Chromosome Segregation–1–like Gene Participates in Ferroptosis in Human Ovarian Granulosa Cells via Nucleocytoplasmic Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. Targeted Panel Sequencing
2.2. Animals and Ethics Statement
2.3. Cell Culture
2.4. Transfection
2.5. RAN–Seq and Analysis
2.6. Immunofluorescence
2.7. Cell Viability Assessment
2.8. Reactive Oxygen Species Assessment
2.9. Malonaldehyde Assessment
2.10. Ferrous Iron Detection
2.11. Transmission Electron Microscopy
2.12. RNA Extraction and qRT–PCR Assay
2.13. Western Blot
2.14. Chromatin Immunoprecipitation Assay
2.15. Coimmunoprecipitation
2.16. Cumulus Cell Monolayer–Denuded Oocyte In Vitro Co–Culture
2.17. Statistical Analysis
3. Results
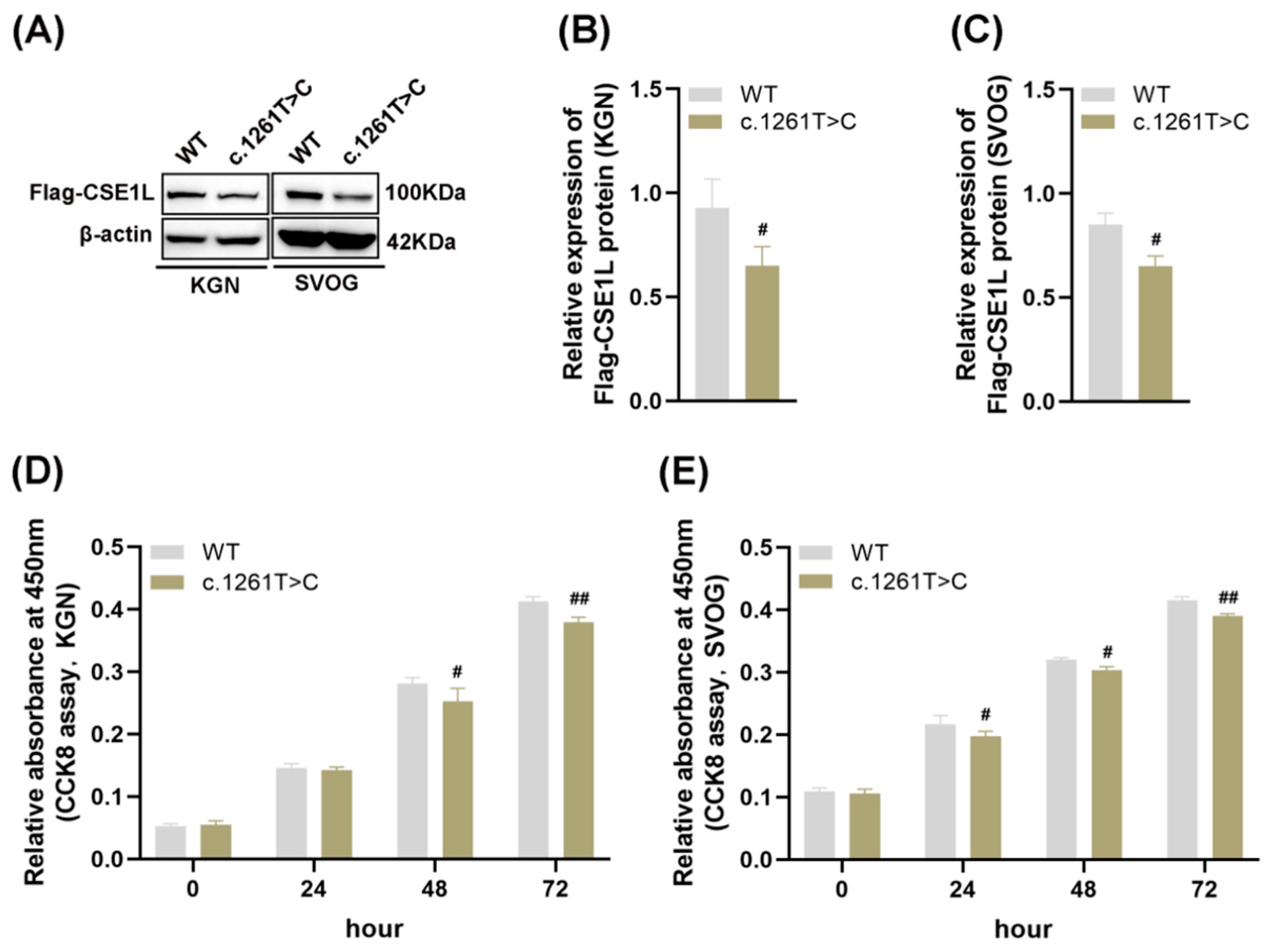
3.1. Pathogenetic Variant of CSE1L Identified in POI Patients
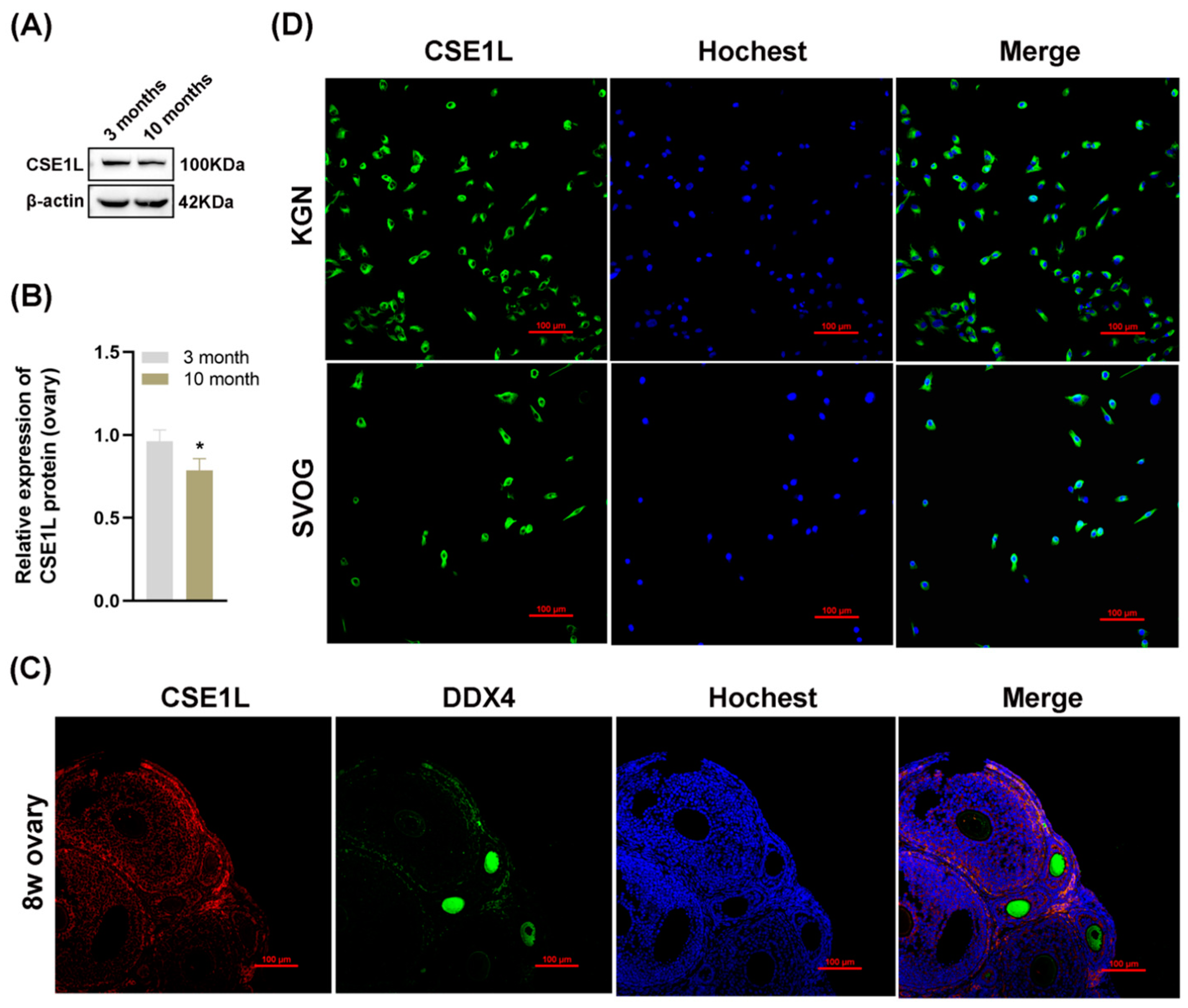
3.2. The Expression of CSE1L was Downregulated in Aged Mice Ovaries
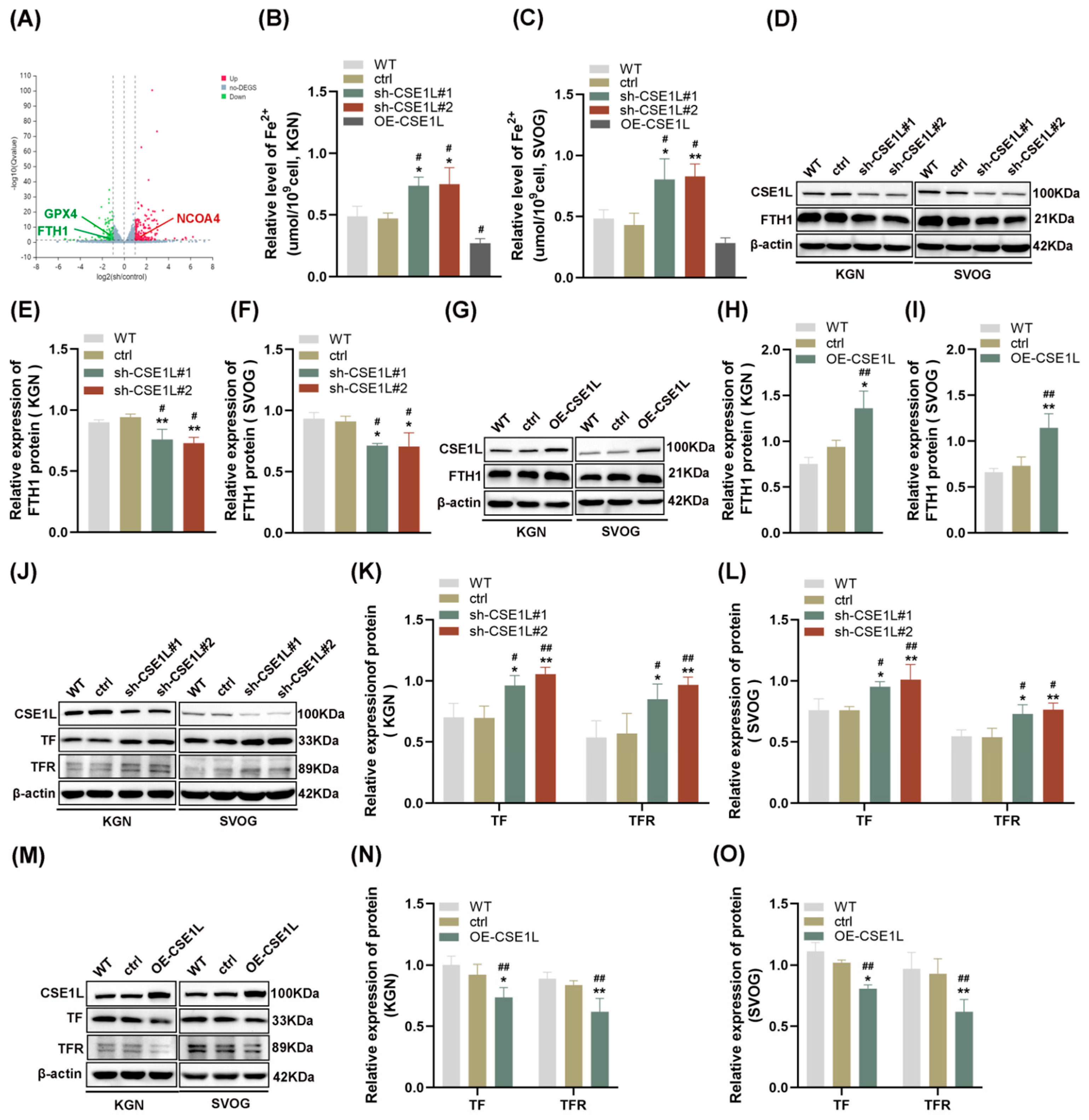
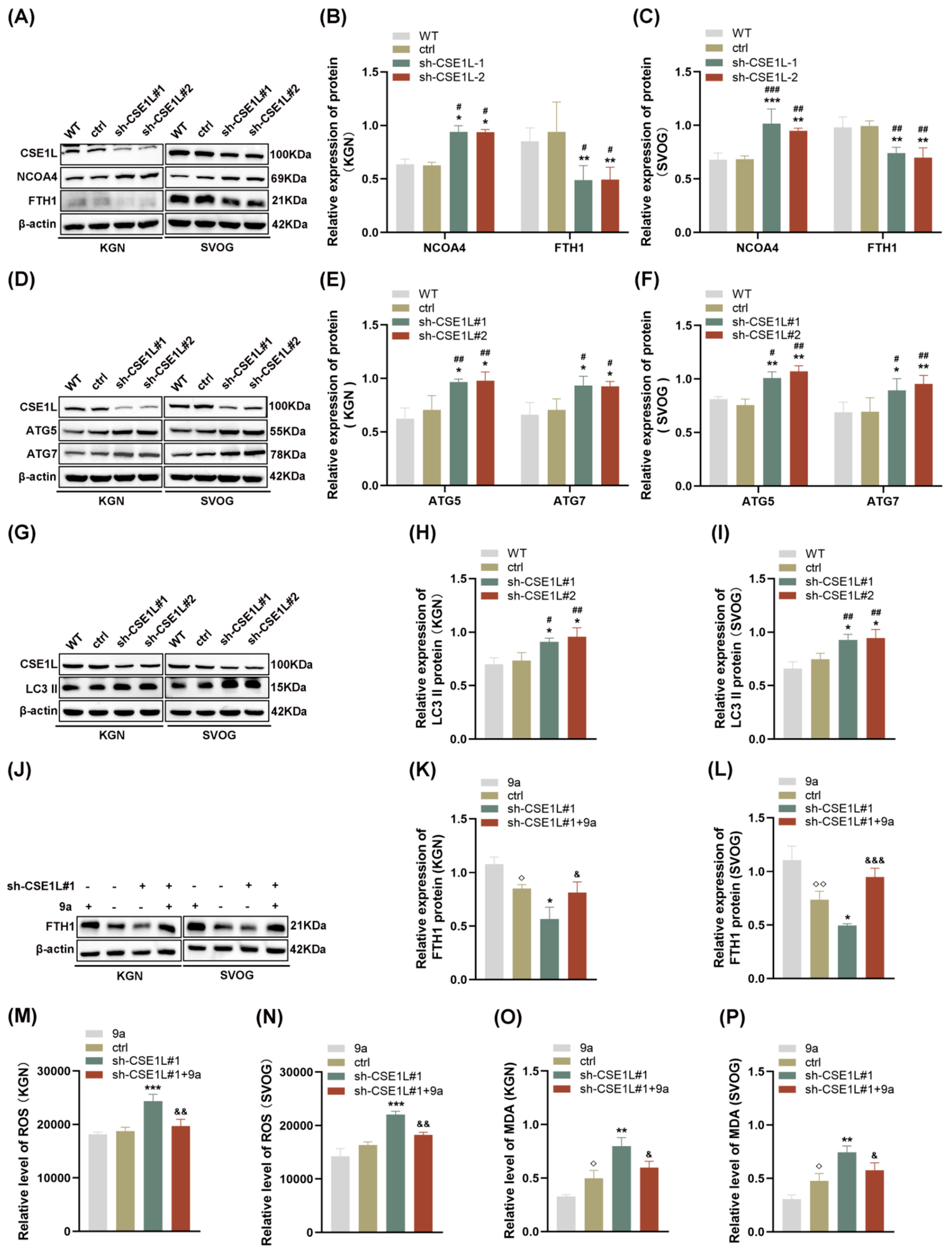
3.3. CSE1L Silence–Affected Iron Metabolism in Human Ovarian Granulosa Cells
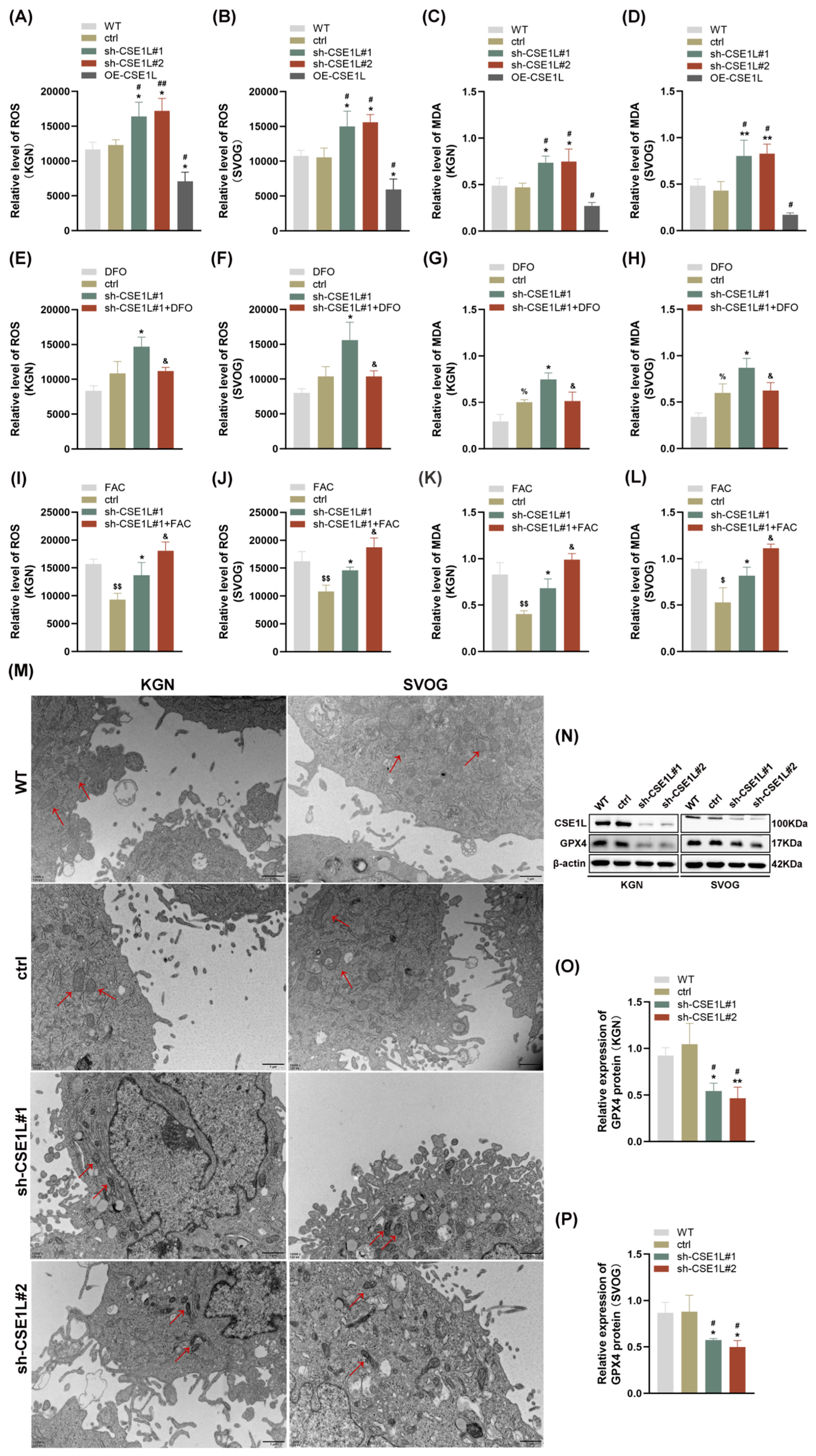
3.4. Intracellular Iron Overload Induced by CSE1L Silence Promoted Ferroptosis in Human Ovarian Granulosa Cells
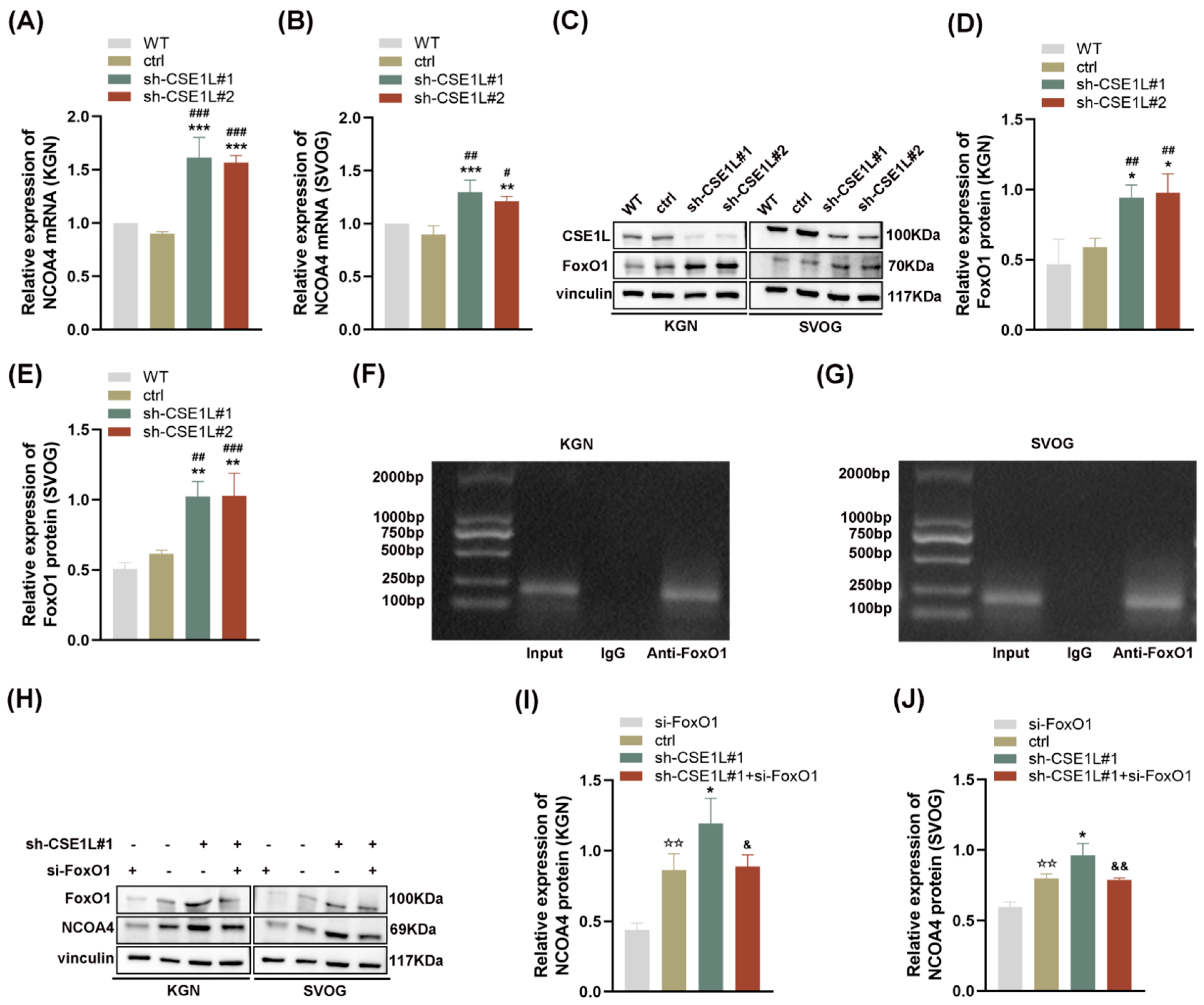
3.5. CSE1L Silence Promoted Ferroptosis via Activating NCOA4–Mediated Ferritinophagy
3.6. CSE1L Participated in Ferritinophagy via Nucleocytoplasmic Transport
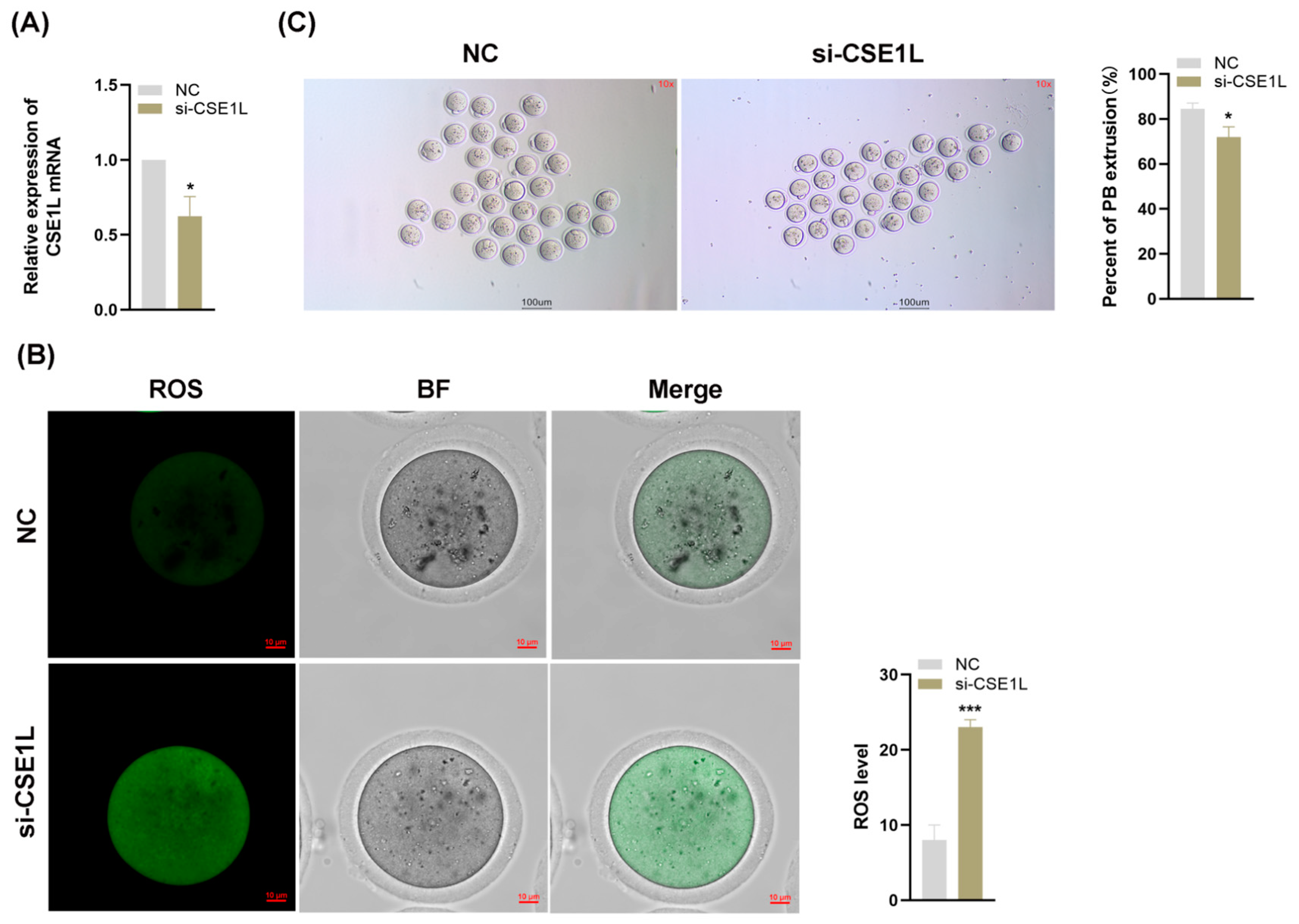
3.7. Inhibition of CSE1L in Cumulus Cell Monolayer Impeded Oocyte Maturation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E.; Janse, F.; et al. ESHRE Guideline: Management of women with premature ovarian insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tan, R.; He, Y.; Wang, H.; Pu, D.; Wu, J. Stem cell therapy for premature ovarian insufficiency: A systematic review and meta-analysis of animal and clinical studies. Arch. Gynecol. Obstet. 2024, 309, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Tang, S.; Guo, T.; Hou, D.; Jiao, X.; Li, S.; Luo, W.; Xu, B.; Zhao, S.; Li, G.; et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat. Med. 2023, 29, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Uddin, M.H.; Mohammad, R.M. The nuclear export protein XPO1—from biology to targeted therapy. Nat. Rev. Clin. Oncol. 2021, 18, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Maruyama, J.; Honda, K.; Kondoh, Y.; Osada, H.; Nawa, M.; Nakahama, K.I.; Ishigami-Yuasa, M.; Kagechika, H.; Sugimura, H.; et al. CSE1L promotes nuclear accumulation of transcriptional coactivator TAZ and enhances invasiveness of human cancer cells. J. Biol. Chem. 2021, 297, 100803. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Li, X.; Wang, C.Z.; Xu, S.; Yuan, G.; Shao, W.; Liu, B.; Zheng, Y.; Wang, H.; Lei, X.; et al. Roles of the CSE1L-mediated nuclear import pathway in epigenetic silencing. Proc. Natl. Acad. Sci. USA 2018, 115, E4013–E4022. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Sepehrimanesh, M. Nucleocytoplasmic Transport: Regulatory Mechanisms and the Implications in Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 4165. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Li, J.; Cheng, D.D.; Zhang, X.; Yu, T.; Zhao, F.Y.; Geng, Q.; Zhu, M.X.; Kong, H.W.; Li, H.; et al. Nuclear export protein CSE1L interacts with P65 and promotes NSCLC growth via NF-κB/MAPK pathway. Mol. Ther. Oncolytics 2021, 21, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.; Young, A.N.; Huntsman, A.C.; Pergande, M.R.; Korkmaz, M.A.; Rathnayake, R.A.; Mize, B.K.; Kinghorn, A.D.; Zhang, X.; Ratia, K.; et al. PHY34 inhibits autophagy through V-ATPase V0A2 subunit inhibition and CAS/CSE1L nuclear cargo trafficking in high grade serous ovarian cancer. Cell Death Dis. 2022, 13, 45. [Google Scholar] [CrossRef]
- Liao, C.F.; Lin, S.H.; Chen, H.C.; Tai, C.J.; Chang, C.C.; Li, L.T.; Yeh, C.M.; Yeh, K.T.; Chen, Y.C.; Hsu, T.H.; et al. CSE1L, a novel microvesicle membrane protein, mediates Ras-triggered microvesicle generation and metastasis of tumor cells. Mol. Med. 2012, 18, 1269–1280. [Google Scholar] [CrossRef]
- Bera, T.K.; Bera, J.; Brinkmann, U.; Tessarollo, L.; Pastan, I. Cse1l is essential for early embryonic growth and development. Mol. Cell. Biol. 2001, 21, 7020–7024. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, J.; Xu, K.; Sun, X.; Zhang, H.; Xiong, C. CSE1L participates in regulating cell mitosis in human seminoma. Cell Prolif. 2019, 52, e12549. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qiao, F.; Ye, M.; Jiang, T.; Liu, J.; Zhang, M.; Xie, G.; Fok, K.L.; Li, X.; Chen, H. CSE1L/CAS regulates cell proliferation through CDK signalling in mouse spermatogenesis. Cell Prolif. 2022, 55, e13334. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhang, J.; Gu, Z.; Chen, Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials 2019, 211, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, K.; Wu, Y. The emerging role of ferroptosis in female reproductive disorders. Biomed. Pharmacother. 2023, 166, 115415. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.A.; Luque-Ramírez, M.; San-Millán, J.L.; Escobar-Morreale, H.F. Body iron stores and glucose intolerance in premenopausal women: Role of hyperandrogenism, insulin resistance, and genomic variants related to inflammation, oxidative stress, and iron metabolism. Diabetes Care 2009, 32, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Luque-Ramírez, M.; Álvarez-Blasco, F.; Alpañés, M.; Escobar-Morreale, H.F. Role of decreased circulating hepcidin concentrations in the iron excess of women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2011, 96, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.M.; López-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Jia, W.; Liu, G.; Zhang, J.; Wang, B.; Li, J.; Cui, P.; Li, X.; Lager, S.; et al. Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J. Endocrinol. 2020, 246, 247–263. [Google Scholar] [CrossRef]
- Du, R.; Cheng, X.; Ji, J.; Lu, Y.; Xie, Y.; Wang, W.; Xu, Y.; Zhang, Y. Mechanism of ferroptosis in a rat model of premature ovarian insufficiency induced by cisplatin. Sci. Rep. 2023, 13, 4463. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Huang, Q.; Fu, X.; Zhang, L.; Gao, F.; Jin, Z.; Wu, L.; Shu, C.; Zhang, X.; et al. Iron overload in endometriosis peritoneal fluid induces early embryo ferroptosis mediated by HMOX1. Cell Death Discov. 2021, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jing, Y.; Zhang, C.; Ma, S.; Zhang, W.; Huang, D.; Zhang, B.; Zuo, Y.; Qin, Y.; Liu, G.H.; et al. Aging hallmarks of the primate ovary revealed by spatiotemporal transcriptomics. Protein Cell 2023, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.C.; Li, Y.Q.; Zhou, B.W.; Xia, Q.C.; Wang, P.X.; Wang, X.X.; Sun, Z.G.; Guo, Y. Transcriptomic profiling of human granulosa cells between women with advanced maternal age with different ovarian reserve. J. Assist. Reprod. Genet. 2023, 40, 2427–2437. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Ni, F.; Jin, J.; Wu, Y.; Huang, Y.; Ye, X.; Shen, X.; Ying, Y.; Chen, J.; et al. BNC1 deficiency-triggered ferroptosis through the NF2-YAP pathway induces primary ovarian insufficiency. Nat. Commun. 2022, 13, 5871. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, X.; Zhao, Y.; Chen, R. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct. Target. Ther. 2023, 8, 115. [Google Scholar] [CrossRef]
- Illés, E.; Patra, S.G.; Marks, V.; Mizrahi, A.; Meyerstein, D. The Fe(II)(citrate) Fenton reaction under physiological conditions. J. Inorg. Biochem. 2020, 206, 111018. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Moura, M.T.; Latorraca, L.B.; Paula-Lopes, F.F. Contextualizing Autophagy during Gametogenesis and Preimplantation Embryonic Development. Int. J. Mol. Sci. 2021, 22, 6313. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Inhibiting Ferroptosis through Disrupting the NCOA4-FTH1 Interaction: A New Mechanism of Action. ACS Cent. Sci. 2021, 7, 980–989. [Google Scholar] [CrossRef]
- Wang, J.J.; Ge, W.; Zhai, Q.Y.; Liu, J.C.; Sun, X.W.; Liu, W.X.; Li, L.; Lei, C.Z.; Dyce, P.W.; De Felici, M.; et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol. 2020, 18, e3001025. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Fu, Y.H.; Han, J.; Shen, M.; Du, C.W.; Li, R.; Ma, X.S.; Liu, H.L. Changes in the expression of Fox O1 and death ligand genes during follicular atresia in porcine ovary. Genet. Mol. Res. GMR 2014, 13, 6638–6645. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zuo, W.; Yan, G.; Wang, S.; Sun, S.; Li, S.; Tang, X.; Li, Y.; Cai, C.; Wang, H.; et al. Granulosa cell mevalonate pathway abnormalities contribute to oocyte meiotic defects and aneuploidy. Nat. Aging 2023, 3, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.J.; Umair, Z.; Yoon, M.S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Li, Y.; Song, D.; Ding, J.; Mei, S.; Sun, S.; Cheng, W.; Yu, J.; Zhou, L.; Kuang, Y.; et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. 2022, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, J.; Zeng, L.; Yang, Q.; Bai, F.; Mai, Q.; Deng, K. Human mesenchymal stem cells derived exosomes improve ovarian function in chemotherapy-induced premature ovarian insufficiency mice by inhibiting ferroptosis through Nrf2/GPX4 pathway. J. Ovarian Res. 2024, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Li, H.; Mu, H.; Zeng, L.; Cai, S.; Su, P.; Li, H.; Zhang, L.; Xiang, W. miR-484 mediates oxidative stress-induced ovarian dysfunction and promotes granulosa cell apoptosis via SESN2 downregulation. Redox Biol. 2023, 62, 102684. [Google Scholar] [CrossRef] [PubMed]
- Sreerangaraja Urs, D.B.; Wu, W.H.; Komrskova, K.; Postlerova, P.; Lin, Y.F.; Tzeng, C.R.; Kao, S.H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Duan, C.C.; Jin, S.; Sheng, C.B.; Wang, Y.S.; Yue, Z.P.; Guo, B. HB-EGF induces mitochondrial dysfunction via estrogen hypersecretion in granulosa cells dependent on cAMP-PKA-JNK/ERK-Ca(2+)-FOXO1 pathway. Int. J. Biol. Sci. 2022, 18, 2047–2059. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, W.; Chen, S.; Wang, H.; Zhong, C.; Gao, L.; Cui, Y.; Pu, D.; Tan, R.; Wu, J. CLPP inhibition triggers apoptosis in human ovarian granulosa cells via COX5A abnormality-Mediated mitochondrial dysfunction. Front. Genet. 2023, 14, 1141167. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Hou, L.; Guo, Z.; Wang, G.; Guo, J.; Xu, J.; Zhang, X.; Guo, F. JNK-JUN-NCOA4 axis contributes to chondrocyte ferroptosis and aggravates osteoarthritis via ferritinophagy. Free Radic. Biol. Med. 2023, 200, 87–101. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Hong, T.; He, Y.; Wang, H.; Cao, J.; Pu, D.; Gao, L.; Gao, C.; Cui, Y.; Wu, J.; et al. Chromosome Segregation–1–like Gene Participates in Ferroptosis in Human Ovarian Granulosa Cells via Nucleocytoplasmic Transport. Antioxidants 2024, 13, 911. https://doi.org/10.3390/antiox13080911
Hu L, Hong T, He Y, Wang H, Cao J, Pu D, Gao L, Gao C, Cui Y, Wu J, et al. Chromosome Segregation–1–like Gene Participates in Ferroptosis in Human Ovarian Granulosa Cells via Nucleocytoplasmic Transport. Antioxidants. 2024; 13(8):911. https://doi.org/10.3390/antiox13080911
Chicago/Turabian StyleHu, Luanqian, Tongtong Hong, Yuheng He, Huiyuan Wang, Jinxiang Cao, Danhua Pu, Li Gao, Chao Gao, Yugui Cui, Jie Wu, and et al. 2024. "Chromosome Segregation–1–like Gene Participates in Ferroptosis in Human Ovarian Granulosa Cells via Nucleocytoplasmic Transport" Antioxidants 13, no. 8: 911. https://doi.org/10.3390/antiox13080911
APA StyleHu, L., Hong, T., He, Y., Wang, H., Cao, J., Pu, D., Gao, L., Gao, C., Cui, Y., Wu, J., & Tan, R. (2024). Chromosome Segregation–1–like Gene Participates in Ferroptosis in Human Ovarian Granulosa Cells via Nucleocytoplasmic Transport. Antioxidants, 13(8), 911. https://doi.org/10.3390/antiox13080911






