Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches
Abstract
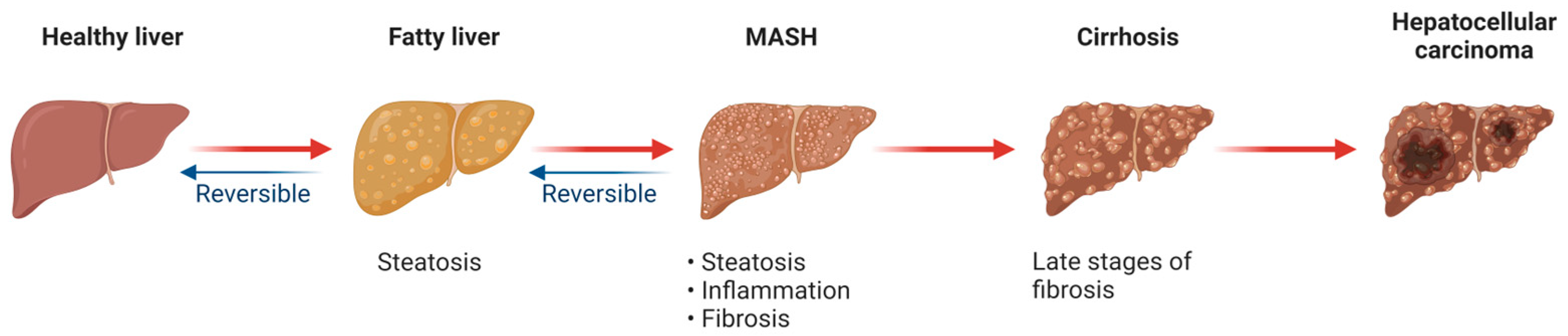
1. Introduction
2. Mitochondrial Biology
2.1. Mitochondria and Oxidative Phosphorylation
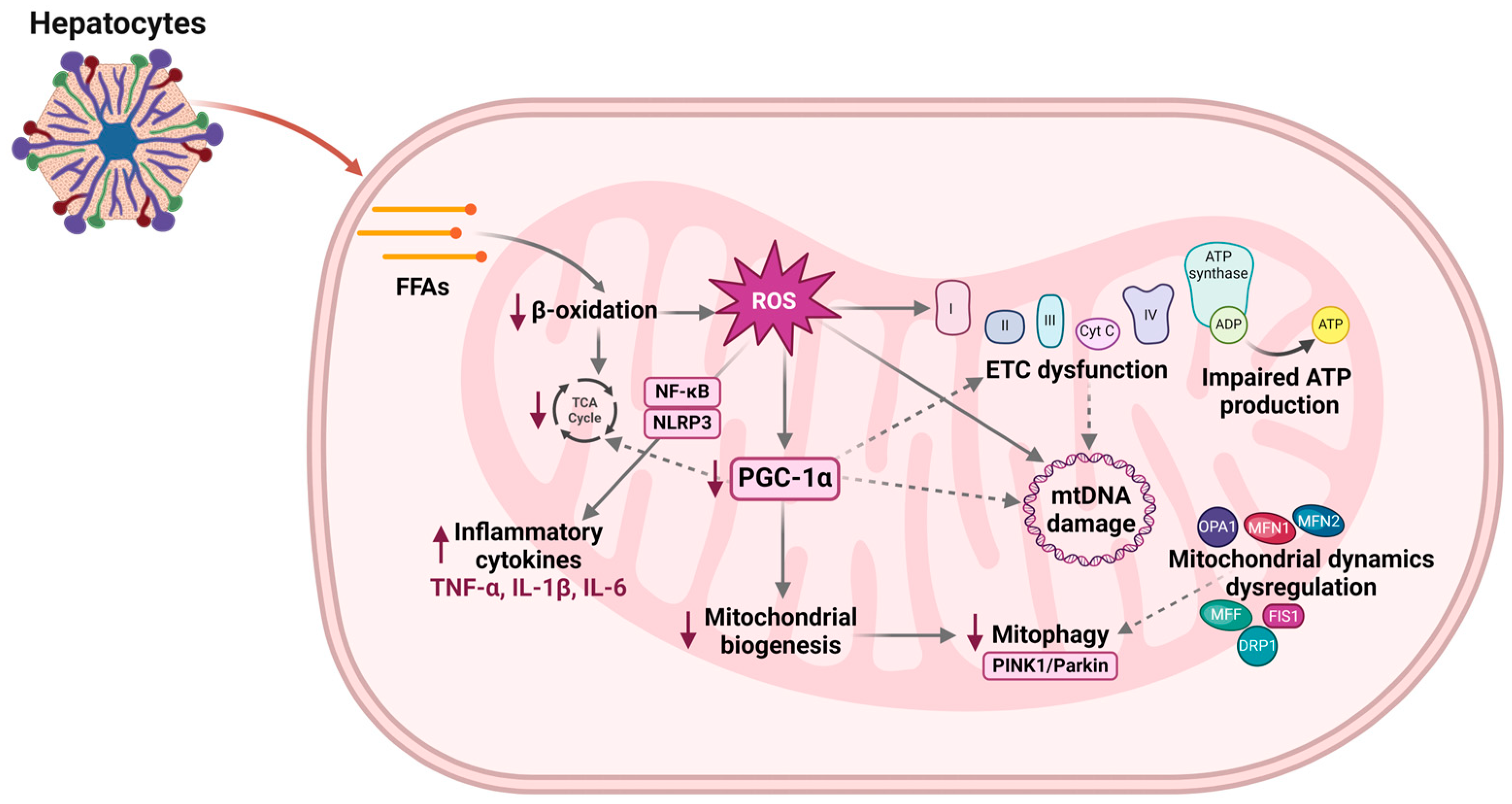
2.2. Mitochondrial Oxidative Stress
2.3. Mitochondria and Cell Death
2.4. Mitochondrial Quality Control
2.4.1. Mitochondrial Biogenesis
2.4.2. Mitochondrial Dynamics
Mitochondrial Fusion
Mitochondrial Fission
2.4.3. Mitophagy
3. Mitochondrial Dysfunction and MASLD
4. Potential Therapeutic Approaches for MASLD
5. Conclusions
| Category | Name | Function | References |
|---|---|---|---|
| PPAR agonists | Elafibranor Thiazolidinediones Pioglitazone Azemiglitazone potassium (MSDC-0602K) | Improves steatosis, inflammation, and fibrosis Potential effects on mitochondria Promotes mitochondrial biogenesis Inhibits the mitochondrial pyruvate carrier (MPC) | [104,105,106] [112,113,114] [115,116,117] |
| SGLT2 inhibitors | Empagliflozin Ipragliflozin Dapagliflozin | Decreases ROS production Up-regulates transcription factors (PGC-1α and TFAM), regulate mitophagy Normalizes mitochondrial size in hepatocytes Increases mtDNA copy number | [133,134] [126,131] [125,135] |
| Biguanides | Metformin | Increases β-oxidation and mitochondrial biogenesis Modulates mitochondrial respiratory chain complexes I and IV | [143] [144,146] |
| Sirtuins (small molecule modulators) | SRT2104 (SIRT1 activator) | Increases the expression of genes involved in mitochondrial biogenesis | [153] |
| Mitochondria-targeted agents | MitoQ MitoTEMPO Elamipretide (SS-31) | Reduces oxidative stress Protective effect on mtDNA Reduces oxidative stress Interacts with cardiolipin, which stabilizes mitochondrial membranes, improves ETC activity and reduces oxidative stress | [88] [89] [96,97,98] [93] |
| Gene therapy | Corrects genetic mutations or defects affecting mitochondrial function | [156] |
Author Contributions
Funding
Conflicts of Interest
References
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Alabdul Razzak, I.; Noureddin, M.; Trivedi, H. From Nonalcoholic Fatty Liver Disease to Metabolic Dysfunction-Associated Steatotic Liver Disease: Out with the Old, in with the New. J. Clin. Med. 2024, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Mellemkjær, A.; Kjær, M.B.; Haldrup, D.; Grønbæk, H.; Thomsen, K.L. Management of Cardiovascular Risk in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. Eur. J. Intern. Med. 2024, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pipitone, R.M.; Ciccioli, C.; Infantino, G.; La Mantia, C.; Parisi, S.; Tulone, A.; Pennisi, G.; Grimaudo, S.; Petta, S. MAFLD: A Multisystem Disease. Ther. Adv. Endocrinol. Metab. 2023, 14, 204201882211455. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ivancovsky Wajcman, D.; Mark, H.E.; Younossi, Z.M.; Kopka, C.J.; Cohen, N.; Bansal, M.B.; Betel, M.; Brennan, P.N. Opportunities and Challenges Following Approval of Resmetirom for MASH Liver Disease. Nat. Med. 2024, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Song, M. New Insights into the Pathogenesis of Metabolic-Associated Fatty Liver Disease (MAFLD): Gut–Liver–Heart Crosstalk. Nutrients 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.; Lee, J.Y.; Kim, J.; Oh, C.-M. Mitochondrial Quality Control: Its Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Obes. Metab. Syndr. 2023, 32, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Gopal, T.; Kalari Kandy, R.R.; Boopathy, L.K.; Perumal, S.K.; Ganesan, M.; Rasineni, K.; Donohue, T.M., Jr.; Osna, N.A.; Kharbanda, K.K. Mitochondrial Dysfunction-Associated Mechanisms in the Development of Chronic Liver Diseases. Biology 2023, 12, 1311. [Google Scholar] [CrossRef]
- Caputo, V.; Tarantino, G.; Santini, S.J.; Fracassi, G.; Balsano, C. The Role of Epigenetic Control of Mitochondrial (Dys)Function in MASLD Onset and Progression. Nutrients 2023, 15, 4757. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It Is All about Energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Oláhová, M. Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. Int. J. Mol. Sci. 2020, 21, 3820. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine Modulates Oxidative Stress, Inflammation, Apoptosis, Autophagy, and Akt/mTOR Signaling in Methionine-Choline Deficiency-Induced Fatty Liver Disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Gu, X.; Zheng, Q.; Wang, J.; Zhu, H. Mitochondrial Mechanisms of Apoptosis and Necroptosis in Liver Diseases. Anal. Cell. Pathol. 2021, 2021, 8900122. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd. Molecular Mechanisms That Differentiate Apoptosis from Programmed Necrosis. Toxicol. Pathol. 2013, 41, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Liu, C.; Wang, Q.; Yan, J.; Hui, L.; Jia, Q.; Shan, H.; Tao, L.; Zhang, M. The Role of Mitophagy in Regulating Cell Death. Oxidative Med. Cell. Longev. 2021, 2021, 6617256. [Google Scholar] [CrossRef]
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-Arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3903. [Google Scholar] [CrossRef]
- Atici, A.E.; Crother, T.R.; Noval Rivas, M. Mitochondrial Quality Control in Health and Cardiovascular Diseases. Front. Cell Dev. Biol. 2023, 11, 1290046. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.-D. Mitochondrial Biogenesis: An Update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Qi, Y.; Tsang, S.-Y. Mitochondrial Biogenesis, Mitochondrial Dynamics, and Mitophagy in the Maturation of Cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef]
- Zhao, F.; Zou, M.-H. Role of the Mitochondrial Protein Import Machinery and Protein Processing in Heart Disease. Front. Cardiovasc. Med. 2021, 8, 749756. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Hossain, T.; Eckmann, D.M. Mitochondrial Dynamics Involves Molecular and Mechanical Events in Motility, Fusion and Fission. Front. Cell Dev. Biol. 2022, 10, 1010232. [Google Scholar] [CrossRef]
- Wang, S.; Tan, J.; Miao, Y.; Zhang, Q. Mitochondrial Dynamics, Mitophagy, and Mitochondria–Endoplasmic Reticulum Contact Sites Crosstalk Under Hypoxia. Front. Cell Dev. Biol. 2022, 10, 848214. [Google Scholar] [CrossRef]
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries in and Out. Trends Cell Biol. 2021, 31, 62–74. [Google Scholar] [CrossRef]
- Zerihun, M.; Sukumaran, S.; Qvit, N. The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy. Int. J. Mol. Sci. 2023, 24, 5785. [Google Scholar] [CrossRef]
- Roca-Agujetas, V.; de Dios, C.; Lestón, L.; Marí, M.; Morales, A.; Colell, A. Recent Insights into the Mitochondrial Role in Autophagy and Its Regulation by Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.-W.; Zhao, G. The Mitophagy Pathway and Its Implications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte Mitochondrial DNA Drives Nonalcoholic Steatohepatitis by Activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Karkucinska-Wieckowska, A.; Simoes, I.C.M.; Kalinowski, P.; Lebiedzinska-Arciszewska, M.; Zieniewicz, K.; Milkiewicz, P.; Górska-Ponikowska, M.; Pinton, P.; Malik, A.N.; Krawczyk, M.; et al. Mitochondria, Oxidative Stress and Nonalcoholic Fatty Liver Disease: A Complex Relationship. Eur. J. Clin. Investig. 2022, 52, e13622. [Google Scholar] [CrossRef]
- Ore, A.; Akinloye, O. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina 2019, 55, 26. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, G.; Heo, S.-Y.; Roh, Y.-S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Legaki, A.-I.; Moustakas, I.I.; Sikorska, M.; Papadopoulos, G.; Velliou, R.-I.; Chatzigeorgiou, A. Hepatocyte Mitochondrial Dynamics and Bioenergetics in Obesity-Related Non-Alcoholic Fatty Liver Disease. Curr. Obes. Rep. 2022, 11, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.C.; El Baradie, K.B.Y.; Hamrick, M.W. Targeting the Mitochondrial Permeability Transition Pore to Prevent Age-Associated Cell Damage and Neurodegeneration. Oxidative Med. Cell. Longev. 2021, 2021, 6626484. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive Oxygen Species (ROS) Scavenging Biomaterials for Anti-Inflammatory Diseases: From Mechanism to Therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, H. Inflammation Initiates a Vicious Cycle between Obesity and Nonalcoholic Fatty Liver Disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef]
- Zheng, P.; Ma, W.; Gu, Y.; Wu, H.; Bian, Z.; Liu, N.; Yang, D.; Chen, X. High-Fat Diet Causes Mitochondrial Damage and Downregulation of Mitofusin-2 and Optic Atrophy-1 in Multiple Organs. J. Clin. Biochem. Nutr. 2023, 73, 61–76. [Google Scholar] [CrossRef]
- Gong, F.; Gao, L.; Ding, T. IDH2 Protects against Nonalcoholic Steatohepatitis by Alleviating Dyslipidemia Regulated by Oxidative Stress. Biochem. Biophys. Res. Commun. 2019, 514, 593–600. [Google Scholar] [CrossRef]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernández, V.; Vasconcelos, N.; et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Suarez, J.; Gawlowski, T.; Han, W.; Wang, H.; Scott, B.T.; Dillmann, W.H. Regulation of Mitochondrial Morphology and Function by O-GlcNAcylation in Neonatal Cardiac Myocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.G.; Rodríguez, J.Z.; Barreto, A.; Sanabria-Barrera, S.; Iglesias, J.; Morales, L. Impact of Acute High Glucose on Mitochondrial Function in a Model of Endothelial Cells: Role of PDGF-C. Int. J. Mol. Sci. 2023, 24, 4394. [Google Scholar] [CrossRef] [PubMed]
- Civiletto, G.; Varanita, T.; Cerutti, R.; Gorletta, T.; Barbaro, S.; Marchet, S.; Lamperti, C.; Viscomi, C.; Scorrano, L.; Zeviani, M. Opa1 Overexpression Ameliorates the Phenotype of Two Mitochondrial Disease Mouse Models. Cell Metab. 2015, 21, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Varanita, T.; Soriano, M.E.; Romanello, V.; Zaglia, T.; Quintana-Cabrera, R.; Semenzato, M.; Menabò, R.; Costa, V.; Civiletto, G.; Pesce, P.; et al. The Opa1-Dependent Mitochondrial Cristae Remodeling Pathway Controls Atrophic, Apoptotic, and Ischemic Tissue Damage. Cell Metab. 2015, 21, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Murata, D.; Kleiner, D.E.; Anders, R.; Rosenberg, A.Z.; Kaplan, J.; Hamilton, J.P.; Aghajan, M.; Levi, M.; Wang, N.-Y.; et al. Prevention and Regression of Megamitochondria and Steatosis by Blocking Mitochondrial Fusion in the Liver. iScience 2022, 25, 103996. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, T.J.; Galloway, C.A.; Zhi, W.; Xiao, W.; de Mesy Bentley, K.L.; Sharma, A.; Teng, Y.; Sesaki, H.; Yoon, Y. The Mitochondrial Fusion Protein OPA1 Is Dispensable in the Liver and Its Absence Induces Mitohormesis to Protect Liver from Drug-Induced Injury. Nat. Commun. 2023, 14, 6721. [Google Scholar] [CrossRef]
- Da Dalt, L.; Moregola, A.; Svecla, M.; Pedretti, S.; Fantini, F.; Ronzio, M.; Uboldi, P.; Dolfini, D.; Donetti, E.; Baragetti, A.; et al. The Inhibition of Inner Mitochondrial Fusion in Hepatocytes Reduces Non-Alcoholic Fatty Liver and Improves Metabolic Profile during Obesity by Modulating Bile Acid Conjugation. Cardiovasc. Res. 2024, 119, 2917–2929. [Google Scholar] [CrossRef]
- Yoo, J.; Cheol Hwang, Y. Regulation of Mitochondrial Dynamics Ameliorates Hepatic Steatosis through TFEB Activation. Diabetes 2023, 72, 1599. [Google Scholar] [CrossRef]
- Xia, W.; Veeragandham, P.; Cao, Y.; Xu, Y.; Rhyne, T.E.; Qian, J.; Hung, C.-W.; Zhao, P.; Jones, Y.; Gao, H.; et al. Obesity Causes Mitochondrial Fragmentation and Dysfunction in White Adipocytes due to RalA Activation. Nat. Metab. 2024, 6, 273–289. [Google Scholar] [CrossRef]
- Galloway, C.A.; Lee, H.; Brookes, P.S.; Yoon, Y. Decreasing Mitochondrial Fission Alleviates Hepatic Steatosis in a Murine Model of Nonalcoholic Fatty Liver Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chang, L.; Luo, Y.; Zhou, Y.; Zhang, J. Mst1 Inhibition Attenuates Non-Alcoholic Fatty Liver Disease via Reversing Parkin-Related Mitophagy. Redox Biol. 2019, 21, 101120. [Google Scholar] [CrossRef] [PubMed]
- Undamatla, R.; Fagunloye, O.G.; Chen, J.; Edmunds, L.R.; Murali, A.; Mills, A.; Xie, B.; Pangburn, M.M.; Sipula, I.; Gibson, G.; et al. Reduced Mitophagy Is an Early Feature of NAFLD and Liver-Specific PARKIN Knockout Hastens the Onset of Steatosis, Inflammation and Fibrosis. Sci. Rep. 2023, 13, 7575. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Zhang, W.; Beaton, M.; Marsboom, G.; Gruber, M.; Simon, M.C.; Hart, J.; Dorn, G.W., II.; Brady, M.J.; Macleod, K.F. BNip3 Regulates Mitochondrial Function and Lipid Metabolism in the Liver. Mol. Cell. Biol. 2012, 32, 2570–2584. [Google Scholar] [CrossRef]
- Kyriakoudi, S.; Drousiotou, A.; Petrou, P.P. When the Balance Tips: Dysregulation of Mitochondrial Dynamics as a Culprit in Disease. Int. J. Mol. Sci. 2021, 22, 4617. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Deen, N.; Zamani, S.; Hasnat, M.A. Mitophagy and the Release of Inflammatory Cytokines. Mitochondrion 2018, 41, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Park, S.; Koyanagi, A.; Jacob, L.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Kim, S.U.; Kim, B.K.; Shin, J.I.; et al. The Effect of Pharmacological Treatment and Lifestyle Modification in Patients with Nonalcoholic Fatty Liver Disease: An Umbrella Review of Meta-analyses of Randomized Controlled Trials. Obes. Rev. 2022, 23, e13464. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease (accessed on 13 April 2024).
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Karim, G.; Bansal, M.B. Resmetirom: An Orally Administered, Small-Molecule, Liver-Directed, β-Selective THR Agonist for the Treatment of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. Eur. Endocrinol. 2023, 19, 60. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, L.; Zhu, X.; Bian, H.; Gao, X.; Xia, M. Advances in Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: From Mechanisms to Therapeutics. Lipids Health Dis. 2024, 23, 95. [Google Scholar] [CrossRef]
- Heinle, J.W.; DiJoseph, K.; Sabag, A.; Oh, S.; Kimball, S.R.; Keating, S.; Stine, J.G. Exercise Is Medicine for Nonalcoholic Fatty Liver Disease: Exploration of Putative Mechanisms. Nutrients 2023, 15, 2452. [Google Scholar] [CrossRef] [PubMed]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of Beneficial Effects of Exercise Training on Non-alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The Effect of Exercise on Cytokines: Implications for Musculoskeletal Health: A Narrative Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef]
- Zhu, W.; Sahar, N.E.; Javaid, H.M.A.; Pak, E.S.; Liang, G.; Wang, Y.; Ha, H.; Huh, J.Y. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 2021, 10, 3306. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef]
- Zou, Y.-Y.; Tang, X.-B.; Chen, Z.-L.; Liu, B.; Zheng, L.; Song, M.-Y.; Xiao, Q.; Zhou, Z.-Q.; Peng, X.-Y.; Tang, C.-F. Exercise Intervention Improves Mitochondrial Quality in Non-Alcoholic Fatty Liver Disease Zebrafish. Front. Endocrinol. 2023, 14, 1162485. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Diniz, M.S.; Tocantins, C.; Grilo, L.F.; Pereira, S.P. The Bitter Side of Sugar Consumption: A Mitochondrial Perspective on Diabetes Development. Diabetology 2022, 3, 583–595. [Google Scholar] [CrossRef]
- Miryan, M.; Darbandi, M.; Moradi, M.; Najafi, F.; Soleimani, D.; Pasdar, Y. Relationship between the Mediterranean Diet and Risk of Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Analysis of the RaNCD. Cohort. Front. Nutr. 2023, 10, 1062008. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol Intake from a Mediterranean Diet Decreases Inflammatory Biomarkers Related to Atherosclerosis: A Substudy of the PREDIMED Trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and Mitochondria: An Update on Their Increasingly Emerging ROS-Scavenging Independent Actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shen, J.; Yuan, R.; Jia, B.; Zhang, Y.; Wang, S.; Zhang, Y.; Liu, M.; Wang, T. Mitochondrial Targeting Therapeutics: Promising Role of Natural Products in Non-Alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 796207. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, J.; Lenart, J.; Gaj, P.; Kolanowska, M.; Jazdzewski, K.; Stepien, P.P.; Buzanska, L. Bezafibrate Upregulates Mitochondrial Biogenesis and Influence Neural Differentiation of Human-Induced Pluripotent Stem Cells. Mol. Neurobiol. 2019, 56, 4346–4363. [Google Scholar] [CrossRef]
- Wang, S.-W.; Sheng, H.; Bai, Y.-F.; Weng, Y.-Y.; Fan, X.-Y.; Lou, L.-J.; Zhang, F. Neohesperidin Enhances PGC-1α-Mediated Mitochondrial Biogenesis and Alleviates Hepatic Steatosis in High Fat Diet Fed Mice. Nutr. Diabetes 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Duarte, F.V.; Gomes, A.P.; Varela, A.T.; Peixoto, F.M.; Rolo, A.P.; Palmeira, C.M. Berberine Reverts Hepatic Mitochondrial Dysfunction in High-Fat Fed Rats: A Possible Role for SirT3 Activation. Mitochondrion 2013, 13, 637–646. [Google Scholar] [CrossRef]
- Karimi, A.; Vajdi, M.; Sanaie, S.; Akhlaghi, S.; Negari, A.; Dashti, F.; Sefidmooye Azar, P. A Comprehensive Insight into the Effect of Berberine on Nonalcoholic Fatty Liver Disease (NAFLD): A Systematic Review. J. Food Biochem. 2023, 2023, 1–22. [Google Scholar] [CrossRef]
- Wang, Y.; Tai, Y.-L.; Zhao, D.; Zhang, Y.; Yan, J.; Kakiyama, G.; Wang, X.; Gurley, E.C.; Liu, J.; Liu, J.; et al. Berberine Prevents Disease Progression of Nonalcoholic Steatohepatitis through Modulating Multiple Pathways. Cells 2021, 10, 210. [Google Scholar] [CrossRef]
- Nie, Q.; Li, M.; Huang, C.; Yuan, Y.; Liang, Q.; Ma, X.; Qiu, T.; Li, J. The Clinical Efficacy and Safety of Berberine in the Treatment of Non-Alcoholic Fatty Liver Disease: A Meta-Analysis and Systematic Review. J. Transl. Med. 2024, 22, 225. [Google Scholar] [CrossRef]
- Lee, Y.M.; Choi, J.S.; Kim, M.H.; Jung, M.H.; Lee, Y.S.; Song, J. Effects of Dietary Genistein on Hepatic Lipid Metabolism and Mitochondrial Function in Mice Fed High-Fat Diets. Nutrition 2006, 22, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Seidemann, L.; Krüger, A.; Kegel-Hübner, V.; Seehofer, D.; Damm, G. Influence of Genistein on Hepatic Lipid Metabolism in an In Vitro Model of Hepatic Steatosis. Molecules 2021, 26, 1156. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, F.; Li, S.; Lu, B. Administration of Silymarin in NAFLD/NASH: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2024, 29, 101174. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Giudetti, A.M.; Gnoni, G.V.; Petrella, A.; Tamborra, R.; Romano, A.D.; Rollo, T.; Vendemiale, G.; Altomare, E. A Silybin-Phospholipid Complex Prevents Mitochondrial Dysfunction in a Rodent Model of Nonalcoholic Steatohepatitis. J. Pharmacol. Exp. Ther. 2010, 332, 922–932. [Google Scholar] [CrossRef] [PubMed]
- García-Berumen, C.I.; Vargas-Vargas, M.A.; Ortiz-Avila, O.; Piña-Zentella, R.M.; Ramos-Gómez, M.; Figueroa-García, M.d.C.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.R.; Saavedra-Molina, A.; Cortés-Rojo, C. Avocado Oil Alleviates Non-Alcoholic Fatty Liver Disease by Improving Mitochondrial Function, Oxidative Stress and Inflammation in Rats Fed a High Fat–High Fructose Diet. Front. Pharmacol. 2022, 13, 1089130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Nie, Y.; Wang, J. The Emerging Significance of Mitochondrial Targeted Strategies in NAFLD Treatment. Life Sci. 2023, 329, 121943. [Google Scholar] [CrossRef]
- Sulaimon, L.A.; Afolabi, L.O.; Adisa, R.A.; Ayankojo, A.G.; Afolabi, M.O.; Adewolu, A.M.; Wan, X. Pharmacological Significance of MitoQ in Ameliorating Mitochondria-Related Diseases. Adv. Redox Res. 2022, 5, 100037. [Google Scholar] [CrossRef]
- Williamson, J.; Hughes, C.M.; Cobley, J.N.; Davison, G.W. The Mitochondria-Targeted Antioxidant MitoQ, Attenuates Exercise-Induced Mitochondrial DNA Damage. Redox Biol. 2020, 36, 101673. [Google Scholar] [CrossRef]
- Chen, W.; Dream, S.; Leung, P.-Y.; Wu, P.-K.; Wong, S.; Park, J.-I. Selpercatinib Combination with the Mitochondria-Targeted Antioxidant MitoQ Effectively Suppresses RET–Mutant Thyroid Cancer. NPJ Precis. Oncol. 2024, 8, 39. [Google Scholar] [CrossRef]
- Rezaei, A.; Bahmani, H.R.; Mafakheri, S.; Farshad, A.; Nazari, P.; Masoudi, R. Protective Effects of Different Doses of MitoQ Separately and Combined with Trehalose on Oxidative Stress and Sperm Function of Cryopreserved Markhoz Goat Semen. Cryobiology 2023, 110, 36–43. [Google Scholar] [CrossRef]
- Oskuye, Z.Z.; Mehri, K.; Mokhtari, B.; Bafadam, S.; Nemati, S.; Badalzadeh, R. Cardioprotective Effect of Antioxidant Combination Therapy: A Highlight on MitoQ plus Alpha-Lipoic Acid Beneficial Impact on Myocardial Ischemia-Reperfusion Injury in Aged Rats. Heliyon 2024, 10, e28158. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl. Sci. 2019, 4, 147–157. [Google Scholar] [CrossRef]
- Allen, M.E.; Pennington, E.R.; Perry, J.B.; Dadoo, S.; Makrecka-Kuka, M.; Dambrova, M.; Moukdar, F.; Patel, H.D.; Han, X.; Kidd, G.K.; et al. The Cardiolipin-Binding Peptide Elamipretide Mitigates Fragmentation of Cristae Networks Following Cardiac Ischemia Reperfusion in Rats. Commun. Biol. 2020, 3, 389. [Google Scholar] [CrossRef] [PubMed]
- Pharaoh, G.; Kamat, V.; Kannan, S.; Stuppard, R.S.; Whitson, J.; Martín-Pérez, M.; Qian, W.-J.; MacCoss, M.J.; Villén, J.; Rabinovitch, P.; et al. The Mitochondrially Targeted Peptide Elamipretide (SS-31) Improves ADP Sensitivity in Aged Mitochondria by Increasing Uptake through the Adenine Nucleotide Translocator (ANT). GeroScience 2023, 45, 3529–3548. [Google Scholar] [CrossRef]
- Shetty, S.; Anushree, U.; Kumar, R.; Bharati, S. Mitochondria-Targeted Antioxidant, Mito-TEMPO Mitigates Initiation Phase of N-Nitrosodiethylamine-Induced Hepatocarcinogenesis. Mitochondrion 2021, 58, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-F.; Xie, K.; Cao, Y.-X.; Zhang, A. Hepatoprotective Effect of Mitochondria-Targeted Antioxidant Mito-TEMPO against Lipopolysaccharide-Induced Liver Injury in Mouse. Mediat. Inflamm. 2022, 2022, 6394199. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Ma, P.-W.; Yuan, H.; Wang, W.-L.; Lu, P.-H.; Ding, X.-R.; Lun, Y.-Q.; Yang, Q.; Lu, L.-J. Mito-TEMPO Attenuates Oxidative Stress and Mitochondrial Dysfunction in Noise-Induced Hearing Loss via Maintaining TFAM-mtDNA Interaction and Mitochondrial Biogenesis. Front. Cell. Neurosci. 2022, 16, 803718. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications—A Review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular Mechanism of PPARα Action and Its Impact on Lipid Metabolism, Inflammation and Fibrosis in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Li, Y.; Wu, S.; Luo, J.; Yang, H.; Subbiah, R.; Chatham, J.; Zhelyabovska, O.; Yang, Q. Peroxisome Proliferator-Activated Receptor δ Is an Essential Transcriptional Regulator for Mitochondrial Protection and Biogenesis in Adult Heart. Circ. Res. 2010, 106, 911–919. [Google Scholar] [CrossRef]
- Corona, J.C.; Duchen, M.R. PPARγ as a Therapeutic Target to Rescue Mitochondrial Function in Neurological Disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Bowlus, C.L.; Levy, C.; Akarca, U.S.; Alvares-da-Silva, M.R.; Andreone, P.; Arrese, M.; Corpechot, C.; Francque, S.M.; Heneghan, M.A.; et al. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N. Engl. J. Med. 2024, 390, 795–805. [Google Scholar] [CrossRef]
- Zhang, M.; Barroso, E.; Ruart, M.; Peña, L.; Peyman, M.; Aguilar-Recarte, D.; Montori-Grau, M.; Rada, P.; Cugat, C.; Montironi, C.; et al. Elafibranor Upregulates the EMT-Inducer S100A4 via PPARβ/δ. Biomed. Pharmacother. 2023, 167, 115623. [Google Scholar] [CrossRef] [PubMed]
- Westerouen Van Meeteren, M.J.; Drenth, J.P.H.; Tjwa, E.T.T.L. Elafibranor: A Potential Drug for the Treatment of Nonalcoholic Steatohepatitis (NASH). Expert Opin. Investig. Drugs 2020, 29, 117–123. [Google Scholar] [CrossRef]
- Boeckmans, J.; Buyl, K.; Natale, A.; Vandenbempt, V.; Branson, S.; De Boe, V.; Rogiers, V.; De Kock, J.; Rodrigues, R.M.; Vanhaecke, T. Elafibranor Restricts Lipogenic and Inflammatory Responses in a Human Skin Stem Cell-Derived Model of NASH. Pharmacol. Res. 2019, 144, 377–389. [Google Scholar] [CrossRef] [PubMed]
- RESOLVE-IT Phase 3 of Elafibranor in NASH: Final Results of the Week 72 Interim Surrogate Efficacy Analysis. Natap.org. Available online: https://www.natap.org/2020/AASLD/AASLD_162.htm (accessed on 17 April 2024).
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diab. Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Ndakotsu, A.; Vivekanandan, G. The Role of Thiazolidinediones in the Amelioration of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e25380. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Genua, I.; Cusi, K. Pharmacological Approaches to Nonalcoholic Fatty Liver Disease: Current and Future Therapies. Diabetes Spectr. 2024, 37, 48–58. [Google Scholar] [CrossRef]
- Fujisawa, K.; Nishikawa, T.; Kukidome, D.; Imoto, K.; Yamashiro, T.; Motoshima, H.; Matsumura, T.; Araki, E. TZDs Reduce Mitochondrial ROS Production and Enhance Mitochondrial Biogenesis. Biochem. Biophys. Res. Commun. 2009, 379, 43–48. [Google Scholar] [CrossRef]
- Skov, V.; Glintborg, D.; Knudsen, S.; Tan, Q.; Jensen, T.; Kruse, T.A.; Beck-Nielsen, H.; Højlund, K. Pioglitazone Enhances Mitochondrial Biogenesis and Ribosomal Protein Biosynthesis in Skeletal Muscle in Polycystic Ovary Syndrome. PLoS ONE 2008, 3, e2466. [Google Scholar] [CrossRef] [PubMed]
- Kalavalapalli, S.; Bril, F.; Koelmel, J.P.; Abdo, K.; Guingab, J.; Andrews, P.; Li, W.-Y.; Jose, D.; Yost, R.A.; Frye, R.F.; et al. Pioglitazone Improves Hepatic Mitochondrial Function in a Mouse Model of Nonalcoholic Steatohepatitis. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hodges, W.T.; Jarasvaraparn, C.; Ferguson, D.; Griffett, K.; Gill, L.E.; Chen, Y.; Ilagan, M.X.G.; Hegazy, L.; Elgendy, B.; Cho, K.; et al. Mitochondrial Pyruvate Carrier Inhibitors Improve Metabolic Parameters in Diet-Induced Obese Mice. J. Biol. Chem. 2022, 298, 101554. [Google Scholar] [CrossRef] [PubMed]
- Kamm, D.R.; Pyles, K.D.; Sharpe, M.C.; Healy, L.N.; Colca, J.R.; McCommis, K.S. Novel Insulin Sensitizer MSDC-0602K Improves Insulinemia and Fatty Liver Disease in Mice, Alone and in Combination with Liraglutide. J. Biol. Chem. 2021, 296, 100807. [Google Scholar] [CrossRef] [PubMed]
- Benova, A.; Ferencakova, M.; Bardova, K.; Funda, J.; Prochazka, J.; Spoutil, F.; Cajka, T.; Dzubanova, M.; Balcaen, T.; Kerckhofs, G.; et al. Novel Thiazolidinedione Analog Reduces a Negative Impact on Bone and Mesenchymal Stem Cell Properties in Obese Mice Compared to Classical Thiazolidinediones. Mol. Metab. 2022, 65, 101598. [Google Scholar] [CrossRef] [PubMed]
- A Study of MSDC-0602K to Assess Glycemic Control and Cardiovascular Outcomes in Patients with Pre-T2D or T2D and NAFLD/NASH. Clinicaltrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03970031 (accessed on 21 April 2024).
- Akbari, R.; Behdarvand, T.; Afarin, R.; Yaghooti, H.; Jalali, M.T.; Mohammadtaghvaei, N. Saroglitazar Improved Hepatic Steatosis and Fibrosis by Modulating Inflammatory Cytokines and Adiponectin in an Animal Model of Non-Alcoholic Steatohepatitis. BMC Pharmacol. Toxicol. 2021, 22, 53. [Google Scholar] [CrossRef]
- Kumar, D.P.; Caffrey, R.; Marioneaux, J.; Santhekadur, P.K.; Bhat, M.; Alonso, C.; Koduru, S.V.; Philip, B.; Jain, M.R.; Giri, S.R.; et al. The PPAR α/γ Agonist Saroglitazar Improves Insulin Resistance and Steatohepatitis in a Diet Induced Animal Model of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 9330. [Google Scholar] [CrossRef] [PubMed]
- Goyal, O.; Nohria, S.; Goyal, P.; Kaur, J.; Sharma, S.; Sood, A.; Chhina, R.S. Saroglitazar in Patients with Non-Alcoholic Fatty Liver Disease and Diabetic Dyslipidemia: A Prospective, Observational, Real World Study. Sci. Rep. 2020, 10, 21117. [Google Scholar] [CrossRef] [PubMed]
- A Phase 3 Study Evaluating Efficacy and Safety of Lanifibranor followed by an Active Treatment Extension in Adult Patients with (NASH) and Fibrosis Stages F2 and F3 (NATiV3). Clinicaltrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04849728 (accessed on 21 April 2024).
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef]
- Young, C.F.; Farnoudi, N.; Chen, J.; Shubrook, J.H. Exploring SGLT-2 Inhibitors: Benefits beyond the Glucose-Lowering Effect—What Is New in 2023? Endocrines 2023, 4, 630–655. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Starinets, V.S.; Belosludtsev, M.N.; Mikheeva, I.B.; Dubinin, M.V.; Belosludtseva, N.V. Chronic Treatment with Dapagliflozin Protects against Mitochondrial Dysfunction in the Liver of C57BL/6NCrl Mice with High-Fat Diet/Streptozotocin-Induced Diabetes Mellitus. Mitochondrion 2021, 59, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. Empagliflozin Attenuates Doxorubicin-Induced Cardiotoxicity by Activating AMPK/SIRT-1/PGC-1α-Mediated Mitochondrial Biogenesis. Toxicol. Res. 2023, 12, 216–223. [Google Scholar] [CrossRef]
- Martínez-Rojas, M.Á.; Balcázar, H.; González-Soria, I.; González-Rivera, J.M.; Rodríguez-Vergara, M.E.; Velazquez-Villegas, L.A.; León-Contreras, J.C.; Pérez-Villalva, R.; Correa, F.; Rosetti, F.; et al. Transient Inhibition of Sodium-Glucose Cotransporter 2 after Ischemia/Reperfusion Injury Ameliorates Chronic Kidney Disease. JCI Insight 2024, 9, e173675. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.; Wang, J.; Zhang, Y.; Wan, B.; Ke, Z.; Ren, Z.; Yang, X.; Lei, M.; Guo, X.; Liu, X.; et al. Dapagliflozin Improves Diabetic Cognitive Impairment via Indirectly Modulating the Mitochondria Homeostasis of Hippocampus in Diabetic Mice. Biofactors 2024, 50, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Huo, J.; Jiang, W.; Yang, W.; Wang, S.; Zhang, S.; Cheng, Y.; Jiang, Z.; Shan, Q. Empagliflozin Ameliorates Cardiac Dysfunction in Heart Failure Mice via Regulating Mitochondrial Dynamics. Eur. J. Pharmacol. 2023, 942, 175531. [Google Scholar] [CrossRef] [PubMed]
- Ala, M.; Khoshdel, M.R.F.; Dehpour, A.R. Empagliflozin Enhances Autophagy, Mitochondrial Biogenesis, and Antioxidant Defense and Ameliorates Renal Ischemia/Reperfusion in Nondiabetic Rats. Oxidative Med. Cell. Longev. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Zhuravlev, A.D.; Kartuesov, A.G.; Borisov, E.E.; Sukhorukov, V.N.; Orekhov, A.N. Mitochondria-Mediated Cardiovascular Benefits of Sodium-Glucose Co-Transporter 2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 5371. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef]
- Gager, G.M.; von Lewinski, D.; Sourij, H.; Jilma, B.; Eyileten, C.; Filipiak, K.; Hülsmann, M.; Kubica, J.; Postula, M.; Siller-Matula, J.M. Effects of SGLT2 Inhibitors on Ion Homeostasis and Oxidative Stress Associated Mechanisms in Heart Failure. Biomed. Pharmacother. 2021, 143, 112169. [Google Scholar] [CrossRef]
- Koizumi, T.; Watanabe, M.; Yokota, T.; Tsuda, M.; Handa, H.; Koya, J.; Nishino, K.; Tatsuta, D.; Natsui, H.; Kadosaka, T.; et al. Empagliflozin Suppresses Mitochondrial Reactive Oxygen Species Generation and Mitigates the Inducibility of Atrial Fibrillation in Diabetic Rats. Front. Cardiovasc. Med. 2023, 10, 1005408. [Google Scholar] [CrossRef]
- Belosludtseva, N.V.; Starinets, V.S.; Belosludtsev, K.N. Effect of Dapagliflozin on the Functioning of Rat Liver Mitochondria In Vitro. Bull. Exp. Biol. Med. 2021, 171, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Morishita, A.; Tadokoro, T.; Fujihara, S.; Iwama, H.; Oura, K.; Fujita, K.; Tani, J.; Takuma, K.; Nakahara, M.; Shi, T.; et al. Ipragliflozin Attenuates Non-Alcoholic Steatohepatitis Development in an Animal Model. PLoS ONE 2022, 17, e0261310. [Google Scholar] [CrossRef]
- Suga, T.; Sato, K.; Ohyama, T.; Matsui, S.; Kobayashi, T.; Tojima, H.; Horiguchi, N.; Yamazaki, Y.; Kakizaki, S.; Nishikido, A.; et al. Ipragliflozin-Induced Improvement of Liver Steatosis in Obese Mice May Involve Sirtuin Signaling. World J. Hepatol. 2020, 12, 350–362. [Google Scholar] [CrossRef]
- Jang, H.; Kim, Y.; Lee, D.H.; Joo, S.K.; Koo, B.K.; Lim, S.; Lee, W.; Kim, W. Outcomes of Various Classes of Oral Antidiabetic Drugs on Nonalcoholic Fatty Liver Disease. JAMA Intern. Med. 2024, 184, 375. [Google Scholar] [CrossRef] [PubMed]
- Inventiva Announces Positive Results from the Phase II, LEGEND, Proof-of-Concept Study Combining Lanifibranor with Empagliflozin in Patients with MASH/NASH and T2D. BioSpace. Available online: https://www.biospace.com/article/releases/inventiva-announces-positive-results-from-the-phase-ii-legend-proof-of-concept-study-combining-lanifibranor-with-empagliflozin-in-patients-with-mash-nash-and-t2d/ (accessed on 23 April 2024).
- Chun, H.J.; Kim, E.R.; Lee, M.; Choi, D.H.; Kim, S.H.; Shin, E.; Kim, J.-H.; Cho, J.W.; Han, D.H.; Cha, B.-S.; et al. Increased Expression of Sodium-Glucose Cotransporter 2 and O-GlcNAcylation in Hepatocytes Drives Non-Alcoholic Steatohepatitis. Metabolism 2023, 145, 155612. [Google Scholar] [CrossRef]
- Cheung, K.S.; Ng, H.Y.; Hui, R.W.H.; Lam, L.K.; Mak, L.Y.; Ho, Y.C.; Tan, J.T.; Chan, E.W.; Seto, W.K.; Yuen, M.F.; et al. Effects of Empagliflozin on Liver Fat in Metabolic-Dysfunction Associated Steatotic Liver Disease Patients without Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]
- Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D’Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F.; et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14, 186. [Google Scholar] [CrossRef]
- Rey, V.; Tamargo-Gómez, I. From Kinases to Diseases: Investigating the Role of AMPK in Human Pathologies. Kinases Phosphatases 2023, 1, 181–205. [Google Scholar] [CrossRef]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Geng, Y.; Hernández Villanueva, A.; Oun, A.; Buist-Homan, M.; Blokzijl, H.; Faber, K.N.; Dolga, A.; Moshage, H. Protective Effect of Metformin against Palmitate-Induced Hepatic Cell Death. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165621. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Butrico, G.M.; Kalpage, H.A.; Goedeke, L.; Hubbard, B.T.; Vatner, D.F.; Gaspar, R.C.; Zhang, X.-M.; Cline, G.W.; Nakahara, K.; et al. Metformin, Phenformin, and Galegine Inhibit Complex IV Activity and Reduce Glycerol-Derived Gluconeogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2122287119. [Google Scholar] [CrossRef]
- de Marañón, A.M.; Díaz-Pozo, P.; Canet, F.; Díaz-Morales, N.; Abad-Jiménez, Z.; López-Domènech, S.; Vezza, T.; Apostolova, N.; Morillas, C.; Rocha, M.; et al. Metformin Modulates Mitochondrial Function and Mitophagy in Peripheral Blood Mononuclear Cells from Type 2 Diabetic Patients. Redox Biol. 2022, 53, 102342. [Google Scholar] [CrossRef]
- Xu, J.; Kitada, M.; Koya, D. The Impact of Mitochondrial Quality Control by Sirtuins on the Treatment of Type 2 Diabetes and Diabetic Kidney Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165756. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, D.; Gong, Q.; Xu, Q.; Pan, D.; Lu, F.; Tang, Q. Elucidation of SIRT-1/PGC-1α-Associated Mitochondrial Dysfunction and Autophagy in Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yan, W.-Y.; Lei, Y.-H.; Wan, Z.; Hou, Y.-Y.; Sun, L.-K.; Zhou, J.-P. SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, T.; Chen, J.; Fan, Z.; Gao, F.; Yu, Z.; Jiang, Y. SIRT3/6: An Amazing Challenge and Opportunity in the Fight against Fibrosis and Aging. Cell. Mol. Life Sci. 2024, 81, 69. [Google Scholar] [CrossRef]
- Chang, N.; Li, J.; Lin, S.; Zhang, J.; Zeng, W.; Ma, G.; Wang, Y. Emerging Roles of SIRT1 Activator, SRT2104, in Disease Treatment. Sci. Rep. 2024, 14, 5521. [Google Scholar] [CrossRef]
- Lambona, C.; Zwergel, C.; Valente, S.; Mai, A. SIRT3 Activation a Promise in Drug Development? New Insights into SIRT3 Biology and Its Implications on the Drug Discovery Process. J. Med. Chem. 2024, 67, 1662–1689. [Google Scholar] [CrossRef]
- Zang, M.; Gao, B. SIRT6: Therapeutic Target for Nonalcoholic Fatty Liver Disease. Trends Endocrinol. Metab. 2022, 33, 801–803. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bezsonov, E.E.; Baig, M.S.; Popkova, T.V.; Nedosugova, L.V.; Starodubova, A.V.; Orekhov, A.N. Mitochondrial Mutations and Genetic Factors Determining NAFLD Risk. Int. J. Mol. Sci. 2021, 22, 4459. [Google Scholar] [CrossRef] [PubMed]
- Newman, N.J.; Yu-Wai-Man, P.; Subramanian, P.S.; Moster, M.L.; Wang, A.-G.; Donahue, S.P.; Leroy, B.P.; Carelli, V.; Biousse, V.; Vignal-Clermont, C.; et al. Randomized Trial of Bilateral Gene Therapy Injection for m.11778G>A MT-ND4 Leber Optic Neuropathy. Brain 2023, 146, 1328–1341. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretić, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches. Antioxidants 2024, 13, 906. https://doi.org/10.3390/antiox13080906
Radosavljevic T, Brankovic M, Samardzic J, Djuretić J, Vukicevic D, Vucevic D, Jakovljevic V. Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches. Antioxidants. 2024; 13(8):906. https://doi.org/10.3390/antiox13080906
Chicago/Turabian StyleRadosavljevic, Tatjana, Milica Brankovic, Janko Samardzic, Jasmina Djuretić, Dusan Vukicevic, Danijela Vucevic, and Vladimir Jakovljevic. 2024. "Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches" Antioxidants 13, no. 8: 906. https://doi.org/10.3390/antiox13080906
APA StyleRadosavljevic, T., Brankovic, M., Samardzic, J., Djuretić, J., Vukicevic, D., Vucevic, D., & Jakovljevic, V. (2024). Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches. Antioxidants, 13(8), 906. https://doi.org/10.3390/antiox13080906








