Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Human Sperm Preparation
2.3. SDS-PAGE and Immunoblotting
2.4. Silver Staining
2.5. Sperm Motility and Viability Analysis
2.6. Nitric Oxide Quantification
2.7. Citrate Quantification
2.8. Assessment of Sperm Acrosome Reaction
2.9. Statistical Analysis
3. Results
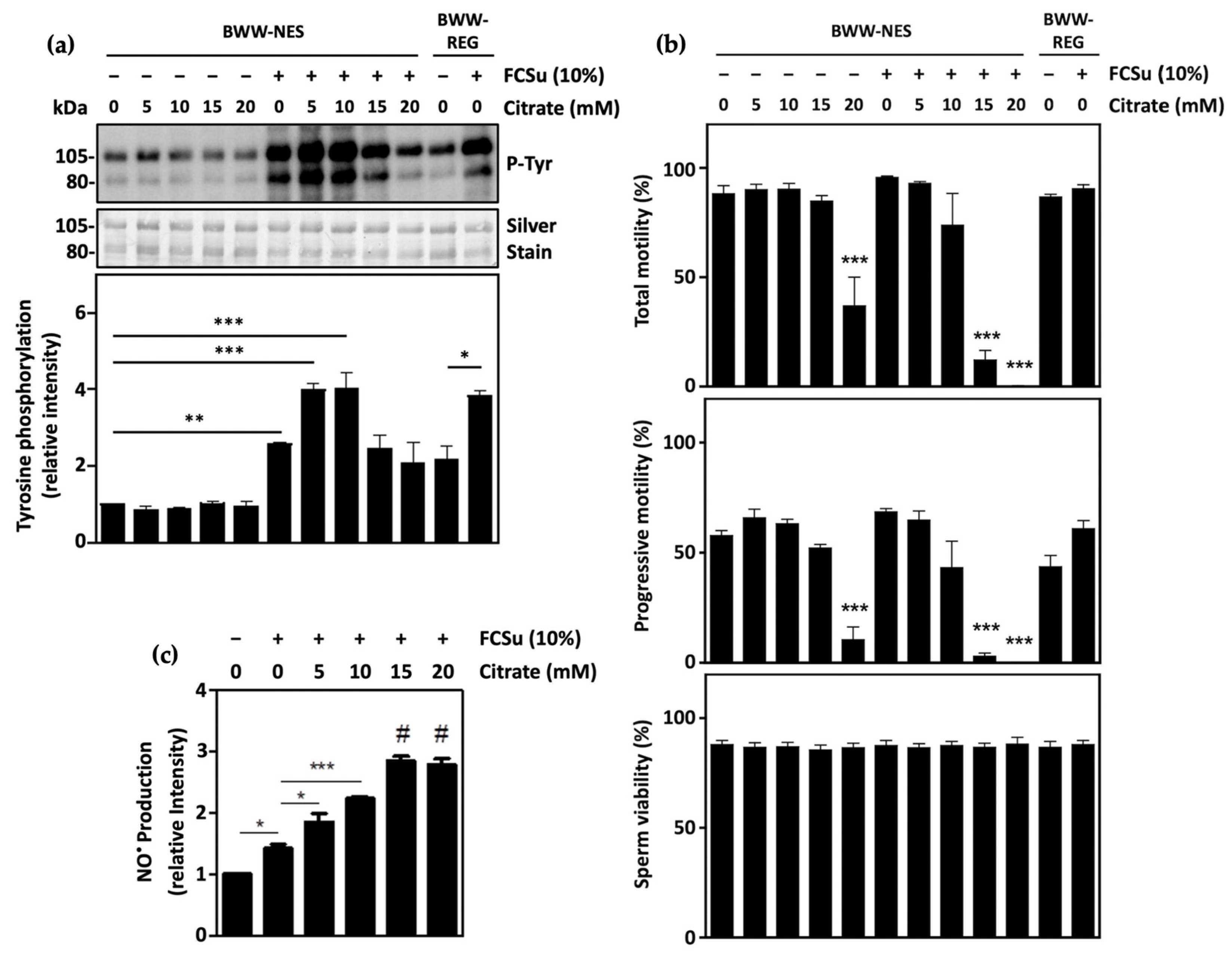
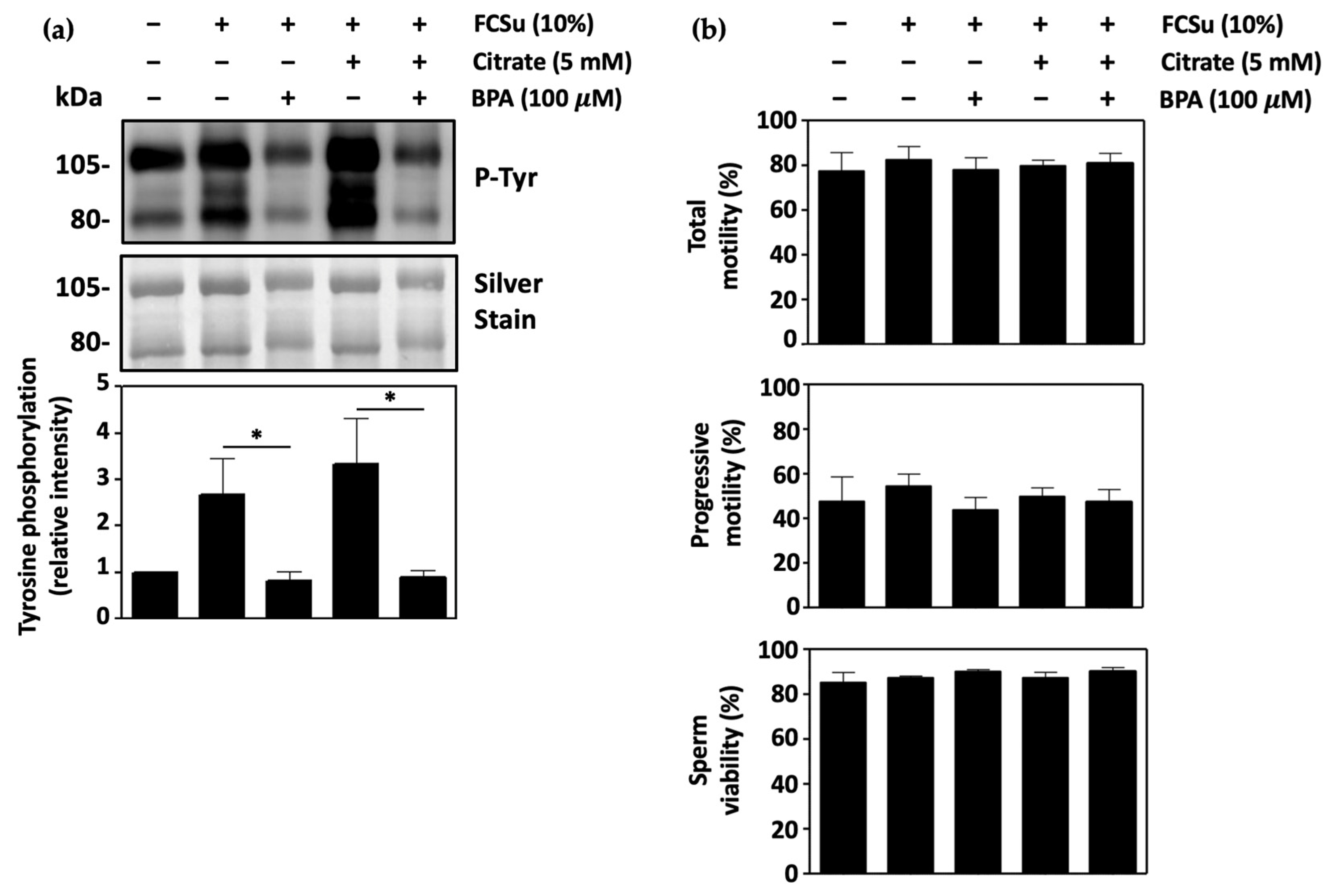
3.1. Citrate Supports Human Sperm Capacitation
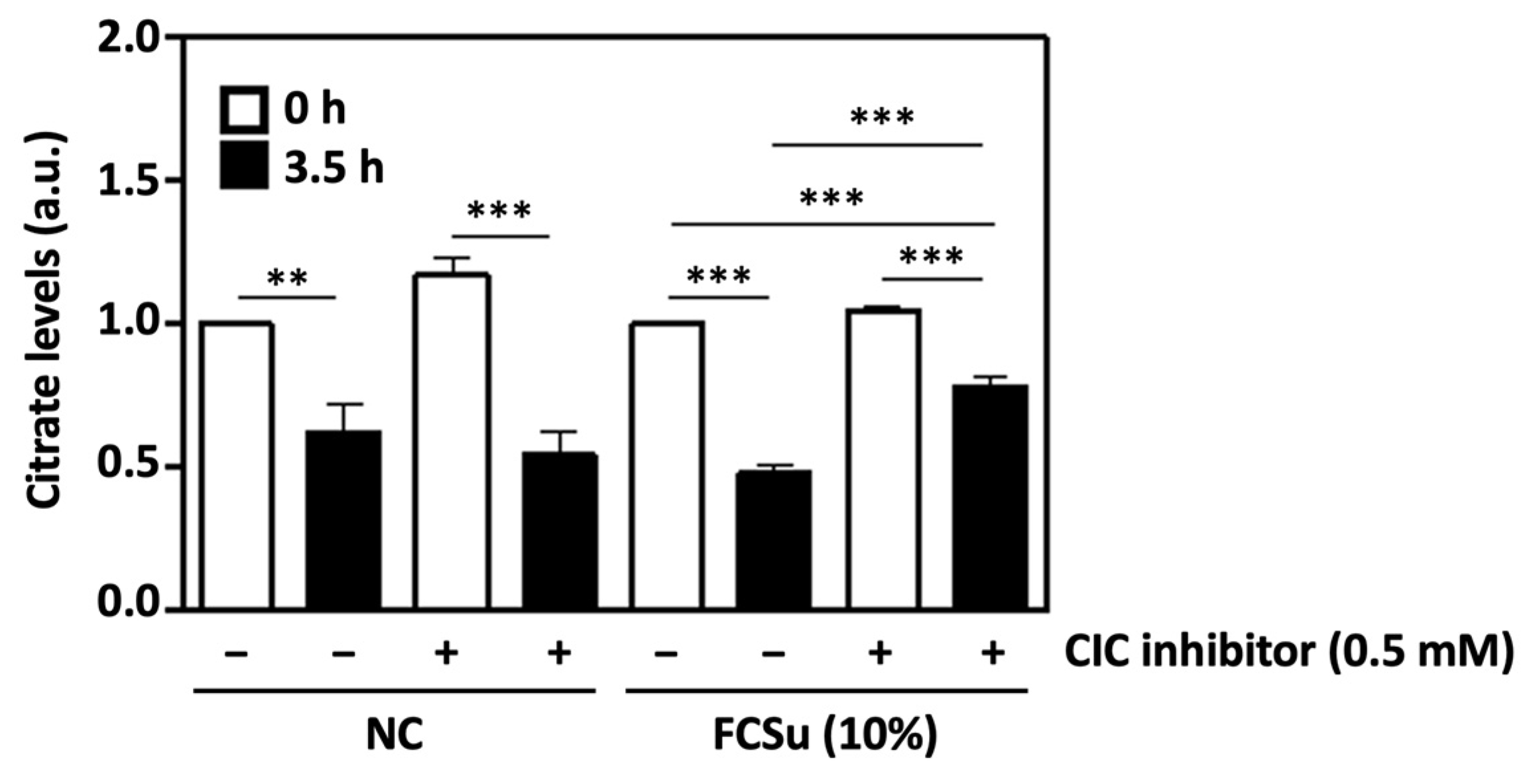
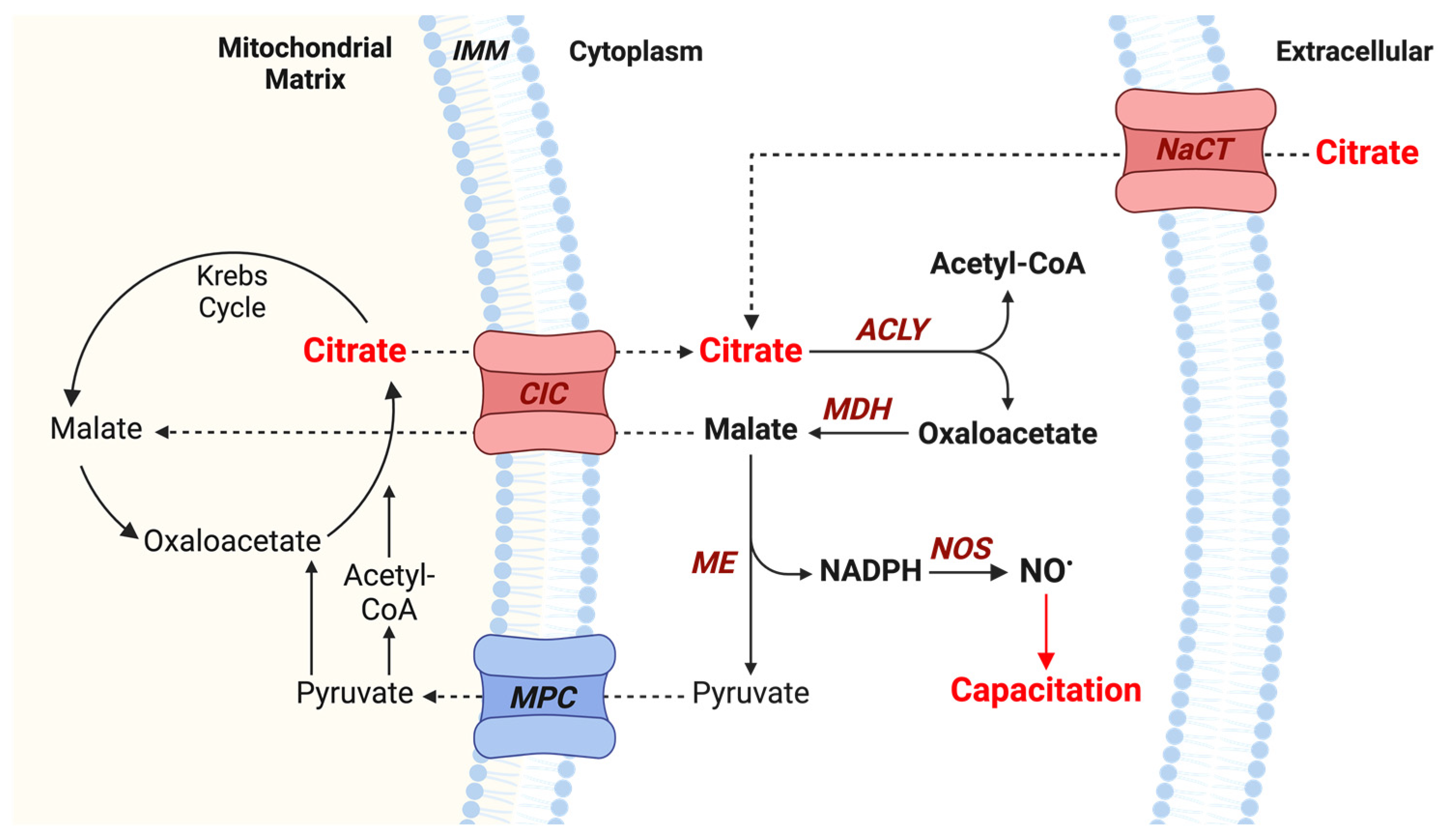
3.2. Cytosolic Citrate Is Consumed during Human Sperm Capacitation In Vitro
3.3. Mitochondrial Citrate Transport Supports Human Sperm Capacitation
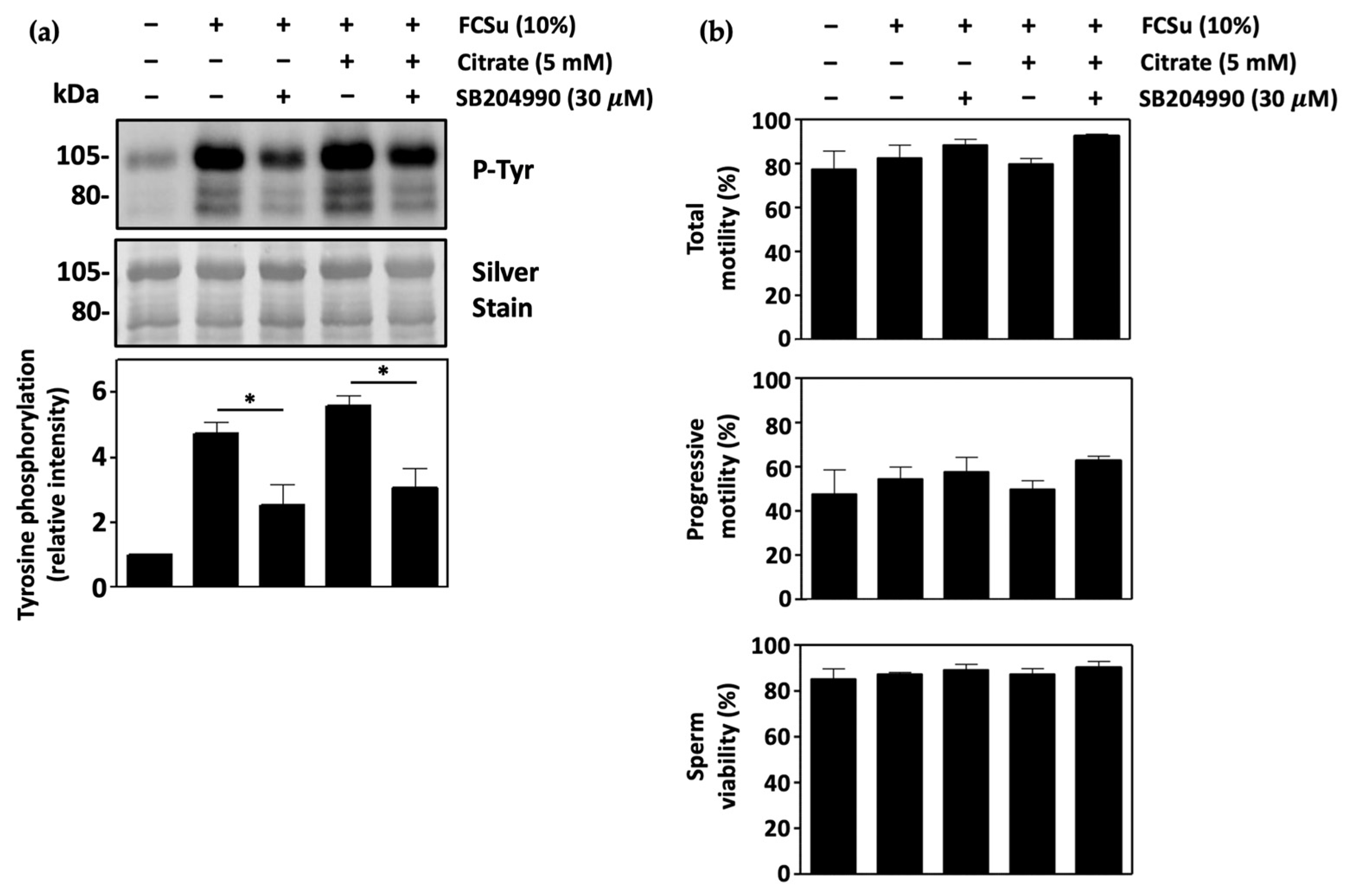
3.4. ATP-Citrate Lyase Supports Human Sperm Capacitation
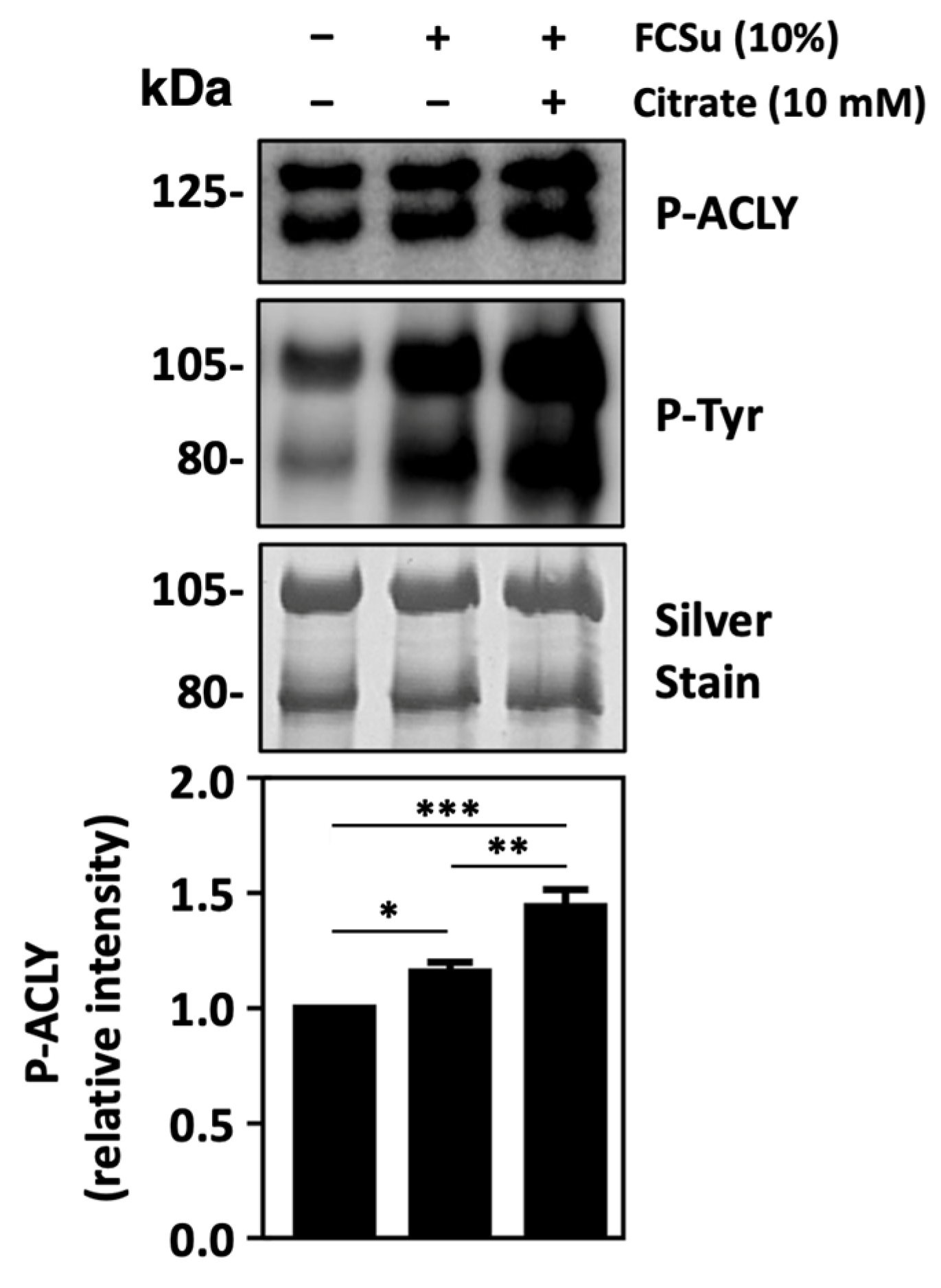
3.5. ATP-Citrate Lyase Is Activated during Sperm Capacitation
3.6. Malic Enzyme Supports Human Sperm Capacitation
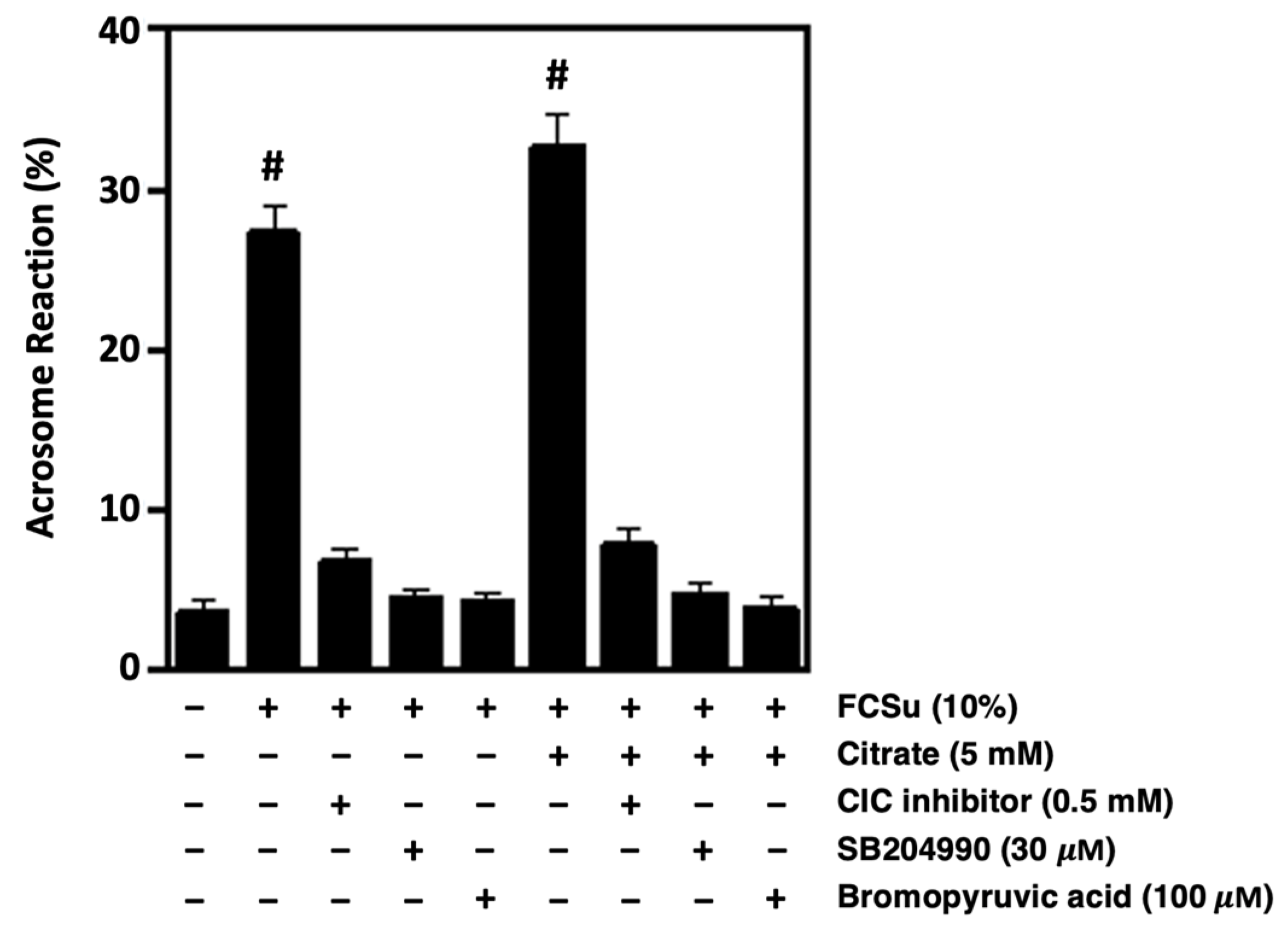
3.7. Citrate Metabolism Supports the Ability of Spermatozoa to Undergo Acrosome Reaction
3.8. Nitric Oxide Production Is Involved in Citrate-Mediated Capacitation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; WHO Press: Geneva, Switzerland, 2023; ISBN 978-92-4-006831-5. [Google Scholar]
- Bushnik, T.; Cook, J.L.; Yuzpe, A.A.; Tough, S.; Collins, J. Estimating the prevalence of infertility in Canada. Hum. Reprod. 2012, 27, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Fallara, G.; Cazzaniga, W.; Boeri, L.; Capogrosso, P.; Candela, L.; Pozzi, E.; Belladelli, F.; Schifano, N.; Ventimiglia, E.; Abbate, C.; et al. Male factor infertility trends throughout the last 10 years: Report from a tertiary-referral academic andrology centre. Andrology 2021, 9, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. Male Infertility; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Longo, V.; Forleo, A.; Provenzano, S.P.; Coppola, L. Seminal VOCs Analysis Investigating Sperm Quality Decline—New Studies to Improve Male Fertility Contrasting Population Ageing. Lect. Notes Electr. Eng. 2019, 544, 501–508. [Google Scholar] [CrossRef]
- Chávez, J.C.; Carrasquel-Martínez, G.; Hernández-Garduño, S.; Matamoros Volante, A.; Treviño, C.L.; Nishigaki, T.; Darszon, A. Cytosolic and Acrosomal pH Regulation in Mammalian Sperm. Cells 2024, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic adenosine 3’,5’monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic. Biol. Med. 1997, 22, 643–656. [Google Scholar] [CrossRef]
- de Lamirande, E.; O’Flaherty, C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Biophys. Acta 2008, 1784, 106–115. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Serafini, S.; O’Flaherty, C. Redox Regulation to Modulate Phosphorylation Events in Human Spermatozoa. Antioxid. Redox Signal. 2022, 37, 437–450. [Google Scholar] [CrossRef]
- Benko, F.; Urminská, D.; Ďuračka, M.; Tvrdá, E. Signaling Roleplay between Ion Channels during Mammalian Sperm Capacitation. Biomedicines 2023, 11, 2519. [Google Scholar] [CrossRef] [PubMed]
- Marín-Briggiler, C.I.; Tezón, J.G.; Miranda, P.V.; Vazquez-Levin, M.H. Effect of incubating human sperm at room temperature on capacitation-related events. Fertil. Steril. 2002, 77, 252–259. [Google Scholar] [CrossRef]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Leclerc, P.; Gagnon, C. Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Mol. Hum. Reprod. 1997, 3, 175–194. [Google Scholar] [CrossRef]
- de Lamirande, E.; Harakat, A.; Gagnon, C. Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J. Androl. 1998, 19, 215–225. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic. Biol. Med. 1995, 18, 487–495. [Google Scholar] [CrossRef]
- Zini, A.; De Lamirande, E.; Gagnon, C. Low levels of nitric oxide promote human sperm capacitation in vitro. J. Androl. 1995, 16, 424–431. [Google Scholar] [CrossRef]
- Herrero, M.B.; de Lamirande, E.; Gagnon, C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol. Reprod. 1999, 61, 575–581. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Lamothe, G. Reactive oxygen-induced reactive oxygen formation during human sperm capacitation. Free Radic. Biol. Med. 2009, 46, 502–510. [Google Scholar] [CrossRef]
- Herrero, M.B.; Pérez Martínez, S.; Viggiano, J.M.; Polak, J.M.; de Gimeno, M.F. Localization by indirect immunofluorescence of nitric oxide synthase in mouse and human spermatozoa. Reprod. Fertil. Dev. 1996, 8, 931–934. [Google Scholar] [CrossRef]
- Lewis, S.E.; Donnelly, E.T.; Sterling, E.S.; Kennedy, M.S.; Thompson, W.; Chakravarthy, U. Nitric oxide synthase and nitrite production in human spermatozoa: Evidence that endogenous nitric oxide is beneficial to sperm motility. Mol. Hum. Reprod. 1996, 2, 873–878. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Concepts of citrate production and secretion by prostate. 1. Metabolic relationships. Prostate 1991, 18, 25–46. [Google Scholar] [CrossRef]
- Zöpfgen, A.; Priem, F.; Sudhoff, F.; Jung, K.; Lenk, S.; Loening, S.A.; Sinha, P. Relationship between semen quality and the seminal plasma components carnitine, alpha-glucosidase, fructose, citrate and granulocyte elastase in infertile men compared with a normal population. Hum. Reprod. 2000, 15, 840–845. [Google Scholar] [CrossRef]
- Gruhl, S.L.; Ho, L.M.; Sim, M.Y.X.; Lee, S.N.; Yu, S.L.; Yong, T.T.; Lim, L.S.; Rajesh, H. Seminal biomarkers and their correlations to semen parameters in subfertile men. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2023, 19, 100229. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mahdi, A.A.; Ahmad, M.K.; Shukla, K.K.; Jaiswer, S.P.; Shankhwar, S.N. 1H NMR spectroscopic studies on human seminal plasma: A probative discriminant function analysis classification model. J. Pharm. Biomed. Anal. 2011, 54, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Seguin, F.; Barthelemy, C.; Akoka, S.; Le Pape, A.; Lansac, J.; Royere, D. 1H nuclear magnetic resonance studies of seminal plasma from fertile and infertile men. J. Reprod. Fertil. 1993, 97, 51–55. [Google Scholar] [CrossRef]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Visconti, P.E. Sperm Bioenergetics in a Nutshell. Biol. Reprod. 2012, 87, 72. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef]
- Herrero, M.B.; Gagnon, C. Nitric oxide: A novel mediator of sperm function. J. Androl. 2001, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Iacobazzi, V.; Menga, A.; Avantaggiati, M.L.; Palmieri, F. A key role of the mitochondrial citrate carrier (SLC25A1) in TNFα- and IFNγ-triggered inflammation. Biochim. Biophys. Acta 2014, 1839, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Iacobazzi, V.; Palmieri, F.; Menga, A. ATP-citrate lyase is essential for macrophage inflammatory response. Biochem. Biophys. Res. Commun. 2013, 440, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Convertini, P.; Cucci, L.; Panaro, M.A.; Di Noia, M.A.; Calvello, R.; Palmieri, F.; Iacobazzi, V. The mitochondrial citrate carrier: A new player in inflammation. Biochem. J. 2011, 438, 433–436. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-0030787. [Google Scholar]
- Biggers, J.D.; Whitten, W.K.; Whittingham, D.G. The culture of mouse embryos in vitro. In Methods in Mammalian Embryology; WH Freeman Co.: San Francisco, CA, USA, 1971. [Google Scholar]
- de Lamirande, E.; Gagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, G.; Karsnas, P. Important parameters in semi-dry electrophoretic transfer. Electrophoresis 1990, 11, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sagare-Patil, V.; Galvankar, M.; Satiya, M.; Bhandari, B.; Gupta, S.K.; Modi, D. Differential concentration and time dependent effects of progesterone on kinase activity, hyperactivation and acrosome reaction in human spermatozoa. Int. J. Androl. 2012, 35, 633–644. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Rodriguez, P.; Srivastava, S. L-arginine promotes capacitation and acrosome reaction in cryopreserved bovine spermatozoa. Biochim. Biophys. Acta 2004, 1674, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef]
- Ramya, T.; Misro, M.M.; Sinha, D.; Nandan, D.; Mithal, S. Altered levels of seminal nitric oxide, nitric oxide synthase, and enzymatic antioxidants and their association with sperm function in infertile subjects. Fertil. Steril. 2011, 95, 135–140. [Google Scholar] [CrossRef]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef]
- Morielli, T.; O’Flaherty, C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction 2015, 149, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Cappello, A.R.; Guido, C.; Santoro, A.; Santoro, M.; Capobianco, L.; Montanaro, D.; Madeo, M.; Andò, S.; Dolce, V.; Aquila, S. The mitochondrial citrate carrier (CIC) is present and regulates insulin secretion by human male gamete. Endocrinology 2012, 153, 1743–1754. [Google Scholar] [CrossRef]
- Koh, H.J.; Lee, S.M.; Son, B.G.; Lee, S.H.; Ryoo, Z.Y.; Chang, K.T.; Park, J.W.; Park, D.C.; Song, B.J.; Veech, R.L.; et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J. Biol. Chem. 2004, 279, 39968–39974. [Google Scholar] [CrossRef]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Jump, D. Mammalian Fatty Acid Elongases. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 579, pp. 375–389. [Google Scholar] [CrossRef]
- Lee, D.; Moawad, A.R.; Morielli, T.; Fernandez, M.C.; O’Flaherty, C. Peroxiredoxins prevent oxidative stress during human sperm capacitation. Mol. Hum. Reprod. 2017, 23, 106–115. [Google Scholar] [CrossRef]
- Thundathil, J.; de Lamirande, E.; Gagnon, C. Nitric oxide regulates the phosphorylation of the threonine-glutamine-tyrosine motif in proteins of human spermatozoa during capacitation. Biol. Reprod. 2003, 68, 1291–1298. [Google Scholar] [CrossRef]
- Cosson, J. ATP: The sperm movement energizer. In Adenosine Triphosphate: Chemical Properties, Biosynthesis and Functions in Cells; Nova Publisher Inc.: New York, NY, USA, 2013; pp. 1–46. [Google Scholar]
- Furse, S.; Kusinski, L.C.; Ray, A.; Glenn-Sansum, C.; Williams, H.E.L.; Koulman, A.; Meek, C.L. Relative Abundance of Lipid Metabolites in Spermatozoa across Three Compartments. Int. J. Mol. Sci. 2022, 23, 11655. [Google Scholar] [CrossRef]
- Amaral, A. Energy metabolism in mammalian sperm motility. WIREs Mech. Dis. 2022, 14, e1569. [Google Scholar] [CrossRef]
- Ritagliati, C.; Luque, G.M.; Stival, C.; Baro Graf, C.; Buffone, M.G.; Krapf, D. Lysine acetylation modulates mouse sperm capacitation. Sci. Rep. 2018, 8, 13334. [Google Scholar] [CrossRef]
- Sun, G.; Jiang, M.; Zhou, T.; Guo, Y.; Cui, Y.; Guo, X.; Sha, J. Insights into the lysine acetylproteome of human sperm. J. Proteom. 2014, 109, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Diao, H.; Wang, C.; Lin, Y.; Yu, F.; Lu, H.; Xu, W.; Li, Z.; Shi, H.; Zhao, S.; et al. Acetylproteomic analysis reveals functional implications of lysine acetylation in human spermatozoa (sperm). Mol. Cell. Proteom. 2015, 14, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Tolwani, A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus 2009, 2, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.C.; Harrison, A. The role of citrate in determining the activity of calcium ions in human semen. Int. J. Androl. 1984, 7, 198–202. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca(2+) signaling in mammalian spermatozoa. Mol. Cell. Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loggia, D.; O’Flaherty, C. Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation. Antioxidants 2024, 13, 885. https://doi.org/10.3390/antiox13080885
Loggia D, O’Flaherty C. Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation. Antioxidants. 2024; 13(8):885. https://doi.org/10.3390/antiox13080885
Chicago/Turabian StyleLoggia, Diego, and Cristian O’Flaherty. 2024. "Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation" Antioxidants 13, no. 8: 885. https://doi.org/10.3390/antiox13080885
APA StyleLoggia, D., & O’Flaherty, C. (2024). Citrate Promotes Nitric Oxide Production during Human Sperm Capacitation. Antioxidants, 13(8), 885. https://doi.org/10.3390/antiox13080885







