Black Goji Berry (Lycium ruthenicum) Juice Fermented with Lactobacillus rhamnosus GG Enhances Inhibitory Activity against Dipeptidyl Peptidase-IV and Key Steps of Lipid Digestion and Absorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BGB Powder
2.3. Fermentation
2.4. Determination of Physicochemical Properties
2.4.1. pH, Total Soluble Solid (TSS), and Color
2.4.2. Total Carbohydrate and Reducing Sugar
2.4.3. Analysis of Lactic Acid
2.4.4. Total Phenolic Content (TPC)
2.4.5. Total Anthocyanin Content (TAC)
2.4.6. Total Flavonoid Content (TFC)
2.4.7. Antioxidant Activities
Ferric-Reducing Antioxidant Power (FRAP)
DPPH Radical Scavenging Activity
Trolox Equivalent Antioxidant Activity (TEAC)
2.5. Non-Volatile Metabolite Profiling Using Liquid Chromatography Coupled with High-Resolution Fourier Transform Mass Spectrometry (LC-HRFTMS)
2.6. Determination of Biological Activities
2.6.1. Bile Acid Binding Activity
2.6.2. Pancreatic Lipase Inhibitory Activity
2.6.3. Cholesterol Micellization Inhibitory Activity
2.6.4. Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Activity
2.7. Microbiological Analysis
2.8. Caco-2 Cell Culture
2.8.1. Cell Viability
2.8.2. Cholesterol Uptake in Caco-2 Cells
2.9. Sensory Evaluation
2.10. Statistical Analyses
3. Results and Discussion
3.1. Alterations in Physicochemical Properties Following Fermentation of BGB
3.2. Alterations in Viable Lactobacilli Following Fermentation of BGB
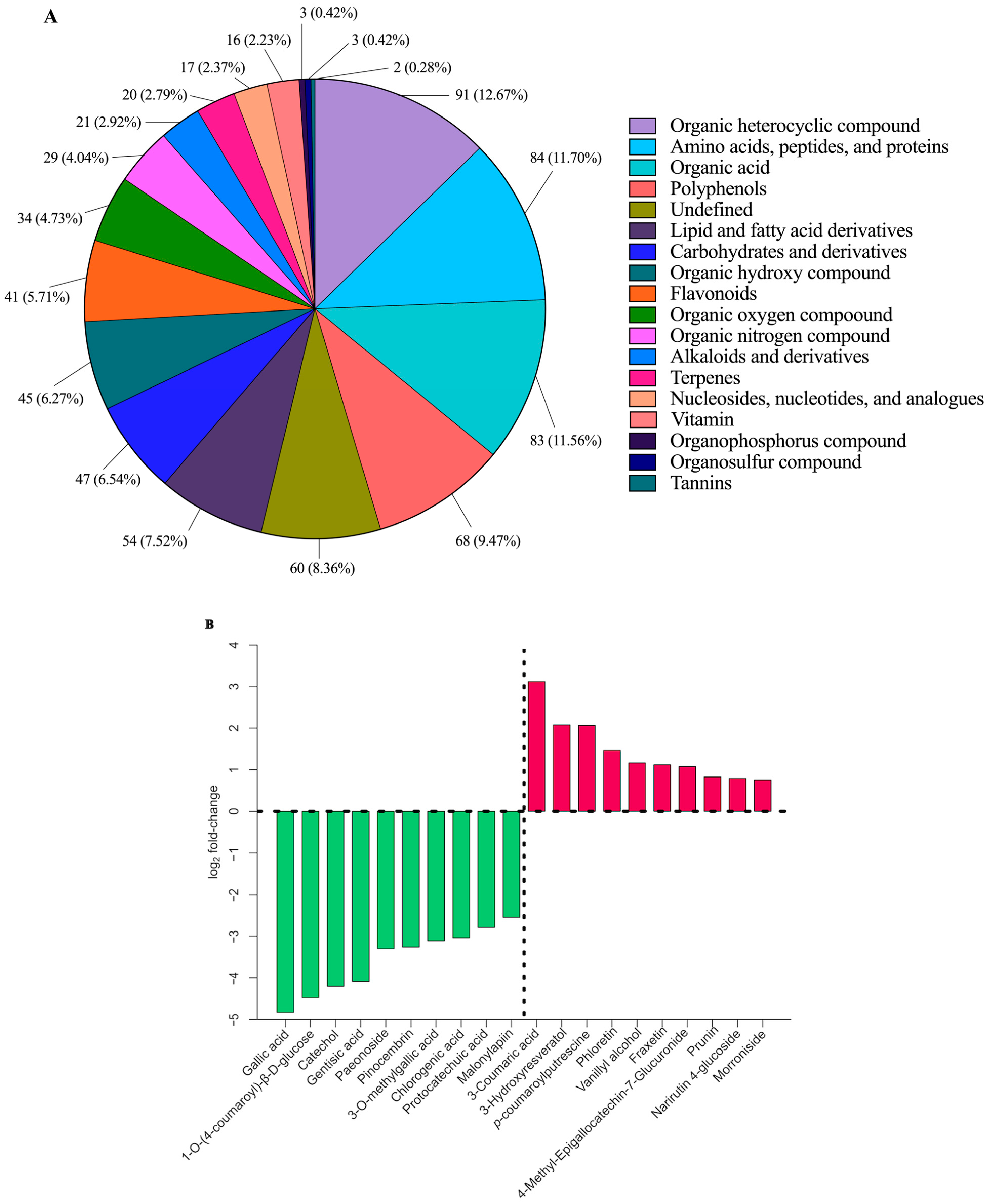
3.3. Alterations in Phytochemical Composition Following Fermentation of BGB
3.4. Alterations in Antioxidant Activities Following Fermentation of BGB
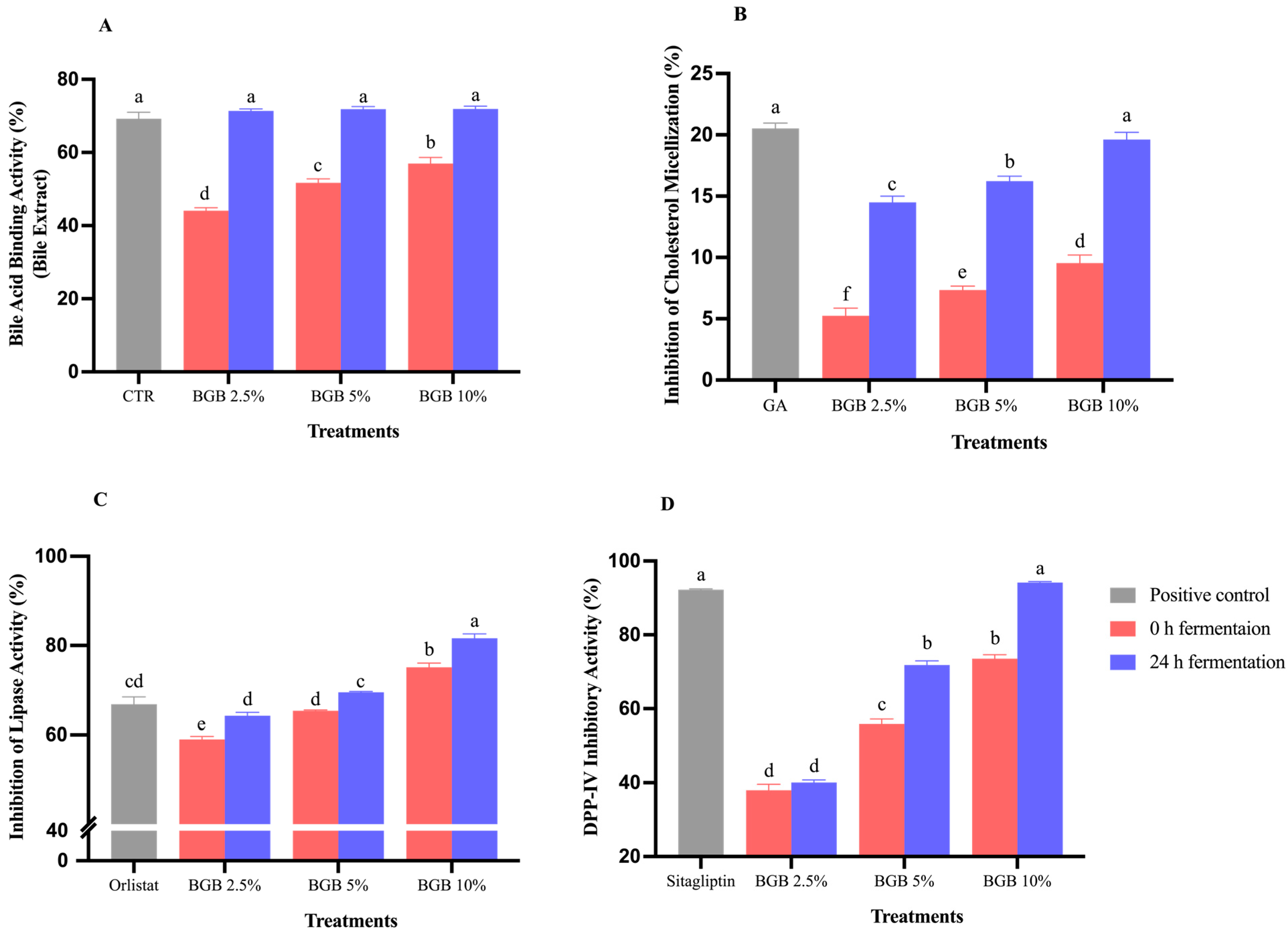
3.5. Alterations in Biological Properties Following Fermentation of BGB
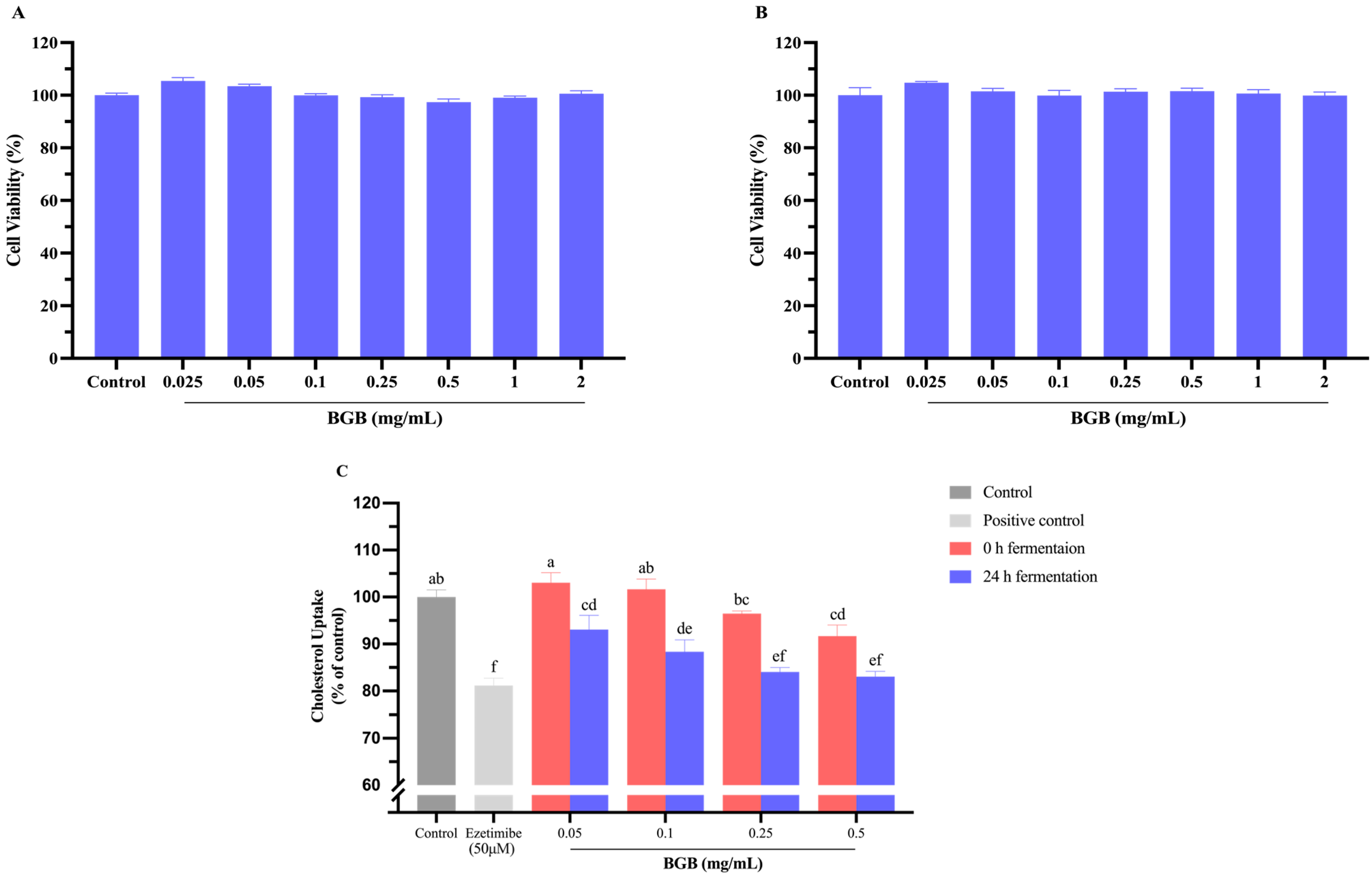
3.6. Effect of BGB on Cell Viability and Cholesterol Uptake in Caco-2 Cells
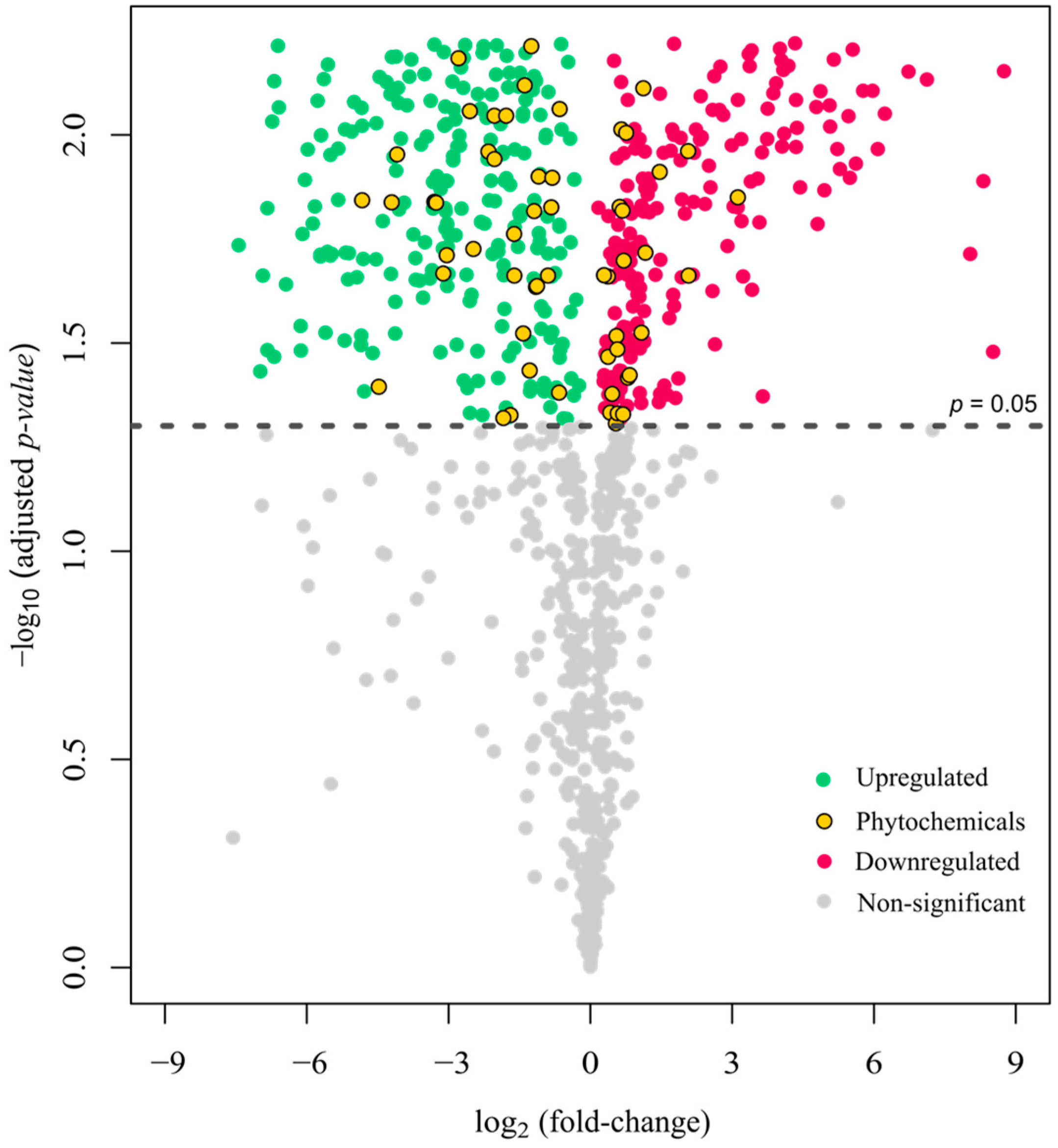
3.7. Non-Volatile Compound Profiling
3.8. Sensory Evaluation
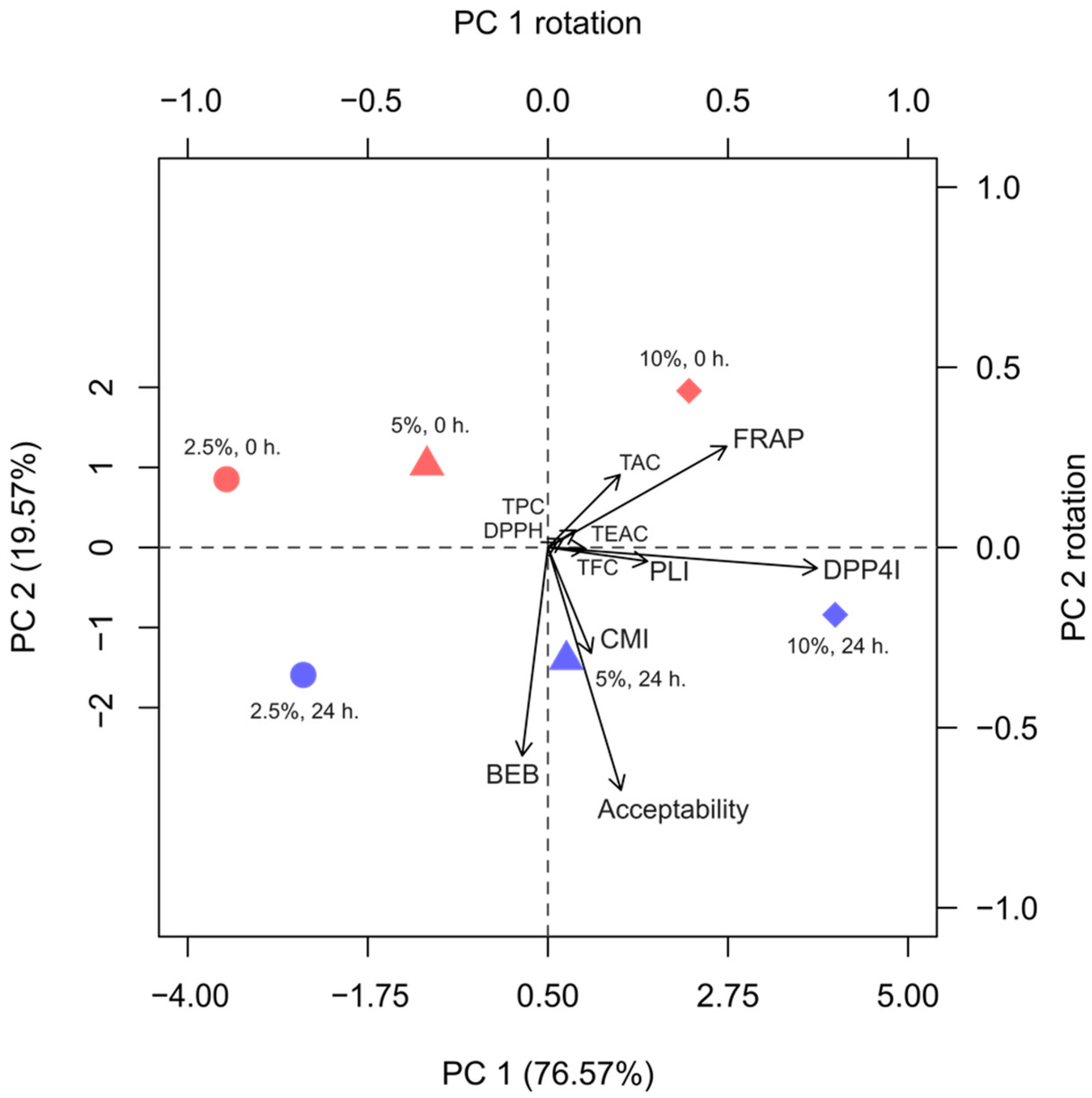
3.9. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Keefe, J.H.; Bell, D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am. J. Card. 2007, 100, 899–904. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Zhang, Y.; Xia, J.; Guo, H.; Hu, H.; Shan, P.; Li, T. The Global Burden of Diseases attributed to high low-density lipoprotein cholesterol from 1990 to 2019. Front. Public Health 2022, 10, 891929. [Google Scholar] [CrossRef]
- Ros, E. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atheroscler 2000, 151, 357–379. [Google Scholar] [CrossRef]
- Singh, A.K. Dipeptidyl peptidase-4 inhibitors: Novel mechanism of actions. Indian J. Endocrinol. Metab. 2014, 18, 753–759. [Google Scholar] [CrossRef]
- Bahmani, M.; Mirhoseini, M.; Shirzad, H.; Sedighi, M.; Shahinfard, N.; Rafieian-Kopaei, M. A review on promising natural agents effective on hyperlipidemia. J. Evid. Based Complement. Altern. Med. 2015, 20, 228–238. [Google Scholar] [CrossRef]
- Panwar, H.; Calderwood, D.; Grant, I.R.; Grover, S.; Green, B.D. Lactobacilli possess inhibitory activity against dipeptidyl peptidase-4 (DPP-4). Ann. Microbiol. 2016, 66, 505–509. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Mannaa, M.; Han, G.; Seo, Y.S.; Park, I. Evolution of food fermentation processes and the use of multi-omics in deciphering the roles of the microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef]
- Emmanuel, E.; Deborah, S. Phytochemical and anti-nutritional studies on some commonly consumed fruits in lokoja, kogi state of Nigeria. Gen. Med. Open 2018, 2, 2–5. [Google Scholar] [CrossRef]
- Idrees, M.; Imran, M.; Atiq, N.; Zahra, R.; Abid, R.; Alreshidi, M.; Roberts, T.; Abdelgadir, A.; Tipu, M.K.; Farid, A.; et al. Probiotics, their action modality and the use of multi-omics in metamorphosis of commensal microbiota into target-based probiotics. Front. Nutr. 2022, 9, 959941. [Google Scholar] [CrossRef]
- Lan, T.; Lv, X.; Zhao, Q.; Lei, Y.; Gao, C.; Yuan, Q.; Sun, X.; Liu, X.; Ma, T. Optimization of strains for fermentation of kiwifruit juice and effects of mono- and mixed culture fermentation on its sensory and aroma profiles. Food Chem. X 2023, 17, 100595. [Google Scholar] [CrossRef]
- Shehata, M.G.; El Sohaimy, S.A.; El-Sahn, M.A.; Youssef, M.M. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016, 61, 65–75. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R.; Gorbach, S.L. Lactobacillus GG: Bacteriology and clinical applications. Gastroenterol. Clin. N. Am. 2005, 34, 483–498. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG -host interactions. Microb Cell Fact. 2014, 13 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Capurso, L. Thirty years of Lactobacillus rhamnosus GG: A review. J. Clin. Gastroenterol. 2019, 53 (Suppl. S1), S1–S41. [Google Scholar] [CrossRef]
- Doriya, K.; Kumar, D.S.; Thorat, B.N. A systematic review on fruit-based fermented foods as an approach to improve dietary diversity. J. Food Process. Preserv. 2022, 46, e16994. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Q.; Sun, Y. Black goji berry (Lycium ruthenicum) tea has higher phytochemical contents and in vitro antioxidant properties than red goji berry (Lycium barbarum) tea. Food Qual. Saf. 2020, 4, 193–201. [Google Scholar] [CrossRef]
- Tang, P.; Giusti, M. Black goji (Lycium ruthenicum Murr.) polyphenols: Potent antioxidants and natural colorants. In Phytochemicals in Goji berries, 1st ed.; Ye, X., Jiang, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 109–134. [Google Scholar]
- Vidovic, B.B.; Milincic, D.D.; Marcetic, M.D.; Djuris, J.D.; Ilic, T.D.; Kostic, A.Z.; Pesic, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, H.; Liu, H.; Cheng, H.; Pan, L.; Hu, M.; Li, X. Goji berry juice fermented by probiotics attenuates dextran sodium sulfate-induced ulcerative colitis in mice. J. Funct. Foods 2021, 83, 104491. [Google Scholar] [CrossRef]
- Jain, A.; Jain, R.; Jain, S. Quantitative analysis of reducing sugars by 3, 5-dinitrosalicylic acid (DNSA method). In Basic Techniques in Biochemistry, Microbiology, and Molecular Biology; Springer: New York, NY, USA, 2020; pp. 181–183. [Google Scholar]
- Marnpae, M.; Chusak, C.; Balmori, V.; Kamonsuwan, K.; Dahlan, W.; Nhujak, T.; Hamid, N.; Adisakwattana, S. Probiotic gac fruit beverage fermented with Lactobacillus paracasei: Physiochemical properties, phytochemicals, antioxidant activities, functional properties, and volatile flavor compounds. LWT 2022, 169, 113986. [Google Scholar] [CrossRef]
- Chusak, C.; Chanbunyawat, P.; Chumnumduang, P.; Chantarasinlapin, P.; Suantawee, T.; Adisakwattana, S. Effect of gac fruit (Momordica cochinchinensis) powder on in vitro starch digestibility, nutritional quality, textural and sensory characteristics of pasta. LWT 2020, 118, 108856. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.; Wrolstad, R. AOAC 2005.02: Total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines- ph differential method. J. AOAC Int. 2005, 88, 37–39. [Google Scholar]
- Adisakwattana, S.; Intrawangso, J.; Hemrid, A.; Chanathong, B.; Mäkynen, K. Extracts of edible plants inhibit pancreatic lipase, cholesterol esterase and cholesterol micellization, and bind bile acids. Food Technol Biotech. 2012, 50, 11–16. [Google Scholar]
- Chayaratanasin, P.; Barbieri, M.A.; Suanpairintr, N.; Adisakwattana, S. Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complement. Altern. Med. 2015, 15, 27. [Google Scholar] [CrossRef]
- Beniddir, M.A.; Kang, K.B.; Genta-Jouve, G.; Huber, F.; Rogers, S.; van der Hooft, J.J.J. Advances in decomposing complex metabolite mixtures using substructure- and network-based computational metabolomics approaches. Nat. Prod. Rep. 2021, 38, 1967–1993. [Google Scholar] [CrossRef]
- Peeler, J.T.; Maturin, L. BAM Chapter 3: Aerobic Plate Count: U.S. Food and Drug Administration. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count (accessed on 3 October 2023).
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complement. Altern. Med. 2019, 19, 242. [Google Scholar]
- Coudeyras, S.; Marchandin, H.; Fajon, C.; Forestier, C. Taxonomic and strain-specific identification of the probiotic strain Lactobacillus rhamnosus 35 within the Lactobacillus casei group. Appl. Environ. Microbiol. 2008, 74, 2679–2689. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by multiple bacterial strains improves the production of bioactive compounds and antioxidant activity of goji juice. Molecules 2019, 24, 3519. [Google Scholar] [CrossRef]
- Balmori, V.; Marnpae, M.; Chusak, C.; Kamonsuwan, K.; Katelakha, K.; Charoensiddhi, S.; Adisakwattana, S. Enhancing phytochemical compounds, functional properties, and volatile flavor profiles of pomelo (Citrus grandis (L.) Osbeck) juices from different cultivars through fermentation with Lacticaseibacillus paracasei. Foods 2023, 12, 4278. [Google Scholar] [CrossRef]
- Enaru, B.; Dretcanu, G.; Pop, T.D.; Stanila, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar]
- Lahteinen, T.; Malinen, E.; Koort, J.M.; Mertaniemi-Hannus, U.; Hankimo, T.; Karikoski, N.; Pakkanen, S.; Laine, H.; Sillanpaa, H.; Soderholm, H.; et al. Probiotic properties of Lactobacillus isolates originating from porcine intestine and feces. Anaerobe 2010, 16, 293–300. [Google Scholar] [CrossRef]
- Fujita, A.; Sarkar, D.; Genovese, M.I.; Shetty, K. Improving anti-hyperglycemic and anti-hypertensive properties of camu-camu (Myriciaria dubia Mc. Vaugh) using lactic acid bacterial fermentation. Process Biochem. 2017, 59, 133–140. [Google Scholar] [CrossRef]
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; de Pascual-Teresa, S. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Hunaefi, D.; Gruda, N.; Riedel, H.; Akumo, D.N.; Saw, N.M.M.T.; Smetanska, I. Improvement of antioxidant activities in red cabbage sprouts by lactic acid bacterial fermentation. Food Biotech. 2013, 27, 279–302. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef]
- Rodriguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; Lopez de Felipe, F.; Gomez-Cordoves, C.; Mancheno, J.M.; Munoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Zhao, M.N.; Zhang, F.; Zhang, L.; Liu, B.J.; Meng, X.H. Mixed fermentation of jujube juice (Ziziphus jujuba Mill.) with L. rhamnosus GG and L. plantarum-1: Effects on the quality and stability. Int. J. Food Sci. Technol. 2019, 54, 2624–2631. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N. Evaluation of antioxidant capacity. What capacity is being measured by which method? IUBMB Life 2000, 50, 323–329. [Google Scholar] [CrossRef]
- Insull, W., Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: A scientific review. South. Med. J. 2006, 99, 257–273. [Google Scholar] [CrossRef]
- Naumann, S.; Schweiggert-Weisz, U.; Eisner, P. Characterisation of the molecular interactions between primary bile acids and fractionated lupin cotyledons (Lupinus angustifolius L.). Food Chem. 2020, 323, 126780. [Google Scholar] [CrossRef]
- De Boever, P.; Verstraete, W. Bile salt deconjugation by Lactobacillus plantarum 80 and its implication for bacterial toxicity. J. Appl. Microbiol. 1999, 87, 345–352. [Google Scholar]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Diaz-Sanchez, A.G.; de la Rosa, L.A.; Nunez-Gastelum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol. Biotech. 2017, 55, 519–530. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Fan, J.; Johnson, M.H.; Lila, M.A.; Yousef, G.; de Mejia, E.G. Berry and citrus phenolic compounds inhibit dipeptidyl peptidase IV: Implications in diabetes management. J. Evid. Based Complement. Altern. Med. 2013, 2013, 479505. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Yue, Y.; Wang, C.; Zhao, L.; Evivie, S.E.; Li, B.; Huo, G. Screening for potential novel probiotics with dipeptidyl peptidase IV-Inhibiting activity for type 2 diabetes attenuation in vitro and in vivo. Front. Microbiol. 2019, 10, 2855. [Google Scholar] [CrossRef]
- Vella, A. Mechanism of action of DPP-4 inhibitors—New insights. J. Clin. Endocrinol. Metab. 2012, 97, 2626–2628. [Google Scholar] [CrossRef]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Ann. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter Niemann-pick C1-like 1 in Caco-2 cells and rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Identification of a novel potential probiotic Lactobacillus plantarum FB003 isolated from salted-fermented shrimp and its effect on cholesterol absorption by regulation of NPC1L1 and PPARα. Probiotics Antimicrob. Proteins 2019, 11, 785–793. [Google Scholar] [CrossRef]
- Kobayashi, S. The Effect of polyphenols on hypercholesterolemia through inhibiting the transport and expression of Niemann-Pick C1-Like 1. Int. J. Mol. Sci. 2019, 20, 4939. [Google Scholar] [CrossRef]
- Filannino, P.; Tlais, A.Z.A.; Morozova, K.; Cavoski, I.; Scampicchio, M.; Gobbetti, M.; Cagno, R.D. Lactic acid fermentation enriches the profile of biogenic fatty acid derivatives of avocado fruit (Persea americana Mill.). Food Chem. 2020, 317, 126384. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Qiao, Y.; Liu, G.; Leng, C.; Zhang, Y.; Lv, Z.; Feng, Z. The nutrient requirements of Lactobacillus rhamnosus GG and their application to fermented milk. J. Dairy Sci. 2019, 102, 5971–5978. [Google Scholar] [CrossRef]
- Braga, A.R.C.; de Souza Mesquita, L.M.; Martins, P.L.G.; Habu, S.; de Rosso, V.V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2018, 69, 162–170. [Google Scholar]
- Lopez de Felipe, F.; Curiel, J.A.; Munoz, R. Improvement of the fermentation performance of Lactobacillus plantarum by the flavanol catechin is uncoupled from its degradation. J. Appl. Microbiol. 2010, 109, 687–697. [Google Scholar] [CrossRef]
- Issaoui, M.; Delgado, A.M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Ouslati, A.; Chammem, N. Phenols, flavors, and the mediterranean diet. J. AOAC Int. 2020, 103, 915–924. [Google Scholar] [CrossRef]
| Samples | pH | TSS (oBrix) | Total Sugar (mg/mL) | Reducing Sugar (mg/mL) | Lactic Acid (mg/mL) | L* | a* | b* | Bacterial Enumeration (LogCFU/mL) |
|---|---|---|---|---|---|---|---|---|---|
| BGB 2.5%, 0 h | 5.37 ± 0.01 Aa | 7.88 ± 0.02 Ca | 65.84 ± 1.21 Ca | 21.46 ± 0.17 Ca | N.D. | 0.77 ± 0.08 Ab | 0.45 ± 0.06 Ab | 0.43 ± 0.05 Bb | 7.43 ± 0.01 Bb |
| BGB 2.5%, 24 h | 3.88 ± 0.01 Bb | 7.56 ± 0.01 Cb | 57.31 ± 1.41 Cb | 19.60 ± 0.26 Cb | 2.66 ± 0.03 C | 1.95 ± 0.10 Aa | 3.30 ± 0.06 Aa | 1.07 ± 0.06 Aa | 8.52 ± 0.07 Aa |
| BGB 5%, 0 h | 5.32 ± 0.01 Ba | 10.22 ± 0.03 Ba | 73.64 ± 0.92 Ba | 46.48 ± 0.51 Ba | N.D. | 0.33 ± 0.03 Bb | 0.16 ± 0.03 Bb | 0.24 ± 0.05 Ab | 7.46 ± 0.01 Bb |
| BGB 5%, 24 h | 3.87 ± 0.00 Bb | 9.86 ± 0.03 Bb | 65.39 ± 1.43 Bb | 40.08 ± 0.05 Bb | 5.17 ± 0.35 B | 0.78 ± 0.01 Ba | 2.10 ± 0.03 Ba | 0.55 ± 0.03 Ba | 8.75 ± 0.02 Aa |
| BGB 10%, 0 h | 5.24 ± 0.01 Ca | 14.36 ± 0.05 Aa | 92.36 ± 1.31 Aa | 95.37 ± 1.38 Aa | N.D. | 0.21 ± 0.02 Bb | 0.07 ± 0.09 Bb | 0.25 ± 0.05 Ab | 7.45 ± 0.00 Bb |
| BGB 10%, 24 h | 3.93 ± 0.01 Ab | 13.90 ± 0.06 Ab | 80.27 ± 1.12 Ab | 82.88 ± 0.46 Ab | 8.11 ± 0.67 A | 0.54 ± 0.01 Ca | 1.35 ± 0.11 Ca | 0.27 ± 0.01 Ca | 8.74 ± 0.08 Aa |
| Samples | TPC (g GAE/100 mL) | TAC (mg C3GE/100 mL) | TFC (mg QE/100 mL) | FRAP (mmol FeSO4/100 mL) | DPPH (mg AAE/100 mL) | TEAC (mg TE/100 mL) |
|---|---|---|---|---|---|---|
| BGB 2.5%, 0 h | 0.13 ± 0.00 Ca | 6.84 ± 0.12 Ca | 2.21 ± 0.16 Cb | 1.18 ± 0.02 Ca | 67.55 ± 1.92 Ca | 637.70 ± 27.94 Ca |
| BGB 2.5%, 24 h | 0.13 ± 0.00 Ca | 6.17 ± 0.07 Cb | 4.43 ± 0.11 Ca | 1.22 ± 0.02 Ca | 68.37 ± 1.92 Ca | 637.22 ± 18.94 Ca |
| BGB 5%, 0 h | 0.25 ± 0.00 Ba | 12.77 ± 0.10 Ba | 5.76 ± 0.25 Bb | 2.36 ± 0.04 Ba | 119.88 ± 0.88 Ba | 810.99 ± 30.59 Ba |
| BGB 5%, 24 h | 0.25 ± 0.00 Ba | 11.40 ± 0.11 Bb | 7.75 ± 0.21 Ba | 2.38 ± 0.02 Ba | 121.42 ± 2.43 Ba | 809.17 ± 18.92 Ba |
| BGB 10%, 0 h | 0.41 ± 0.00 Aa | 22.79 ± 0.28 Aa | 9.42 ± 0.05 Ab | 4.47 ± 0.01 Aa | 230.77 ± 5.30 Ab | 1158.33 ± 22.69 Aa |
| BGB 10%, 24 h | 0.42 ± 0.00 Aa | 18.92 ± 0.09 Ab | 10.36 ± 0.18 Aa | 4.59 ± 0.05 Aa | 240.19 ± 3.31 Aa | 1160.86 ± 15.55 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamonsuwan, K.; Balmori, V.; Marnpae, M.; Chusak, C.; Thilavech, T.; Charoensiddhi, S.; Smid, S.; Adisakwattana, S. Black Goji Berry (Lycium ruthenicum) Juice Fermented with Lactobacillus rhamnosus GG Enhances Inhibitory Activity against Dipeptidyl Peptidase-IV and Key Steps of Lipid Digestion and Absorption. Antioxidants 2024, 13, 740. https://doi.org/10.3390/antiox13060740
Kamonsuwan K, Balmori V, Marnpae M, Chusak C, Thilavech T, Charoensiddhi S, Smid S, Adisakwattana S. Black Goji Berry (Lycium ruthenicum) Juice Fermented with Lactobacillus rhamnosus GG Enhances Inhibitory Activity against Dipeptidyl Peptidase-IV and Key Steps of Lipid Digestion and Absorption. Antioxidants. 2024; 13(6):740. https://doi.org/10.3390/antiox13060740
Chicago/Turabian StyleKamonsuwan, Kritmongkhon, Vernabelle Balmori, Marisa Marnpae, Charoonsri Chusak, Thavaree Thilavech, Suvimol Charoensiddhi, Scott Smid, and Sirichai Adisakwattana. 2024. "Black Goji Berry (Lycium ruthenicum) Juice Fermented with Lactobacillus rhamnosus GG Enhances Inhibitory Activity against Dipeptidyl Peptidase-IV and Key Steps of Lipid Digestion and Absorption" Antioxidants 13, no. 6: 740. https://doi.org/10.3390/antiox13060740
APA StyleKamonsuwan, K., Balmori, V., Marnpae, M., Chusak, C., Thilavech, T., Charoensiddhi, S., Smid, S., & Adisakwattana, S. (2024). Black Goji Berry (Lycium ruthenicum) Juice Fermented with Lactobacillus rhamnosus GG Enhances Inhibitory Activity against Dipeptidyl Peptidase-IV and Key Steps of Lipid Digestion and Absorption. Antioxidants, 13(6), 740. https://doi.org/10.3390/antiox13060740







