Responses of Digestive, Antioxidant, Immunological and Metabolic Enzymes in the Intestines and Liver of Largemouth Bass (Micropterus salmoides) under the Biofloc Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Feeding and Management of Experimental Fish
2.3. Experimental Carbon Source and Addition Method
2.4. Sample Collection
2.5. Growth Performance and Survival
2.6. Liver and Intestinal Biochemical Analyses
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Feed Utilization
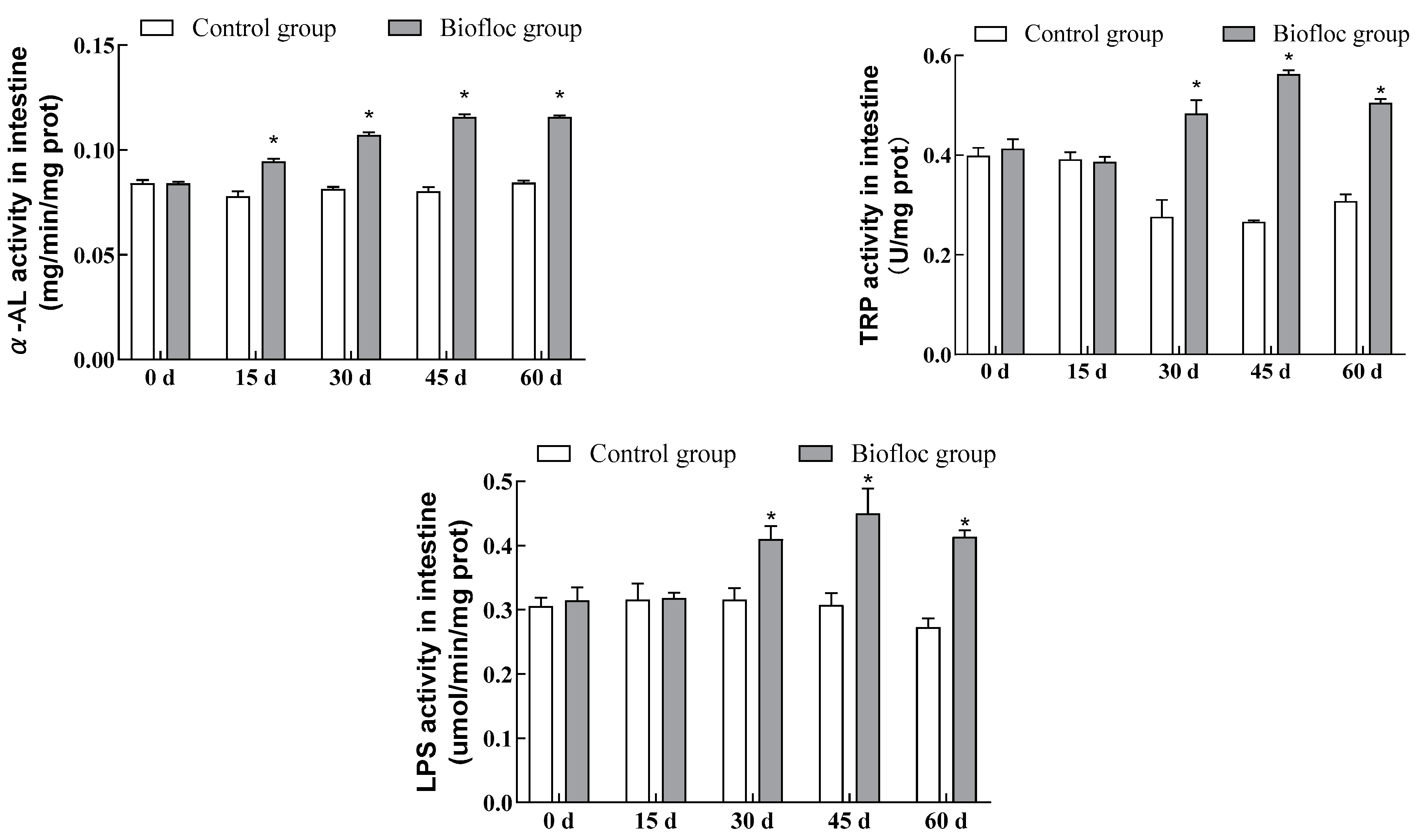
3.2. Digestive Enzymatic Activities in the Intestines
3.3. Antioxidant Enzymatic Activities in the Liver
3.4. Immunoenzymatic Activities in the Liver
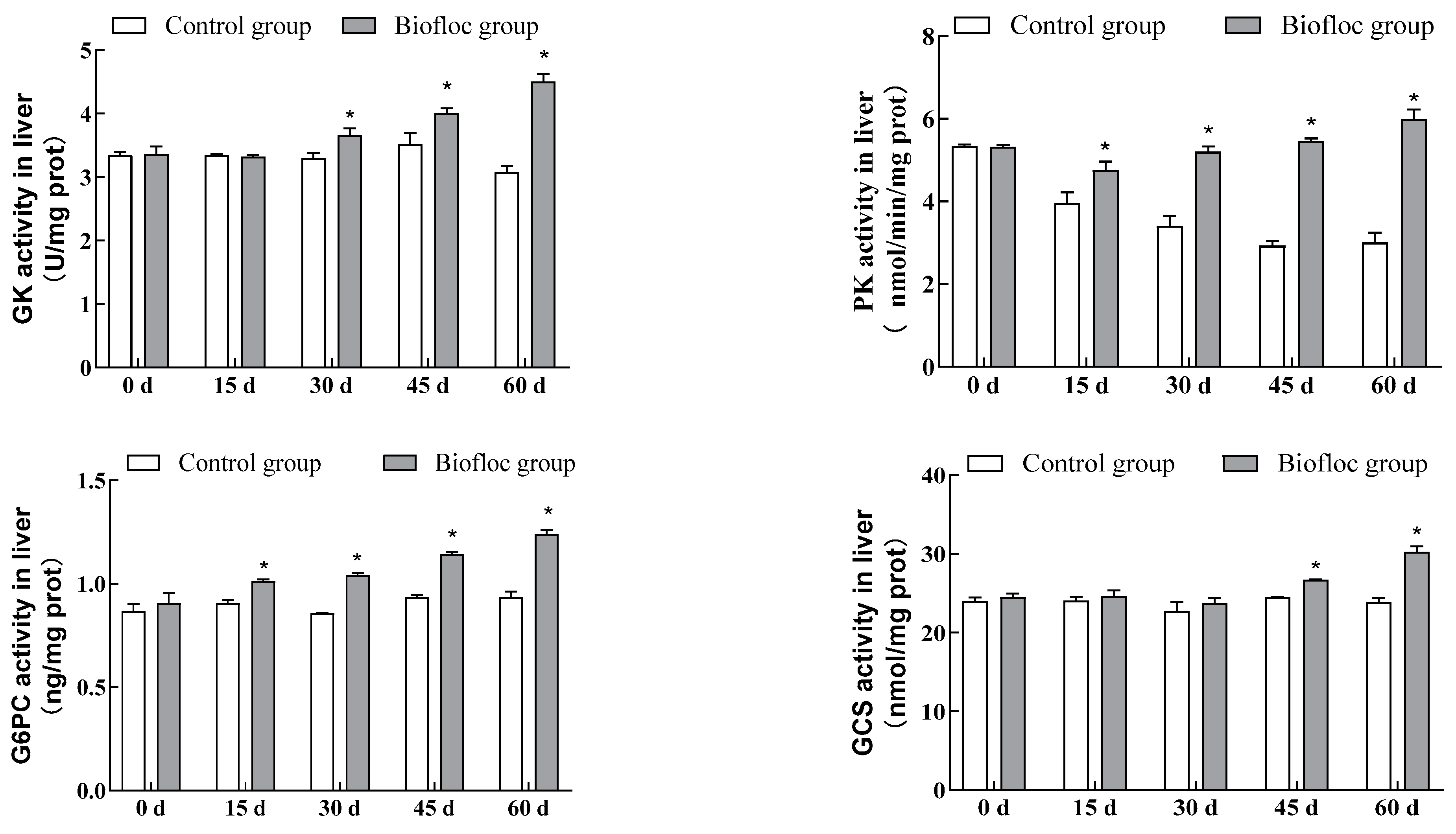
3.5. Metabolic Enzymatic Activities in the Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, J.J.; Lutz-Carrillo, D.J.; Quan, Y.C.; Liang, S.X. Taxonomic status and genetic diversity of cultured largemouth bass (Micropterus salmoides) in China. Aquaculture 2008, 278, 27–30. [Google Scholar] [CrossRef]
- Wang, Q.C.; Ye, W.; Tao, Y.F.; Li, Y.; Lu, S.Q.; Xu, P.; Qiang, J. Transport Stress Induces Oxidative Stress and Immune Response in Juvenile Largemouth Bass (Micropterus salmoides): Analysis of Oxidative and Immunological Parameters and the Gut Microbiome. Antioxidants 2023, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Vinatea, L.; Malpartida, J.; Carbó, R.; Andree, K.B.; Gisbert, E.; Estévez, A. A comparison of recirculation aquaculture systems versus biofloc technology culture system for on-growing of fry of Tinca tinca (Cyprinidae) and fry of grey Mugil cephalus (Mugilidae). Aquaculture 2018, 482, 155–161. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bioflocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Jia, S.P.; Wang, L.; Zhang, J.M.; Zhang, L.; Ma, F.R.; Huang, M.L.; Liu, S.S.; Gong, J.H.; Zhang, M.; Yu, M.; et al. Comparative study on the morphological characteristics and nutritional quality of largemouth bass (Micropterus salmoides) cultured in an aquaculture system using land-based container with recycling water and a traditional pond system. Aquaculture 2022, 549, 737721. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, Y.; Xu, D.H.; Qiao, G.; Zhang, J.L.; Qi, Z.T.; Li, Q. Effect of different water biofloc contents on the growth and immune response of gibel carp cultured in zero water exchange and no feed addition system. Aquac. Res. 2018, 49, 1647–1656. [Google Scholar] [CrossRef]
- Deb, S.; Noori, M.T.; Rao, P.S. Application of biofloc technology for Indian major carp culture (polyculture) along with water quality management. Aquac. Eng. 2020, 91, 102106. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 2012, 356–357, 147–152. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q. Evaluation of dietary protein level on selected parameters of immune and antioxidant systems, and growth performance of juvenile Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquaculture 2014, 426–427, 181–188. [Google Scholar] [CrossRef]
- Panigrahi, A.; Saranya, C.; Sundaram, M.; Vinoth Kannan, S.R.; Das, R.R.; Satish Kumar, R.; Rajesh, P.; Otta, S.K. Carbon: Nitrogen (C:N) ratio level variation influences microbial community of the system and growth as well as immunity of shrimp (Litopenaeus vannamei) in biofloc based culture system. Fish Shellfish Immunol. 2018, 81, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, L.; Zhu, R.; Li, M.; Duan, J.; Wang, J.Y.; Liu, Y.H.; Wu, L.F. Monitoring of growth, digestive enzyme activity, immune response and water quality parameters of Golden crucian carp (Carassius auratus) in zero-water exchange tanks of biofloc systems. Aquac. Rep. 2020, 16, 100283. [Google Scholar] [CrossRef]
- Mansour, A.T.; Esteban, M.Á. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 64, 202–209. [Google Scholar] [CrossRef]
- Rind, K.H.; Habib, S.S.; Ujan, J.A.; Fazio, F.; Naz, S.; Batool, A.I.; Ullah, M.; Attaullah, S.; Khayyam, K.; Khan, K. The Effects of Different Carbon Sources on Water Quality, Growth Performance, Hematology, Immune, and Antioxidant Status in Cultured Nile Tilapia with Biofloc Technology. Fishes 2023, 8, 512. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology—A Practical Guide Book; The World Aquaculture Society: Sorrento, LA, USA, 2012. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, E.; Xie, J.; Yu, D.; Li, Z.; Luo, W.; Qiu, L.; Zheng, Z. Effect of C/N ratio on water quality in zero-water exchange tanks and the biofloc supplementation in feed on the growth performance of crucian carp, Carassius auratus. Aquaculture 2015, 443, 98–104. [Google Scholar] [CrossRef]
- Ren, W.; Li, L.; Dong, S.; Tian, X.; Xue, Y. Effects of C/N ratio and light on ammonia nitrogen uptake in Litopenaeus vannamei culture tanks. Aquaculture 2019, 498, 123–131. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sundaram, M.; Saranya, C.; Satish Kumar, R.; Syama Dayal, J.; Saraswathy, R.; Otta, S.K.; Shyne Anand, P.S.; Nila Rekha, P.; Gopal, C. Influence of differential protein levels of feed on production performance and immune response of pacific white leg shrimp in a biofloc–based system. Aquaculture 2019, 503, 118–127. [Google Scholar] [CrossRef]
- Liu, G.; Ye, Z.Y.; Liu, D.Z.; Zhao, J.; Sivaramasamy, E.; Deng, Y.L.; Zhu, S.M. Influence of stocking density on growth, digestive enzyme activities, immune responses, antioxidant of Oreochromis niloticus fingerlings in biofloc systems. Fish Shellfish Immunol. 2018, 81, 416–422. [Google Scholar] [CrossRef]
- Yu, Z.; Li, L.; Zhu, R.; Li, M.; Wu, L.-F. Effects of bioflocs with different C/N ratios on growth, immunological parameters, antioxidants and culture water quality in Opsariichthys kaopingensis Dybowski. Aquac. Res. 2020, 51, 805–815. [Google Scholar] [CrossRef]
- Jin, Y.Q.; Meng, S.l.; Xu, H.M.; Song, C.; Fan, L.M.; Qiu, L.P.; Li, D.D. Study on aquatic environment and nitrogen and phosphorus balance in Micropterus salmoides culture under biofloc model. Prog. Fish. Sci. 2024, 45. [Google Scholar]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tokmechi, A.; Rahmani Farah, K.; Shalizar Jalali, A. Growth performance, haematology, antioxidant status, immune response and histology of common carp (Cyprinus carpio L.) fed biofloc grown on different carbon sources. Aquac. Res. 2018, 49, 393–403. [Google Scholar] [CrossRef]
- Fauji, H.; Budiardi, T.; Ekasari, J. Growth performance and robustness of African Catfish Clarias gariepinus (Burchell) in biofloc-based nursery production with different stocking densities. Aquac. Res. 2018, 49, 1339–1346. [Google Scholar] [CrossRef]
- Anand, P.S.S.; Kohli, M.P.S.; Kumar, S.; Sundaray, J.K.; Roy, S.D.; Venkateshwarlu, G.; Sinha, A.; Pailan, G.H. Effect of dietary supplementation of biofloc on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture 2014, 418–419, 108–115. [Google Scholar] [CrossRef]
- Long, L.; Yang, J.; Li, Y.; Guan, C.W.; Wu, F. Effect of biofloc technology on growth, digestive enzyme activity, hematology, and immune response of genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2015, 448, 135–141. [Google Scholar] [CrossRef]
- Li, J.H.; Li, J.Y.; Li, W.; Sun, Y.; Liu, X.F.; Liu, M.M.; Cheng, Y.X. Juvenile Procambarus clarkii farmed using biofloc technology or commercial feed in zero-water exchange indoor tanks: A comparison of growth performance, enzyme activity and proximate composition. Aquac. Res. 2019, 50, 1834–1843. [Google Scholar] [CrossRef]
- Ge, H.L.; Zhu, J.Y.; Zhao, C.Z.; Miao, S.Y. Effects of Biofloc on the body composition of giant freshwater prawn, Macrobrachium rosenbergii. Freshw. Fish. 2017, 47, 66–72. [Google Scholar] [CrossRef]
- Sun, S.M.; GE, X.P.; Zhu, J.; Jiang, X.J.; Zhang, W.X. Effects of Bioflocs on Growth Performance, Digestive Enzyme and Immunity Enzyme Activities in Blunt Snout Bream(Megalobrama amblycephala). Prog. Fish. Sci. 2016, 37, 49–55. [Google Scholar] [CrossRef]
- Wasielesky, W.; Atwood, H.; Stokes, A.; Browdy, C.L. Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture 2006, 258, 396–403. [Google Scholar] [CrossRef]
- Chen, J.H.; Ren, Y.C.; Li, Y.Q.; Xia, B. Regulation of growth, intestinal microbiota, non-specific immune response and disease resistance of sea cucumber Apostichopus japonicus (Selenka) in biofloc systems. Fish Shellfish Immunol. 2018, 77, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, J.C. Effects of sub-chronic exposure to lead (Pb) and ascorbic acid in juvenile rockfish: Antioxidant responses, MT gene expression, and neurotransmitters. Chemosphere 2017, 171, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, J.C. Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium (Cr6+) exposure. Ecotoxicol. Environ. Saf. 2016, 125, 78–84. [Google Scholar] [CrossRef]
- Xu, C.; Gong, H.H.; Niu, L.L.; Li, T.Y.; Guo, H.Q.; Hu, C.J.; Sun, X.H.; Li, L.; Liu, W.P. Maternal exposure to dietary uranium causes oxidative stress and thyroid disruption in zebrafish offspring. Ecotoxicol. Environ. Saf. 2023, 265, 115501. [Google Scholar] [CrossRef]
- Shourbela, R.M.; Khatab, S.A.; Hassan, M.M.; Van Doan, H.; Dawood, M.A.O. The Effect of Stocking Density and Carbon Sources on the Oxidative Status, and Nonspecific Immunity of Nile tilapia (Oreochromis niloticus) Reared under Biofloc Conditions. Animals 2021, 11, 184. [Google Scholar] [CrossRef]
- Nageswari, P.; Verma, A.K.; Gupta, S.; Jeyakumari, A.; Hittinahalli, C.M. Haematological, serum biochemical and antioxidative enzymes responses of sutchi catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila using various carbon sources in biofloc system. Aquac. Res. 2022, 53, 1851–1861. [Google Scholar] [CrossRef]
- Haridas, H.; Verma, A.; Rathore, G.; Prakash, C.; Banerjee Sawant, P.; Asanaru Majeedkutty, B. Enhanced growth and immuno-physiological response of Genetically Improved Farmed Tilapia in indoor biofloc units at different stocking densities. Aquac. Res. 2017, 48, 4346–4355. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.M.; Liu, D.; Ye, Z.Y. Effect of the C/N ratio on inorganic nitrogen control and the growth and physiological parameters of tilapias fingerlings, Oreochromis niloticu reared in biofloc systems. Aquac. Res. 2018, 49, 2429–2439. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, Y.Y.; Jiang, N.; Zhang, A.Z.; Li, M.Y. Bioflocs attenuates lipopolysaccharide-induced inflammation, immunosuppression and oxidative stress in Channa argus. Fish Shellfish Immunol. 2021, 114, 218–228. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.M.; Liu, D.Z.; Guo, X.S.; Ye, Z.Y. Effects of stocking density of the white shrimp Litopenaeus vannamei (Boone) on immunities, antioxidant status, and resistance against Vibrio harveyi in a biofloc system. Fish Shellfish Immunol. 2017, 67, 19–26. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Yang, H.L.; Pan, W.; Sun, Y.Z. Advances in the interactions between intestinal microorganisms and host immune system in fish. Acta Microbiol. Sin. 2021, 61, 3046–3058. [Google Scholar] [CrossRef]
- Ackerman, P.A.; Iwama, G.K.; Thornton, J.C. Physiological and immunological effects of adjuvanted Aeromonas salmonicida vaccines on juvenile rainbow trout. J. Aquat. Anim. Health 2000, 12, 157–164. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Jiang, W.D.; Wu, P.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Liu, Y.; et al. Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-κB, TOR and Nrf2 signaling molecules in the gills of fish: Preventive role of arginine. Aquat. Toxicol. 2015, 158, 125–137. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Verma, A.K.; Babitha Rani, A.M.; Rathore, G.; Saharan, N.; Gora, A.H. Growth, non-specific immunity and disease resistance of Labeo rohita against Aeromonas hydrophila in biofloc systems using different carbon sources. Aquaculture 2016, 457, 61–67. [Google Scholar] [CrossRef]
- Yu, Y.B.; Choi, J.H.; Lee, J.H.; Jo, A.H.; Lee, K.M.; Kim, J.H. Biofloc Technology in Fish Aquaculture: A Review. Antioxidants 2023, 12, 398. [Google Scholar] [CrossRef]
- Hwihy, H.M.; Zeina, A.M.; Husien, M.; El-Damhougy, K.A. Impact of Biofloc technology on growth performance and biochemical parameters of Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2021, 25, 761–774. [Google Scholar] [CrossRef]
- Zhou, X.X.; Tian, Z.Q.; Wang, Y.B.; Li, W.F. Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol. Biochem. 2010, 36, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ekasari, J.; Angela, D.; Waluyo, S.H.; Bachtiar, T.; Surawidjaja, E.H.; Bossier, P.; De Schryver, P. The size of biofloc determines the nutritional composition and the nitrogen recovery by aquaculture animals. Aquaculture 2014, 426–427, 105–111. [Google Scholar] [CrossRef]
- Ren, X.; Wang, L.X.; Chen, Z.L.; Hou, D.Z.; Xue, Y.; Diao, X.M.; Shen, Q. Foxtail Millet Improves Blood Glucose Metabolism in Diabetic Rats through PI3K/AKT and NF-κB Signaling Pathways Mediated by Gut Microbiota. Nutrients 2021, 13, 1837. [Google Scholar] [CrossRef]
- Bou, M.; Todorčević, M.; Fontanillas, R.; Capilla, E.; Gutiérrez, J.; Navarro, I. Adipose tissue and liver metabolic responses to different levels of dietary carbohydrates in gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 175, 72–81. [Google Scholar] [CrossRef]
- Che, M.X.; Zhang, X.J.; Lu, Z.Y.; Chi, S.Y.; Tan, B.P. Effects of Different Carbohydrate Sources on Growth Performance, Glucose and Lipid Metabolism and Antioxidant Capacity of Largemouth Bass (Micropterus salmoides). Chin. J. Anim. Nutr. 2023, 35, 1169–1181. [Google Scholar] [CrossRef]
- Cowey, C.B.; Knox, D.; Walton, M.J.; Adron, J.W. The regulation of gluconeogenesis by diet and insulin in rainbow trout (Salmo gairdneri). Br. J. Nutr. 1977, 38, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Enes, P.; Sanchez-Gurmaches, J.; Navarro, I.; Gutiérrez, J.; Oliva-Teles, A. Role of insulin and IGF-I on the regulation of glucose metabolism in European sea bass (Dicentrarchus labrax) fed with different dietary carbohydrate levels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Shi, C.M.; Mu, M.M.; Chen, Y.J.; Luo, L. Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2018, 78, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zierler, K. Whole body glucose metabolism. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, E409–E426. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, X.Y.; Zhang, Y.; Li, S.L.; Lu, L.Q.; Xu, D.; Liu, X.W. Effects of dietary carbohydrate levels on the growth, glycometabolism, antioxidant capacity and metabolome of largemouth bass (Micropterus salmoides). Aquac. Res. 2022, 53, 3748–3758. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Xie, S.W.; Wei, H.L.; Zheng, L.; Liu, Z.L.; Fang, H.H.; Xie, J.J.; Liao, S.Y.; Tian, L.X.; Liu, Y.J.; et al. High dietary starch impaired growth performance, liver histology and hepatic glucose metabolism of juvenile largemouth bass. Micropterus salmoides. Aquac. Nutr. 2020, 26, 1083–1095. [Google Scholar] [CrossRef]


| Parameters | Control Group | Biofloc Group |
|---|---|---|
| IW (g) | 33.32 ± 1.30 | 33.18 ± 1.07 |
| FW (g) | 99.06 ± 5.39 | 97.15 ± 6.17 |
| WGR (%) | 196.46 ± 3.71 | 193.47 ± 6.65 |
| SGR (% day−1) | 1.81 ± 0.02 | 1.79 ± 0.04 |
| SR (%) | 93.33 ± 2.89 | 95.00 ± 0.00 |
| FCR | 1.48 ± 0.03 a | 1.14 ± 0.05 b |
| PER (%) | 1.47 ± 0.03 b | 1.90 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Meng, S.; Xu, H.; Song, C.; Fan, L.; Qiu, L.; Li, D. Responses of Digestive, Antioxidant, Immunological and Metabolic Enzymes in the Intestines and Liver of Largemouth Bass (Micropterus salmoides) under the Biofloc Model. Antioxidants 2024, 13, 736. https://doi.org/10.3390/antiox13060736
Jin Y, Meng S, Xu H, Song C, Fan L, Qiu L, Li D. Responses of Digestive, Antioxidant, Immunological and Metabolic Enzymes in the Intestines and Liver of Largemouth Bass (Micropterus salmoides) under the Biofloc Model. Antioxidants. 2024; 13(6):736. https://doi.org/10.3390/antiox13060736
Chicago/Turabian StyleJin, Yuqin, Shunlong Meng, Huimin Xu, Chao Song, Limin Fan, Liping Qiu, and Dandan Li. 2024. "Responses of Digestive, Antioxidant, Immunological and Metabolic Enzymes in the Intestines and Liver of Largemouth Bass (Micropterus salmoides) under the Biofloc Model" Antioxidants 13, no. 6: 736. https://doi.org/10.3390/antiox13060736






