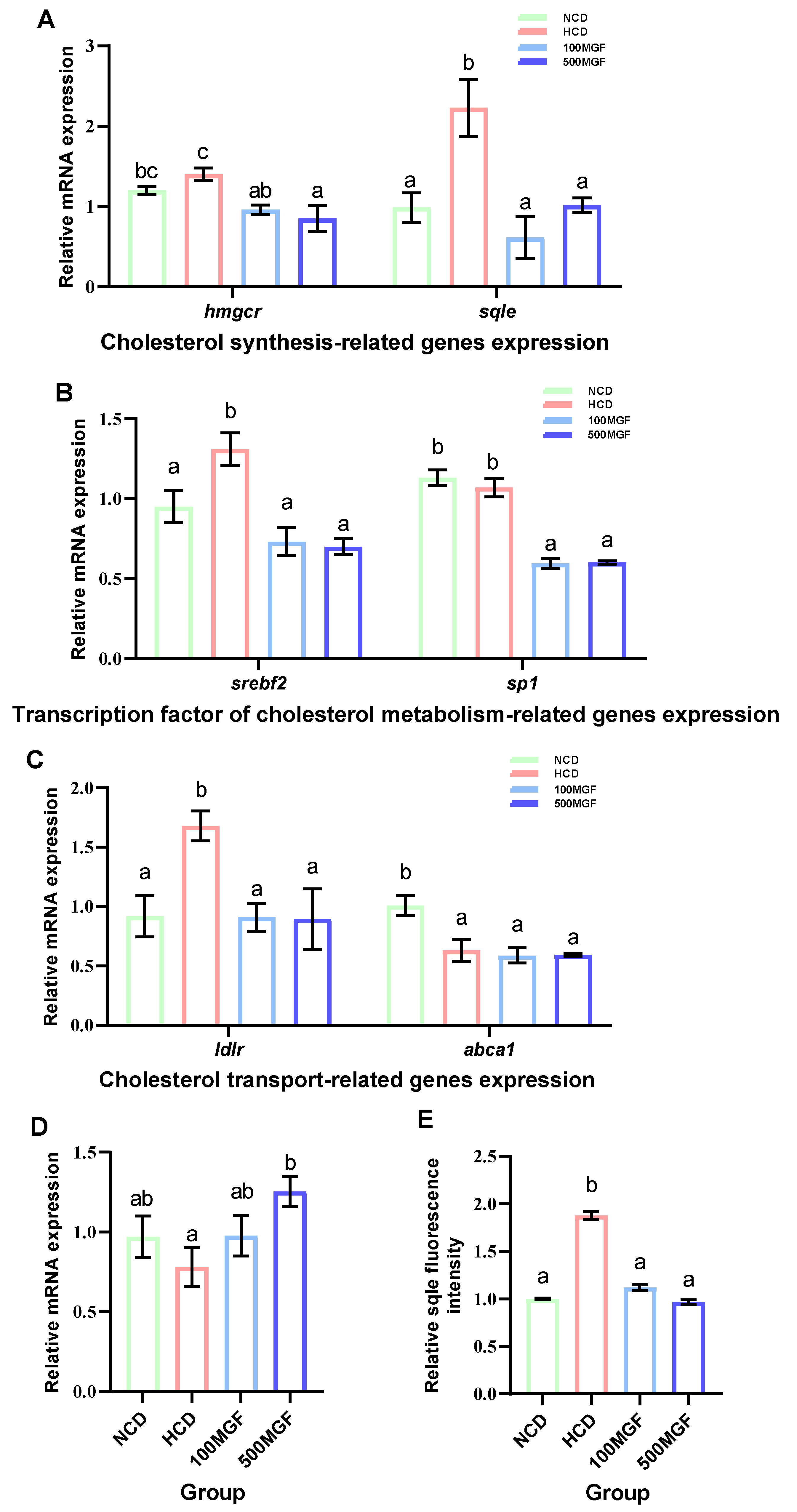
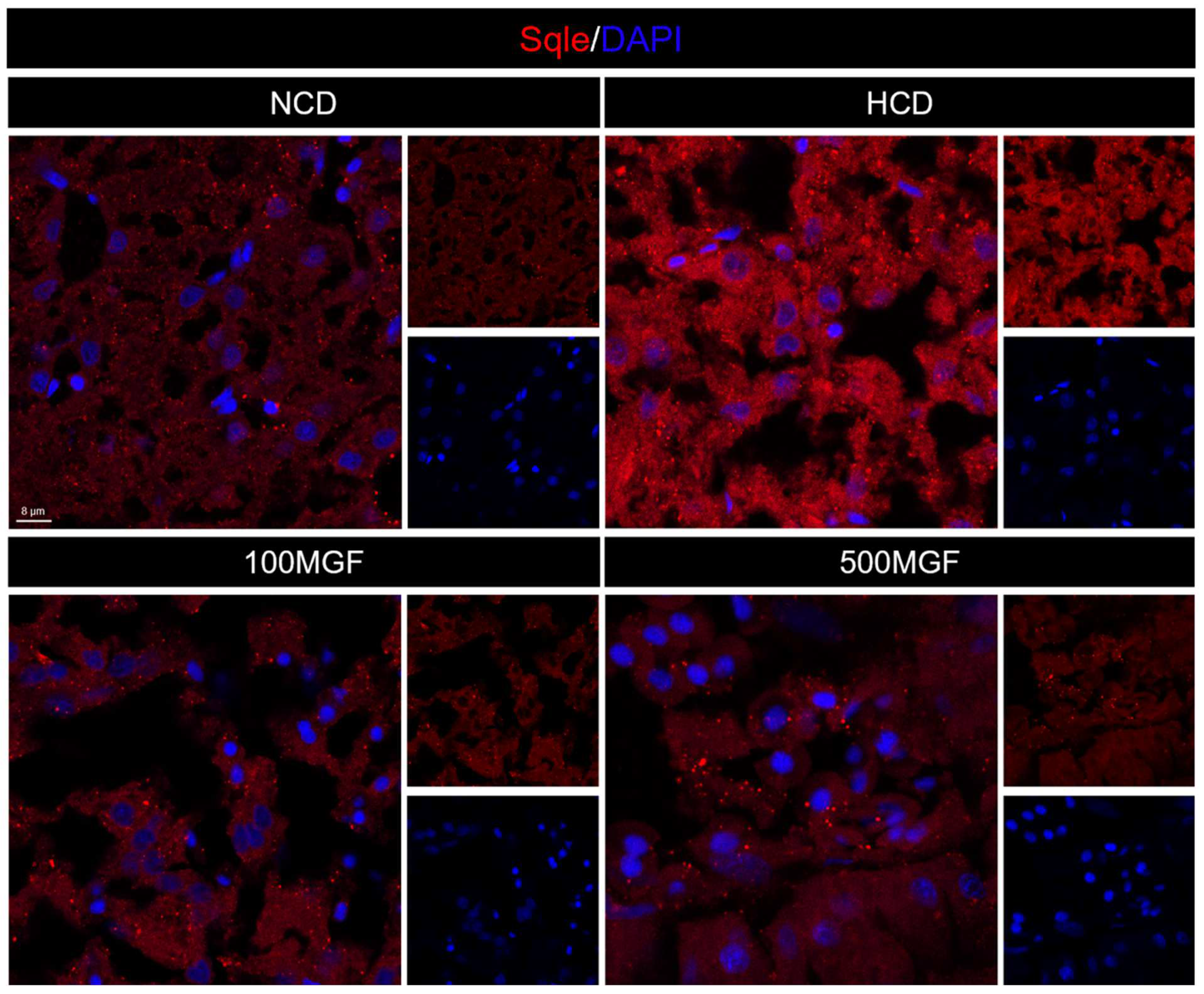
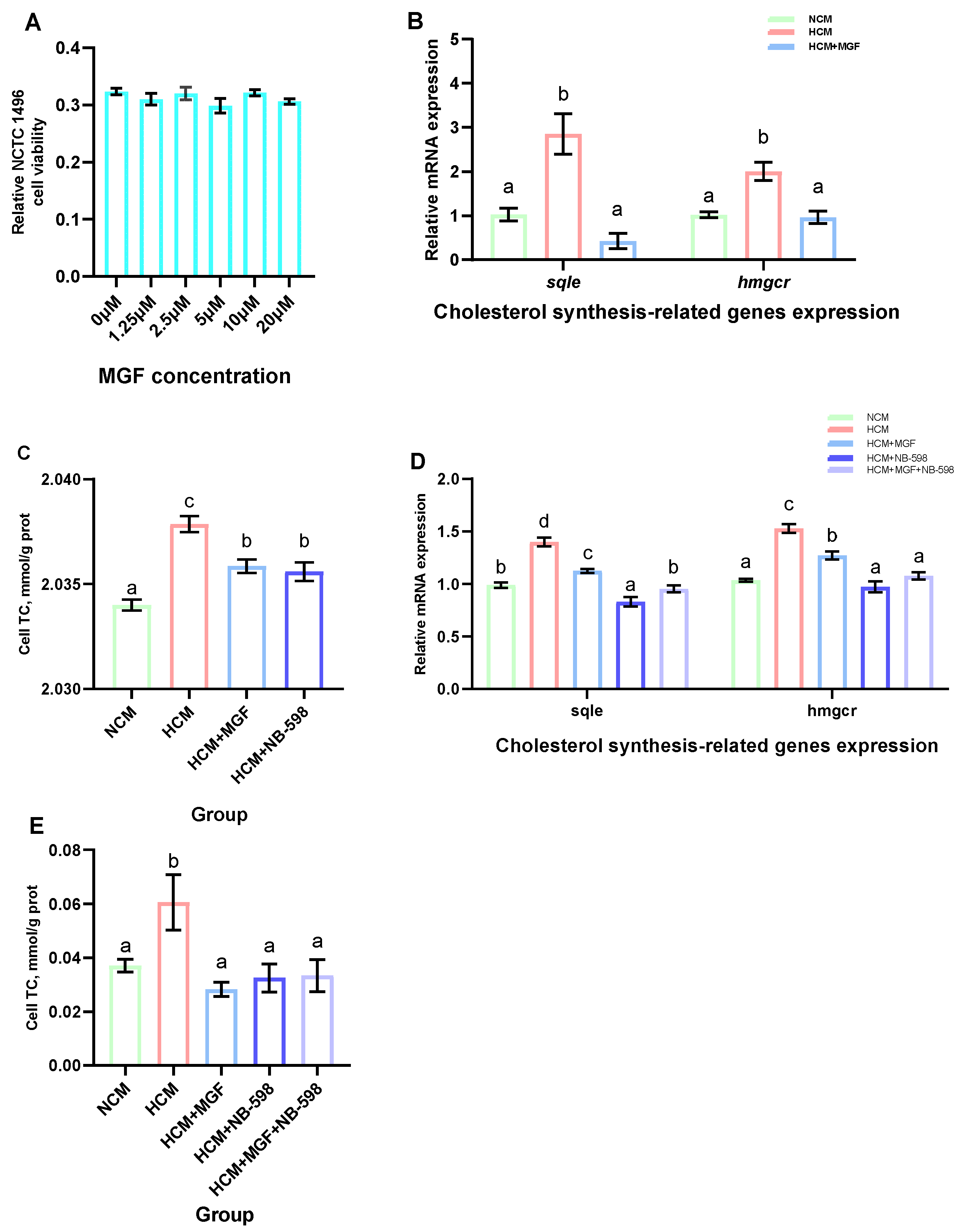
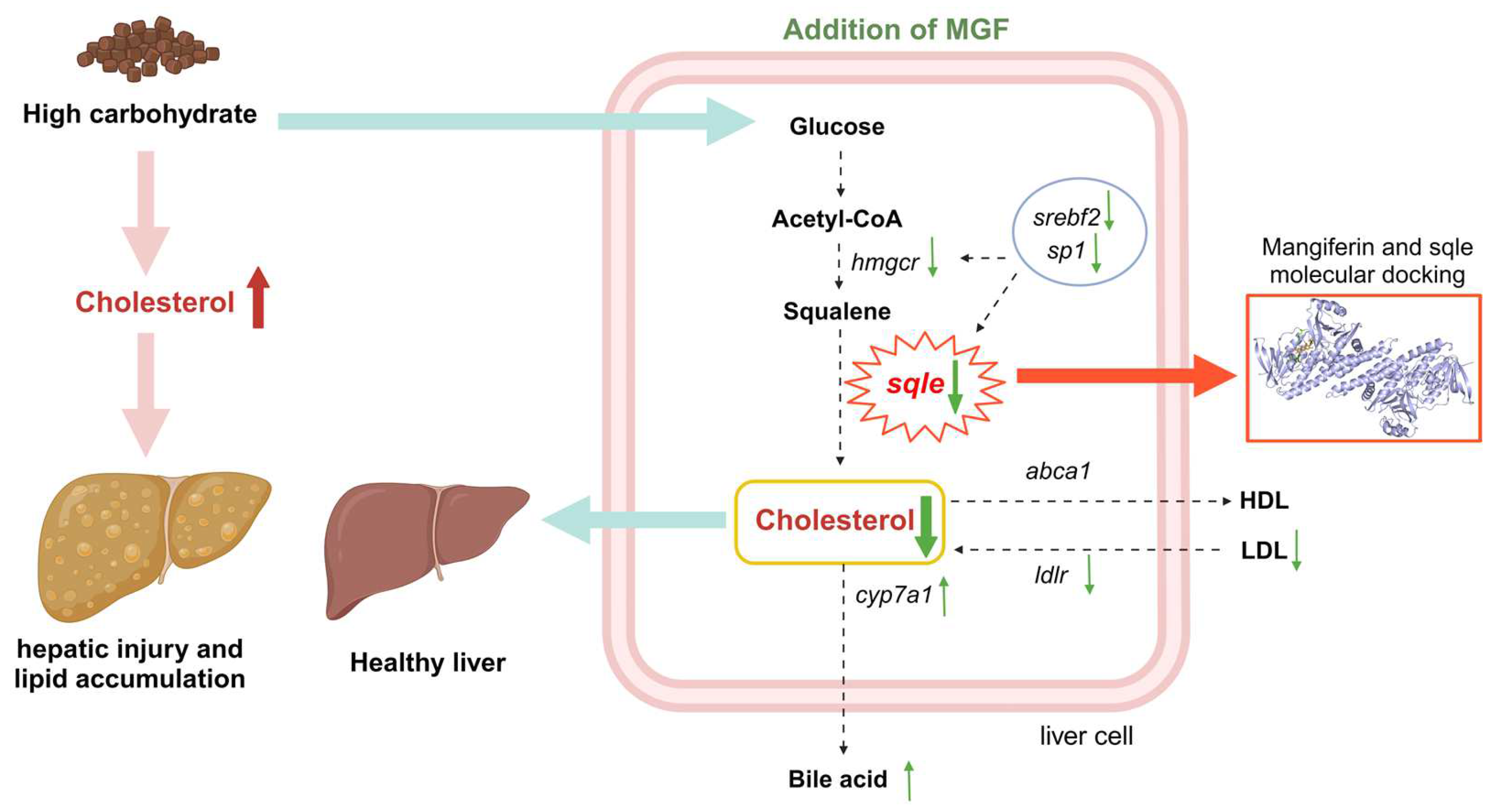
3.6. MGF Regulates Hepatic Cholesterol Metabolism
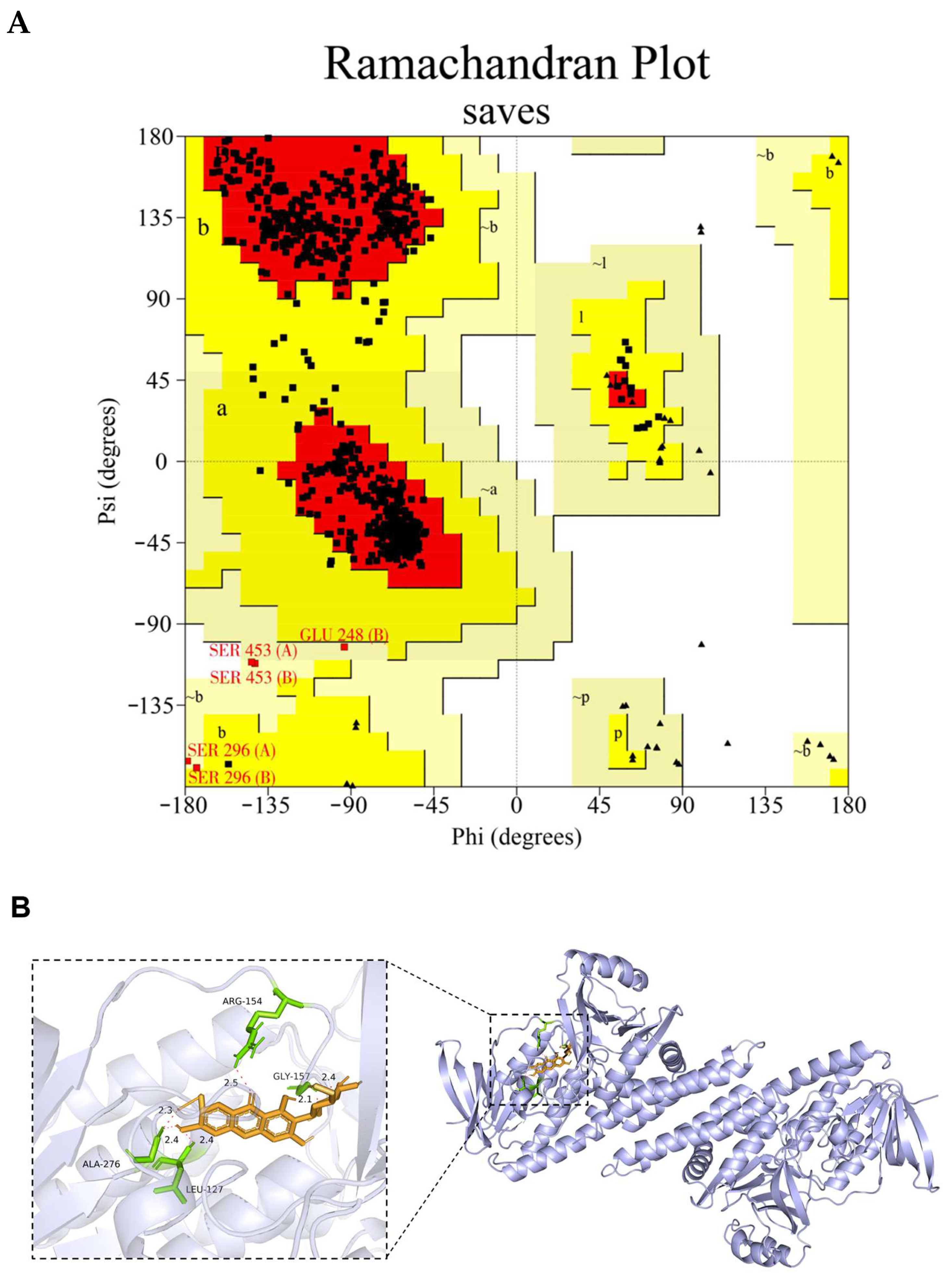
3.7. Molecular Docking Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, X.; Luo, Z.; Liu, Y. Progress in the study of fish utilisation of carbohydrates in feedstuffs. China Feed 2007, 6, 19–23, (In Chinese with English Abstract). [Google Scholar]
- Kari, Z.; Sukri, S.; Rusli, N.; Mat, K.; Mahmud, M.; Zakaria, N.; Wee, W.; Hamid, N.; Kabir, M.; Ariff, N.; et al. Recent Advances, Challenges, Opportunities, Product Development and Sustainability of Main Agricultural Wastes for the Aquaculture Feed Industry—A Review. Ann. Anim. Sci. 2023, 23, 25–38. [Google Scholar] [CrossRef]
- Kamalam, B.; Médale, F.; Panserat, S. Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Bai, J.; Li, C.; Tang, Z.; Wu, C.; Wei, Z. Comparative study of carbohydrate levels on growth, oxidative stress and glucolipid metabolism of hybrid fish between Megalobrama amblycephala (♀) × Culter alburnus (♂) and Culter alburnus. Reprod. Breed. 2023, 3, 131–142. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Zhong, H.; Zhang, J.; Liu, X.; Peng, M.; Fu, G.; Hu, Y. Taurine supplements in high-carbohydrate diets increase growth performance of Monopterus albus by improving carbohydrate and lipid metabolism, reducing liver damage, and regulating intestinal microbiota. Aquaculture 2022, 554, 738150. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, L.; Tang, X.; Liang, J.; Liu, Q.; Luo, W.; Adam, A.; Luo, J.; Li, Z.; Yang, S.; et al. High-carbohydrate diet altered conversion of metabolites, and deteriorated health in juvenile largemouth bass. Aquaculture 2022, 549, 737816. [Google Scholar] [CrossRef]
- Burris, T.P. Cholesterol and Other Steroids. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, pp. 233–240. [Google Scholar]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrin. Met. 2016, 27, 84–95. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Ou, X.; Ouyang, P.; Tang, C. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G. Beyond reasonable doubt: Who is the culprit in lipotoxicity in NAFLD/NASH? Hepatology 2012, 55, 2030–2032. [Google Scholar] [CrossRef]
- Kaurav, M.; Kanoujia, J.; Gupta, M.; Goyal, P.; Pant, S.; Rai, S.; Sahu, K.; Bhatt, P.; Ghai, R. In-depth analysis of the chemical composition, pharmacological effects, pharmacokinetics, and patent history of mangiferin. Phytomedicine Plus 2023, 3, 100445. [Google Scholar] [CrossRef]
- Nagul Kumar, S.; Darvin, S.; Toppo, E.; Porchezhian, V.; Pandikumar, P.; Paulraj, M.; Ignacimuthu, S. Ameliorative effect of mangiferin on high fat diet—Diethylnitrosamine induced non-alcoholic steatohepatitis rats. Biocatal. Agr. Biotech. 2022, 41, 102312. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Wang, L.; Teng, T.; Zhou, M.; Wang, S.; Tian, Y.; Du, L.; Yin, X.; Sun, Y. Mangiferin ameliorates fatty liver via modulation of autophagy and inflammation in high-fat-diet induced mice. Biomed. Pharmacother. 2017, 96, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Lee, H.; Lee, B. Mangiferin Ameliorates Obesity-Associated Inflammation and Autophagy in High-Fat-Diet-Fed Mice: In Silico and In Vivo Approaches. Int. J. Mol. Sci. 2022, 23, 15329. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Liu, Z.; Apontes, P.; Feng, D.; Pessin, J.; Sauve, A.; Angeletti, R.; Chi, Y. Dual mode action of mangiferin in mouse liver under high fat diet. PLoS ONE 2014, 9, e90137. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.; Song, B. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wong, C.; Zhou, Y.; Li, C.; Chen, H.; Ji, F.; Go, M.; Wang, F.; Su, H.; Wei, H.; et al. Squalene Epoxidase Induces Nonalcoholic Steatohepatitis Via Binding to Carbonic Anhydrase III and is a Therapeutic Target. Gastroenterology 2021, 160, 2467–2482.e3. [Google Scholar] [CrossRef]
- Gill, S.; Stevenson, J.; Kristiana, I.; Brown, A.J. Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase. Cell Metab. 2011, 13, 260–273. [Google Scholar] [CrossRef]
- Chugh, A.; Ray, A.; Gupta, J. Squalene epoxidase as hypocholesterolemic drug target revisited. Prog. Lipid Res. 2003, 42, 37–50. [Google Scholar] [CrossRef]
- Gupta, N.; Porter, T. Garlic and garlic-derived compounds inhibit human squalene monooxygenase. J. Nutr. 2001, 131, 1662–1667. [Google Scholar] [CrossRef]
- Hu, X.; Qin, F.; Lu, X.; Zhang, L.; Cheng, Y. Three New Polyynes from Codonopsis pilosula and Their Activities on Lipid Metabolism. Molecules 2018, 23, 887. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Chen, Q.; Chen, X.; Zhou, G.; Sun, H. Curcumin sensitizes response to cytarabine in acute myeloid leukemia by regulating intestinal microbiota. Cancer Chemoth Pharm. 2022, 89, 243–253. [Google Scholar] [CrossRef]
- Abe, I.; Seki, T.; Noguchi, H. Potent and selective inhibition of squalene epoxidase by synthetic galloyl esters. Biochem. Biophys. Res. Commun. 2000, 270, 137–140. [Google Scholar] [CrossRef]
- Bureau MOAA. 2023 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2023; Volume 2, p. 25. [Google Scholar]
- Jiang, X.; Zhang, S.; Huang, K.; Wu, H.; Jiang, H. Effects of different dietary carbohydrate levels on growth and physiological functions of Channel catfish (Ictalurus punctatus). Guangdong Agric. Sci. 2014, 17, 111–118, (In Chinese with English Abstract). [Google Scholar]
- Gong, Y.; Lu, Q.; Xi, L.; Liu, Y.; Yang, B.; Su, J.; Liu, H.; Jin, J.; Zhang, Z.; Yang, Y.; et al. F6P/G6P-mediated ChREBP activation promotes the insulin resistance-driven hepatic lipid deposition in zebrafish. J. Nutr. Biochem. 2023, 122, 109452. [Google Scholar] [CrossRef]
- Yan, Z.; Cao, X.; Sun, S.; Sun, B.; Gao, J. Inhibition of GSK3B phosphorylation improves glucose and lipid metabolism disorder. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166726. [Google Scholar] [CrossRef]
- AOAC, Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Gaithersburgs, MD, USA, 2006; Volume 16. [Google Scholar]
- Chen, W.; Gao, S.; Chang, K.; Zhao, X.; Niu, B. Dietary sodium butyrate supplementation improves fish growth, intestinal microbiota composition, and liver health in largemouth bass (Micropterus salmoides) fed high-fat diets. Aquaculture 2022, 564, 739040. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, L.; Yan, H.; Tang, X.; He, K.; Liu, Q.; Luo, J.; Du, Z.; Chen, S.Y.; Zhang, X.; et al. Physiological responses to acute hypoxia in the liver of largemouth bass by alteration of mitochondrial function and Ca2+ exchange. Aquat. Toxicol. 2023, 256, 106436. [Google Scholar] [CrossRef]
- Horie, M.; Tsuchiya, Y.; Hayashi, M.; Iida, Y.; Iwasawa, Y.; Nagata, Y.; Sawasaki, Y.; Fukuzumi, H.; Kitani, K.; Kamei, T. NB-598: A potent competitive inhibitor of squalene epoxidase. J. Biol. Chem. 1990, 265, 18075–18078. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; He, Y.; Yuan, Z.; Chen, M.; Xu, K.; Li, K.; Guo, A.; Cai, K.; Chen, L. Osteoimmunomodulation mediating improved osteointegration by OGP-loaded cobalt-metal organic framework on titanium implants with antibacterial property. Chem. Eng. J. 2021, 423, 130176. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Qi, F.; Xia, T.; Xia, Y.; Xu, J.; Zhang, X. An Activatable Host-Guest Conjugate as a Nanocarrier for Effective Drug Release through Self-Inclusion. ACS Appl. Mater. 2021, 13, 33962–33968. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Meng, X.; Lin, X.; Zhang, L.; Mi, H.; Deng, J. Effects of dietary pectin on the growth performance, intestinal barrier, and antioxidant status of juvenile rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2024, 36, 102055. [Google Scholar] [CrossRef]
- Liu, Y.; Hong, Y.; Yao, C.; Zhang, C.; Zhao, Z.; Zhang, W.; Lai, W.; Zhang, J.; Li, Y.; Mai, K.; et al. Role of sodium octanoate in regulating survival, growth, intestinal development, digestive and absorptive capacities, antioxidant capacity and innate immunity of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2023, 582, 740476. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, S.; Zhang, J.; Li, X.; Xu, Y.; Liu, Q.; Du, J.; Li, Y.; Mai, K.; Ai, Q. Effects of nuciferine on growth performance, body composition, lipid metabolism and antioxidant capacity of juvenile large yellow croaker (Larmichthys crocea) fed diets with high palm oil level. Aquaculture 2024, 586, 740821. [Google Scholar] [CrossRef]
- Olson, B. Assays for Determination of Protein Concentration. Curr. Protoc. Pharmacol. 2007, 73, A.3A.1–A.3A.32. [Google Scholar]
- Wang, Y.; Gao, J.; Zhang, D.; Zhang, J.; Ma, J.; Jiang, H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J. Hepatol. 2010, 53, 132–144. [Google Scholar] [CrossRef]
- He, J.; Zhao, K.; Zheng, L.; Xu, Z.; Gong, W.; Chen, S.; Shen, X.; Huang, G.; Gao, M.; Zeng, Y.; et al. Upregulation of microRNA-122 by farnesoid X receptor suppresses the growth of hepatocellular carcinoma cells. Mol. Cancer. 2015, 14, 163. [Google Scholar] [CrossRef]
- Yang, B.; Maddison, L.; Zaborska, K.; Dai, C.; Yin, L.; Tang, Z.; Zang, L.; Jacobson, D.; Powers, A.; Chen, W. RIPK3-mediated inflammation is a conserved β cell response to ER stress. Sci. Adv. 2020, 6, eabd7272. [Google Scholar] [CrossRef]
- Cardoso, A.; Santos, W.; Gomes, J.; Martins, M.; Coura, R.; Oliveira, M.; Salaro, A.; Ferreira, P.; Carneiro, A.; Zuanon, J.A. Ginger oil, Zingiber officinale, improve palatability, growth and nutrient utilisation efficiency in Nile tilapia fed with excess of starch. Anim. Feed. Sci. Technol. 2020, 272, 114756. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, X.; Yang, L.; Qin, C.; Pang, P.; Yang, B.; Nie, G. Effects of high-carbohydrate diet supplemented with sea buckthorn powder on the growth, antioxidant ability and non-specific immunity of Yellow River carp (Cyprinus carpio haematopterus). J. Fish. China 2023, 10, 113–127, (In Chinese with English Abstract). [Google Scholar]
- Song, Y.; Liu, W.; Tang, K.; Zang, J.; Li, D.; Gao, H. Mangiferin Alleviates Renal Interstitial Fibrosis in Streptozotocin-Induced Diabetic Mice through Regulating the PTEN/PI3K/Akt Signaling Pathway. J. Diabetes Res. 2020, 2020, 9481720. [Google Scholar] [CrossRef]
- Peter, N.; Majumdar, J.; Biswas, G.; Pawar, H.; Mitra, A.; Mitra, A. Effects of mangiferin isolated from Mangifera indica leaves and evaluation of biologic activities of β-cyclodextrin-mangiferin complex particularly its anti-diabetic and hypolipidaemic propertieson Type 1 diabetes rat model. Int. J. Herb. Med. 2017, 5, 92–98. [Google Scholar]
- Elshazly, S. Ameliorative effect of nicorandil on high fat diet induced non-alcoholic fatty liver disease in rats. Eur. J. Pharmacol. 2015, 748, 123–132. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, W.; Yang, Z.; Kong, Q.; Liu, P.; Liao, H.; Cui, Z.; Tang, H. Dietary Lactobacillus plantarum can alleviate high starch diet-induced liver lipid deposition, tissue damage and oxidative stress in largemouth bass (Micropterus salmoides). Aquac. Rep. 2024, 35, 101955. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta. 2015, 1852, 1765–1778. [Google Scholar] [CrossRef]
- Chowdhury, A.; Lu, J.; Zhang, R.; Nabila, J.; Gao, H.; Wan, Z.; Adelusi Temitope, I.; Yin, X.; Sun, Y. Mangiferin ameliorates acetaminophen-induced hepatotoxicity through APAP-Cys and JNK modulation. Biomed. Pharmacother. 2019, 117, 109097. [Google Scholar] [CrossRef]
- Min, H.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.; Sanyal, A. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, L.; Chen, W.; Sun, M.; Liu, W.; Li, X. Resveratrol modulates the redox response and Bile acid metabolism to maintain the cholesterol homeostasis in fish Megalobrama amblycephala offered a high-carbohydrate Diet. Antioxidants 2023, 12, 121. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Yang, T.; Li, M.; Qin, L.; Wu, L.; Liu, T. Oral administration of mangiferin ameliorates diabetes in animal models: A meta-analysis and systematic review. Nutr. Res. 2020, 87, 57–69. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhang, M.; Lin, C.; Wang, H.; Yao, J.; Wei, Q.; Lu, Y.; Chen, Z.; Xing, G.; et al. OSBPL2 deficiency upregulate SQLE expression increasing intracellular cholesterol and cholesteryl ester by AMPK/SP1 and SREBF2 signalling pathway. Exp. Cell Res. 2019, 383, 111512. [Google Scholar] [CrossRef]
- Li, T.; Guo, R.; Zong, Q.; Ling, G. Application of molecular docking in elaborating molecular mechanisms and interactions of supramolecular cyclodextrin. Carbohydr. Polym. 2021, 276, 118644. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, Q.; Li, Y.; Zhang, D.; Yi, S.; Li, X.; Li, J. Study on the interactions between the screened polyphenols and Penaeus vannamei myosin after freezing treatment. Int. J. Biol. Macromol. 2022, 217, 701–713. [Google Scholar] [CrossRef]
- Somani, S.; Zambad, S.; Modi, P. Mangiferin attenuates DSS colitis in mice: Molecular docking and in vivo approach. Chem-Biol. Interact. 2016, 253, 18–26. [Google Scholar] [CrossRef]


| Ingredients | Experimental Diets | |||
|---|---|---|---|---|
| NCD | HCD | 100 MGF | 500 MGF | |
| 1 Fish meal | 12.00 | 12.00 | 12.00 | 12.00 |
| 2 Cottonseed protein concentrate | 28.00 | 28.00 | 28.00 | 28.00 |
| 3 Soybean meal | 14.00 | 14.00 | 14.00 | 14.00 |
| 4 Soybean oil | 2.20 | 2.20 | 2.20 | 2.20 |
| 5 Fish oil | 2.20 | 2.20 | 2.20 | 2.20 |
| 6 Corn starch | 18.00 | 36.00 | 36.00 | 36.00 |
| 7 Microcrystalline cellulose | 18.00 | 0.00 | 0.00 | 0.00 |
| 8 Sodium carboxymethylcellulose | 2.79 | 2.79 | 2.78 | 2.74 |
| 9 Monocalcium phosphate | 1.50 | 1.50 | 1.50 | 1.50 |
| 10 Vitamin and mineral premix | 1.20 | 1.20 | 1.20 | 1.20 |
| 11 Choline chloride | 0.11 | 0.11 | 0.11 | 0.11 |
| 12 Mangiferin | 0.00 | 0.00 | 0.01 | 0.05 |
| Proximate composition (% dry basis) | ||||
| Crude protein | 33.52 | 33.89 | 33.24 | 33.63 |
| Crude lipid | 4.97 | 4.81 | 4.79 | 5.09 |
| Crude fiber | 22.33 | 9.91 | 9.95 | 9.92 |
| Moisture | 10.96 | 7.74 | 6.15 | 7.02 |
| Ash | 6.55 | 6.64 | 7.00 | 6.88 |
| 13 Nitrogen free extract | 21.67 | 37.01 | 38.87 | 37.46 |
| Target Genes | Forward (5′-3′) | Reverse (5′-3′) | Accession Number |
|---|---|---|---|
| sqle | GTGTAGTGCTCAACGATGTCC | AGCAGGCTCTTCTTAACTGGT | XM_017453059.3 |
| hmgcr | CATCTTCTTTGAGCAGGTGGA | GGGTCACTTCCTTCCTGTAAC | XM_017451441.3 |
| srebf2 | ACGGCGGAGGATTTATAATGG | GGGTCGTCAAACAGATCAGAA | XM_053684143.1 |
| sp1 | TGTCCACATCAGAGTGTGTCA | TGGATGTTCCTTGAAGGAGCT | XM_017450095.3 |
| ldlr | CTGGGTGGACTCTAAACTGCAT | GGAGATGTAAGGTGTTCTGCCA | XM_017492528.3 |
| abca1 | TTACTGGTGCGGAGAGAAATG | GATTCTCGATGTAGACACCGG | XM_017460992.1 |
| cyp7a1 | CATCTTCGCGTTCTGCTACAA | CTGTGGCCACTCTTGAATACG | XM_053687284.1 |
| β-actin | GGATCTGTATGCCAACACTGT | CAGGTGGGGCAATGATCTTAA | XM_017454668.3 |
| ef1α | CACTCCTGGCCTATACGCTG | CAAATGCCACGGTTTCAGGG | XM_053679746.1 |
| Target Genes | Forward (5′-3′) | Reverse (5′-3′) | Accession Number |
|---|---|---|---|
| sqle | CGGATATTCTCTCTGCTTTGC | AGCTGCTCCTGTTAATGTCGT | NM_009270.3 |
| hmgcr | CAGAGAAAGGTGCAAAGTTCC | CACACCACGTTCATGAGTTTC | XM_036157854.1 |
| β-actin | ACGTTGACATCCGTAAAGACC | ATCGTACTCCTGCTTGCTGAT | NM_007393.5 |
| ef1α | CATGCTCTTCTGGCTTACACC | AGGTGCTGACTTCCTTAACGA | NM_010106.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Lu, Q.; Cao, J.; Liu, Y.; Liu, H.; Jin, J.; Zhang, Z.; Yang, Y.; Zhu, X.; Han, D.; et al. Supplementation of Mangiferin to a High-Starch Diet Alleviates Hepatic Injury and Lipid Accumulation Potentially through Modulating Cholesterol Metabolism in Channel Catfish (Ictalurus punctatus). Antioxidants 2024, 13, 722. https://doi.org/10.3390/antiox13060722
Zheng Y, Lu Q, Cao J, Liu Y, Liu H, Jin J, Zhang Z, Yang Y, Zhu X, Han D, et al. Supplementation of Mangiferin to a High-Starch Diet Alleviates Hepatic Injury and Lipid Accumulation Potentially through Modulating Cholesterol Metabolism in Channel Catfish (Ictalurus punctatus). Antioxidants. 2024; 13(6):722. https://doi.org/10.3390/antiox13060722
Chicago/Turabian StyleZheng, Yutong, Qisheng Lu, Jingyue Cao, Yulong Liu, Haokun Liu, Junyan Jin, Zhimin Zhang, Yunxia Yang, Xiaoming Zhu, Dong Han, and et al. 2024. "Supplementation of Mangiferin to a High-Starch Diet Alleviates Hepatic Injury and Lipid Accumulation Potentially through Modulating Cholesterol Metabolism in Channel Catfish (Ictalurus punctatus)" Antioxidants 13, no. 6: 722. https://doi.org/10.3390/antiox13060722
APA StyleZheng, Y., Lu, Q., Cao, J., Liu, Y., Liu, H., Jin, J., Zhang, Z., Yang, Y., Zhu, X., Han, D., & Xie, S. (2024). Supplementation of Mangiferin to a High-Starch Diet Alleviates Hepatic Injury and Lipid Accumulation Potentially through Modulating Cholesterol Metabolism in Channel Catfish (Ictalurus punctatus). Antioxidants, 13(6), 722. https://doi.org/10.3390/antiox13060722








