Mechanism of Action and Therapeutic Implications of Nrf2/HO-1 in Inflammatory Bowel Disease
Abstract
:1. Introduction
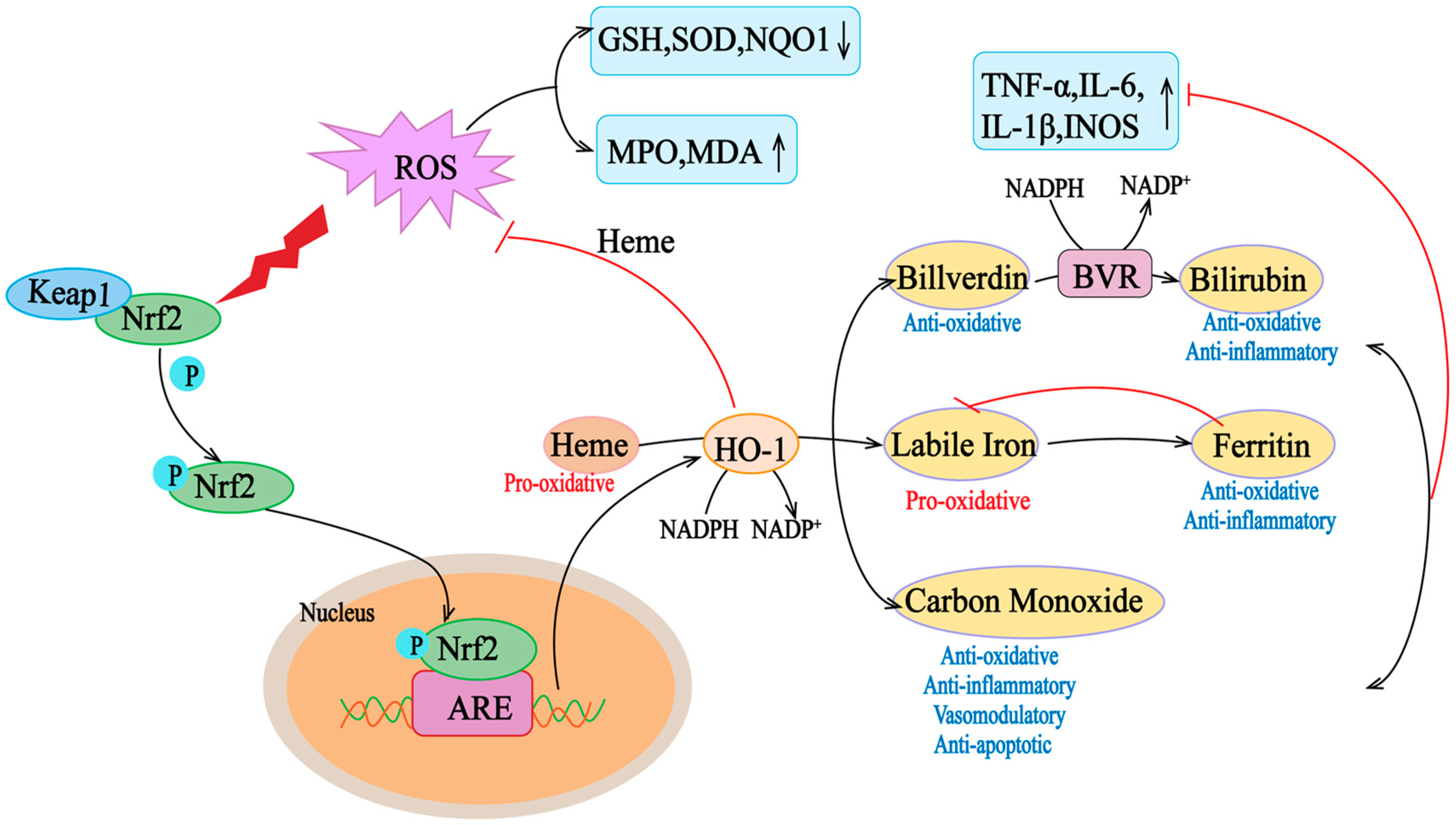
2. Antioxidant Nrf2/HO-1 Signaling Pathway
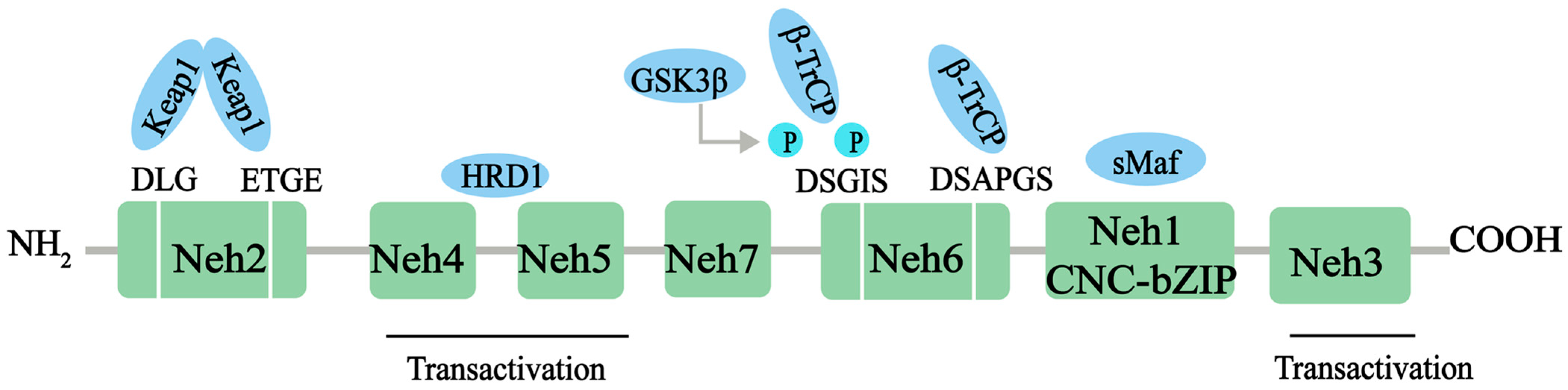
2.1. Structure and Function of Nrf2
2.2. Structure and Function of HO-1
2.3. Antioxidant Effect of the Nrf2/HO-1 Signal
3. Role of Nrf2/HO-1 in Normal Gut Development and Normal Gut Function
3.1. Role of Nrf2/HO-1 in Normal Intestinal Development
3.2. Role of Nrf2/HO-1 in Normal Intestinal Function
4. Role of Nrf2/HO-1 in IBD and IBD Complications
4.1. Role of Nrf2/HO-1 in IBD
4.1.1. Nrf2/HO-1 Attenuates Intestinal Inflammation and Injury by Controlling Oxidative Stress
4.1.2. Nrf2/HO-1 Promotes the Maintenance of the Intestinal Epithelial Barrier by Regulating Intestinal Microbiota
4.1.3. Nrf2/HO-1 Prevents Ferroptosis in Enterocytes
4.2. Role of Nrf2/HO-1 in the Complications of IBD (Intestinal Fibrosis and CRC)
4.2.1. Role of the Nrf2/HO-1 Pathway in Intestinal Fibrosis in IBD
4.2.2. Role of the Nrf2/HO-1 Pathway in IBD-Associated Colorectal Cancer
5. Phytochemicals Targeting the Nrf2/HO-1 Signaling Pathway in the Treatment of IBD
6. Conclusions and Opinions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, R.; Mohammed, Y.S.; Akula, S.B.; Aramin, M.A.S.; Naradasu, P.R.; Patel, S.; Desai, H.D. Inflammatory Bowel Disease in Youth under 20 from 1990–2019: A Global Perspective on Regional and National Variations from the GBD 2019 Analysis. Gastroenterology 2024, 166, S99. [Google Scholar] [CrossRef]
- Gorospe, J.; Windsor, J.; Hracs, L.; Coward, S.; Buie, M.; Quan, J.; Caplan, L.; Markovinovic, A.; Cummings, M.; Goddard, Q.; et al. Trends in Inflammatory Bowel Disease Incidence and Prevalence across Epidemiologic Stages: A Global Systematic Review with Meta-Analysis. Inflamm. Bowel Dis. 2024, 30, S42. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Z.; Wan, X. Global, regional, and national burden of inflammatory bowel disease and its associated anemia, 1990 to 2019 and predictions to 2050: An analysis of the global burden of disease study 2019. Autoimmun. Rev. 2024, 23, 103498. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, S.; Bourgonje, A.R.; Fagundes, R.R.; Gacesa, R.; Weersma, R.K.; Van Goor, H.; Mann, G.E.; Dijkstra, G.; Faber, K.N. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 2023, 29, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Han, R.; Yuan, Y.; Xing, Y.; Zhang, W.; Sun, Z.; Liu, Y.; Li, J.; Mao, T. The role of Akkermansia muciniphila in inflammatory bowel disease: Current knowledge and perspectives. Front. Immunol. 2022, 13, 1089600. [Google Scholar] [CrossRef]
- Hirten, R.P.; Sands, B.E. New Therapeutics for Ulcerative Colitis. Annu. Rev. Med. 2021, 72, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; Van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative Stress and DNA Damage: Implications in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Lan, S. Implications of Antioxidant Systems in Inflammatory Bowel Disease. Biomed Res. Int. 2018, 2018, 1290179. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid. Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, P.; Fan, Z.; He, X.; Liu, J.; Li, M.; Chen, F. Dandelion polysaccharide treatment protects against dextran sodium sulfate-induced colitis by suppressing NF-κB/NLRP3 inflammasome-mediated inflammation and activating Nrf2 in mouse colon. Food Sci. Nutr. 2023, 11, 7271–7282. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przęczek, A.; Feelisch, M.; Cuadrado, A.; Van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Lin, J.; Zhou, J.; Du, H.; Chen, X.; Liu, M.; Qu, Q.; Lv, W.; Guo, S. Polyphenol Rich Forsythia suspensa Extract Alleviates DSS-Induced Ulcerative Colitis in Mice through the Nrf2-NLRP3 Pathway. Antioxidants 2022, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Huang, X.L.; Chen, R.R.; Li, T.; Ye, H.J.; Xie, W.; Huang, Z.M.; Cao, G.Z. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J. Honokiol Ameliorates DSS-Induced Mouse Colitis by Inhibiting Inflammation and Oxidative Stress and Improving the Intestinal Barrier. Oxid. Med. Cell. Longev. 2022, 2022, 1755608. [Google Scholar] [CrossRef]
- Wang, J.; Behl, T.; Rana, T.; Sehgal, A.; Wal, P.; Saxena, B.; Yadav, S.; Mohan, S.; Anwer, M.K.; Chigurupati, S.; et al. Exploring the pathophysiological influence of heme oxygenase-1 on neuroinflammation and depression: A study of phytotherapeutic-based modulation. Phytomedicine 2024, 127, 155466. [Google Scholar] [CrossRef]
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; León, R. KEAP1-NRF2 protein-protein interaction inhibitors: Design, pharmacological properties and therapeutic potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef]
- Chowdhry, S.; Zhang, Y.; Mcmahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Moosavi-Movahedi, F.; Saso, L.; Habibi-Rezaei, M.; Khatibi, A.; Hong, J.; Moosavi-Movahedi, A.A. Modulation of Nrf2/HO-1 by Natural Compounds in Lung Cancer. Antioxidants 2023, 12, 735. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Sadeghi, M.; Fathi, M.; Gholizadeh Navashenaq, J.; Mohammadi, H.; Yousefi, M.; Hojjat-Farsangi, M.; Namdar, A.; Movasaghpour Akbari, A.A.; Jadidi-Niaragh, F. The prognostic and therapeutic potential of HO-1 in leukemia and MDS. Cell Commun. Signal. CCS 2023, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Igarashi, K.; Immenschuh, S.; Shibahara, S.; Tyrrell, R.M. Regulation of heme oxygenase-1 gene transcription: Recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase. Antioxid. Redox Signal. 2004, 6, 924–933. [Google Scholar]
- Puentes-Pardo, J.D.; Moreno-Sanjuan, S.; Carazo, Á.; León, J. Heme Oxygenase-1 in Gastrointestinal Tract Health and Disease. Antioxidants 2020, 9, 1214. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef]
- Kang, J.Y.; Xu, M.M.; Sun, Y.; Ding, Z.X.; Wei, Y.Y.; Zhang, D.W.; Wang, Y.G.; Shen, J.L.; Wu, H.M.; Fei, G.H. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Niu, Z.; Li, X.; Yang, X.; Sun, Z. Protective effects of sinomenine against dextran sulfate sodium-induced ulcerative colitis in rats via alteration of HO-1/Nrf2 and inflammatory pathway. Inflammopharmacology 2024, 32, 2007–2022. [Google Scholar] [CrossRef]
- Liu, R.; Yang, J.; Li, Y.; Xie, J.; Wang, J. Heme oxygenase-1: The roles of both good and evil in neurodegenerative diseases. J. Neurochem. 2023, 167, 347–361. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, Y.M. Beneficial and Detrimental Roles of Heme Oxygenase-1 in the Neurovascular System. Int. J. Mol. Sci. 2022, 23, 7041. [Google Scholar] [CrossRef]
- Lu, Y.; Xie, X.; Luo, L. Ferroptosis crosstalk in anti-tumor immunotherapy: Molecular mechanisms, tumor microenvironment, application prospects. Apoptosis 2024. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Chiang, S.K.; Chen, S.E.; Yu, Y.L.; Chou, R.H.; Chang, W.C. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018, 416, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Adedoyin, O.; Boddu, R.; Traylor, A.; Lever, J.M.; Bolisetty, S.; George, J.F.; Agarwal, A. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2018, 314, F702–F714. [Google Scholar] [CrossRef]
- Fahrer, J.; Wittmann, S.; Wolf, A.C.; Kostka, T. Heme Oxygenase-1 and Its Role in Colorectal Cancer. Antioxidants 2023, 12, 1989. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 Signaling and Redox Homeostasis in Physiopathological Conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Lee, D.Y.; Song, M.Y.; Kim, E.H. Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals. Antioxidants 2021, 10, 743. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Q.; Bao, L.; Li, M.; Chang, K.; Yi, X. Cytoprotective Role of Heme Oxygenase-1 in Cancer Chemoresistance: Focus on Antioxidant, Antiapoptotic, and Pro-Autophagy Properties. Antioxidants 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yan, C.; Gu, J.; Yuan, Y.; Zou, H.; Liu, Z.; Bian, J. Resveratrol Alleviates Zearalenone-Induced Intestinal Dysfunction in Mice through the NF-κB/Nrf2/HO-1 Signalling Pathway. Foods 2024, 13, 1217. [Google Scholar] [CrossRef]
- Yang, S.; Liu, G.; Xia, X.; Gan, D.; Xiang, S.; Xiang, M. α-Mangostin suppresses ethanol-induced gastric ulceration by regulating the Nrf2/HO-1 and NF-κB/NLRP3/caspase-1 signaling pathways and gut microbiota. Heliyon 2024, 10, e24339. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Sharaf, H.; Saber, S.; Youssef, M.E.; Abdelhamid, A.M.; Mourad, A.A.E.; Ibrahim, S.; Allam, S.; Elgharabawy, R.M.; El-Ahwany, E.; et al. Ambroxol, a mucolytic agent, boosts HO-1, suppresses NF-κB, and decreases the susceptibility of the inflamed rat colon to apoptosis: A new treatment option for treating ulcerative colitis. FASEB J. 2022, 36, e22496. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Klimczyk, D.; Kopec, M.; Jozkowicz, A.; Piechota-Polanczyk, A. Nrf2 Transcriptional Activity Governs Intestine Development. Int. J. Mol. Sci. 2022, 23, 6175. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Chartoumpekis, D.V.; Kensler, T.W. Crosstalk between Nrf2 and Notch signaling. Free Radic. Biol. Med. 2015, 88 Pt B, 158–167. [Google Scholar] [CrossRef]
- Gregorieff, A.; Clevers, H. Wnt signaling in the intestinal epithelium: From endoderm to cancer. Genes Dev. 2005, 19, 877–890. [Google Scholar] [CrossRef]
- Koch, S. Extrinsic control of Wnt signaling in the intestine. Differentiation 2017, 97, 1–8. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Jiang, X.; Du, H.; Shi, Y.; Xiu, M.; Liu, Y.; He, J. Natural products targeting Nrf2/ARE signaling pathway in the treatment of inflammatory bowel disease. Biomed. Pharmacother. 2023, 164, 114950. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef] [PubMed]
- Long, M.J.; Lin, H.Y.; Parvez, S.; Zhao, Y.; Poganik, J.R.; Huang, P.; Aye, Y. β-TrCP1 Is a Vacillatory Regulator of Wnt Signaling. Cell Chem. Biol. 2017, 24, 944–957.e7. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Lu, R.; Chang, J.C.; Kan, Y.W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA 1996, 93, 13943–13948. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Huang, J.; Ayansola, H.; Masatoshi, H.; Zhang, B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front. Immunol. 2020, 11, 623691. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Shen, L.; Yu, X.; Zhang, L.; Xu, K.; Xia, Y.; Zha, L.; Wu, J.; Luo, H. The role of Nrf2 in the pathogenesis and treatment of ulcerative colitis. Front. Immunol. 2023, 14, 1200111. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Akagi, R.; Mori, M.; Tsuchiya, T.; Sassa, S. Marked developmental changes in heme oxygenase-1 (HO-1) expression in the mouse placenta: Correlation between HO-1 expression and placental development. Placenta 2004, 25, 387–395. [Google Scholar] [CrossRef]
- Bainbridge, S.A.; Smith, G.N. HO in pregnancy. Free Radic. Biol. Med. 2005, 38, 979–988. [Google Scholar] [CrossRef]
- Bishop, A.; Yet, S.F.; Lee, M.E.; Perrella, M.A.; Demple, B. A key role for heme oxygenase-1 in nitric oxide resistance in murine motor neurons and glia. Biochem. Biophys. Res. Commun. 2004, 325, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, Y.S.; Wang, J.M.; Ding, Y.; Li, Y.; Wu, W. Neurotrophin-3 improves retinoic acid-induced neural differentiation of skin-derived precursors through a p75NTR-dependent signaling pathway. Neurosci. Res. 2009, 64, 170–176. [Google Scholar] [CrossRef]
- Kedinger, M.; Simon-Assmann, P.; Bouziges, F.; Arnold, C.; Alexandre, E.; Haffen, K. Smooth muscle actin expression during rat gut development and induction in fetal skin fibroblastic cells associated with intestinal embryonic epithelium. Differentiation 1990, 43, 87–97. [Google Scholar] [CrossRef]
- Lai, Y.L.; Lin, C.Y.; Jiang, W.C.; Ho, Y.C.; Chen, C.H.; Yet, S.F. Loss of heme oxygenase-1 accelerates mesodermal gene expressions during embryoid body development from mouse embryonic stem cells. Redox Biol. 2018, 15, 51–61. [Google Scholar] [CrossRef]
- Lin, C.Y.; Peng, C.Y.; Huang, T.T.; Wu, M.L.; Lai, Y.L.; Peng, D.H.; Chen, P.F.; Chen, H.F.; Yen, B.L.; Wu, K.K.; et al. Exacerbation of oxidative stress-induced cell death and differentiation in induced pluripotent stem cells lacking heme oxygenase-1. Stem Cells Dev. 2012, 21, 1675–1687. [Google Scholar] [CrossRef]
- Brigham, K.L. Oxidant stress and adult respiratory distress syndrome. Eur. Respir. J. Suppl. 1990, 11, 482s–484s. [Google Scholar] [PubMed]
- Li, M.; Wang, B.; Sun, X.; Tang, Y.; Wei, X.; Ge, B.; Tang, Y.; Deng, Y.; He, C.; Yuan, J.; et al. Upregulation of Intestinal Barrier Function in Mice with DSS-Induced Colitis by a Defined Bacterial Consortium Is Associated with Expansion of IL-17A Producing Gamma Delta T Cells. Front. Immunol. 2017, 8, 824. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, B.; Gao, Y. Tight junction proteins related to blood-brain barrier and their regulatory signaling pathways in ischemic stroke. Biomed. Pharmacother. 2023, 165, 115272. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wu, H.; Wang, X.; He, J.; He, S.; Yin, Y. Resveratrol Attenuates Oxidative Stress-Induced Intestinal Barrier Injury through PI3K/Akt-Mediated Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7591840. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, Z.; Xu, X.; Chao, H.; Lin, C.; Li, Z.; Liu, Y.; Wang, X.; You, Y.; Liu, N.; et al. Extracellular Signal-Regulated Kinase/Nuclear Factor-Erythroid2-like2/Heme Oxygenase-1 Pathway-Mediated Mitophagy Alleviates Traumatic Brain Injury-Induced Intestinal Mucosa Damage and Epithelial Barrier Dysfunction. J. Neurotrauma 2017, 34, 2119–2131. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Xu, Y.; Li, X.; Zhang, J.; Su, Y.; Guo, L. Resveratrol attenuates intestinal epithelial barrier dysfunction via Nrf2/HO-1 pathway in dextran sulfate sodium-induced Caco-2 cells. Immun. Inflamm. Dis. 2024, 12, e1193. [Google Scholar] [CrossRef]
- Yang, J.; Mo, J.; Dai, J.; Ye, C.; Cen, W.; Zheng, X.; Jiang, L.; Ye, L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxid. Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, Y.; Fang, Y.; Djukic, Z.; Yamamoto, M.; Shaheen, N.J.; Orlando, R.C.; Chen, X. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut 2014, 63, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yin, L.; Dong, R. Inhibition of IEC-6 Cell Proliferation and the Mechanism of Ulcerative Colitis in C57BL/6 Mice by Dandelion Root Polysaccharides. Foods 2023, 12, 3800. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhang, Y.; Fan, Y.; Liu, J.; Zhu, K.; Jiang, S.; Duan, J. Lizhong decoction ameliorates ulcerative colitis by inhibiting ferroptosis of enterocytes via the Nrf2/SLC7A11/GPX4 pathway. J. Ethnopharmacol. 2024, 326, 117966. [Google Scholar] [CrossRef]
- Ismail Abo El-Fadl, H.M.; Mohamed, M.F.A. Targeting endoplasmic reticulum stress, Nrf-2/HO-1, and NF-κB by myristicin and its role in attenuation of ulcerative colitis in rats. Life Sci. 2022, 311 Pt B, 121187. [Google Scholar] [CrossRef]
- Puppala, E.R.; Yalamarthi, S.S.; Aochenlar, S.L.; Prasad, N.; Syamprasad, N.P.; Singh, M.; Nanjappan, S.K.; Ravichandiran, V.; Tripathi, D.M.; Gangasani, J.K.; et al. Mesua assamica (King&Prain) kosterm. Bark ethanolic extract attenuates chronic restraint stress aggravated DSS-induced ulcerative colitis in mice via inhibition of NF-κB/STAT3 and activation of HO-1/Nrf2/SIRT1 signaling pathways. J. Ethnopharmacol. 2023, 301, 115765. [Google Scholar]
- Tang, J.; Song, X.; Zhao, M.; Chen, H.; Wang, Y.; Zhao, B.; Yu, S.; Ma, T.; Gao, L. Oral administration of live combined Bacillus subtilis and Enterococcus faecium alleviates colonic oxidative stress and inflammation in osteoarthritic rats by improving fecal microbiome metabolism and enhancing the colonic barrier. Front. Microbiol. 2022, 13, 1005842. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.R.; Lin, C.H.; Lin, W.S.; Pan, M.H. L-Glutamine Substantially Improves 5-Fluorouracil-Induced Intestinal Mucositis by Modulating Gut Microbiota and Maintaining the Integrity of the Gut Barrier in Mice. Mol. Nutr. Food Res. 2024, 68, e2300704. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, P.; Chen, W.; Chen, G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol. Lett. 2020, 225, 9–15. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, F.; Zhou, M.; Guo, X.; Yang, Y.; Qiu, W.; Liao, C.; Chen, Y.; Tang, C. Electroacupuncture Reduces Inflammatory Bowel Disease in Obese Mice by Activating the Nrf2/HO-1 Signaling Pathways and Repairing the Intestinal Barrier. Diabetes Metab. Syndr. Obes. 2024, 17, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Uchiyama, K.; Yoshikawa, T. The role of heme oxygenase and carbon monoxide in inflammatory bowel disease. Redox Rep. 2010, 15, 193–201. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Mizushima, K.; Nukigi, Y.; Okada, H.; Suzuki, T.; Hirata, I.; Omatsu, T.; Okayama, T.; Handa, O.; et al. Increased intestinal expression of heme oxygenase-1 and its localization in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. 2), S229–S233. [Google Scholar] [CrossRef]
- Onyiah, J.C.; Sheikh, S.Z.; Maharshak, N.; Otterbein, L.E.; Plevy, S.E. Heme oxygenase-1 and carbon monoxide regulate intestinal homeostasis and mucosal immune responses to the enteric microbiota. Gut Microbes 2014, 5, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Bataille, F.; Obermeier, F.; Bock, J.; Klebl, F.; Strauch, U.; Lochbaum, D.; Rümmele, P.; Farkas, S.; Schölmerich, J.; et al. Analysis of intestinal haem-oxygenase-1 (HO-1) in clinical and experimental colitis. Clin. Exp. Immunol. 2005, 140, 547–555. [Google Scholar] [CrossRef]
- Wang, W.P.; Guo, X.; Koo, M.W.; Wong, B.C.; Lam, S.K.; Ye, Y.N.; Cho, C.H. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G586–G594. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Osburn, W.O.; Karim, B.; Dolan, P.M.; Liu, G.; Yamamoto, M.; Huso, D.L.; Kensler, T.W. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int. J. Cancer 2007, 121, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, M.; Stadelmaier, J.; Eble, J.; Grummich, K.; Szczerba, E.; Kiesswetter, E.; Schlesinger, S.; Schwingshackl, L. Substitution of animal-based with plant-based foods on cardiometabolic health and all-cause mortality: A systematic review and meta-analysis of prospective studies. BMC Med. 2023, 21, 404. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Youssef, M.E.; Cavalu, S.; Mostafa-Hedeab, G.; Youssef, A.; Elazab, S.T.; Ibrahim, S.; Allam, S.; Elgharabawy, R.M.; El-Ahwany, E.; et al. Carbocisteine as a Modulator of Nrf2/HO-1 and NFκB Interplay in Rats: New Inspiration for the Revival of an Old Drug for Treating Ulcerative Colitis. Front. Pharmacol. 2022, 13, 887233. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xiang, Q.; Ge, W.; Wang, Y.; Xu, F.; Shi, G. Network pharmacology mechanisms and experimental verification of licorice in the treatment of ulcerative colitis. J. Ethnopharmacol. 2024, 324, 117691. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Zhang, Q.; Qin, Y.; Sun, X.; Liu, L.; Liu, H.; Mao, L.; Yan, Y.; Liao, W.; Zha, L.; et al. Sodium Butyrate Inhibits Oxidative Stress and NF-κB/NLRP3 Activation in Dextran Sulfate Sodium Salt-Induced Colitis in Mice with Involvement of the Nrf2 Signaling Pathway and Mitophagy. Dig. Dis. Sci. 2023, 68, 2981–2996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, F.; Li, D.; Wang, C.Z.; Yao, H.; Wan, J.Y.; Yuan, C.S. Emerging insights into inflammatory bowel disease from the intestinal microbiota perspective: A bibliometric analysis. Front. Immunol. 2023, 14, 1264705. [Google Scholar] [CrossRef] [PubMed]
- Koboziev, I.; Reinoso Webb, C.; Furr, K.L.; Grisham, M.B. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic. Biol. Med. 2014, 68, 122–133. [Google Scholar] [CrossRef]
- Zhu, L.; Qiao, L.; Dou, X.; Song, X.; Chang, J.; Zeng, X.; Xu, C. Lactobacillus casei ATCC 393 combined with vasoactive intestinal peptide alleviates dextran sodium sulfate-induced ulcerative colitis in C57BL/6 mice via NF-κB and Nrf2 signaling pathways. Biomed. Pharmacother. 2023, 165, 115033. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, W.; Wang, X.; Guo, F.; Cheng, Y.; Cao, L.; Zhu, W.; Sun, Y.; Xiong, H. Industrially Produced Rice Protein Ameliorates Dextran Sulfate Sodium-Induced Colitis via Protecting the Intestinal Barrier, Mitigating Oxidative Stress, and Regulating Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4952–4965. [Google Scholar] [CrossRef]
- Yang, W.; Huang, Z.; Xiong, H.; Wang, J.; Zhang, H.; Guo, F.; Wang, C.; Sun, Y. Rice Protein Peptides Alleviate Dextran Sulfate Sodium-Induced Colitis via the Keap1-Nrf2 Signaling Pathway and Regulating Gut Microbiota. J. Agric. Food Chem. 2022, 70, 12469–12483. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, X.; Chen, J.; Luo, D.; Xie, J.; Su, Z.; Huang, X.; Yi, X.; Wei, L.; Cai, J.; et al. Protective Effect of Bruguiera gymnorrhiza (L.) Lam. Fruit on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice: Role of Keap1/Nrf2 Pathway and Gut Microbiota. Front. Pharmacol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Wu, T.; Wang, X.; Xiong, H.; Deng, Z.; Peng, X.; Xiao, L.; Jiang, L.; Sun, Y. Bioactives and their metabolites from Tetrastigma hemsleyanum leaves ameliorate DSS-induced colitis via protecting the intestinal barrier, mitigating oxidative stress and regulating the gut microbiota. Food Funct. 2021, 12, 11760–11776. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites 2023, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, S.; Ji, Z.; Meng, J.; Mou, Y.; Wu, X.; Yang, X.; Xiong, P.; Li, M.; Guo, Y. Ferroptosis: An important mechanism of disease mediated by the gut-liver-brain axis. Life Sci. 2024, 347, 122650. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Lu, Y.; Peng, G.; Li, J.; Li, W.; Li, M.; Wang, H.; Liu, L.; Zhao, Q. Furin inhibits epithelial cell injury and alleviates experimental colitis by activating the Nrf2-Gpx4 signaling pathway. Dig. Liver Dis. 2021, 53, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, F.; Zhong, S.; Ding, R.; Su, J.; Li, X. Astaxanthin attenuates ferroptosis via Keap1-Nrf2/HO-1 signaling pathways in LPS-induced acute lung injury. Life Sci. 2022, 311 Pt A, 121091. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Li, J.; Zhu, J.; Wang, R.; Xi, Q.; Wu, H.; Shi, T.; Chen, W. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur. J. Pharmacol. 2021, 911, 174518. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Sriramaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Chen, Z.; Liu, Y.; Pei, C.; Huang, H.; Hou, S.; Ning, W.; Liang, J. Gingerenone A Alleviates Ferroptosis in Secondary Liver Injury in Colitis Mice via Activating Nrf2-Gpx4 Signaling Pathway. J. Agric. Food Chem. 2022, 70, 12525–12534. [Google Scholar] [CrossRef]
- Guo, M.; Du, X.; Wang, X. Inhibition of ferroptosis: A new direction in the treatment of ulcerative colitis by traditional Chinese medicine. J. Ethnopharmacol. 2024, 324, 117787. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Mediators of Intestinal Fibrosis. J. Crohn′s Colitis 2017, 11, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- D’alessio, S.; Ungaro, F.; Noviello, D.; Lovisa, S.; Peyrin-Biroulet, L.; Danese, S. Revisiting fibrosis in inflammatory bowel disease: The gut thickens. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Mukherjee, P.K.; Massey, W.J.; Wang, Y.; Fiocchi, C. Fibrosis in IBD: From pathogenesis to therapeutic targets. Gut 2024, 73, 854–866. [Google Scholar] [CrossRef]
- Giuffrida, P.; Pinzani, M.; Corazza, G.R.; Di Sabatino, A. Biomarkers of intestinal fibrosis—One step towards clinical trials for stricturing inflammatory bowel disease. United Eur. Gastroenterol. J. 2016, 4, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Pellino, G.; Pallante, P.; Selvaggi, F. Novel biomarkers of fibrosis in Crohn’s disease. World J. Gastrointest. Pathophysiol. 2016, 7, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Dong, Y.; Xie, W.; Jin, T.; Xu, D.; Liu, L. Cod (Gadus) skin collagen peptide powder reduces inflammation, restores mucosal barrier function, and inhibits fibrosis in dextran sodium sulfate-induced colitis in mice. J. Ethnopharmacol. 2023, 316, 116728. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Di Sabatino, A. Intestinal fibrosis. Mol. Asp. Med. 2019, 65, 100–109. [Google Scholar] [CrossRef]
- Medina, C.; Santos-Martinez, M.J.; Santana, A.; Paz-Cabrera, M.C.; Johnston, M.J.; Mourelle, M.; Salas, A.; Guarner, F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J. Pathol. 2011, 224, 461–472. [Google Scholar] [CrossRef]
- Biel, C.; Faber, K.N.; Bank, R.A.; Olinga, P. Matrix metalloproteinases in intestinal fibrosis. J. Crohn′s Colitis 2024, 18, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar] [CrossRef]
- Luther, J.; Gala, M.; Patel, S.J.; Dave, M.; Borren, N.; Xavier, R.J.; Ananthakrishnan, A.N. Loss of Response to Anti-Tumor Necrosis Factor Alpha Therapy in Crohn’s Disease Is Not Associated with Emergence of Novel Inflammatory Pathways. Dig. Dis. Sci. 2018, 63, 738–745. [Google Scholar] [CrossRef]
- Barberio, B.; D’incà, R.; Facchin, S.; Dalla Gasperina, M.; Fohom Tagne, C.A.; Cardin, R.; Ghisa, M.; Lorenzon, G.; Marinelli, C.; Savarino, E.V.; et al. Matrix Metalloproteinase 3 Predicts Therapeutic Response in Inflammatory Bowel Disease Patients Treated with Infliximab. Inflamm. Bowel Dis. 2020, 26, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Kashima, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Inaba, Y.; Moriichi, K.; Tanabe, H.; Ikuta, K.; Ohtake, T.; Kohgo, Y. Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis. Transl. Res. 2015, 166, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Latella, G. Redox Imbalance in Intestinal Fibrosis: Beware of the TGFβ-1, ROS, and Nrf2 Connection. Dig. Dis. Sci. 2018, 63, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Sferra, R.; Gaudio, E.; Viscido, A.; Frieri, G.; Vetuschi, A.; Latella, G. Can Nrf2 Modulate the Development of Intestinal Fibrosis and Cancer in Inflammatory Bowel Disease? Int. J. Mol. Sci. 2019, 20, 4061. [Google Scholar] [CrossRef]
- Guan, Y.; Tan, Y.; Liu, W.; Yang, J.; Wang, D.; Pan, D.; Sun, Y.; Zheng, C. NF-E2-Related Factor 2 Suppresses Intestinal Fibrosis by Inhibiting Reactive Oxygen Species-Dependent TGF-β1/SMADs Pathway. Dig. Dis. Sci. 2018, 63, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, D.; Wang, H.; Wang, T.; Weng, Y.; Zhang, Y.; Luo, Y.; Lu, Y.; Wang, Y. Therapeutic Targeting of Nrf2 Signaling by Maggot Extracts Ameliorates Inflammation-Associated Intestinal Fibrosis in Chronic DSS-Induced Colitis. Front. Immunol. 2021, 12, 670159. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Yang, H.; Chen, S.; Zheng, D.; Liu, X.; Jiang, Q.; Chen, Y. Metformin Ameliorates Chronic Colitis-Related Intestinal Fibrosis via Inhibiting TGF-β1/Smad3 Signaling. Front. Pharmacol. 2022, 13, 887497. [Google Scholar] [CrossRef]
- Laudadio, I.; Bastianelli, A.; Fulci, V.; Carissimi, C.; Colantoni, E.; Palone, F.; Vitali, R.; Lorefice, E.; Cucchiara, S.; Negroni, A.; et al. ZNF281 Promotes Colon Fibroblast Activation in TGFβ1-Induced Gut Fibrosis. Int. J. Mol. Sci. 2022, 23, 10261. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Swierczynski, M.; Kasprzak, Z.; Makaro, A.; Salaga, M. Regulators of G-Protein Signaling (RGS) in Sporadic and Colitis-Associated Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 577. [Google Scholar] [CrossRef]
- Bye, W.A.; Ma, C.; Nguyen, T.M.; Parker, C.E.; Jairath, V.; East, J.E. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2018, 113, 1801–1809. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Khare, V.; Paul, G.; Lang, M.; Ferk, F.; Knasmüller, S.; Beer, A.; Oberhuber, G.; Gasche, C. Overt Increase of Oxidative Stress and DNA Damage in Murine and Human Colitis and Colitis-Associated Neoplasia. Mol. Cancer Res. MCR 2018, 16, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Kim, K.C.; Kang, H.K.; Chang, W.Y.; Park, I.C.; Keum, Y.S.; Surh, Y.J.; Hyun, J.W. Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: Involvement of TET-dependent DNA demethylation. Cell Death Dis. 2014, 5, e1183. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhang, Y.F.; Xia, Y.F.; Zhou, Z.M.; Cao, Y.Q. Promoter demethylation of nuclear factor-erythroid 2-related factor 2 gene in drug-resistant colon cancer cells. Oncol. Lett. 2015, 10, 1287–1292. [Google Scholar] [CrossRef]
- Polimeno, L.; Viggiani, M.T.; Giorgio, F.; Polimeno, L.; Fratantonio, D.; Di Domenico, M.; Boccellino, M.; Ballini, A.; Topi, S.; Di Leo, A.; et al. Possible role of nuclear factor erythroid 2-related factor 2 in the progression of human colon precancerous lesions. Dig. Liver Dis. 2022, 54, 1716–1720. [Google Scholar] [CrossRef]
- Gobert, A.P.; Asim, M.; Smith, T.M.; Williams, K.J.; Barry, D.P.; Allaman, M.M.; Mcnamara, K.M.; Hawkins, C.V.; Delgado, A.G.; Zhao, S.; et al. Electrophilic reactive aldehydes as a therapeutic target in colorectal cancer prevention and treatment. Oncogene 2023, 42, 1685–1691. [Google Scholar] [CrossRef]
- Furfaro, A.L.; Traverso, N.; Domenicotti, C.; Piras, S.; Moretta, L.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. The Nrf2/HO-1 Axis in Cancer Cell Growth and Chemoresistance. Oxid. Med. Cell. Longev. 2016, 2016, 1958174. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, M.; Aslan, A.; Tuzcu, Z.; Yabas, M.; Bahcecioglu, I.H.; Ozercan, I.H.; Kucuk, O.; Sahin, K. Tomato powder impedes the development of azoxymethane-induced colorectal cancer in rats through suppression of COX-2 expression via NF-κB and regulating Nrf2/HO-1 pathway. Mol. Nutr. Food Res. 2012, 56, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Hong, E.M.; Kim, M.; Kim, J.H.; Jang, J.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; et al. Simvastatin induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2) activation through ERK and PI3K/Akt pathway in colon cancer. Oncotarget 2016, 7, 46219–46229. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Lim, J.H.; Nam, S.I.; Park, J.W.; Kwon, T.K. Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through reactive oxygen species (ROS) dependent and PKC delta-independent pathway in human colon cancer HT29 cells. Biochimie 2010, 92, 110–115. [Google Scholar] [CrossRef]
- Dong, M.; Liu, H.; Cao, T.; Li, L.; Sun, Z.; Qiu, Y.; Wang, D. Huoxiang Zhengqi alleviates azoxymethane/dextran sulfate sodium-induced colitis-associated cancer by regulating Nrf2/NF-κB/NLRP3 signaling. Front. Pharmacol. 2022, 13, 1002269. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; He, C.N.; Li, X.X.; Zhou, L.Y.; Liu, R.J.; Zhang, S.; Li, G.Q.; Chen, Z.C.; Zhang, P.F. Ginnalin A from Kujin tea (Acer tataricum subsp. ginnala) exhibits a colorectal cancer chemoprevention effect via activation of the Nrf2/HO-1 signaling pathway. Food Funct. 2018, 9, 2809–2819. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, D.; Yang, Y.T.; Li, X.Y.; Li, H.N.; Zhang, X.P.; Long, J.Y.; Lu, Y.Q.; Liu, L.; Yang, G.; et al. Suppression of microRNA-222-3p ameliorates ulcerative colitis and colitis-associated colorectal cancer to protect against oxidative stress via targeting BRG1 to activate Nrf2/HO-1 signaling pathway. Front. Immunol. 2023, 14, 1089809. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.P.; Jena, G.B.; Tikoo, K.B.; Kumar, V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol. Carcinog. 2016, 55, 255–267. [Google Scholar] [CrossRef]
- Liang, J.; Yang, C.; Li, P.; Zhang, M.; Xie, X.; Xie, X.; Chen, Y.; Wang, Q.; Zhou, L.; Luo, X. Astragaloside IV inhibits AOM/DSS-induced colitis-associated tumorigenesis via activation of PPARγ signaling in mice. Phytomedicine 2023, 121, 155116. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.; Oh, J.; Kim, J.S. Glyceollins Modulate Tumor Development and Growth in a Mouse Xenograft Model of Human Colon Cancer in a p53-Dependent Manner. J. Med. Food 2019, 22, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Goulart, A.; Ferreira, C.; Rodrigues, A.; Coimbra, B.; Sousa, N.; Leão, P. The correlation between serum vascular endothelial growth factor (VEGF) and tumor VEGF receptor 3 in colorectal cancer. Ann. Surg. Treat. Res. 2019, 97, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, J.; Ma, D.; Li, D.; Sun, Y. Angiogenesis in primary colorectal cancer and matched metastatic tissues: Biological and clinical implications for anti-angiogenic therapies. Oncol. Lett. 2020, 19, 3558–3566. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Hur, E.G.; Kang, S.J.; Kim, J.A.; Thapa, D.; Lee, Y.M.; Ku, S.K.; Jung, Y.; Kwak, M.K. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Cancer Res. 2011, 71, 2260–2275. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Mimura, K.; Nakajima, S.; Okayama, H.; Saito, K.; Nakajima, T.; Kikuchi, T.; Onozawa, H.; Fujita, S.; Sakamoto, W.; et al. M2 tumor-associated macrophages resist to oxidative stress through heme oxygenase-1 in the colorectal cancer tumor microenvironment. Cancer Immunol. Immunother. CII 2023, 72, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Tasaki, Y.; Shirai, S.; Hisatomi, H. Upregulation of nuclear factor (erythroid-derived 2)-like 2 protein level in the human colorectal adenocarcinoma cell line DLD-1 by a heterocyclic organobismuth(III) compound: Effect of organobismuth(III) compound on NRF2 signaling. Biomed. Pharmacother. 2020, 125, 109928. [Google Scholar] [CrossRef]
- Fang, R.; Wu, R.; Zuo, Q.; Yin, R.; Zhang, C.; Wang, C.; Guo, Y.; Yang, A.Y.; Li, W.; Lin, L.; et al. Sophora flavescens Containing-QYJD Formula Activates Nrf2 Anti-Oxidant Response, Blocks Cellular Transformation and Protects against DSS-Induced Colitis in Mouse Model. Am. J. Chin. Med. 2018, 46, 1609–1623. [Google Scholar] [CrossRef]
- Kabel, A.M.; Ashour, A.M.; Ali, D.A.; Arab, H.H. The immunomodulatory effects of topiramate on azoxymethane-induced colon carcinogenesis in rats: The role of the inflammatory cascade, vascular endothelial growth factor, AKT/mTOR/MAP kinase signaling and the apoptotic markers. Int. Immunopharmacol. 2021, 98, 107830. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Forbes-Hernández, T.Y.; Gasparrini, M.; Amici, A.; Cianciosi, D.; Quiles, J.L.; Battino, M. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic. Biol. Med. 2018, 126, 41–54. [Google Scholar] [CrossRef]
- Waghela, B.N.; Vaidya, F.U.; Pathak, C. Upregulation of NOX-2 and Nrf-2 Promotes 5-Fluorouracil Resistance of Human Colon Carcinoma (HCT-116) Cells. Biochem. Biokhimiia 2021, 86, 262–274. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updates 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, L.; Chen, Y.; Zheng, X.; Wang, R.; Liu, B.; Zhang, S.; Wang, H. Quercetin reverses 5-fluorouracil resistance in colon cancer cells by modulating the NRF2/HO-1 pathway. Eur. J. Histochem. EJH 2023, 67, 3719. [Google Scholar] [CrossRef]
- Luo, P.; Wu, S.; Ji, K.; Yuan, X.; Li, H.; Chen, J.; Tian, Y.; Qiu, Y.; Zhong, X. LncRNA MIR4435-2HG mediates cisplatin resistance in HCT116 cells by regulating Nrf2 and HO-1. PLoS ONE 2020, 15, e0223035. [Google Scholar] [CrossRef]
- Chian, S.; Li, Y.Y.; Wang, X.J.; Tang, X.W. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X.; Zhang, R.; Liu, S.; Xiang, Y.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; Feng, J.; et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics 2020, 10, 5107–5119. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Chen, C.; Sui, X.; Yang, J.; Wang, L.; Zhou, J. The crosstalk between autophagy and ferroptosis: What can we learn to target drug resistance in cancer? Cancer Biol. Med. 2019, 16, 630–646. [Google Scholar] [CrossRef]
- Wei, R.; Zhao, Y.; Wang, J.; Yang, X.; Li, S.; Wang, Y.; Yang, X.; Fei, J.; Hao, X.; Zhao, Y.; et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int. J. Biol. Sci. 2021, 17, 2703–2717. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Z.; Lin, W.; Wu, Q.; Wu, Y.; Hong, Y.; Tong, H.; Wang, C.; Zhang, Y. Esculin induces endoplasmic reticulum stress and drives apoptosis and ferroptosis in colorectal cancer via PERK regulating eIF2α/CHOP and Nrf2/HO-1 cascades. J. Ethnopharmacol. 2024, 328, 118139. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, C.; Zhao, Y.; Zhang, X.; Chen, W.; Zhou, Q.; Hu, B.; Gao, D.; Raatz, L.; Wang, Z.; et al. Quiescin sulfhydryl oxidase 1 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by driving EGFR endosomal trafficking and inhibiting NRF2 activation. Redox Biol. 2021, 41, 101942. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pi, D.; Zhou, S.; Yi, Z.; Dong, Y.; Wang, W.; Ye, H.; Chen, Y.; Zuo, Q.; Ouyang, M. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim. Biophys. Sin. 2023, 55, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Sai, X.; Gai, L.; Huang, G.; Chen, X.; Tu, X.; Ding, Z. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: A HuGE review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wen, D.; Shen, L.; Feng, Y.; Xiong, Q.; Li, P.; Zhao, Z. Cepharanthine Exerts Antioxidant and Anti-Inflammatory Effects in Lipopolysaccharide (LPS)-Induced Macrophages and DSS-Induced Colitis Mice. Molecules 2023, 28, 6070. [Google Scholar] [CrossRef]

| Categories | Natural Product | Experiment Models | Effective Dose | Associated Phenotypes | Potential Mechanism | PMID |
|---|---|---|---|---|---|---|
| Single drug | Licorice | DSS induced in mouse model | 160 mg/kg | IL-1β↓, IL-6↓, IL-17↓, TNF-α↓, SOD↑, GPX↑, MDA↓, Nrf2↑, PINK1↑, Parkin↑, HO-1↑, P62↑, LC3↑ | Nrf2/PINK1 pathway | 36705402 |
| Licorice | DSS induced in mouse model | 160 mg/kg | MDA↓, IL-1β↓, IL-6↓, TNF-α↓, SOD↑, GSH-PX↑, IL-10↑, Nrf2↑, PINK1↑, Parkin↑, HO-1↑, ZO-1↑, Occludin↑, P62↑ | Nrf2/PINK1 signaling pathway | 38176667 | |
| Crocus sativus | TNBS and DSS induced in mouse model | 20 mg/kg | Colon length↑, HO-1↑, GPX-2↑, TNF-α↓, IFN-γ↓ | Ahr/Nrf2 pathway | 34275090 | |
| Tetrastigma hemsleyanum leaves | DSS induced in mouse model | - | Colon length↑, ZO-1↑, Occludin↑, Claudin-1↑, IL-1β↓, IL-6↓, TNF-α↓, SOD↑, CAT↑, HO-1↑, NQO1↑, GCLC↑, MPO↓, MDA↓, Nrf2↑ | Keap1/Nrf2 signaling pathway | 34747421 | |
| Ficus pandurate Hance | DSS induced in mouse model | 480 mg/kg | MPO↓, ZO-1↑, Occludin↑, SOD↑, GSH-Px↑, NRF2↑, HO-1↑, NQO1↑, MDA↓, Keap1↓, NOX2↓ | Nrf2 pathway | 34966476 | |
| Polyphenols | Paeoniflorin | LPS induced in Caco-2 cell | 100 μm | COX-2↓, INOS↓, TNF-α↓, IL-6↓, Occludin↑, ZO-1↑, Claudin-5↑ | Nrf2/HO-1 signaling pathway | 31473900 |
| Puerarin | DSS induced in mouse model | 50 mg/kg | MPO↓, TNF-α↓, IL-1β↓, IL-6↓, IFN-r↓, Nrf2↑, HO-1↑, NQO1↑, MDA↓, CAT↑, GSH↑, SOD↑, ZO-1↑, Occludin↑, Claudin-1↑, COX-2↓, INOS↓ | Nrf2 pathway | 31981944 | |
| Honokiol | DSS induced in mouse model | 40 mg/kg | IL-1β↓, IL-6↓, TNF-α↓, INOS↓, COX-2↓, ZO-1↑, Occludin↑, Claudin-1↑ | Nrf2/HO-1 signaling pathway | 36578522 | |
| Myristicin | AA induced in rat model | 150 mg/kg | MPO↓, TNF-α↓, IL-1β↓, COX-2↓, IL-10↑, Nrf2↑, HO-1↑ | Nrf2/HO-1 signaling pathway | 36403646 | |
| Dieckol | DSS induced in mouse model | 15 mg/kg | TNF-α↓, IL-1β↓, MDA↓, MPO↓, Nrf2↑, HO-1↑, Colon length↑ | Nrf2/HO-1 signaling pathway | 33331035 | |
| Resveratrol | DSS induced in mouse model | 100 mg/kg | IL-6↓, IL-1β↓, TNF-α↓, IL-10↑, ZO-1↑, Occludin↑, Nrf2↑, HO-1↑ | Nrf2/HO-1 pathway | 38488031 | |
| coumaric acid and syringic acid | AA induced in rat model | 150 mg/kg, 50 mg/kg | TNF-α↓, IL-1β↓, HO-1↑, Nrf2↑, NQO1↑ | Nrf2/HO-1 pathway | 37182450 | |
| Luteolin | DSS induced in mouse model | 50 mg/kg | Colon length↑, INOS↓, TNF-α↓, IL-6↓, MDA↓, SOD↑, CAT↑, Nrf2↑, HO-1↑, NQO1↑ | Nrf2/HO-1 pathway | 27569028 | |
| Rosmarinic Acid-Loaded Nanovesicles | DSS induced in mouse model | 20 mg/kg | MPO↓, TNF-α↓, IL-1β↓, NLRP3↓, ASC↓, caspase-1↓, Nrf2↑, HO-1↑ | NLRP3 pathway | 33530569 | |
| Synthetic Imine Resveratrol Analog 2-Methoxyl-3,6-Dihydroxyl-IRA | DSS induced in mouse model | 200 mg/kg | Colon length↑, IL-6↓, TNF-α↓, HO-1↑, NLRP3↑ | Nrf2 pathway | 31885813 | |
| Oligonol | DSS induced in mouse model | 100 mg/kg | Colon length↑, IL-1β↓, IL-6↓, TNF-α↓, HO-1↑, NQO-1↑ | Nrf2 pathway | 30149369 | |
| Licochalcone A | DSS induced in mouse model | 80 mg/kg | Colon length↑, MPO↓, TNF-α↓, IL-1β↓, IL-6↓, COX-2↓, GSH↑, SOD↑, NO↓, Nrf2↑, HO-1↑, GCL↑, Keap-1↓ | Nrf2 pathway | 29710547 | |
| Cyanidin-3-glucoside and resveratrol | Cytokine induced in HT-29 intestinal cells | 25 μM | Nrf2↑, HO-1↑, GSH/GSSG↑ | Nrf2 pathway | 27818126 | |
| Carnosic acid | DSS induced in mouse model | 100 mg/kg | Colon length↑, TNF-α↓, IL-6↓, IFN-γ↓, IL-1β↓, IL-18↓, HO-1↑, GPX2↑, SOD↑, Nrf2↑, GSH↑, SOD↑, MDA↓, INOS↓ | Keap1/Nrf2 pathway | 28887507 | |
| Sesamin | DSS induced in mouse model | 100 mg/kg | Colon length↑, IL-6↓, IL-1β↓, TNF-α↓, Nrf2↑, GSH↑, SOD↑, MDA↓, HO-1↑ | Nrf2 pathway | 31534619 | |
| Polysaccharide | Astragalus polysaccharide | DSS induced in mouse model | 300 mg/kg | Histological damage↓, IFN-γ↓, IL-6↓, TNF-α↓, IL-1β↓, MDA↓, GSH↑ | Nrf2/HO-1 pathway | 34562468 |
| Dandelion polysaccharide | DSS induced in mouse model | 300 mg/kg | Nrf2↑, HO-1↑, NQO-1↑, GSH↑, MDA↓, MPO↓, IL-1β↓, IL-6↓, TNF-α↓, INOS↓, Occludin↑, ZO-1↑ | Nrf2 pathway | 37893693 | |
| Polysaccharides from garlic | DSS induced in mouse model | 300 mg/kg | Colon length↑, INOS↓, COX2↓, ZO-1↑, Occludin↑, MUC2↑, IL-1β↓, IL18↓, MDA↓, Keap-1↓, GPX4↑, SOD↑, HO-1↑, NQO1↑, Nrf2↑ | Nrf2 pathway | 36017235 | |
| Aloe polysaccharides | DSS induced in mouse model | 100 mg/kg | Colon length↑, GSH↑, CAT↑,SOD↑, MPO↓, IFN-γ↓, MDA↓, TNF-α↓, IFN-γ↓, IL-1β↓, IL-6↓, IL-8↓, IL-17↓, ZO-1↑, Occludin↑, Nrf2↑, HO-I↑, NQO-1↑, IL-10↑, ZO-1↑, Claudin-1↑ | Nrf2/HO-1 signaling pathway | 34229016 | |
| Dandelion polysaccharide | DSS induced in mouse model | 300 mg/kg | IL-1β↓, IL-6↓, TNF-α↓, INOS↓, ZO-1↑, Occludin↑, MDA↓, MPO↓ | Nrf2 pathway | 37893693 | |
| Fucoidan | AA induced in rat model | 150 mg/kg | Colon length↑, IL-22↑, Nrf2↑, HO-1↑ | Nrf2 pathway | 35870906 | |
| Terpenoids | Geniposide | DSS induced in mouse model | 60 mg/kg | Colon length↑, Histopathologic scores↓, ZO-1↑, Occludin↑, IL-1β↓, IL-6↓, TNF-α↓, MDA↓, NQO1↑, HO-1↑ | Nrf2/ARE signaling pathway | 37187359 |
| Melianodiol | DSS induced in mouse model | 200 mg/kg | IL-10↑, IL-1β↓, TNF-α↓, MDA↓, NO↓, GSH↑, SOD↑, Nrf2↑, Keap-1↓, HO-1↑ | Nrf2 signaling pathway | 36312760 | |
| Ruscogenin | TNBS induced in mouse model | 2 mg/mouse | TNF-α↓, IFN-γ↓, Nrf2↑, NQO1↑ | Nrf2/HO-1 signaling pathway | 35308175 | |
| Crocin | AA induced in rat model | 20 mg/kg | Weight/length index↓, SOD↑, GSH↑, TAC↑, CAT↑, MDA↓, TNF-α↓, Nrf2↑, HO-1↑ | Nrf2/HO-1 signaling pathway | 30530041 | |
| Ginsenoside Rg1 | DSS induced in mouse model | 200 mg/kg | Colon length↑, IL-1β↓, IL-6↓, TNF-α↓, SOD↑, MDA↓, MPO↓ | Nrf2/HO-1/NF-kB pathway | 38215064 | |
| Triptolide | DSS induced in mouse model | 0.02 mg/kg | Colon length↑, Claudin-1↑, Occludin↑, IL-1β↓, IL-6↓, ROS↓ | NRF2/HO-1 signaling pathway | 33240279 | |
| Masticadienonic acid | DSS induced in mouse model | 100 mg/kg | TNF-α↓, IL-1β↓, IL-6↓, MAPK↓, NF-κB↓, ZO-1↑, Occludin↑ | Nrf2/HO-1 and MAPK/NF-κB signaling pathway | 36403513 | |
| Geniposide | DSS induced in mouse model | 40 mg/kg | IL-6↓, IL-1β↓, TNF-α↓, MDA↓, SOD↑, MPO↓, Nrf2↑, HO-1↑, p-NF-κBp65↓, p-IκBα↓ | Nrf2/HO-1/NF-κB pathway | 32787366 | |
| Huzhangoside C | DSS induced in mouse model | - | Colon length↑, INOS↓, MDA↓, NO↓, Nrf2↑, SOD↑, GSH↑ | Nrf2 pathway | 38156815 | |
| Nerolidol | DSS induced in mouse model | 150 mg/kg | MPO↓, IL-6↓, IL-1β↓, TNF-α↓, COX-2↓, INOS↓, Keap-1↓, Nrf2↑, SOD↑, HO-1↑ | Nrf2 pathway | 32650602 | |
| Tussilagone | DSS induced in mouse model | 2.5 mg/kg | Colon length↑, MPO↓, INOS↓, COX-2↓, TNF-α↓, IL-6↓, Nrf2↑, HO-1↑ | Nrf2 pathway | 30142311 | |
| Asperuloside | DSS induced in mouse model | 500 μg/kg | Colon length↑, Histopathological score↓, MPO↓, SOD↑, GSH-Px↑, MDA↓, Nrf2↑, HO-1↑, NQO1↑, TNF-α↓, IL-6↓, IL-10↑ | Nrf2/HO-1 pathway | 33974900 | |
| D-Pinitol | DSS induced in mouse model | 40 mg/kg | Colon length↑, HO-1↑, NQO1↑, GSH↑, SOD↑, CAT↑, MPO↓, MDA↓, TNF-α↓, IFN-γ↓, IL-6↓, IL-1β↓, INOS↓, COX-2↓, IL-10↑ | Nrf2/ARE pathway | 33625409 | |
| Sericic acid | DSS induced in mouse model | 50 mg/kg | Colon length↑, NO↓, TNF-α↓, IL-6↓, IL-1β↓, MDA↓, SOD↑, Nrf2↑, HO-1↑ | Nrf2 pathway | 36173058 | |
| Loganic acid | DSS induced in mouse model | 30 mg/kg | Colon length↑, MDA↓, NO↓, MPO↓, GSH↑, IL-1β↓, IL-6↓, TNF-α↓, TLR4↓, NF-κB↓, MPO↓, IFN-γ↓, Nrf2↑, HO-1↑, SOD↑ | TLR4/NF-κB and SIRT1/Nrf2 pathway | 37421777 | |
| Toosendanin | DSS induced in mouse model | 1 mg/kg | Colon length↑, MPO↓, TNF-α↓, IL-1β↓, IL-6↓, SOD↑, GSH↑, MDA↓, Nrf2↑, HO-1↑, ZO-1↑, Occludin↑ | Nrf2/HO-1 pathway | 31520988 | |
| RH-F/C-NPs | DSS induced in mouse model | 25 mg/kg | Colon length↑, MPO↓, TNF-α↓, IL-1β↓, IL-6↓, TLR4↓, NF-κB↓, GSH-PX↑, SOD↑, MDA↓, Nrf2↑, HO-1↑, ZO-1↑, Claudin-1↑, Occludin↑ | TLR4/NF-κB and Nrf2/HO-1 signaling pathway | 36326017 | |
| American ginseng, panaxynol, hexane fraction | DSS induced in mouse model | 75 mg/kg, 1 mg/kg, 75 mg/kg | COX2↓, HO-1↑ | Nrf2 pathway | 32575883 | |
| Alkaloid | Oxyberberine | TNBS induced in rat model | 50 mg/kg | IL-4↑,TNF-α↓, IL-2↓, IL-8↓,IL-22↓, HO-1↑, GCLM↑, GCLC↑, NQO-1↑, SOD↑, GSH↑, MDA↓, ROS↓, CAT↑, Keap1↓, Nrf2↑ | Keap1/Nrf2/NF-κB pathway | 37247589 |
| Oleracein E | TNBS induced in rat model | 20 mg/kg | IL-6↓, IL-1β↓, TNF-α↓, CAT↑, MPO↓, ROS↓, ZO-1↑, Occludin↑, Claudin-2↑, Nrf2↑, HO-1↑ | Nrf2/HO-1 pathway | 37395238 | |
| Berberine | AA induced in rat model | 50 mg/kg | IL-1β↓, IL-6↓, TNF-α↓, MPO↓, GSH↑, SOD↑, CAT↑, GPx↑, MDA↓, NO↓, Nrf2↑, HO-1↑ | Nrf2/HO-1 pathway | 33061833 | |
| Sinomenine | DSS induced in rat model | 40 mg/kg | Colon length↑, NO↓, MPO↓, SOD↑, CAT↑, GPx↑, MDA↓, TNF-α↓, IL-1β↓, IL-6↓, IL-10↑, HO-1↑, Nrf2↑, COX-2↓, INOS↓ | HO-1/Nrf2 pathway | 38573363 | |
| Corynoline | DSS induced in mouse model | 30 mg/kg | Colon length↑, Histological scores↓, MPO↓, IL-1β↓, IL-6↓, TNF-α↓, SOD↑, CAT↑, ROS↓, Nrf2↑, HO-1↑, IκBα↓, NF-κB p65↓ | Nrf2/HO-1/NF-κB pathway | 35980837 | |
| 8-Oxypalmatine | DSS induced in mouse model | 50 mg/kg | Colon length↑, Histological scores↓, MPO↓, TNF-α↓, IL-1β↓, IFN-γ↓, IL-6↓, SOD↑, GSH↑, CAT↑, GSH-Px↑, MDA↓, NLRP3↓, Nrf2↑, HO-1↑ | Nrf2 signaling pathway | 35779424 | |
| Leonurine | DSS induced in mouse model | 30 mg/kg | Colon length↑, TNF-α↓, IL-6↓, IL-1β↓, MDA↓, ROS↓, SOD↑, GSH↑, TLR4↓, p-NF-κB↓, Nrf2↑, HO-1↑ | Nrf2/HO-1 and TLR4/NF-κB pathway | 35253649 | |
| Sanguinarine | NSAIDs-induced small intestinal inflammation in rat model | 3.3 mg/kg | TDI↓, CMDI↓, LDH↓, ZO-1↑, TNF-α↓, IL-6↓, IL-1β↓, SOD↑, MDA↓, Nrf2↑, HO-1↑, Keap-1↓, P-p65↓ | Nrf2/NF-κB pathways | 36304153 | |
| Flavonoid | Galangin | DSS induced in mouse model | 40 mg/kg | TNF-α↓, IL-6↓, IL-10↑, MPO↓, SOD↑, COX-2↓, INOS↓, Nrf2↑, HO-1↑, GST↑, GSH↑, SOD↑ | Nrf2 pathway | 31147743 |
| Cardamonin | TNBS and DSS induced in mouse model | 60 mg/kg | Colon length↑, Nrf2↑, NQO1↑, Trx1↑, SOD↑, HO-1↑, MPO↓, IL-1β↓, TNF-α↓, IL-6↓ | AhR/Nrf2/NQO1 pathway | 30071202 | |
| Flavonoid isoliquiritigenin | TNF-α-induced HT-29 cells | 20 uM | Nrf2↑, HO-1↑, NQO1↑, IL-8↓, IL-1β↓, COX-2↓ | Nrf2 pathway | 28012970 | |
| LL202 | TNBS induced in mouse model | 30 mg/kg | IL-1β↓, IL-6↓, TNF-α↓, SOD↑, GSH↑, TAC↑, MDA↓, Nrf2↑, HO-1↑ | Nrf2/HO-1 pathway | 30426485 | |
| Alpinetin | DSS induced in mouse model | 100 mg/kg | Colon length↑, MPO↓, Occludin↑, ZO-1↑, Claudin-2↑, MDA↓, SOD↑, Nrf2↑, HO-1↑ | Nrf2/HO-1 signaling pathway | 29661352 | |
| Diosmetin (3′,5,7-trihydroxy-4′-methoxy flavone) | DSS induced in mouse model | 50 mg/kg | Occludin↑, Claudin-1↑, ZO-1↑, IL-1β↓, IL-6↓, TNF-α↓, COX-2↓, GSH-Px↑, SOD↑, MDA↓, GSH↑, ROS↓, Nrf2↑, HO-1↑ | Nrf2 pathway | 34262136 | |
| Diosmin | DSS induced in mouse model | 200 mg/kg | Colon length↑, GSH↑, CAT↑, Nrf2↑, HO-1↑, MDA↓, NO↓, Mucin-2↑, ZO-1↑, Histopathological score↓, NF-κB↓, TNF-α↓, IL-6↓, Claudin-1↑, Occludin↑ | NF-κB/Nrf2 pathway | 38500992 | |
| Quercetin nanoparticles | DSS induced in mouse model | 20 mg/kg | GSH-Px↑, SOD↑, CAT↑, ROS↓, NO↓, MDA↓, IL-6↓, TNF-α↓, IFN-γ↓, IL-10↑, MUC-2↑, Occludin↑, Nrf2↑, HO-1↑, INOS↓, COX2↓ | Nrf2 pathway | 35884960 | |
| Hyperoside | DSS induced in mouse model | 120 mg/kg | Colon length↑, MDA↓, TNF-α↓, IL-6↓, COX-2↓, IL-10↑, MDA↓, Nrf2↑, HO-1↑, SOD↑ | Nrf2 pathway | 29162986 | |
| Sulforaphane | AA induced in rat model | 15 mg/kg | Colon length↑, Nrf2↑, HO-1↑, NO↓, MDA↓, GSH-Px↑, GSH↑ | Nrf2 pathway | 35754320 | |
| Genistein | AA induced in rat model | 25 mg/kg | Nrf2↑, HO-1↑, Caspase-3↑ | Nrf2/HO-1 pathway | 37084167 | |
| Diclofenac and eugenol hybrid | DSS induced in mouse model | 40 mg/kg | Colon length↑, p-NF-κB↓, NF-κB↓, HO-1↑, ROS↓, Nrf2↑, INOS↓ | Nrf2/HO-1 and INOS/NF-κB signaling pathway | 37517204 | |
| Coumarins | Imperatorin | TNBS induced in rat model | 60 mg/kg | TNF-α↓, IL-6↓., Nrf2↑, ARE↑, HO-1↑ | Nrf2/ARE/HO-1 pathway | 33098052 |
| Isofraxidin | DSS induced in mouse model | 80 mg/kg | Colon length↑, IL-8↓, TNF-α↓, MPO↓, ZO-1↑, IL-1β↓, IL18↓, ROS↓, Nrf2↑, HO-1↑, MDA↓, SOD↑ | Nrf2 pathway | 38280336 |
| Natural Extract | Experiment Models | Effective Dose | Associated Phenotypes | Potential Mechanism | PMID | |
|---|---|---|---|---|---|---|
| Aucklandia lappa Decne extract | DSS induced in mouse model | 182 mg/kg | Colon length↑, TNF-α↓, IL-6↓, IL-1β↓ | Nrf2-HO-1 signaling pathway | 35623504 | |
| Lotus Leaf Extract | LPS induced in mouse model | 200 mg/kg | ZO-1↑, Occludin↑, Claudin-1↑, IL-1β↓, IL-6↓, TNF-α↓, Nrf2↑, HO-1↑ | Nrf2/HO-1 signaling pathway | 38526570 | |
| Grape seed proanthocyanidin extract | DSS induced in mouse model | - | IL-1β↓, IL-6↓, TNF-α↓, NO↓, MDA↓, SOD↑, NF-κB↓, Keap-1↓, Nrf2↑, HO-1↑ | NF-κB and Nrf2 pathway | 35692132 | |
| Green pea hull | DSS induced in mouse model | 600 mg/kg | Colon length↑, MPO↓, Claudin-1↑, Occludin↑, ZO-1↑, MDA↓, SOD↑, CAT↑, TNF-α↓, IL-1β↓, IL-6↓, IL-10↑, Keap1↓, Nrf2↑, GCLC↑, HO-1↑, NQO1↑ | Keap1-Nrf2-ARE signaling pathway | 34829046 | |
| Moringa seed extract | DSS induced in mouse model | 150 mg/kg | Colon length↑, TNF-α↓, NO↓, MPO↓, IL-1β↓, IL-6↓, TNF-α↓, INOS↓, Claudin-1↑, ZO-1↑, NQO1↑, HO-1↑ | Nrf2 Pathway | 28922365 | |
| Extract of Rhus chinensis Mill. fruits | DSS induced in mouse model | 600 mg/kg | Colon length↑, MDA↓, MPO↓, TNF-α↓, IL-1β↓, IL-6↓, SOD↑, GSH↑, Occludin↑, ZO-1↑, Claudin-1↑, Nrf2↑, NQO1↑, HO-1↑, COX-2↓, INOS↓, | Nrf2 pathway | 34494061 | |
| Bruguiera gymnorrhiza fruit | DSS induced in mouse model | 100 mg/kg | Colon length↑, MDA↓, TNF-α↓, IL-6↓, IL-1β↓, IFN-γ↓, IL-10↑, SOD↑, GSH↑, Nrf2↑, HO-1↑, NQO1↑, Keap1↓ | Keap1/Nrf2 pathway | 32116661 | |
| ROS extract | DSS induced in mouse model | 500 mg/kg | Colon length↑, INOS↓, MPO↓, MDA↓, p-NF-κB↓, p-IKKα/β↓, Keap1↓, Nrf2↑, HO-1↑, SOD↑, TNF-α↓, IL-6↓, IL-1β↓ | Nrf2/NF-κB pathway | 35692958 | |
| Perilla frutescens extract | DSS induced in mouse model | 100 mg/kg | COX-2↓, INOS↓, HO-1↑, Nrf2↑, TNF-α↓, IL-10↑ | Nrf2 pathway | 28848431 | |
| Artemisia argyi extract | DSS induced in mouse model | 200 mg/kg | IL-6↓, IL-1β↓, TNF-α↓, p-IκBα↓, p-NF-κB↓, Cox2↓, Nrf2↑, HO-1↑, MPO↓, INOS↓ | NF-κB/Nrf2 pathway | 35277165 | |
| Maggot extract | DSS induced in mouse model | 1000 mg/kg | Colon length↑, pIκB↓, IL-6↓, IL-1β↓, NFκB p65↓, TNF-α↓, Nrf2↑, HO-1↑, TGF-β1↓, SMADs↓ | TGF-β1/SMAD pathway | 34456904 | |
| Iziphus spina-christi fruit extract | AA induced in rat model | 400 mg/kg | Colon length↑, NO↓, MPO↓, GSH↑, SOD↑, CAT↑, GPx↑, Nrf2↑, HO-1↑, IL-1β↓, INOS↓, TNF-α↓, COX-2↓ | Nrf2/HO-1 pathway | 29518435 | |
| Extract of Mesua Assamica (King & prain) Kosterm. | DSS induced in mouse model | 200 mg/kg | Colon length↑, NO↓, MDA↓, MPO↓, GSH↑, IL-1β↓, IL-6↓, TNF-α↓, Nrf2↑, HO-1↑, SOD↑, | HO-1/Nrf2/SIRT1 signaling pathway | 36195303 | |
| 24Z-masticadienonic acid | DSS induced in mouse model | 100 mg/kg | Colon length↑, IL-1β↓, IL-6↓, TNF-α↓, Occludin↑, ZO-1↑, Nrf2↑, HO-1↑, NFκB p65↓, pIκB↓ | Nrf2/HO-1 and NF-κB pathway | 36403513 | |
| Gingerenone A | DSS induced in mouse model | 20 mg/kg | IL-1β↓, IL-6↓, TNF-α↓, MDA↓, GSH↑, Nrf2↑, HO-1↑, NQO1↑, GPX4↑ | Nrf2–GPX4 signaling pathway | 36135333 | |
| Natural Extract | Experiment Models | Effective Dose | Associated Phenotypes | Potential Mechanism | PMID |
|---|---|---|---|---|---|
| Linagliptin | TNBS induced in rat model | 1.5 mg/kg | IL-6↓, TNF-α↓, MPO↓, IL-10↑, IL-6↓, TNF-α↓, IL-10↑, GSH↑, GPx↑, TAC↑, Nrf2↑, HO-1↑ | Nrf2/HO-1 pathway | 33667522 |
| Carbocisteine | AA induced in rat model | 500 mg/kg | Histopathological score↓, TAC↑, HO-1↑, MPO↓, IL-6↓, TNF-α↓, IL-10↑, TLR4↓, Nrf2↑, NF-κB p-65↓ | Nrf2/HO-1 and NFκB pathway | 35754464 |
| Olmesartan | AA induced in rat model | 10 mg/kg | Weight/length ratio↓, IL-6↓, TNF-α↓, IL-1β↓, TGF-β↓, IL-10↑, Nrf2↑, HO-1↑, NFĸB p65↓, MPO↓, TAC↑, SOD↑, GSH↑, CAT↑, MDA↓, IL-10↑ | NFκB and Nrf2/HO-1 pathway | 30594690 |
| Nadroparin sodium | AA induced in rat model | 500 units/kg | NF-κB↓, AP-1↓, COX-2↓, IL-6↓, TNF-α↓, HO-1↑, Nrf2↑, MDA↓ | Nrf2/HO-1 and NF-κB pathway | 22350949 |
| Levetiracetam | AA induced in mouse model | 100 mg/kg | Colon length↑, TNF-α↓, IL-6↓, IL-1β↓, IFN-γ↓, IL-10↑, TGF-β↑, INOS↓, NO↓, GSH↑, SOD↑, CAT↑, MDA↓, MPO↓, Nrf2↑, HO-1↑, p-NF-κB↓ | Nrf2/HO-1 and NF-κB pathway | 37068340 |
| Ambroxol | AA induced in rat model | 200 mg/kg | Weight/length ratio↓, Nrf2↑, HO-1↑, IL-6↓, TNF-α↓, IL-10↑, CAT↑, TAC↑, MPO↓, IL-6↓, TNF-α↓, IL-10↑, IκBα↓, p65↓ | NF-κB/Nrf2 pathway | 35947115 |
| Vildagliptin | AA induced in rat model | 10 mg/kg | Nrf2↑, HO-1↑, NQO1↑, TNF-α↓, NF-κB↓, IL-1β↓, PI3K↓, Akt↓ | PI3K/Akt/NFκB and Nrf2 signaling pathway | 33434756 |
| Dapagliflozin | TNBS induced in rat model | 5 mg/kg | Weight/length ratio↓, TNF-α↓, MPO↓, IL-10↑, GSH↑, GPx↑, Nrf2↑, HO-1↑ | Nrf2/HO-1 pathway | 33412153 |
| Sodium butyrate | DSS induced in mouse model | 10 mg/kg | Colon length↑, MPO↓, MDA↓, GSH↑, COX-2↓, Nrf2↑, HO-1↑, IL-6↓, TNF-α↓, NF-κB↓, IL-1β↓, IL-18↓, NF-κB p65↓, NLRP3↓ | NF-κB/NLRP3 and COX-2/Nrf2/HO-1 pathway | 36867295 |
| N-benzyl-N-methyldecan-1-amine; decyl-(4-methoxy-benzyl)-methyl-amine | DNBS induced in rat model | 0.4 mg/kg | TNF-α↓, IL-1β↓, IL-6↓, HO1↑, Nrf2↑, MPO↓ | Nrf2 pathway | 37153778 |
| NPs-PEG-FA/6-shogaol | DSS induced in mouse model | 15 mg/kg | MPO↓, TNF-α↓, IL-6↓, IL-1β↓, INOS↓, Nrf2↑, HO-1↑ | Nrf2 pathway | 28961808 |
| PEA/Polydatin | DNBS induced in rat model | 10 mg/kg | MPO↓, MDA↓, IL-1β↓, TNF-α↓, NF-κB p65↓, IκB-α↑, INOS↓, SIRT-1↑, HO-1↑, Nrf2↑ | NF-κB/SIRT1/Nrf2 pathway | 33809584 |
| Mo3Se4 nanoflakes | DSS induced in mouse model | 100 mg/kg | Histopathological scores↓, MPO↓, ROS↓, MDA↓, CAT↑, GPx↑, SOD↑, GSH↑, Keap1↓, Nrf2↑, NQO1↑, HO-1↑, IL-1β↓, IL-6↓, TNF-α↓, IFN-β↓, TLR4↓, p-NF-κB↓, IκBα↑, MUC-2↑, Claudin-1↑, Occludin↑, ZO-1↑ | Nrf2-Keap1 and TLR4/NF-кB pathway | 35985164 |
| I.e. diselenide-bridged hyaluronic acid nanogel | DSS and TNBS induced in mouse model | 50 mg/kg | Colon length↑, MPO↓, MDA↓, SOD↑, HO-1↑, Nrf2↑, INOS↓, IL-6↓, TNF-a↓, IL-1β↓ | Nrf2/HO-1 pathway | 35861614 |
| FA-97 | DSS induced in mouse model | 10 mg/kg | Colon length↑, MPO↓, INOS↓, IL-1β↓, IL-6↓, TNF-α↓, IL-12↓, IL-17↓, MDA↓, ROS↓, HO-1↑, NQO-1↑, Nrf2↑ | Nrf2/HO-1 pathway | 31969881 |
| Mesalazine | TNBS induced in rat model | 30 mg/kg | Nrf2↑, HO-1↑, MPO↓, COX-2↓, INOS↓ | Nrf2-HO-1 pathway | 28473247 |
| Telmisartan | DSS induced in rat model | 7 mg/kg | IL-1β↓, IL-6↓, TNF-α↓, Nrf2↑, HO-1↑, IL-10↑ | NF-κB pathway | 31326516 |
| Coenzyme Q10 | AA induced in rat model | 100 mg/kg | Colon weight/colon length ratio↓, GSH↑, SOD↑, CAT↑, Nrf2↑, HO-1↑, MDA↓, TNF-α↓, Caspase-3↓ | Nrf2/HO-1 pathway | 28050757 |
| Spermidine | DSS and TNBS induced in mouse model | 20 mM | TNF-α↓, IL-6↓, Occludin↑, Claudin-1↑, Claudin-3↑, Nrf2↑, HO1↑, NQO1↑, SOD↑ | AhR-Nrf2 pathway | 37104918 |
| Miconazole | AA induced in rat model | 40 mg/kg | Histopathological score↓, MDA↓, SOD↑, GSH↑, IL-6↓, TNF-α↓, Nrf2↑, HO-1↑, IL-10↑, INOS↓, COX2↑, Caspase-3↓ | Nrf2/HO-1 signaling pathway | 35205169 |
| Oat peptides | DSS induced in mouse model | 500 mg/kg | IL-6↓, IL-1β↓,TNF-α↓, MDA↓, SOD↑, ZO-1↑, Occludin↑, Claudin-1↑, Nrf2↑, Keap1↓, NQO1↑, HO-1↑ | Keap1-Nrf2 pathway | 38140314 |
| Dimethyl fumarate | DSS induced in mouse model | 60 mg/kg | Colon length↑, MPO↓, INOS↓, IL-1β↓, IL-6↓, TNF-α↓, Nrf2↑, HO-1↑, NQO1↑ | Nrf2/ARE pathway | 27184504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Wang, Y.; Li, N.; Yang, X.; Sun, X.; Tian, H.; Zhang, Y. Mechanism of Action and Therapeutic Implications of Nrf2/HO-1 in Inflammatory Bowel Disease. Antioxidants 2024, 13, 1012. https://doi.org/10.3390/antiox13081012
Yuan L, Wang Y, Li N, Yang X, Sun X, Tian H, Zhang Y. Mechanism of Action and Therapeutic Implications of Nrf2/HO-1 in Inflammatory Bowel Disease. Antioxidants. 2024; 13(8):1012. https://doi.org/10.3390/antiox13081012
Chicago/Turabian StyleYuan, Lingling, Yingyi Wang, Na Li, Xuli Yang, Xuhui Sun, Huai’e Tian, and Yi Zhang. 2024. "Mechanism of Action and Therapeutic Implications of Nrf2/HO-1 in Inflammatory Bowel Disease" Antioxidants 13, no. 8: 1012. https://doi.org/10.3390/antiox13081012




