Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Smart-Seq2 Omics Data Analysis and Candidate Drug Identification
2.2. In Vitro Culture of Ovine Oocytes
2.3. Preliminary Screening of Drugs and Selection of Working Concentrations
2.4. qPCR Detection of Gene Expression
2.5. Reactive Oxygen Species Immunofluorescence Detection
3. Results
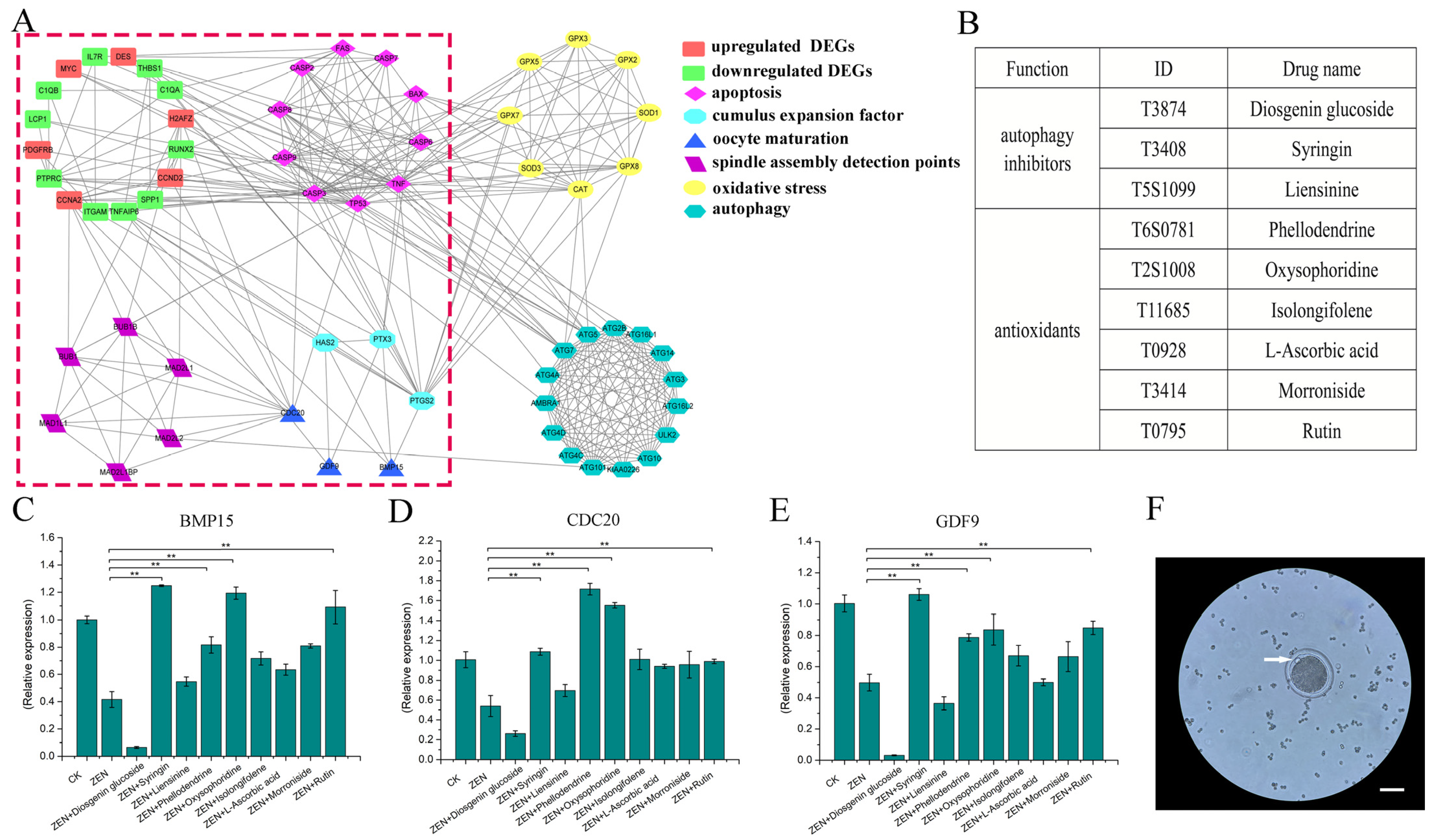
3.1. Preliminary Screening of Drugs
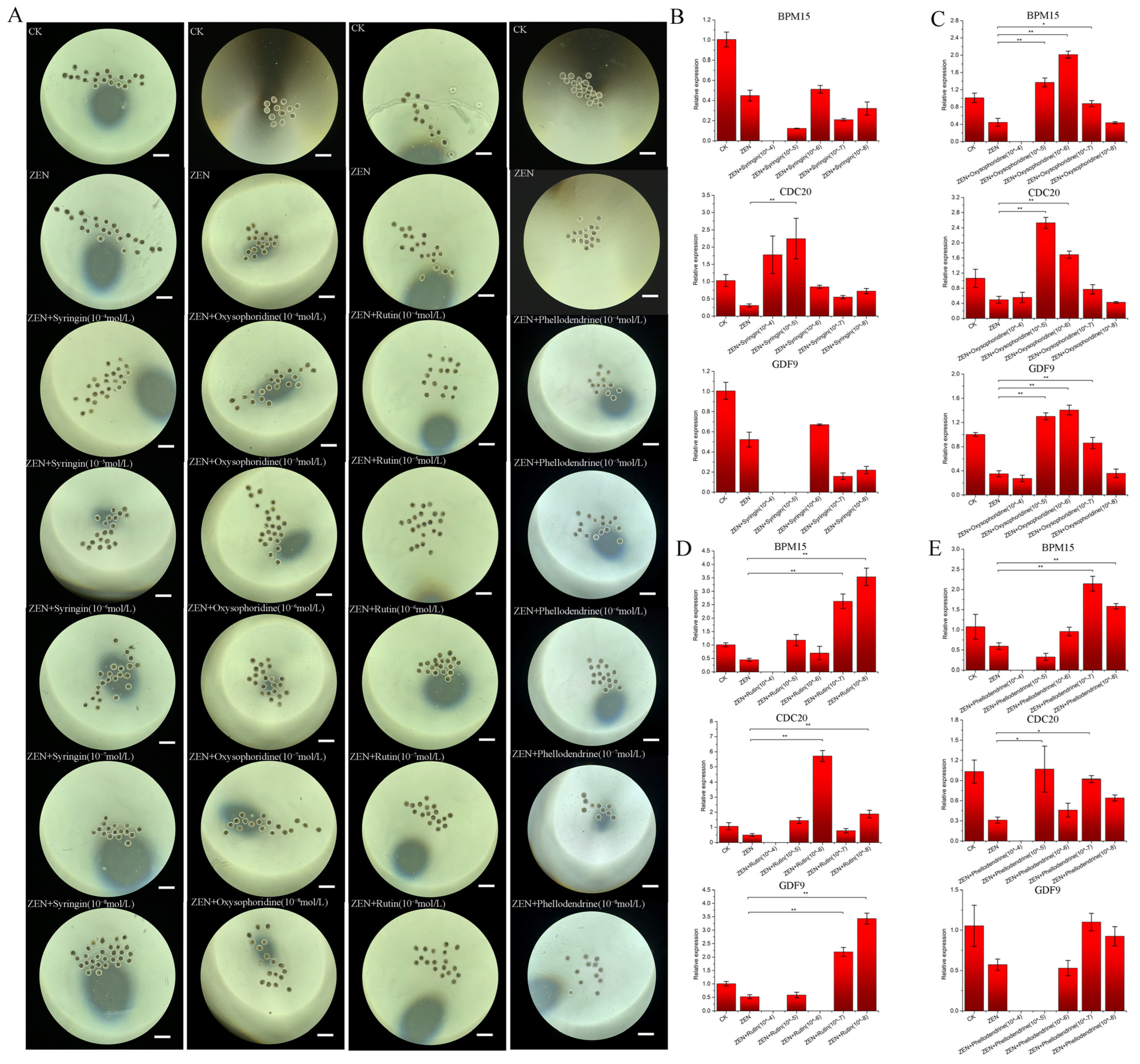
3.2. Concentration Determination of the Selected Drugs
3.3. Detection of Genes Related to Oocyte Maturation
3.4. Expression of Genes Related to Cumulus Expansion Factors
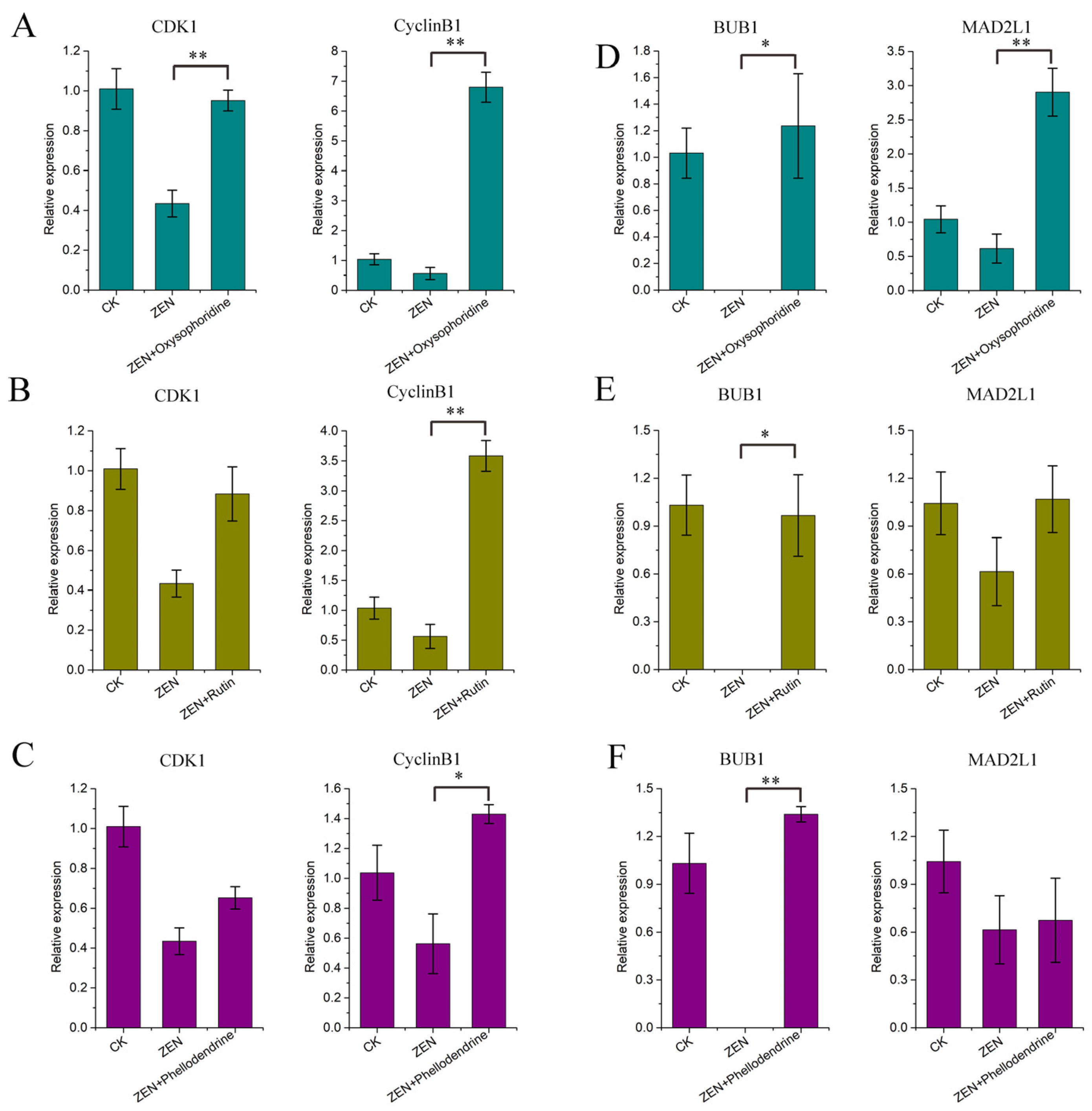
3.5. Expression of Cell Cycle-Related Genes
3.6. Expression of Spindle Checkpoint-Related Genes
3.7. Expression of Oxidative Stress-Related Genes
3.8. Expression of Autophagy-Related Genes
3.9. Expression of Apoptosis-Related Genes
3.10. Diagram of the Mechanism of Three Drugs Reducing the Reproductive Toxicity of ZEN on Sheep Oocyte IVM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stob, M.; Baldwin, R.; Tuite, J.; Andrews, F.; Gillette, K. Isolation of an anabolic, uterotrophic compound from corn infected with Gibberella zeae. Nature 1962, 196, 1318. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ren, C.; Gong, Y.; Gao, X.; Rajput, S.A.; Qi, D.; Wang, S. The insensitive mechanism of poultry to zearalenone: A review. Anim. Nutr. 2021, 7, 587–594. [Google Scholar] [CrossRef]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Golge, O.; Kabak, B. Occurrence of deoxynivalenol and zearalenone in cereals and cereal products from Turkey. Food Control 2020, 110, 106982. [Google Scholar] [CrossRef]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Açar, Y.; Akbulut, G. Evaluation of Aflatoxins Occurrence and Exposure in Cereal-Based Baby Foods: An Update Review. Curr. Nutr. Rep. 2024, 13, 59–68. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Ma, T.; Lv, C.; Li, Y.; Duan, H.; Zhao, X.; Wang, J.; Zhang, Y. Smart-seq2 Technology Reveals a Novel Mechanism That Zearalenone Inhibits the In Vitro Maturation of Ovine Oocytes by Influencing TNFAIP6 Expression. Toxins 2023, 15, 617. [Google Scholar] [CrossRef]
- Ji, Y.M.; Zhang, K.H.; Pan, Z.N.; Ju, J.Q.; Zhang, H.L.; Liu, J.C.; Wang, Y.; Sun, S.C. High-dose zearalenone exposure disturbs G2/M transition during mouse oocyte maturation. Reprod. Toxicol. 2022, 110, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.Z.; Cheng, H.; Zhang, J.; Gong, S.; Tian, X.D.; Pan, C.J.; Luo, M.J.; Tan, J.H. Invivo zearalenone exposure dose-dependently compromises mouse oocyte competence by impairing chromatin configuration and gene transcription. Reprod. Fertil. Dev. 2021, 33, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Q.; Wang, J.J.; Li, M.H.; Tian, Y.; Zhao, A.H.; Li, L.; De Felici, M.; Shen, W. Impaired primordial follicle assembly in offspring ovaries from zearalenone-exposed mothers involves reduced mitochondrial activity and altered epigenetics in oocytes. Cell. Mol. Life Sci. 2022, 79, 258. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, L.; He, M.; Zhang, S.; Gao, J.; Wu, C.; Zhang, D.; Dai, J. Resveratrol Protects against Zearalenone-Induced Mitochondrial Defects during Porcine Oocyte Maturation via PINK1/Parkin-Mediated Mitophagy. Toxins 2022, 14, 641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, C.H.; Chen, S.; Sun, S.C. Zearalenone exposure impairs organelle function during porcine oocyte meiotic maturation. Theriogenology 2022, 177, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.P.; Brito, D.C.C.; Silva, T.E.S.; Silva, R.F.; Guedes, M.I.F.; Silva, J.Y.G.; Rodrigues, A.P.R.; Santos, R.R.; Figueiredo, J.R. In vitro exposure of sheep ovarian tissue to the xenoestrogens zearalenone and enterolactone: Effects on preantral follicles. Theriogenology 2021, 174, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Bochkov, V.; Mihovilovic, M.D.; Kopp, B.; Bauer, R.; Dirsch, V.M.; Stuppner, H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Liu, S.; Tu, Y.; Shan, T. Toxicity, biodegradation, and nutritional intervention mechanism of zearalenone. Sci. Total Environ. 2024, 911, 168648. [Google Scholar] [CrossRef]
- Jing, S.; Liu, C.; Zheng, J.; Dong, Z.; Guo, N. Toxicity of zearalenone and its nutritional intervention by natural products. Food Funct. 2022, 13, 10374–10400. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Yang, M.; Li, J.; Wu, Y.; Fan, H.; Kong, X.; Ning, C.; Wang, S.; Xiao, W.; et al. Betulinic acid alleviates zearalenone-induced uterine injury in mice. Environ. Pollut. 2023, 316 Pt 1, 120435. [Google Scholar] [CrossRef]
- Kang, J.; Li, Y.; Ma, Z.; Wang, Y.; Zhu, W.; Jiang, G. Protective effects of lycopene against zearalenone-induced reproductive toxicity in early pregnancy through anti-inflammatory, antioxidant and anti-apoptotic effects. Food Chem. Toxicol. 2023, 179, 113936. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, J.; Wang, S.; Cheng, J.; Chen, H.; Zhang, Z.; Yang, L.; Hua, R.; Li, Q. Isorhamnetin protects porcine oocytes from zearalenone-induced reproductive toxicity through the PI3K/Akt signaling pathway. J. Anim. Sci. Biotechnol. 2023, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wei, W.; Cao, R.; Lu, L.; Liang, S.; Xiong, M.; Zhang, C.; Liang, X.; Ma, Y. Resveratrol alleviates zea-induced decidualization disturbance in human endometrial stromal cells. Ecotoxicol. Environ. Saf. 2021, 207, 111511. [Google Scholar] [CrossRef] [PubMed]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Sulyok, M.; Artavia, J.I.; Flores-Quiroz, S.I.; Garzón-Pérez, C.; Castillo-Lopez, E.; Zavala, L.; Orozco, J.D.; Faas, J.; Krska, R.; et al. Mixtures of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Whole-Plant Corn Silages and Total Mixed Rations of Dairy Farms in Central and Northern Mexico. Toxins 2023, 15, 153, Erratum in Toxins 2024, 16, 62. [Google Scholar] [CrossRef]
- Goyal, J.; Verma, P.K. An Overview of Biosynthetic Pathway and Therapeutic Potential of Rutin. Mini Rev. Med. Chem. 2023, 23, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, A.; Virk, P.; Almoqhem, R.; Alsharidah, A.; Al-Ghadi, M.Q.; Aljabr, W.; Alasmari, F.; Albasher, G. The Efficacy of Hispidin and Magnesium Nanoparticles against Zearalenone-Induced Fungal Toxicity Causing Polycystic Ovarian Syndrome in Rats. Biomedicines 2024, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Chen, L.; Liu, Y.; Peng, T. Oxysophoridine rescues spinal cord injury via anti-inflammatory, anti-oxidative stress and anti-apoptosis effects. Mol. Med. Rep. 2018, 17, 2523–2528. [Google Scholar] [CrossRef]
- Meng, C.; Liu, C.; Liu, Y.; Wu, F. Oxysophoridine attenuates the injury caused by acute myocardial infarction in rats through anti-oxidative, anti-inflammatory and anti-apoptotic pathways. Mol. Med. Rep. 2015, 11, 527–532. [Google Scholar] [CrossRef]
- Rahmani, S.; Naraki, K.; Roohbakhsh, A.; Hayes, A.W.; Karimi, G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Sci. Nutr. 2022, 11, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wang, X.; Xi, X.; Zhu, L.; Chen, Q.; Zhang, H.; Qin, Y.; Yang, B.; Che, N.; Cao, H.; et al. Phellodendrine promotes autophagy by regulating the AMPK/mTOR pathway and treats ulcerative colitis. J. Cell. Mol. Med. 2021, 25, 5707–5720. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Wu, N.; Zhao, X.; Cai, D. Utilizing network pharmacology and experimental validation to investigate the underlying mechanism of phellodendrine on inflammation. PeerJ 2022, 10, e13852. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, T.; Tian, C.; Xiao, Y.; Kou, S.; Zhou, X.; Liu, S.; Ye, X.; Li, X. The defensive effect of phellodendrine against AAPH-induced oxidative stress through regulating the AKT/NF-κB pathway in zebrafish embryos. Life Sci. 2016, 157, 97–106. [Google Scholar] [CrossRef] [PubMed]







| Primers | Primer Sequences | Product Length (bp) | Annealing Temp (°C) | Gene Accession Number |
|---|---|---|---|---|
| BMP15 | GGACACCCTAGGGAAAACCG | 101 | 60 | NM_001114767.2 |
| TGTATGTGCCAGGAGCCTCT | ||||
| CDC20 | GGCTGAGCTGAAAGGTCACA | 214 | 60 | XM_004023553.6 |
| AACACCGTGAGGAGTTGGTC | ||||
| GDF9 | TGACAGAGCTTTGCGCTACA | 166 | 61 | NM_001142888.2 |
| TGATGGAAAGGTTCCTGCCG | ||||
| HAS2 | GGAGACATATCGCTGCTGCT | 217 | 61 | XM_004011666.5 |
| ACCCACATAAAGCATGGCTAGT | ||||
| PTGS2 | GACCATGGTAGAAGCCGGAG | 294 | 61 | NM_001009432.1 |
| AGTTCGGTTGAACGCTCCTT | ||||
| TNFAIP6 | GCTATGGGAAGAGGCTCACG | 149 | 61 | NM_001009432.1 |
| ATTCACACACTGCCTTCGCT | ||||
| CDK1 | ATGGCTTGGATCTGCTCTCGAA | 154 | 61 | NM_001142508.1 |
| TGCTCTTGACACAACACAGGA | ||||
| CyclinB1 | GCTTGGAGACATCGGTAACA | 129 | 60 | XM_060414226.1 |
| GGAGCCTTTTCCAGAGGTTTTG | ||||
| BUB1 | ACGCCTCACTGAAACCCATT | 195 | 61 | XM_012173898.5 |
| GTGATCACCCTTTGTTCCCCT | ||||
| MAD2L1 | CCTTTTGAAACGAGTGGCGG | 167 | 60 | XM_004009585.5 |
| GAGAAGAACTCGGCCACGAT | ||||
| GPX | CAGTTTGGGCATCAGGAAAAC | 100 | 61 | XM_004018462.5 |
| CGAAGAGCATGAAATTGGGC | ||||
| SOD1 | GGCAATGTGAAGGCTGACAA | 130 | 61 | NM_001145185.2 |
| TGCCCAAGTCATCTGGTCTT | ||||
| SOD2 | GGACAAATCTGAGCCCCAAC | 180 | 61 | NM_001280703.1 |
| CAATCTGTAAGCGTCCCTGC | ||||
| ATG3 | CCCGGTCCTCAAGGAATCAA | 103 | 61 | XM_004002919.6 |
| TTGCCATGTTGGACAGTGGT | ||||
| LC3 | ACGCCTCTCAGGAGACTTTTG | 121 | 61 | XM_004014953.4 |
| ACCTCAGTTGGTAACATCCCT | ||||
| ULK2 | GTGAAGCAAGGTTCAAGCCG | 132 | 61 | XM_060395488.1 |
| TCAATGTCTGCTGGGTCCTG | ||||
| BAX | TTCCGACGGCAACTTCAACT | 260 | 61 | XM_027978594.3 |
| CCATGTGGGTGTCCCAAAGT | ||||
| CAS3 | ACGGAAGCAAATCAGTGGAC | 167 | 61 | XM_060406953.1 |
| GGTTTCCCTGAGGTTTGCTG | ||||
| CAS8 | AGTGAGTTGCAGACATCCGA | 172 | 61 | XM_060410232.1 |
| AGGTCTTGTCCAAAGCCTCT | ||||
| CAS9 | AGAGTGATGAAGCAGGACCC | 195 | 61 | XM_060396599.1 |
| CAGATCGGCATTTCCCTTGG | ||||
| P53 | TCTTCAGATCCGTGGGCGTA | 158 | 61 | NM_001009403.1 |
| TTTTATGGCAGGAGGGAGAAGG | ||||
| GAPDH | AGATGGTGAAGGTCGGAGTG | 188 | 60 | XM_060411595.1 |
| GTTCTCTGCCTTGACTGTGC |
| Group | First Time | Second Time | Third Time | Maturation Rate/% | |||
|---|---|---|---|---|---|---|---|
| Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | ||
| CK | 17 | 14 | 16 | 14 | 17 | 15 | 51.26984 ± 5.35229 |
| ZEN (20 μmol/L) | 17 | 15 | 16 | 14 | 17 | 15 | 29.68254 ± 5.22365 |
| ZEN (20 μmol/L) + Diosgenin glucoside (10−6 mol/L) | 17 | 15 | 16 | 15 | 17 | 14 | 27.14286 ± 5.96665 |
| ZEN (20 μmol/L) + Syringin (10−6 mol/L) | 17 | 15 | 16 | 13 | 17 | 15 | 46.83761 * ± 6.92466 |
| ZEN (20 μmol/L) + Liensinine (10−6 mol/L) | 17 | 16 | 16 | 14 | 17 | 16 | 32.44048 ± 4.58179 |
| ZEN (20 μmol/L) + Phellodendrine (10−6 mol/L) | 17 | 14 | 16 | 15 | 17 | 15 | 50.15873 ** ± 6.04843 |
| ZEN (20 μmol/L) + Oxysophoridine (10−6 mol/L) | 17 | 15 | 16 | 14 | 17 | 14 | 55.71429 ** ± 5.15079 |
| ZEN (20 μmol/L) + Isolongifolene (10−6 mol/L) | 17 | 15 | 16 | 14 | 17 | 13 | 45.22589 * ± 2.06735 |
| ZEN (20 μmol/L) + L-Ascorbic acid (10−6 mol/L) | 17 | 14 | 16 | 15 | 17 | 15 | 45.55556 * ± 5.09175 |
| ZEN (20 μmol/L) + Morroniside (10−6 mol/L) | 18 | 15 | 16 | 14 | 17 | 15 | 47.61905 * ± 5.30263 |
| ZEN (20 μmol/L) + Rutin (10−6 mol/L) | 18 | 16 | 18 | 16 | 19 | 16 | 47.91667 * ± 3.60844 |
| Drug Group | Grouping of Each Drug | First Time | Second Time | Third Time | Maturation Rate/% | |||
|---|---|---|---|---|---|---|---|---|
| Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | |||
| Syringin | CK | 25 | 20 | 21 | 17 | 20 | 16 | 49.4363 ± 5.98998 |
| ZEN (20 μmol/L) | 25 | 23 | 23 | 21 | 20 | 18 | 31.9646 ± 3.69757 | |
| ZEN (20 μmol/L) + Syringin (10−4 mol/L) | 25 | 24 | 23 | 19 | 20 | 15 | 29.8068 ± 3.62705 | |
| ZEN (20 μmol/L) + Syringin (10−5 mol/L) | 25 | 20 | 23 | 20 | 20 | 17 | 36.7647 ± 2.80570 | |
| ZEN (20 μmol/L) + Syringin (10−6 mol/L) | 25 | 22 | 23 | 22 | 20 | 16 | 38.8258 ± 6.23276 | |
| ZEN (20 μmol/L) + Syringin (10−7 mol/L) | 25 | 21 | 23 | 18 | 20 | 17 | 34.2515 ± 5.23716 | |
| ZEN (20 μmol/L) + Syringin (10−8 mol/L) | 32 | 30 | 23 | 22 | 23 | 20 | 28.6869 ± 5.42359 | |
| Oxysophoridine | CK | 19 | 17 | 22 | 18 | 20 | 18 | 49.0196 ± 5.80928 |
| ZEN (20 μmol/L) | 19 | 18 | 22 | 19 | 20 | 18 | 34.6004 ± 3.81613 | |
| ZEN (20 μmol/L) + Oxysophoridine (10−4 mol/L) | 19 | 15 | 22 | 17 | 20 | 16 | 35.6373 ± 5.53444 | |
| ZEN (20 μmol/L) + Oxysophoridine (10−5 mol/L) | 19 | 17 | 22 | 22 | 20 | 17 | 48.4848 ** ± 3.94177 | |
| ZEN (20 μmol/L) + Oxysophoridine (10−6 mol/L) | 19 | 17 | 22 | 16 | 20 | 17 | 51.9608 ** ± 1.69809 | |
| ZEN (20 μmol/L) + Oxysophoridine (10−7 mol/L) | 19 | 17 | 22 | 20 | 20 | 15 | 46.2418 * ± 1.09318 | |
| ZEN (20 μmol/L) + Oxysophoridine (10−8 mol/L) | 17 | 12 | 20 | 15 | 22 | 19 | 39.5029 ± 2.45039 | |
| Rutin | CK | 22 | 20 | 18 | 16 | 23 | 20 | 45.0000 ± 5.00000 |
| ZEN (20 μmol/L) | 22 | 20 | 18 | 15 | 23 | 21 | 28.9683 ± 4.18081 | |
| ZEN (20 μmol/L) + Rutin (10−4 mol/L) | 22 | 16 | 20 | 17 | 23 | 18 | 35.2533 ± 5.23434 | |
| ZEN (20 μmol/L) + Rutin (10−5 mol/L) | 22 | 21 | 20 | 16 | 23 | 19 | 35.3958 ± 3.64461 | |
| ZEN (20 μmol/L) + Rutin (10−6 mol/L) | 22 | 17 | 20 | 20 | 23 | 20 | 34.8039 ± 5.29684 | |
| ZEN (20 μmol/L) + Rutin (10−7 mol/L) | 22 | 17 | 20 | 17 | 23 | 17 | 45.0980 ** ± 3.39618 | |
| ZEN (20 μmol/L) + Rutin (10−8 mol/L) | 20 | 18 | 20 | 16 | 22 | 19 | 48.7939 ** ± 4.56198 | |
| Phellodendrine | CK | 25 | 18 | 24 | 20 | 18 | 16 | 50.1852 ± 5.28021 |
| ZEN (20 μmol/L) | 25 | 18 | 24 | 22 | 18 | 14 | 31.7701 ± 3.96847 | |
| ZEN (20 μmol/L) + Phellodendrine (10−4 mol/L) | 31 | 29 | 24 | 19 | 18 | 17 | 30.3370 ± 5.68084 | |
| ZEN (20 μmol/L) + Phellodendrine (10−5 mol/L) | 25 | 25 | 24 | 20 | 18 | 16 | 32.7500 ± 1.98431 | |
| ZEN (20 μmol/L) + Phellodendrine (10−6 mol/L) | 25 | 21 | 24 | 22 | 18 | 15 | 39.7403 ± 3.25454 | |
| ZEN (20 μmol/L) + Phellodendrine (10−7 mol/L) | 25 | 18 | 24 | 21 | 18 | 16 | 52.6455 ** ± 2.78721 | |
| ZEN (20 μmol/L) + Phellodendrine (10−8 mol/L) | 25 | 19 | 22 | 19 | 22 | 19 | 43.8596 ± 6.07737 | |
| Group | First Time | Second Time | Third Time | Fourth Time | Maturation Rate/% | ||||
|---|---|---|---|---|---|---|---|---|---|
| Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | Initial Quantity (pcs) | Sample Quantity Received (pcs) | ||
| CK | 20 | 16 | 21 | 19 | 23 | 17 | 21 | 17 | 49.0954 ± 4.47271 |
| ZEN (20 μmol/L) | 20 | 17 | 21 | 15 | 23 | 17 | 21 | 17 | 28.9216 ± 6.27757 |
| ZEN (20 μmol/L) + Oxysophoridine (10−6 mol/L) | 20 | 17 | 21 | 19 | 23 | 18 | 21 | 16 | 54.3446 ** ± 1.82583 |
| ZEN (20 μmol/L) + Rutin (10−8 mol/L) | 20 | 18 | 21 | 17 | 23 | 19 | 21 | 19 | 50.5805 ** ± 5.05156 |
| ZEN (20 μmol/L) + Phellodendrine (10−7 mol/L) | 20 | 13 | 21 | 19 | 23 | 18 | 23 | 18 | 51.6925 ** ± 3.70242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Ma, T.; Liu, Y.; Liu, W.; Zhao, X.; Zhang, G.; Wang, J.; Zhang, Y. Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes. Antioxidants 2024, 13, 752. https://doi.org/10.3390/antiox13060752
Li Z, Ma T, Liu Y, Liu W, Zhao X, Zhang G, Wang J, Zhang Y. Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes. Antioxidants. 2024; 13(6):752. https://doi.org/10.3390/antiox13060752
Chicago/Turabian StyleLi, Zongshuai, Tian Ma, Yali Liu, Wanruo Liu, Xingxu Zhao, Gaiping Zhang, Jianlin Wang, and Yong Zhang. 2024. "Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes" Antioxidants 13, no. 6: 752. https://doi.org/10.3390/antiox13060752
APA StyleLi, Z., Ma, T., Liu, Y., Liu, W., Zhao, X., Zhang, G., Wang, J., & Zhang, Y. (2024). Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes. Antioxidants, 13(6), 752. https://doi.org/10.3390/antiox13060752







