GLS and GLS2 Glutaminase Isoenzymes in the Antioxidant System of Cancer Cells
Abstract
1. Introduction
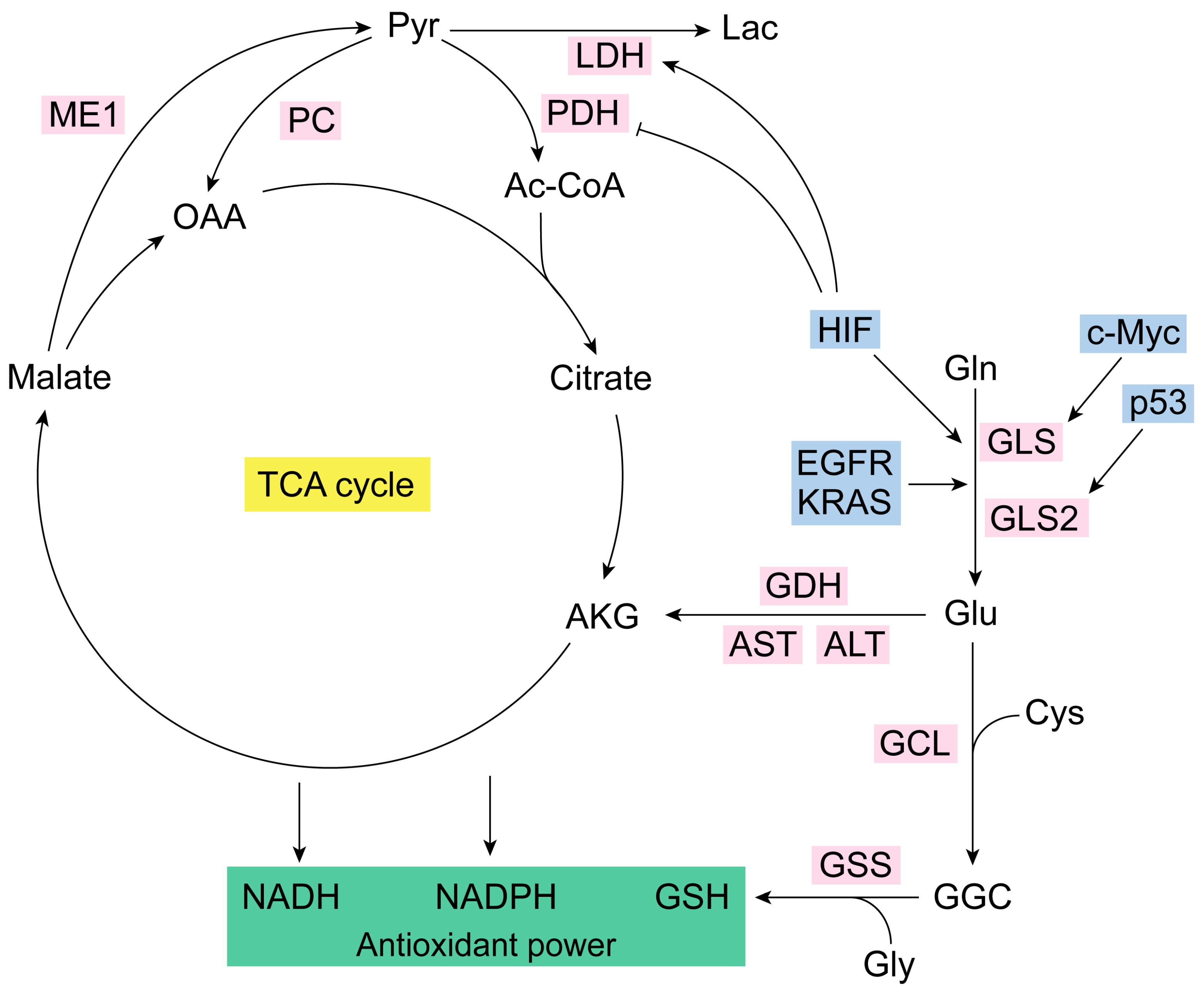
2. Glutaminase Isoenzymes in the Control of Cancer Redox Homeostasis
3. Mitochondrial Metabolism of Glutamine in Cancer: Redox Balance
Glutaminase can Trigger Reductive Carboxylation in Cancer
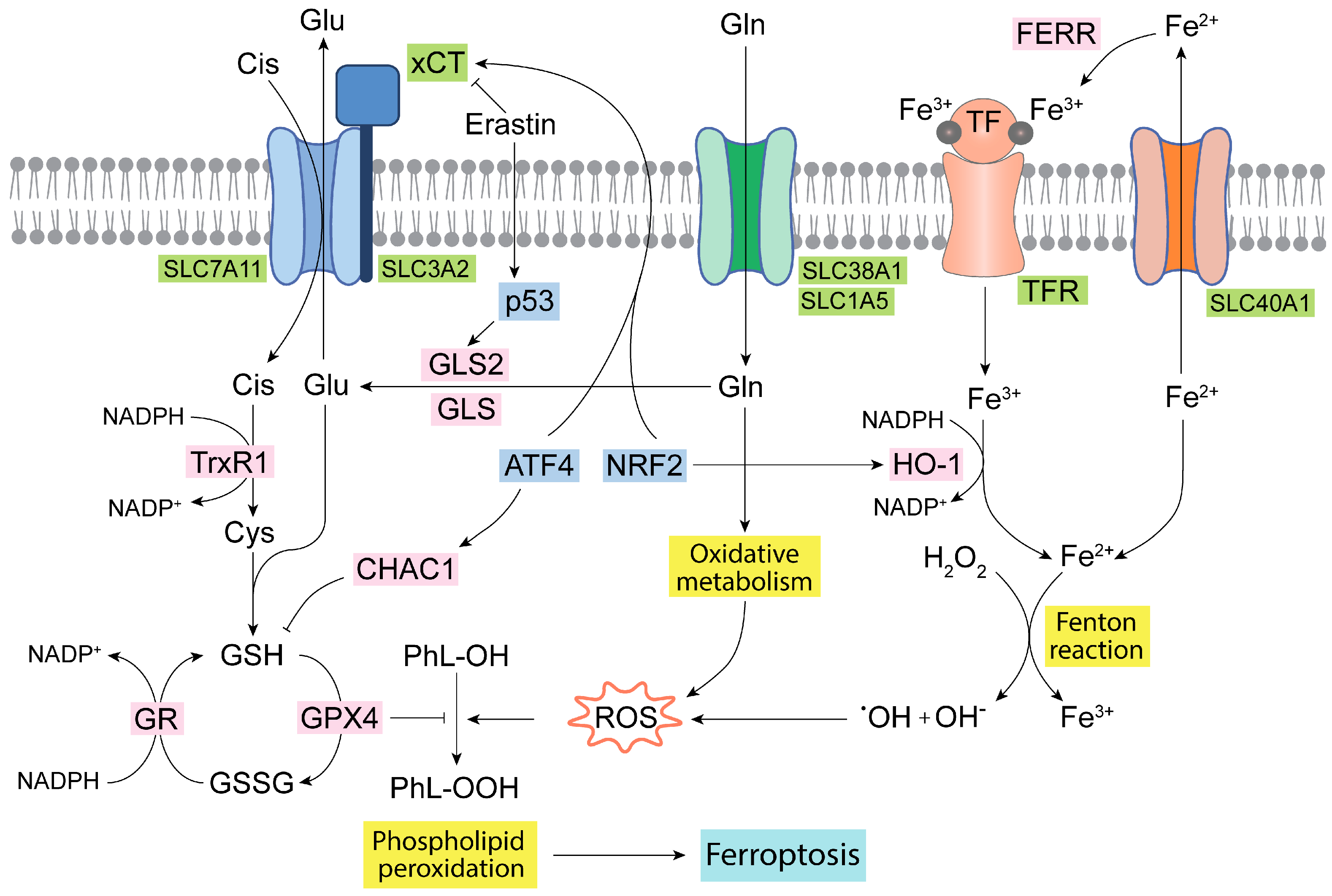
4. Glutaminases, Ferroptosis, and ROS
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Matés, J.M.; Campos-Sandoval, J.A.; De Los Santos-Jiménez, J.; Márquez, J. Glutaminases regulate glutathione and oxidative stress in cancer. Arch. Toxicol. 2020, 94, 2603–2623. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef]
- Matés, J.M.; Di Paola, F.J.; Campos-Sandoval, J.A.; Mazurek, S.; Márquez, J. Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer. Semin. Cell Dev. Biol. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Matés, J.M.; Campos-Sandoval, J.A.; Santos-Jiménez, J.L.; Márquez, J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019, 467, 29–39. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Katt, W.P.; Cerione, R.A. Alone and together: Current approaches to targeting glutaminase enzymes as part of anti-cancer therapies. Future Drug Discov. 2023, 4, FDD79. [Google Scholar] [CrossRef]
- Matés, J.M.; Campos-Sandoval, J.A.; Márquez, J. Glutaminase isoenzymes in the metabolic therapy of cancer. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 158–164. [Google Scholar] [CrossRef]
- Fox, D.B.; Garcia, N.M.G.; McKinney, B.J.; Lupo, R.; Noteware, L.C.; Newcomb, R.; Liu, J.; Locasale, J.W.; Hirschey, M.D.; Alvarez, J.V. NRF2 activation promotes the recurrence of dormant tumour cells through regulation of redox and nucleotide metabolism. Nat. Metab. 2020, 2, 318–334. [Google Scholar] [CrossRef]
- Singh, B.; Tai, K.; Madan, S.; Raythatha, M.R.; Cady, A.M.; Braunlin, M.; Irving, L.R.; Bajaj, A.; Lucci, A. Selection of metastatic breast cancer cells based on adaptability of their metabolic state. PLoS ONE 2012, 7, e36510. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, S.; Safi, R.; Liu, X.; Baldi, R.; Liu, W.; Liu, J.; Locasale, J.W.; Chang, C.Y.; McDonnell, D.P. Inhibition of ERRalpha Prevents Mitochondrial Pyruvate Uptake Exposing NADPH-Generating Pathways as Targetable Vulnerabilities in Breast Cancer. Cell Rep. 2019, 27, 3587–3601.e4. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Zhang, Y.; Wang, J.; Chen, Z.; Wen, T. Zeolitic imidazolate framework-based nanoparticles for the cascade enhancement of cancer chemodynamic therapy by targeting glutamine metabolism. Nanoscale 2022, 14, 8727–8743. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Owada, S.; Inagaki, Y.; Shida, Y.; Tatemichi, M. Metabolic reprogramming sustains cancer cell survival following extracellular matrix detachment. Redox Biol. 2020, 36, 101643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Xu, C.; Hua, A.; Wang, C.; Xiong, Y.; Deng, Q.; Chen, X.; Yang, T.; Wan, J.; et al. A novel lonidamine derivative targeting mitochondria to eliminate cancer stem cells by blocking glutamine metabolism. Pharmacol. Res. 2023, 190, 106740. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, A.; McCullough, C.R.; Lu, Z.; Zacharias, N.M.; Kelderhouse, L.E.; Gray, J.; Yang, H.; Engel, B.J.; Wang, Y.; Mao, W.; et al. Induction of autophagy by ARHI (DIRAS3) alters fundamental metabolic pathways in ovarian cancer models. BMC Cancer 2016, 16, 824. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Shah, A.; Summers, F.; Mathew, R.; Huang, J.; Hsieh, T.C.; Wu, J.M. NRH:quinone oxidoreductase 2 (NQO2) and glutaminase (GLS) both play a role in large extracellular vesicles (LEV) formation in preclinical LNCaP-C4-2B prostate cancer model of progressive metastasis. Prostate 2018, 78, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Mukha, A.; Kahya, U.; Linge, A.; Chen, O.; Löck, S.; Lukiyanchuk, V.; Richter, S.; Alves, T.C.; Peitzsch, M.; Telychko, V.; et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics 2021, 11, 7844–7868. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Khivansara, V.; Leach, A.; Gill, J.G.; Martin-Sandoval, M.; Yang, C.; Kasitinon, S.Y.; Bezwada, D.; Tasdogan, A.; Gu, W.; et al. Loss of glucose 6-phosphate dehydrogenase function increases oxidative stress and glutaminolysis in metastasizing melanoma cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2120617119. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ju, R.; Gao, H.; Huang, Y.; Guo, L.; Zhang, D. Targeting glutamine utilization to block metabolic adaptation of tumor cells under the stress of carboxyamidotriazole-induced nutrients unavailability. Acta Pharm. Sin. B 2022, 12, 759–773. [Google Scholar] [CrossRef]
- Hamada, S.; Matsumoto, R.; Tanaka, Y.; Taguchi, K.; Yamamoto, M.; Masamune, A. Nrf2 Activation Sensitizes K-Ras Mutant Pancreatic Cancer Cells to Glutaminase Inhibition. Int. J. Mol. Sci. 2021, 22, 1870. [Google Scholar] [CrossRef]
- Tong, Y.; Guo, D.; Lin, S.H.; Liang, J.; Yang, D.; Ma, C.; Shao, F.; Li, M.; Yu, Q.; Jiang, Y.; et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol. Cell 2021, 81, 2303–2316.e8. [Google Scholar] [CrossRef]
- Zhang, J.; Han, Z.Q.; Wang, Y.; He, Q.Y. Alteration of mitochondrial protein succinylation against cellular oxidative stress in cancer. Mil. Med. Res. 2022, 9, 6. [Google Scholar] [CrossRef]
- Ammar, N.; Hildebrandt, M.; Geismann, C.; Röder, C.; Gemoll, T.; Sebens, S.; Trauzold, A.; Schäfer, H. Monocarboxylate Transporter-1 (MCT1)-Mediated Lactate Uptake Protects Pancreatic Adenocarcinoma Cells from Oxidative Stress during Glutamine Scarcity Thereby Promoting Resistance against Inhibitors of Glutamine Metabolism. Antioxidants 2023, 12, 1818. [Google Scholar] [CrossRef] [PubMed]
- Abu Aboud, O.; Habib, S.L.; Trott, J.; Stewart, B.; Liang, S.; Chaudhari, A.J.; Sutcliffe, J.; Weiss, R.H. Glutamine Addiction in Kidney Cancer Suppresses Oxidative Stress and Can Be Exploited for Real-Time Imaging. Cancer Res. 2017, 77, 6746–6758. [Google Scholar] [CrossRef] [PubMed]
- Teng, R.; Liu, Z.; Tang, H.; Zhang, W.; Chen, Y.; Xu, R.; Chen, L.; Song, J.; Liu, X.; Deng, H. HSP60 silencing promotes Warburg-like phenotypes and switches the mitochondrial function from ATP production to biosynthesis in ccRCC cells. Redox Biol. 2019, 24, 101218. [Google Scholar] [CrossRef]
- Tronci, L.; Caria, P.; Frau, D.V.; Liggi, S.; Piras, C.; Murgia, F.; Santoru, M.L.; Pibiri, M.; Deiana, M.; Griffin, J.L.; et al. Crosstalk between Metabolic Alterations and Altered Redox Balance in PTC-Derived Cell Lines. Metabolites 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Jayachandran, K.; Zhang, J.; Menon, V.; Muhammad, N.; Zahner, M.; Ruiz, F.; Zhang, S.; Cho, K.; Wang, Y.; et al. Glutaminase Inhibitors Induce Thiol-Mediated Oxidative Stress and Radiosensitization in Treatment-Resistant Cervical Cancers. Mol. Cancer Ther. 2020, 19, 2465–2475. [Google Scholar] [CrossRef]
- Wicker, C.A.; Hunt, B.G.; Krishnan, S.; Aziz, K.; Parajuli, S.; Palackdharry, S.; Elaban, W.R.; Wise-Draper, T.M.; Mills, G.B.; Waltz, S.E.; et al. Glutaminase inhibition with telaglenastat (CB-839) improves treatment response in combination with ionizing radiation in head and neck squamous cell carcinoma models. Cancer Lett. 2021, 502, 180–188. [Google Scholar] [CrossRef]
- Romero, R.; Sayin, V.I.; Davidson, S.M.; Bauer, M.R.; Singh, S.X.; LeBoeuf, S.E.; Karakousi, T.R.; Ellis, D.C.; Bhutkar, A.; Sánchez-Rivera, F.J.; et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017, 23, 1362–1368. [Google Scholar] [CrossRef]
- LeBoeuf, S.E.; Wu, W.L.; Karakousi, T.R.; Karadal, B.; Jackson, S.R.; Davidson, S.M.; Wong, K.K.; Koralov, S.B.; Sayin, V.I.; Papagiannakopoulos, T. Activation of Oxidative Stress Response in Cancer Generates a Druggable Dependency on Exogenous Non-essential Amino Acids. Cell Metab. 2020, 31, 339–350.e4. [Google Scholar] [CrossRef]
- Bruntz, R.C.; Belshoff, A.C.; Zhang, Y.; Macedo, J.K.A.; Higashi, R.M.; Lane, A.N.; Fan, T.W. Inhibition of Anaplerotic Glutaminolysis Underlies Selenite Toxicity in Human Lung Cancer. Proteomics 2019, 19, e1800486. [Google Scholar] [CrossRef]
- Ulanet, D.B.; Couto, K.; Jha, A.; Choe, S.; Wang, A.; Woo, H.K.; Steadman, M.; DeLaBarre, B.; Gross, S.; Driggers, E.; et al. Mesenchymal phenotype predisposes lung cancer cells to impaired proliferation and redox stress in response to glutaminase inhibition. PLoS ONE 2014, 9, e115144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Nabel, C.S.; Li, D.; O’Connor, R.Í.; Crosby, C.R.; Chang, S.M.; Hao, Y.; Stanley, R.; Sahu, S.; Levin, D.S.; et al. Histone Deacetylase 6 Inhibition Exploits Selective Metabolic Vulnerabilities in LKB1 Mutant, KRAS Driven NSCLC. J. Thorac. Oncol. 2023, 18, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, D.; Yang, L.; Wang, N.; Shen, J.; Wang, J.; Zhang, L.; Chen, L.; Gao, S.; Zong, W.X.; et al. Activation of polyamine catabolism promotes glutamine metabolism and creates a targetable vulnerability in lung cancer. Proc. Natl. Acad. Sci. USA 2024, 121, e2319429121. [Google Scholar] [CrossRef] [PubMed]
- Moreira Franco, Y.E.; Alves, M.J.; Uno, M.; Moretti, I.F.; Trombetta-Lima, M.; de Siqueira Santos, S.; Dos Santos, A.F.; Arini, G.S.; Baptista, M.S.; Lerario, A.M.; et al. Glutaminolysis dynamics during astrocytoma progression correlates with tumor aggressiveness. Cancer Metab. 2021, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, G.; Oh, T.I.; Kim, B.M.; Shim, D.W.; Lee, K.H.; Kim, Y.J.; Lim, B.O.; Lim, J.H. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int. J. Oncol. 2016, 48, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Martín-Rufián, M.; Nascimento-Gomes, R.; Higuero, A.; Crisma, A.R.; Campos-Sandoval, J.A.; Gómez-García, M.C.; Cardona, C.; Cheng, T.; Lobo, C.; Segura, J.A.; et al. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J. Mol. Med. 2014, 92, 277–290. [Google Scholar] [CrossRef] [PubMed]
- de Los Santos-Jiménez, J.; Campos-Sandoval, J.A.; Márquez-Torres, C.; Urbano-Polo, N.; Brøndegaard, D.; Martín-Rufián, M.; Lobo, C.; Peñalver, A.; Gómez-García, M.C.; Martín-Campos, J.; et al. Glutaminase isoforms expression switches microRNA levels and oxidative status in glioblastoma cells. J. Biomed. Sci. 2021, 28, 14. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Bai, R.; Liu, Y.; Bi, H.; Shi, X.; Qu, C. Long Non-Coding RNA ATXN8OS Promotes Ferroptosis and Inhibits the Temozolomide-Resistance of Gliomas through the ADAR/GLS2 Pathway. Brain Res. Bull. 2022, 186, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Markert, E.K.; Rufini, A.; Antonov, A.V.; Sayan, B.S.; Tucci, P.; Agostini, M.; Mineo, T.C.; Levine, A.J.; Melino, G. p73 regulates serine biosynthesis in cancer. Oncogene 2014, 33, 5039–5046. [Google Scholar] [CrossRef]
- Chen, G.Y.; Chao, H.C.; Liao, H.W.; Tsai, I.L.; Kuo, C.H. Rapid quantification of glutaminase 2 (GLS2)-related metabolites by HILIC-MS/MS. Anal. Biochem. 2017, 539, 39–44. [Google Scholar] [CrossRef]
- Suzuki, S.; Venkatesh, D.; Kanda, H.; Nakayama, A.; Hosokawa, H.; Lee, E.; Miki, T.; Stockwell, B.R.; Yokote, K.; Tanaka, T.; et al. GLS2 Is a Tumor Suppressor and a Regulator of Ferroptosis in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 3209–3222. [Google Scholar] [CrossRef]
- Buczkowska, J.; Szeliga, M. Two Faces of Glutaminase GLS2 in Carcinogenesis. Cancers 2023, 15, 5566. [Google Scholar] [CrossRef]
- Márquez, J.; Matés, J.M.; Alonso, F.J.; Martín-Rufián, M.; Lobo, C.; Campos-Sandoval, J.A. Canceromis studies unravel tumor’s glutamine addiction after metabolic reprogramming. In Tumor Cell Metabolism: Pathways, Regulation and Biology; Mazurek, S., Shoshan, M., Eds.; Springer Verlag: Wien, Austria, 2015; pp. 257–286. [Google Scholar]
- Hu, W.; Zhang, C.; Wu, R.; Sun, Y.; Levine, A.; Feng, Z. Glutaminase 2, a Novel P53 Target Gene Regulating Energy Metabolism and Antioxidant Function. Proc. Natl. Acad. Sci. USA 2010, 107, 7455–7460. [Google Scholar] [CrossRef]
- Suzuki, S.; Tanaka, T.; Poyurovsky, M.V.; Nagano, H.; Mayama, T.; Ohkubo, S.; Lokshin, M.; Hosokawa, H.; Nakayama, T.; Suzuki, Y.; et al. Phosphate-Activated Glutaminase (GLS2), a P53-Inducible Regulator of Glutamine Metabolism and Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2010, 107, 7461–7466. [Google Scholar] [CrossRef]
- Xiao, D.; Ren, P.; Su, H.; Yue, M.; Xiu, R.; Hu, Y.; Liu, H.; Qing, G. Myc Promotes Glutaminolysis in Human Neuroblastoma through Direct Activation of Glutaminase 2. Oncotarget 2015, 6, 40655–40666. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xie, G.; Liu, C.; Zhou, J.; Chen, J.; Yu, S.; Li, J.; Pang, X.; Shi, H.; Liang, H. Knock-down of Glutaminase 2 Expression Decreases Glutathione, NADH, and Sensitizes Cervical Cancer to Ionizing Radiation. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013, 1833, 2996–3005. [Google Scholar] [CrossRef]
- Dias, M.M.; Adamoski, D.; dos Reis, L.M.; Ascenção, C.F.R.; de Oliveira, K.R.S.; Mafra, A.C.P.; da Silva Bastos, A.C.; Quintero, M.; Cassago, C.d.G.; Ferreira, I.M.; et al. GLS2 Is Protumorigenic in Breast Cancers. Oncogene 2020, 39, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Peña, E.; Arnold, J.; Shivakumar, V.; Joseph, R.; Vidhya Vijay, G.; den Hollander, P.; Bhangre, N.; Allegakoen, P.; Prasad, R.; Conley, Z.; et al. The Epithelial to Mesenchymal Transition Promotes Glutamine Independence by Suppressing GLS2 Expression. Cancers 2019, 11, 1610. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- Yuneva, M.; Zamboni, N.; Oefner, P.; Sachidanandam, R.; Lazebnik, Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 2007, 178, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef]
- Wise, D.R.; Ward, P.S.; Shay, J.E.; Cross, J.R.; Gruber, J.J.; Sachdeva, U.M.; Platt, J.M.; DeMatteo, R.G.; Simon, M.C.; Thompson, C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 2011, 108, 19611–19616. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jun, S.A.; Tsukamoto, T.; Fathi, A.T.; Minden, M.D.; Dang, C.V. Inhibition of glutaminase selectively suppresses the growth of primary acute myeloid leukemia cells with IDH mutations. Exp. Hematol. 2014, 42, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Chu, C.; Li, W.; Wang, C.; Sang, N. ErbB2 activation upregulates glutaminase 1 expression which promotes breast cancer cell proliferation. J. Cell. Biochem. 2014, 115, 498–509. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Deberardinis, R.J.; Sayed, N.; Ditsworth, D.; Thompson, C.B. Brick by brick: Metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008, 18, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; Núñez de Castro, I.; Asenjo, M.; Márquez, J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2002, 34, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Campos-Sandoval, J.A.; Lobo, C.; Alonso, L.; Alonso, F.J.; Márquez, J. Glutamine homeostasis and mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009, 41, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; Yang, M.; Prag, H.A.; Blanco, G.R.; Nikitopoulou, E.; Segarra-Mondejar, M.; Powell, C.A.; Young, T.; Burger, N.; Miljkovic, J.L.; et al. Disruption of the TCA cycle reveals an ATF4-dependent integration of redox and amino acid metabolism. Elife 2021, 10, e72593. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Alberghina, L.; Gaglio, D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014, 5, e1561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Deberardinis, R.J. Cancer metabolism: When more is less. Nature 2012, 489, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Sudderth, J.; Yang, C.; Mullen, A.R.; Jin, E.S.; Matés, J.M.; DeBerardinis, R.J. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8674–8679. [Google Scholar] [CrossRef]
- Mullen, A.R.; Hu, Z.; Shi, X.; Jiang, L.; Boroughs, L.K.; Kovacs, Z.; Boriack, R.; Rakheja, D.; Sullivan, L.B.; Linehan, W.M.; et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014, 7, 1679–1690. [Google Scholar] [CrossRef]
- Sellers, K.; Fox, M.P.; Bousamra, M., 2nd; Slone, S.P.; Higashi, R.M.; Miller, D.M.; Wang, Y.; Yan, J.; Yuneva, M.O.; Deshpande, R.; et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Investig. 2015, 125, 687–698. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Chen, J.; Xie, Z.; Chen, Z. Wild-Type Isocitrate Dehydrogenase-Dependent Oxidative Decarboxylation and Reductive Carboxylation in Cancer and Their Clinical Significance. Cancers 2022, 14, 5779. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2011, 481, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, D.; Fan, H.; Wu, R.; Tu, J.; Zhang, F.Q.; Wang, M.; Zheng, H.; Qu, C.K.; Elf, S.E.; et al. Cellular signals converge at the NOX2-SHP-2 axis to induce reductive carboxylation in cancer cells. Cell Chem. Biol. 2022, 29, 1200–1208.e6. [Google Scholar] [CrossRef]
- Itsumi, M.; Inoue, S.; Elia, A.J.; Murakami, K.; Sasaki, M.; Lind, E.F.; Brenner, D.; Harris, I.S.; Chio, I.I.C.; Afzal, S.; et al. Idh1 protects murine hepatocytes from endotoxin-induced oxidative stress by regulating the intracellular NADP(+)/NADPH ratio. Cell Death Differ. 2015, 22, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Losman, J.A.; Kaelin, W.G., Jr. What a difference a hydroxyl makes: Mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev. 2013, 27, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Jin, G.; Karoly, E.D.; Spasojevic, I.; Yang, J.; Kinzler, K.W.; He, Y.; Bigner, D.D.; Vogelstein, B.; Yan, H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc. Natl. Acad. Sci. USA 2011, 108, 3270–3275. [Google Scholar] [CrossRef]
- McBrayer, S.K.; Mayers, J.R.; DiNatale, G.J.; Shi, D.D.; Khanal, J.; Chakraborty, A.A.; Sarosiek, K.A.; Briggs, K.J.; Robbins, A.K.; Sewastianik, T.; et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell 2018, 175, 101–116.e25. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, Z.; Wang, G.; Wang, Q.A.; DeBerardinis, R.J.; Jiang, L. FASN deficiency induces a cytosol-to-mitochondria citrate flux to mitigate detachment-induced oxidative stress. Cell Rep. 2023, 42, 112971. [Google Scholar] [CrossRef]
- Delgir, S.; Bastami, M.; Ilkhani, K.; Safi, A.; Seif, F.; Alivand, M.R. The pathways related to glutamine metabolism, glutamine inhibitors and their implication for improving the efficiency of chemotherapy in triple-negative breast cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108366. [Google Scholar] [CrossRef]
- Jiang, L.; Shestov, A.A.; Swain, P.; Yang, C.; Parker, S.J.; Wang, Q.A.; Terada, L.S.; Adams, N.D.; McCabe, M.T.; Pietrak, B.; et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 2016, 532, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, Z.; Wang, Q.A.; Chan, D.; Jiang, L. Metabolic reprogramming in the OPA1-deficient cells. Cell. Mol. Life Sci. 2022, 79, 517. [Google Scholar] [CrossRef]
- Dai, W.; Xu, L.; Yu, X.; Zhang, G.; Guo, H.; Liu, H.; Song, G.; Weng, S.; Dong, L.; Zhu, J.; et al. OGDHL silencing promotes hepatocellular carcinoma by reprogramming glutamine metabolism. J. Hepatol. 2020, 72, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, A.; Gameiro, P.A.; Christodoulou, D.; Laviollette, L.; Schneider, M.; Chaves, F.; Stemmer-Rachamimov, A.; Yazinski, S.A.; Lee, R.; Stephanopoulos, G.; et al. Glutaminase and poly(ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J. Clin. Investig. 2017, 127, 1631–1645. [Google Scholar] [CrossRef]
- Sigoillot, F.D.; Berkowski, J.A.; Sigoillot, S.M.; Kotsis, D.H.; Guy, H.I. Cell cycle-dependent regulation of pyrimidine biosynthesis. J. Biol. Chem. 2003, 278, 3403–3409. [Google Scholar] [CrossRef]
- De Los Santos-Jiménez, J.; Rosales, T.; Ko, B.; Campos-Sandoval, J.A.; Alonso, F.J.; Márquez, J.; DeBerardinis, R.J.; Matés, J.M. Metabolic Adjustments following Glutaminase Inhibition by CB-839 in Glioblastoma Cell Lines. Cancers 2023, 15, 531. [Google Scholar] [CrossRef] [PubMed]
- Motooka, Y.; Toyokuni, S. Ferroptosis As Ultimate Target of Cancer Therapy. Antioxid. Redox Signal. 2023, 39, 206–223. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, S.; Wang, L.; Li, H.; Zhang, L.; Xu, S.; Zheng, X. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 2020, 235, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, S.; Xu, W.; Zhang, Y.; Yang, R.; Ma, K.; Zhang, J.; Xu, J. Piperlongumine Inhibits Thioredoxin Reductase 1 by Targeting Selenocysteine Residues and Sensitizes Cancer Cells to Erastin. Antioxidants 2022, 11, 710. [Google Scholar] [CrossRef]
- Hu, Q.; Dai, J.; Zhang, Z.; Yu, H.; Zhang, J.; Zhu, X.; Qin, Y.; Zhang, L.; Zhang, P. ASS1-Mediated Reductive Carboxylation of Cytosolic Glutamine Confers Ferroptosis Resistance in Cancer Cells. Cancer Res. 2023, 83, 1646–1665. [Google Scholar] [CrossRef]
- Jennis, M.; Kung, C.P.; Basu, S.; Budina-Kolomets, A.; Leu, J.I.; Khaku, S.; Scott, J.P.; Cai, K.Q.; Campbell, M.R.; Porter, D.K.; et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016, 30, 918–930. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.Y.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zheng, J.; Liang, Q.; Liu, Y.; Yang, Y.; Wang, R.; Wang, M.; Zhang, Q.; Xuan, Z.; Sun, H.; et al. Identification and Validation of a Novel Ferroptotic Prognostic Genes-Based Signature of Clear Cell Renal Cell Carcinoma. Cancers 2022, 14, 4690. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Wang, S.F.; Hsu, C.Y.; Yin, P.H.; Yeh, T.S.; Lee, H.C.; Tseng, L.M. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 2017, 8, 114588. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Morrow, J.D.; Burk, R.F. Thioredoxin reductase reduces lipid hydroperoxides and spares alpha-tocopherol. Biochem. Biophys. Res. Commun. 2002, 292, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Choi, H.; Gupta, M.; Hensley, C.; Lee, H.; Lu, Y.T.; Pantel, A.; Mankoff, D.; Zhou, R. Disruption of redox balance in glutaminolytic triple negative breast cancer by inhibition of glutamate export and glutaminase. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, H.; Fan, J.; Wang, F.; Xu, C.; Li, Y.; Tu, J.; Nephew, K.P.; Long, X. Glutamine metabolism in breast cancer and possible therapeutic targets. Biochem. Pharmacol. 2023, 210, 115464. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Lin, R.; Huang, S.; Miao, H.; Zou, L.; Liu, K.; Cui, X.; Wang, Z.; Zhang, Y.; et al. Lithocholic acid inhibits gallbladder cancer proliferation through interfering glutaminase-mediated glutamine metabolism. Biochem. Pharmacol. 2022, 205, 115253. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. A comprehensive overview of recent developments on the mechanisms and pathways of ferroptosis in cancer: The potential implications for therapeutic strategies in ovarian cancer. Cancer Drug Resist. 2023, 6, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H. Recent advances in understanding the metabolic plasticity of ovarian cancer: A systematic review. Heliyon 2022, 8, e11487. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, F.; Qi, Q.R.; Yue, B.S.; Zhai, B.T. Targeted drug delivery systems for elemene in cancer therapy: The story thus far. Biomed. Pharmacother. 2023, 166, 115331. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Blatt, E.B.; Parra, K.; Neeb, A.; Buroni, L.; Bogdan, D.; Yuan, W.; Gao, Y.; Gilbreath, C.; Paschalis, A.; Carreira, S.; et al. Critical role of antioxidant programs in enzalutamide-resistant prostate cancer. Oncogene 2023, 42, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Guo, L.; Nancolas, B.; Nelson, D.S.; Shestov, A.A.; Lee, S.-C.; Roman, J.; Zhou, R.; Leeper, D.B.; Halestrap, A.P.; et al. Mechanism of antineoplastic activity of lonidamine. BBA Rev. Cancer 2016, 1866, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Erez, I.; Issacson, C.; Lavi, Y.; Shaco-Levy, R.; Milam, J.; Laster, B.; Gheber, L.A.; Rapaport, H. Antitumor Effect of Lonidamine-Polypeptide-Peptide Nanoparticles in Breast Cancer Models. ACS Appl. Mater. Interfaces 2019, 11, 32670–32678. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.; Posadas, E.; Ellis, L.; Freedland, S.J.; Vizio, D.D.; Freeman, M.R.; Theodorescu, D.; Figlin, R.; Gong, J. Targeting Glutamine Metabolism in Prostate Cancer. Front. Biosci. 2023, 15, 2. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Guo, X.; Du, Y.; Zhu, Q.; Wang, X.; Duan, C. FDX1 can Impact the Prognosis and Mediate the Metabolism of Lung Adenocarcinoma. Front. Pharmacol. 2021, 12, 749134. [Google Scholar] [CrossRef]

| Molecular Mechanism(s)/Impact on Cancer | Cancer Type(s) | In Vitro Model(s) | In Vivo Model(s) | Reference |
|---|---|---|---|---|
| NRF2 1 caused tumor growth through a mechanism that includes GLS overexpression | Breast | BT474, SKBR3 | Nude mice in orthotopic models | [8] |
| Gln-independent cells lowered GLS, increased oxidative stress, and enhanced resistance to drugs undergoing EMT 2 | Breast (TNBC 3) | MDA-MB-231, SUM149, 4T1 | Female athymic nude xenografted mice | [9] |
| GLS inhibition by CB-839 synergistically worked with the inhibition of the ERRα 4, blocking NADPH 5 synthesis and decreasing tumor growth | Breast (TNBC 3) | MDA436 | None | [10] |
| Iron oxidative nanoparticles coupled to the GLS inhibitor CB-839 both increased ROS and decreased GSH, boosting DNA oxidative damage and cancer cell death | Breast (TNBC 3) | MDA-MB-231 | Mice injected with tumor cells and iron-CB-839 nanoparticles | [11] |
| AMPK 6 activated NRF2 1 and its target proteins to allow tumor cells to grow and maintain their redox status through GLS, supporting anchorage-independent cancer cell survival | TNBC 3, Liver, Pancreas, Skin | MDA-MB-231, HepG2, BxPC-3, HT-1080, HaCaT | None | [12] |
| HYL001, a new drug with a low IC50 against cancer cells and minimal toxicity toward normal cells and healthy mice, repressed GLS, reduced GSH, enhanced ROS, and blunted the TCA 7 cycle and OXPHOS 8 | TNBC 3, Liver (HCC 9) | 4T1, H22 | 4T1 metastatic and orthotopic models in BALB/c mice | [13] |
| Induction of ARHI 10 resulted in oxidative stress, which was augmented following GLS inhibition by BPTES | Ovarium | SKOv3 | Xenografted mice bearing SKOv3 cells | [14] |
| BPTES prevents the interaction between NQO2 11 and caveolin-1 of cancer cells that induce their metastatic activity | Prostate | LNCaP, C4, C4-2 | None | [15] |
| Inhibition of GLS increased DNA oxidative damage and boosted susceptibility to ionizing radiation | Prostate | DU145, LNCaP | PC3 injected into nude NSG 12 mice | [16] |
| Mutant G6PD 13 melanoma cells increased glutaminolysis, which correlated with higher ROS levels, decreased NADPH, and lower GSH/GSSG ratios | Skin (melanoma) | M481, M214, A375 | Melanoma cell lines injected into nude NSG 11 mice | [17] |
| GLS inhibition by CB-839 increased mitochondrial ROS, lowered the GSH/GSSG ratio, enhanced apoptosis, and diminished cancer growth in vitro and in vivo | Colon (CRC 14) | HCT116, C26 | CRC 14 in BALB/c mice and CRC 14 from patients in NSG 12 mice | [18] |
| KRAS-mutant cells increased sensitivity to GLS inhibition by CB-839 through NRF2 1 | Pancreas | BxPC3, Panc-1, MiaPaC2 | None | [19] |
| Oxidative stress increased glutaminolysis and the production of NADPH 5 and GSH | Pancreas (PDAC 15) | SW1990 | Nude BALB/c mice | [20] |
| GLS succinylation was essential to maintain redox homeostasis measured as NADPH and GSH levels, as well as ROS formation | Pancreas (PDAC 15) | SW1990 | Male athymic nude BALB/c mice | [21] |
| Lactate imported by MCT1 16 maintained redox homeostasis via NRF2 1 and thereby cell viability following GLS inhibition by CB-839, which shortened GSH and increased ROS | Pancreas (PDAC 15) | T3M4, A818-6 | PDAC 15 patients with a tumor disease at stage T3N1M0 | [22] |
| Following GLS inhibition by BPTES or CB-839, cancer cells showed decreased survival and more apoptosis associated with a lowered GSH/GSSG ratio, increased NRF2 1, and higher oxidative DNA damage | Kidney | SN12, 786-O | Mice with orthotopic injections and treated with CB-839 | [23] |
| HSP60 silencing activated the MEK/ERK/c-MYC axis to evoke Gln addiction while increasing susceptibility to oxidative stress and GLS inhibition by BPTES | Kidney | 786-O, 769-P | None | [24] |
| More aggressive tumors showed higher GLS activity, increased ROS levels, enhanced GSSG/GSH ratios, and accumulation of NAD+ and NADP+ | Thyroid | B-CPAP, K1, TPC-1 | None | [25] |
| GLS inhibition by CB-839 induced oxidative stress, i.e., lowering GSH/GSSG, enhancing TrxR1 17, and diminishing tumor growth | Uterine, Cervix | CaSki, SiHa | Nude mice were xenografted with SiHa cells | [26] |
| CB-839 in combination with radiation increased oxidative stress and boosted DNA oxidative damage | HNSCC 18 | CAL-27, FaDu, HN5 | Nude mice xenografted with CAL-27/HN5 | [27] |
| Keap1-mutant cells displayed a robust sensitivity to GLS inhibition by BPTES and CB-839 through NRF2 1, increasing survival ratios | Lung | Human adenocarcinomas | Mice xenografted, treated with CB-839 | [28] |
| Oxidative stress, by NRF2 1 malfunction, depleted Glu, which was lowered by GLS inhibition by CB-839, blocking cancer growth | Lung | LKR10/13 | Mice subcutaneously injected with tumor cells | [29] |
| Selenite impaired GLS expression and increased the GSSG/GSH ratio | NSCLC 19 | A549 | Ex vivo NSCLC 18 | [30] |
| TGFβ 20 induced EMT 2 that evoked sensitivity to BPTES, which reduced citrate levels and OXPHOS 8, lowering the cells’ antioxidant capacity | NSCLC 19 | A427, NCI-H358 | None | [31] |
| Lower levels of NADH, GSH, and GSSG were concomitants to longer survival after using a combination of an HDAC6 21 inhibitor + CB-839 in in vitro and in vivo KRAS/LKB1 22 models (displaying high GLS activity) | NSCLC 19 | H23, H358 | C57BL/6 mice inoculated with KRAS/TP53 or KRAS/LKB1 cancer cells | [32] |
| Activation of SAT1 23 increased GLS activity and GSH synthesis, ameliorating oxidative stress to support lung cancer cell proliferation, which was blocked by inhibition of GLS, resulting in ROS accumulation | NSCLC 19 | A549, PC9, H1650, H1792, H358, H1944 | PC9 cells were subcutaneously injected into BALB/c nude mice | [33] |
| GAC isoform was highly expressed versus KGA isoform in GBM 24 and more malignant astrocytomas | Brain | U87MG | Astrocytomas of different malignancies | [34] |
| Molecular Mechanism(s)/Impact on Cancer | Cancer Type(s) | In Vitro Model(s) | In Vivo Model(s) | Reference |
|---|---|---|---|---|
| Inhibition of GA by the compound 968 in apigenin-treated cells decreased NADPH and increased intracellular ROS levels, boosting apoptosis | Lung | H1299, H660 | None | [35] |
| GLS silencing and GLS2 overexpression induced oxidative stress, increased apoptosis, and decreased cell migration | Brain (GBM 1) | SFxL, LN229, T98G | None | [36] |
| Silencing of GLS or overexpression of GLS2 decreased oxidative status and boosted antioxidant enzymes | Brain (GBM 1) | LN229, T98G | None | [37] |
| GLS2 reduced the TMZ 2 resistance of GBM 1 in vitro and in vivo through the long noncoding RNA ATXN8OS, which mediated ferroptosis and increased oxidative damage to lipids | Brain (GBM 1) | U251, U251TR | U251 + GLS2-transfected cells were injected into the brains of nude mice | [38] |
| p73 Transcriptionally activated GLS2, increased serine and diminished oxidative stress | NSCLC 3, Osteosarcoma | H1299, SaOs-2 | None | [39] |
| GLS2 overexpression shortened oxidative stress by a GSH-independent mechanism | NSCLC 3 | CL1-0 | None | [40] |
| Knockout of GLS2, a tumor suppressor in this study, reduced the GSH/GSSG ratio and increased oxidative damage to lipids during ferroptosis | Liver (HCC 4) | HepG2, HepG3, SKHep1 | Injections of SKHep1 cells on the flanks of NSG 5 mice | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De los Santos-Jiménez, J.; Campos-Sandoval, J.A.; Alonso, F.J.; Márquez, J.; Matés, J.M. GLS and GLS2 Glutaminase Isoenzymes in the Antioxidant System of Cancer Cells. Antioxidants 2024, 13, 745. https://doi.org/10.3390/antiox13060745
De los Santos-Jiménez J, Campos-Sandoval JA, Alonso FJ, Márquez J, Matés JM. GLS and GLS2 Glutaminase Isoenzymes in the Antioxidant System of Cancer Cells. Antioxidants. 2024; 13(6):745. https://doi.org/10.3390/antiox13060745
Chicago/Turabian StyleDe los Santos-Jiménez, Juan, José A. Campos-Sandoval, Francisco J. Alonso, Javier Márquez, and José M. Matés. 2024. "GLS and GLS2 Glutaminase Isoenzymes in the Antioxidant System of Cancer Cells" Antioxidants 13, no. 6: 745. https://doi.org/10.3390/antiox13060745
APA StyleDe los Santos-Jiménez, J., Campos-Sandoval, J. A., Alonso, F. J., Márquez, J., & Matés, J. M. (2024). GLS and GLS2 Glutaminase Isoenzymes in the Antioxidant System of Cancer Cells. Antioxidants, 13(6), 745. https://doi.org/10.3390/antiox13060745









