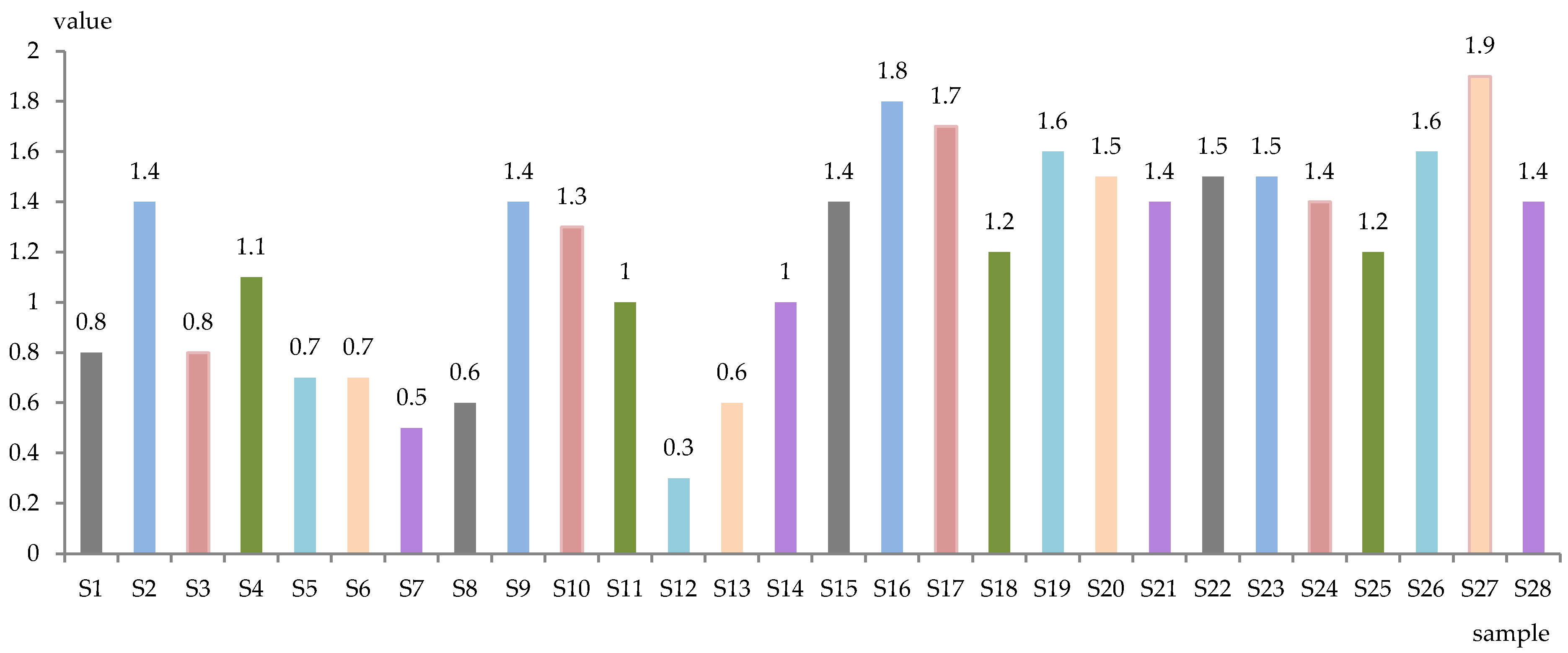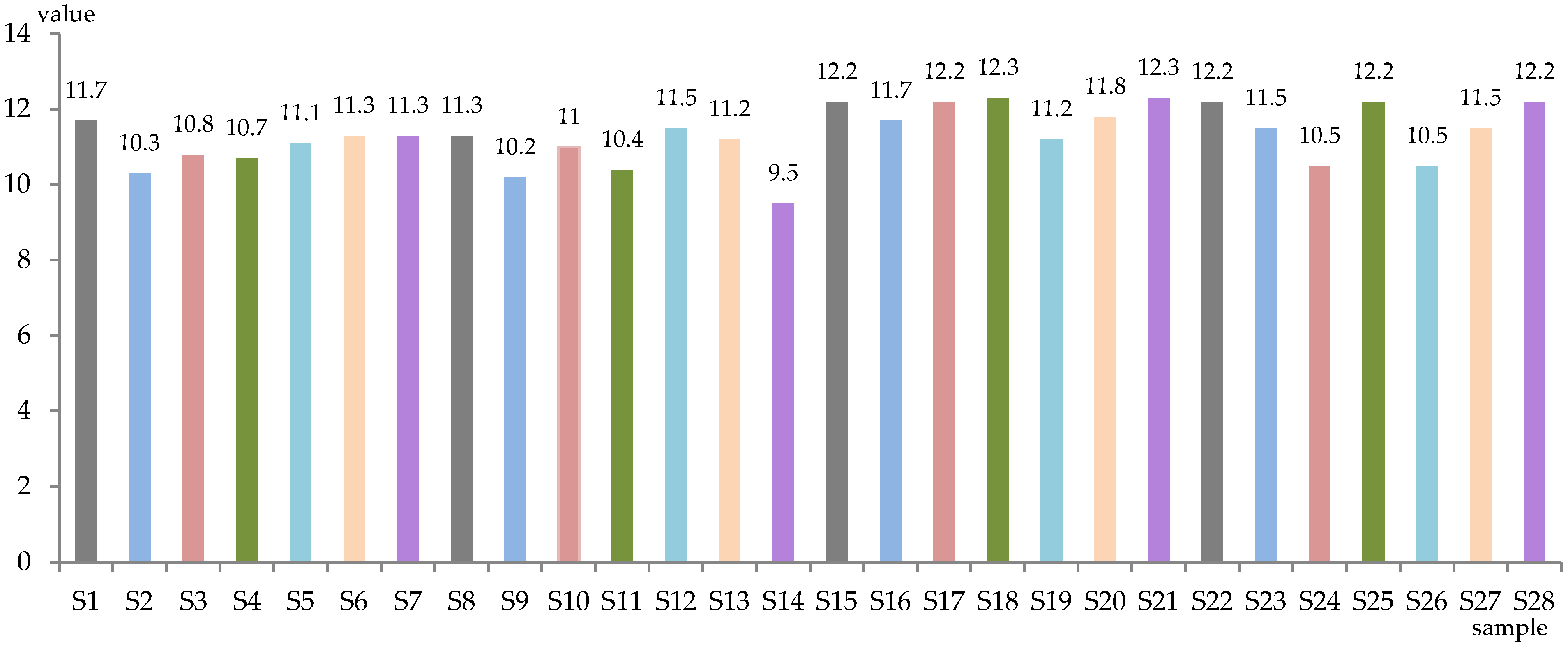Abstract
Melatonin is commonly found in various fruits, juices, and some fermented beverages. Its concentration in wine is influenced by soil properties, climatic factors, and yeast activity. Even if it is found in fermented beverages in relatively low proportions, melatonin still holds significant nutritional value, giving anti-aging properties, anti-inflammatory actions, and antidepressant effects. In this context, this article focuses on evaluating the impact of different Saccharomyces and non-Saccharomyces yeast species on the formation of melatonin and its contribution to wines’ total antioxidant capacity. Considering that the antioxidant activity of wine is usually related to the content of phenolic compounds, ten such compounds were analyzed. The evaluation of bioactive compounds was performed using high-performance liquid chromatography (HPLC) coupled with mass spectrometry. The total antioxidant capacity of wine samples was evaluated by the ABTS+ method. The administration of bâtonnage products increased the efficiency of non-Saccharomyces yeasts. The mixtures of Saccharomyces and non-Saccharomyces yeasts generated higher values for melatonin. The results confirm a significant impact from the grape variety and the specific yeast strains on the melatonin concentration. Also, a strong dependence between antioxidant activity and melatonin levels was observed. Given the limited existing studies on the presence of melatonin in wines, new perspectives are needed for future exploration and understanding.
1. Introduction
Melatonin, which is typically synthesized from tryptophan, has received great attention in recent years due to its potential antioxidant activity. In plants, melatonin promotes growth and has shown anti-senescence effects, while in the human body, it helps to improve circadian rhythms, sustain eye health and neurological activity, and has manifested anti-inflammatory, anti-cancer, anti-diabetes, and anti-aging properties. Moreover, melatonin contributes to a general state of well-being, reducing anxiety and depression [1]. The ability to synthesize melatonin in the human body diminishes with age and is influenced by the adopted lifestyle. Thus, diet is an essential factor in the synthesis of different bioactive compounds, including melatonin [2]. This compound can be found in a variety of seeds, cereals, and fruits, including strawberries, blueberries, cherries, sour cherries, and table grapes. It was also identified in drinks, including fruit juices, coffee, tea, beer, and wine [3,4,5,6]. However, relatively few studies have focused on the melatonin content of wine. Although the nutraceutical effects of wine consumption are a controversial subject due to the alcohol content, wine is made up of numerous classes of compounds, many of which have positive effects on the human body. Therefore, the tendency is to produce wines with an improved nutritional value, to counterbalance the negative impact of alcohol. In this context, Boban et al. [7] demonstrated the protective action of wine on the cardiovascular system. In this sense, following an induced myocardial infarction, the survival rate was significantly higher in rats that received reduced amounts of white wine (72.2%), compared to those that received water (47.8%). This effect can be attributed to the numerous biologically active compounds, especially phenolic compounds and melatonin [8,9].
The majority of the existing studies correlate the antiradical activity in wines with their phenolic compound profile [10]. Some research articles that focused on the impact of moderate consumption (1–2 glasses/day) of wine reported a significant increase in the antioxidant capacity in plasma, high-density lipoprotein levels, in parallel with an important decrease in oxidative stress, cardiovascular diseases and cancer cells [11,12]. Different Saccharomyces spp. yeasts (Saccharomyces cerevisiae, Saccharomyces uvarum) and non-Saccharomyces spp. (Candida colliculosa, Candida stellata, Metschnikowia pulcherima, Torulospora delbrueckii, Kloeckera thermotolerans, Kloeckera apiculata, Hanseniaspora uvarum) were isolated in wine fermentation [13]. In general, non-Saccharomyces types of yeast cannot complete alcoholic fermentation and so are frequently used in combination with Saccharomyces yeasts. Non-Saccharomyces species are able to reduce the initial ethanol level by 1–2% v/v. Gomez et al. [14] studied the evolution of melatonin and its isomer in the Malbec grape variety using an UHPLC-MS/MS system. Melatonin was identified in the grape extract while its isomer was present in musts and wines. The results confirmed that Saccharomyces cerevisiae plays an essential role in the production of melatonin and its isomer in wine. Fernández-Cruz et al. [15] studied melatonin and derived tryptophan metabolites produced during alcoholic fermentation by different Saccharomyces and non-Saccharomyces (Torulaspora delbrueckii and Metschnikowia pulcherrima) yeast strains. In Romania, Albu et al. [16] reported for the first time the analysis of melatonin and its precursors. Their study focuses on the development and validation of a sensitive and selective HPLC-MS/MS method for the simultaneous analysis of melatonin, serotonin, and tryptophan in wine samples. Rodriguez-Naranjo et al. [17] did not identify melatonin in Cabernet Sauvignon, Merlot, Syrah, Tempranillo, Tintilla de Rota, Petit Verdot, Pedro Ximénez, Nebbiolo, Palomino Fino. Also, the same results were presented for Flame Seedless, Red Globe, Moscatel Italica, and Superior Seedless, all table grapes from Spain. In a previous study published by the authors [18], melatonin was found in important amounts in table grapes from Romania (Timpuriu de Pietroasa, Coarnă neagră selecționată, Paula). In general, dosages of 0.5 to 5 mg are well tolerated and have no side effects. Although melatonin is naturally present in plants, the quantities are extremely low, making it impossible to obtain concentrations that exceed the maximum permissible concentration, which is the limit for harmful effects on the human body. [19]. Indeed, the concentration of melatonin in fermented beverages is usually low (pg/mL to ng/L), but manifests an important contribution to their nutritional value, which has been less studied in white wines [20]. The increase in the consumption of white wine at the global level requires the development of additional research on the biological effects of white wine [7]. There is increased interest in the finding of natural sources of melatonin and studies are not sufficient in this area. The goal of the present research is to obtain wines that support balanced diets, with high antioxidant capacity. For this reason, this study focuses on optimizing the production technology of some wines by monitoring the influence of different yeasts (Saccharomyces and non-Saccharomyces yeasts) on the production of melatonin. Since most authors report the antioxidant activity of wines according to the presence of phenolic compounds, ten such compounds were analyzed. To amplify the yeasts’ activity, some bâtonnage products were also applied. The topic is up-to-date and presents novelty through the chosen varieties, but also through the diversity of the inoculated yeasts and applied technology.
2. Materials and Methods
2.1. Wine Sample
For this experiment, two types of white wines were analyzed: the first category was obtained from an Aligoté + Fetească albă grape blend and the second category from a Sauvignon blanc variety. The grapes were processed using white wine technology, with some particularities. After pressing, the grape juice was transferred into a stainless steel container (600 L) and a dose of 5 g/hL enzyme preparation (Lafase® fruit, Laffort®, Bordeaux, France), 80 g/L fining product (Polymust® press, Laffort®, Bordeaux, France) and 1 mL/L SO2 solution (6%). After 1 day, the lees sediment was evacuated (515 L remained). Different yeast preparations were inoculated in a dose of 20 g/hL, following the producers recommendations: Lachancea thermotolerans yeast (CONCERTOTM, CHR Hansen, Hørsholm, Denmark); Saccharomyces cerevisiae yeast (ZYMAFLORE® X16, Laffort®, Bordeaux, France); Torulaspora delbrueckii yeast (PRELUDETM, CHR Hansen, Hørsholm, Denmark); Pichia kluyveri yeast (FROOTZEN®, CHR Hansen, Hørsholm, Denmark); Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts (SYMPHONY®, CHR Hansen, Hørsholm, Denmark); Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts (MELODYTM, CHR Hansen, Hørsholm, Denmark). After 14 days of fermentation, all the samples were divided into two aliquots. One was directly bottled, while for the second, different bâtonnage products (OENOZYM® TH, Lamothe-Abiet, Canéjan, France—0.06 mL/L; AROMA PROTECT®, Lamothe-Abiet, Canéjan, France—0.3 g/L; AROMA T’N’T®, Lamothe-Abiet, France—0.3 g/L) were administered and then bottling ensued. The samples were obtained in triplicate and recorded as shown in Table 1.

Table 1.
Sample codifications.
The level of total sugars (g/L) and alcoholic strength (% vol. alc.) of the obtained samples is represented in Figure 1 and Figure 2. The wines were dry, with total sugar content between 0.3 g/L (S12) and 1.9 g/L (S27), while the alcoholic concentration was between 9.5% (S14) and 12.3% (S18, S21) alc. vol.

Figure 1.
Total sugar of experimental wines (g/L).

Figure 2.
Alcoholic strength of experimental samples (% vol. alc.).
2.2. Laboratory Analysis
For laboratory analysis, standard solutions and different reagents (e.g., melatonin, trans-resveratrol, cis-resveratrol, epicatechin, catechin, gallic acid, protocatechuic acid, caftaric acid, caffeic acid, p-coumaric acid, ferulic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid-Trolox solution, 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonate–ABTS+ solution, acetonitrile, methanol, isopropanol, acetone, formic acid, triethylamine, hydrogen peroxide, etc.) were purchased from Merck (Darmstadt, Germany).
2.2.1. Phenolic Compound Evaluation
The principal phenolic compounds were determined using an Agilent 1100 HPLC Series system (Agilent, Santa Clara, CA, USA) coupled with an Agilent Ion Trap VL mass spectrometer (Agilent, Santa Clara, USA), following the method presented in our team’s previous papers [21,22]. Wine samples were filtered using 0.45 μm sterile filters. Determinations were performed in triplicate and the results are presented as means, including standard deviations. For the analyzed phenolic compounds, the detection limit (LOD) was 0.04 µg/mL, while the quantification limit (LOQ) was 0.2 µg/mL. Some analytical conditions are presented in Table 2.

Table 2.
Analytical parameters applied for the detection and quantification of phenolic compounds [21,22].
2.2.2. Identification and Quantification of Melatonin
This analysis was performed according to the method presented in the previous paper [18], with some modifications and using a Transcend XT Ultimate 3000 UHPLC system (Thermo Scientific TM, Waltham, MA, USA) coupled with a TSQ Access Max mass spectrometer. The elution of compounds was carried out using an Agilent Poroshell C18 column (Agilent, USA) (4.6 × 100 mm, 1.8 μm). The transfer was achieved using a 30% water (TA) and 70% methanol (TD) mixture (v/v) (in loop). The TX column was prepared using a mixture (v/v/v) of acetonitrile (45%), isopropanol (45%), and acetone (10%) (TB), followed by triethylamine (0.05%) solution prepared in acetonitrile (TC). For separating melatonin from the internal standard, a mobile phase of 0.1% formic acid in water (LA) and 0.1% formic acid in methanol (LB) was used. Regarding the mass spectrometry analysis, a heated electrospray ionization source (in positive mode) was used for ionization, while a collision cell within Q2 was engaged in fragmenting and separating particular ions to allow accurate identification. The ionization conditions were set with an ionization potential of 3 kV, an ionization source temperature of 350 °C, a nebulization gas pressure of 35 psi, and an auxiliary gas pressure of 10 psi. The capillary tube was kept at 350 °C, while the polarity was positive [18].
For the standard solutions, a quantity of 10 mg of melatonin was dissolved in methanol and diluted to 10 mL in the same solvent. A volume of 0.5 mL solution was diluted to 10 mL with methanol. A volume of 0.1 mL from the previous solution was diluted with 10 mL of methanol. An internal standard was prepared in water by dissolving a quantity of 6 mg of tryptophan in 10 mL of solvent. A volume of 1.66 mL was diluted to 5 mL with methanol. A series of concentrations of 0.5, 2.0, 10.0, 25.0, and 100 ppb were produced by taking corresponding volumes of the stock solutions, a 0.05 mL internal standard, and dilution to 1 mL with a mixture of water: methanol (50%:50% (v/v)). For sample preparation, a volume of 2.5 mL of wine was filtered through a 0.45 µm nylon filter; a portion of 1 mL of clear filtrate was spiked with 0.05 mL of internal standard and subjected to analysis according to the method. For the control samples, the second volume of 1.0 mL of filtrate was subjected to analysis to subtract the tryptophan content from the sample. The areas of tryptophan were subtracted from the control samples and used further for the calculation of melatonin in the wine samples. The method’s selectivity was confirmed using blank solutions, showing no interference. Melatonin detection was based on the specific transition of m/z 233⟶174, with a retention time at 3.28 min. For the internal standard, the detection used the transition of m/z 205.1⟶146, with a retention time at 2.50 min. A supplementary chromatographic peak was observed in the melatonin chromatogram, likely due to a tryptophan impurity, but it did not interfere with melatonin determination. The method demonstrated linearity in the range of 0.05 ppb to 100 ppb, with calibration points at 0.5, 2.0, 10.0, 25.0, and 100 ppb. The regression correlation coefficient was 0.9945. The back-calculation of standard concentrations using the regression equation showed values within 85% to 115% of the expected concentrations. The highest deviation was 5.85% at 0.5 ppb, and the lowest was 0.7% at 100 ppb. The standard relative deviation for three series of samples under the same conditions was 1.5%. The LOD and LOQ were calculated using the standard deviation of the intercept and the slope, multiplied by 3.3 for LOD and 10 for LOQ. The LOQ was 0.12 ppb, and the LOD was 0.059 ppb, confirmed by the signal-to-noise ratio. Accuracy and precision were evaluated using standard method addition at concentrations of 1 ppb, 25 ppb, and 100 ppb in representative wine samples. Recovery rates were within 85% to 115%: 87.3% at 1 ppb, 92% at 25 ppb, and 93.2% at 100 ppb. Repeatability was assessed with three concentrations within the linearity range, achieving values within 98% to 102% of the target. Inter-day and intra-day precision showed values of 8.5% for the lowest concentration and 6.5% for the highest concentration. The samples were analyzed in triplicate and the results are presented as arithmetic means and standard deviations. The concentration of melatonin is expressed in µg/L [18].
2.2.3. Total Antioxidant Capacity
Total antioxidant capacity of wine samples was evaluated by ABTS+ method (also known as Trolox equivalent antioxidant capacity (TEAC) assay), which relies on the ability of antioxidants to diminish the blue-green color of ABTS+ in correspondence with their concentrations and scavenging properties. Initially, the reduced ABTS molecule is converted by oxidation to ABTS+ using hydrogen peroxide–H2O2 in an acidic medium of 30 mmol/L acetate buffer solution (pH = 3.6). In the acetate buffer solution, the concentrate (deep green) ABTS+ molecules persist for a long time. Another solution of 0.4 mmol/L acetate buffer (pH = 5.8) was prepared and used for the dilution of the initial medium. The color of ABTS+ molecules was spontaneously and gradually decolorized. The decolorizing rate is proportional to the concentrations in different antioxidant compounds. The absorbance was monitored at 660 nm and the antioxidant capacity is inversely related to the decolorizing rate of the mixture. The calibration curve was made with Trolox solution and the results are expressed as mmol Trolox equivalent per liter [23].
2.3. Statistical Tests
The statistical analysis of the data was carried out using XLStat (Luminevo, Denver, CO, USA) and aimed at the analysis of variance (ANOVA) which reveals the existence of a statistically significant difference (p-value < 0.05) between the analyzed samples. Student-t test highlights pairs of samples that are significantly different from each other (p-value < 0.05). The possible existing correlations between the analyzed bioactive compounds were highlighted by principal components analysis (PCA). Linear regression analysis highlighted the influence of the analyzed bioactive compounds on the antioxidant capacity value.
3. Results and Discussion
3.1. Effect of Different Yeasts on Wine Bioactive Compounds
According to Table 3 and Table 4, the samples showed different values of bioactive compounds in relation to the specificity of the grape varieties and the applied technology (different species of inoculated yeasts and various bâtonnage products). Tables S1–S3 contains the differences between each pair of samples, for each bioactive compound.

Table 3.
Bioactive compounds in wine samples obtained from Aligoté + Fetească albă wines.

Table 4.
Bioactive compounds in Sauvignon blanc wine samples.
In general, caftaric acid is the main representative in samples obtained from the mix of Aligote + Fetească albă grapes, without bâtonnage (from 16.14 ± 0.15 µg/L in samples with Lachancea thermotolerans yeast—S2 to 19.90 ± 0.15 µg/L in S3—Saccharomyces cerevisiae). This compound is caffeic acid’s ethyl ester. The results are in accordance to Peréz-Navarro [24] that presented caftaric acid as one of the predominant phenolic acids in white wines. Its concentrations decreased by up to eight times in the case of samples with bâtonnage (from 2.49 ± 0.01 µg/L in S10—Saccharomyces cerevisiae yeast + bâtonnage products to 3.28 ± 0.00 µg/L in S11—Torulaspora delbrueckii yeast + bâtonnage products). Indeed, bâtonnage (inactivated Saccharomyces cerevisiae yeasts, glutathione and pectolytic enzymes) can increase the wines’ complexity and mouthfeel by favoring yeast autolysis and releasing aroma constituents. Other factors that can cause the reduction in phenolic compounds are different chemical and physical processes that can occur, including oxidation, binding to lees, polymerization and precipitation. Certain phenolic molecules have the potential to react with sulfur dioxide, creating more stable complexes [25]. Following bâtonnage application to this category of samples, caffeic acid became predominant in most samples (from 9.31 ± 0.12 µg/L in S11—Torulaspora delbrueckii yeast to 10.32 ± 0.01 µg/L in S13—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products). This compound usually derives from p-coumaric acid (which results from cinnamic acid), but free forms of caffeic acid can arise due to esterase activity, too [24]. Contrary to these results, this compound was not identified in the white wine samples studied by Onache et al. [26], while a strong positive correlation of caffeic acid with catechin, epicatechin and trans-resveratrol was shown by the authors. In the present article, only the positive correlation between epicatechin and catechin was confirmed by principal component analysis, for both Aligoté + Fetească regală (r = 0.937) and Sauvignon blanc wines (r = 0.762).
The concentration of caftaric acid was significantly modified with the inoculation of the analyzed yeast preparations. Significant differences are shown between both yeast and control samples, but each yeast preparation led to significantly different results. For caffeic acid, significantly different concentrations between the following pairs were obtained: S2–S6 (Lachancea thermotolerans vs. Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts), S4–S5 (Torulaspora delbrueckii vs. Pichia kluyveri yeasts), S5–S6 (Pichia kluyveri vs. Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts) and S5–S7 (Pichia kluyveri vs. Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts). The differences increased with the application of bâtonnage products.
Gallic acid showed the highest values in samples obtained from the Sauvignon blanc variety. Garrido and Borges [27] suggested that gallic acid was a significant phenolic compound due to its important scavenging activity. While it can originate from the grape, its presence may also stem from chemical transformations occurring during fermentation. Thus, enzymes and acids present in the grape and microbial activity may catalyze the hydrolysis of hydrolysable and condensed tannins, leading to the release of gallic acid [28]. In this category, samples subjected to bâtonnage (from 20.65 ± 0.03 in S27—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products to 22.96 ± 0.08 in S23—Lachancea thermotolerans yeast + bâtonnage products) with various oenological products indicating slightly higher concentrations compared to those without bâtonnage (from 18.85 ± 0.02 in S16—Lachancea thermotolerans yeast, to 20.70 ± 0.20 in S21—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts). If gallic, caffeic, and caftaric acids are predominant in samples without bâtonnage treatment, samples with bâtonnage showed the highest values of gallic acid, caffeic acid, and p-coumaric acid. In a separate study conducted by our team [21], it was observed that enzyme preparations had a notable impact on the generation of various phenolic compounds in Sauvignon blanc wines. Among these compounds, protocatechuic acid and caftaric acid were found to be most predominant.
The values recorded in the case of gallic acid are significantly influenced by the applied treatments. Gallic acid is in general influenced by the inoculated yeasts, but minor differences were recorded between S15 and S20 (control sample vs. Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts), S15 and S21 (control sample vs. Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts), S17 and S21 (Saccharomyces cerevisiae vs. Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts), S20 and S21 (Saccharomyces cerevisiae + Kluyveromyces thermotolerans vs. Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts). With the exception of samples S5 (Pichia kluyveri) and S6 (Saccharomyces cerevisiae + Kluyveromyces thermotolerans), all yeast preparations generated significant differences compared to the control sample (S20), in the category of variants without bâtonnage.
The bâtonnage products in some cases modified gallic acid content, generating significant differences between the control sample and S9 (Lachancea thermotolerans yeast + bâtonnage products), as well as S12 (Pichia kluyveri yeast + bâtonnage products), S13 (Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products) variants.
The analyzed phenolic acids emerged as significant indicators for distinguishing the analyzed varieties across the diverse wine-growing regions in Romania [29]. Lengyel [30] also noted comparable concentrations of phenolic compounds in wines derived from Sauvignon blanc varieties.
The content of the samples in bioactive compounds was also evaluated after the application of the bâtonnage products, and Table S3 highlights the differences between the pairs of samples with and without this treatment. Therefore, for Aligoté + Fetească albă wines, the most differences were between the variants S1–S8 (control samples) and S2–S9 (Lachancea thermotolerans vs. Lachancea thermotolerans yeasts + bâtonnage products). Sauvignon blanc samples displayed most differences between the S18–S25 (Torulaspora delbrueckii) and S19–S26 (Pichia kluyveri) pairs.
Certain strains of yeast have the ability to synthesize melatonin from tryptophan during fermentation, although other microorganisms such as bacteria and fungi may also contribute to its synthesis through enzymatic processes [31,32]. From Table 3 and Table S1, it can certainly be confirmed that the applied technology (different yeasts, application of bâtonnage) influences the melatonin concentration in Aligote + Fetească albă wines. Yeasts administered in samples S2 (Lachancea thermotolerans yeast) and S7 (Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae) did not show significant differences compared to the control sample. With the application of the bâtonnage products, the S14 variant (Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts + bâtonnage products) presented a significant difference from S8 (control sample, with bâtonnage, no exogenous yeasts).
Table 4 shows that S15 variant (Sauvignon blanc grape–control sample, no bâtonnage, no exogenous yeasts) presented important levels of melatonin (7.81 ± 0.15 µg/L), followed by S20 (2.71 ± 0.15 µg/L, Sauvignon blanc grapes–Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts) and S13 (1.58 ± 0.15 µg/L, Aligoté + Fetească albă grapes–Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products), while no melatonin was identified in the S25 sample (Sauvignon blanc grapes-–Torulaspora delbrueckii yeasts + bâtonnage products). According to the obtained results, the yeast mix formed by Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts yielded favorable results in increasing melatonin content for the studied grape varieties. However, it seems that in Sauvignon blanc wines, the control sample showed the maximum identified value (S15—Sauvignon blanc grape control sample, no bâtonnage, no exogenous yeasts). Significant differences were obtained for most pairs of experimental variants, except for S16–S18 (Lachancea thermotolerans vs. Torulaspora delbrueckii yeasts), S16–C21 (Lachancea thermotolerans vs. Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts), S17–S19 (Saccharomyces cerevisiae vs. Pichia kluyveri yeasts) and S18–S21 (Torulaspora delbrueckii vs. Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts).
According to Fernández-Cruz et al. [15], each yeast type has the ability to produce melatonin at different growth stages. Alcohol content can influence the dilution and release of phenolic compounds and melatonin [31]. For the analyzed samples, minor differences in alcoholic strength were registered. The correlation between melatonin production and growth phase suggests that melatonin may play a role in the yeast’s adaptability to the changing conditions of alcoholic fermentation. Also, melatonin–protein binding for some yeast species should be taken into consideration (not analyzed in this paper) [32]. Fracassetti et al. [33] identified between 0.038 µg/L and 0.063 µg/L melatonin in red wines, being in accordance with the results presented by Vitalini et al. [34]. This compound was found in great amounts (0.011–0.019 µg/mL) in Riesling wines from Romania (commercial samples), analyzed by Albu et al. [16]. In another study, Eremia et al. [8] reported 0.74–0.84 ng/mL melatonin in Fetească neagră and Cabernet sauvignon red wine samples, comparable with the team’s results for white samples. It is clear that different yeasts can synthetize different amounts of bioactive compounds [17]. In accordance with Sunyer-Figueres et al. [35], melatonin acts as a modulator of the biosynthesis of different phenolic compounds. In correlation with Morcillo-Parra et al. [32], melatonin increases the survival of non-Saccharomyces species when fermentation is carried out using a mixed inoculum, which is either solely or co-inoculated with non-Saccharomyces and Saccharomyces. Valera et al. [36] suggested that yeast cells become more fermentative in the presence of melatonin, completing the fermentation a day or two sooner. The authors observed that when melatonin was added to the synthetic must, Torulaspora delbrueckii and Saccharomyces bacillaris remained until the completion of the fermentation, but Metschnikowia pulcherrima and Hanseniaspora uvarum only showed up at the start of the process. Therefore, variations in melatonin–protein interactions between non-Saccharomyces species may be explained by variations in sugar metabolism and enzyme activity. In another study, Rodriguez-Naranjo et al. [17] evaluated the ability of several Saccharomyces and non-Saccharomyces yeasts to produce melatonin. Different strains exhibited varying degrees of production; the non-Saccharomyces yeast with the greatest concentration was Starmerella bacillaris. However, depending on the yeast strain, extracellular melatonin was found at various stages of the fermentation process. Nevertheless, the same authors also postulated that melatonin requires tryptophan to be present.
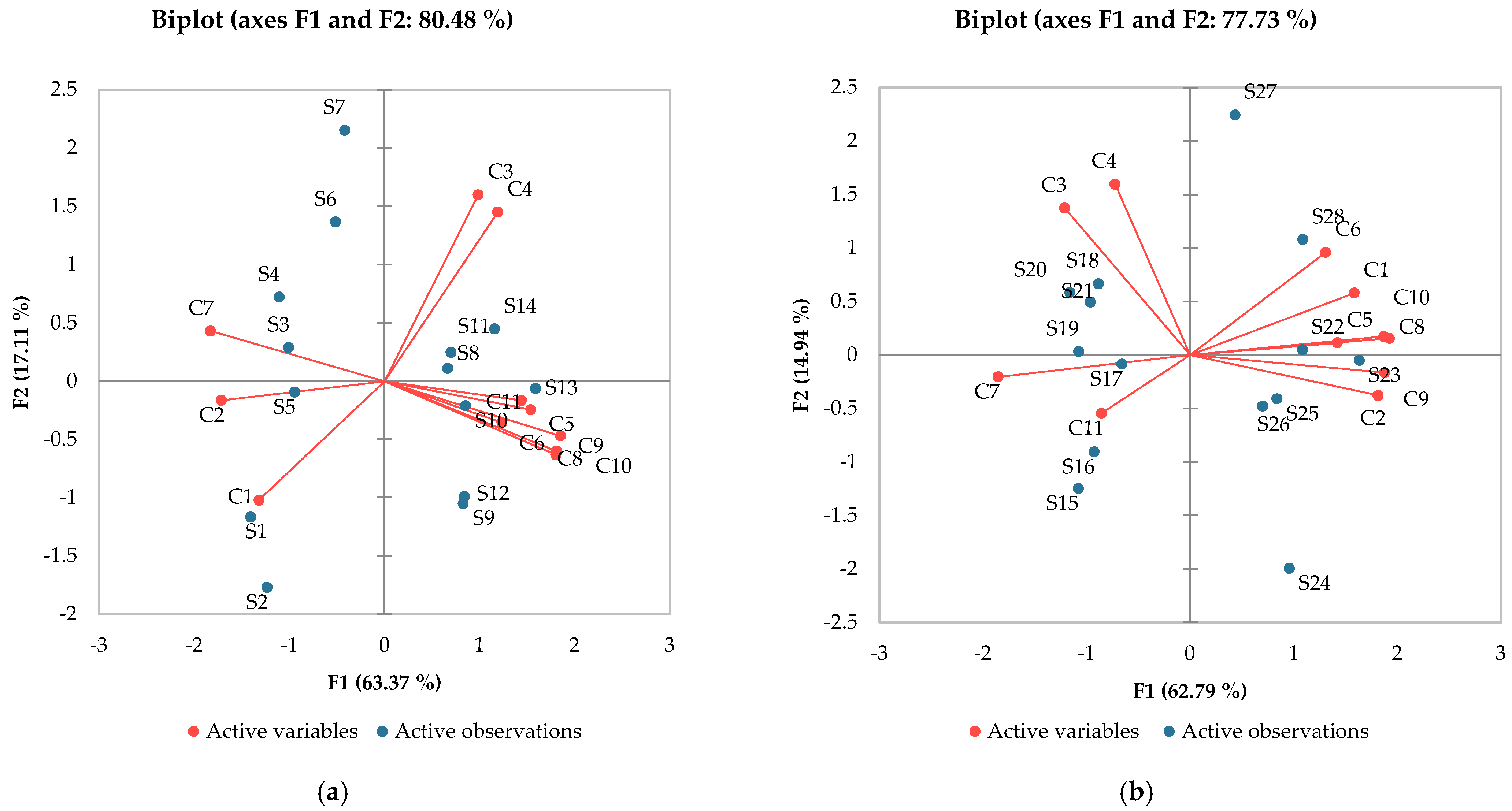
For another perspective, principal component analysis (Figure 3) helps to identify the directions of the variation in the results and marks possible correlations between samples and the analyzed compounds. So, as far as melatonin is concerned, the influence of varietal variability was clear. Very high correlations (r > 0.9) between ferulic, p-coumaric and caffeic acid could be observed. High correlations (r > 7) were presented by gallic, caffeic, and ferulic acids, while a medium correlation of melatonin and protocatechuic acid was registered (r = 0.610).
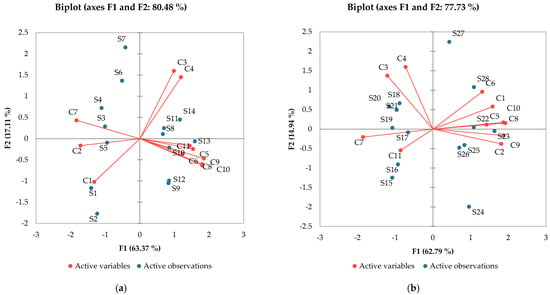
Figure 3.
Principal component analysis: (a) Aligoté + Fetească albă; (b) Sauvignon blanc. C1—trans-resveratrol; C2—cis-resveratrol; C3—epicatechin; C4—catechin; C5—gallic acid; C6—protocatechuic acid; C7—caftaric acid; C8—caffeic acid; C9—p-coumaric acid; C10—ferulic acid; C11—melatonin. S1–S14—Aligoté + Fetească albă: S1—control sample, no bâtonnage, no exogenous yeasts; S2—Lachancea thermotolerans yeast; S3—Saccharomyces cerevisiae yeast; S4—Torulaspora delbrueckii yeast; S5—Pichia kluyveri yeast; S6—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts; S7—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts; S8—control sample, with bâtonnage, no exogenous yeasts; S9—Lachancea thermotolerans yeast + bâtonnage products; S10—Saccharomyces cerevisiae yeast + bâtonnage products; S11—Torulaspora delbrueckii yeast + bâtonnage products; S12—Pichia kluyveri yeast + bâtonnage products; S13—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products; S14—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts + bâtonnage products. S15-S28—Sauvignon blanc: S15—control sample, no bâtonnage, no exogenous yeasts; S16—Lachancea thermotolerans yeast; S17—Saccharomyces cerevisiae yeast; S18—Torulaspora delbrueckii yeast; S19—Pichia kluyveri yeast; S20—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts; S21—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts; S22—control sample, with bâtonnage, no exogenous yeasts; S23—Lachancea thermotolerans yeast + bâtonnage products; S24—Saccharomyces cerevisiae yeast + bâtonnage products; S25—Torulaspora delbrueckii yeast + bâtonnage products; S26—Pichia kluyveri yeast + bâtonnage products; S27—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products; S28—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts + bâtonnage products. The results are significantly influenced by the applied technology when p-value is less than 0.05.
The effects of yeasts on the chemical composition of wines have been intensively studied; numerous studies followed the influence of similar oenological products [37,38], but few studies focused on the variation in melatonin content. In general, samples with a high content of melatonin also show higher antioxidant activity, which confirms the results obtained in other studies [18].
3.2. Total Antioxidant Activity
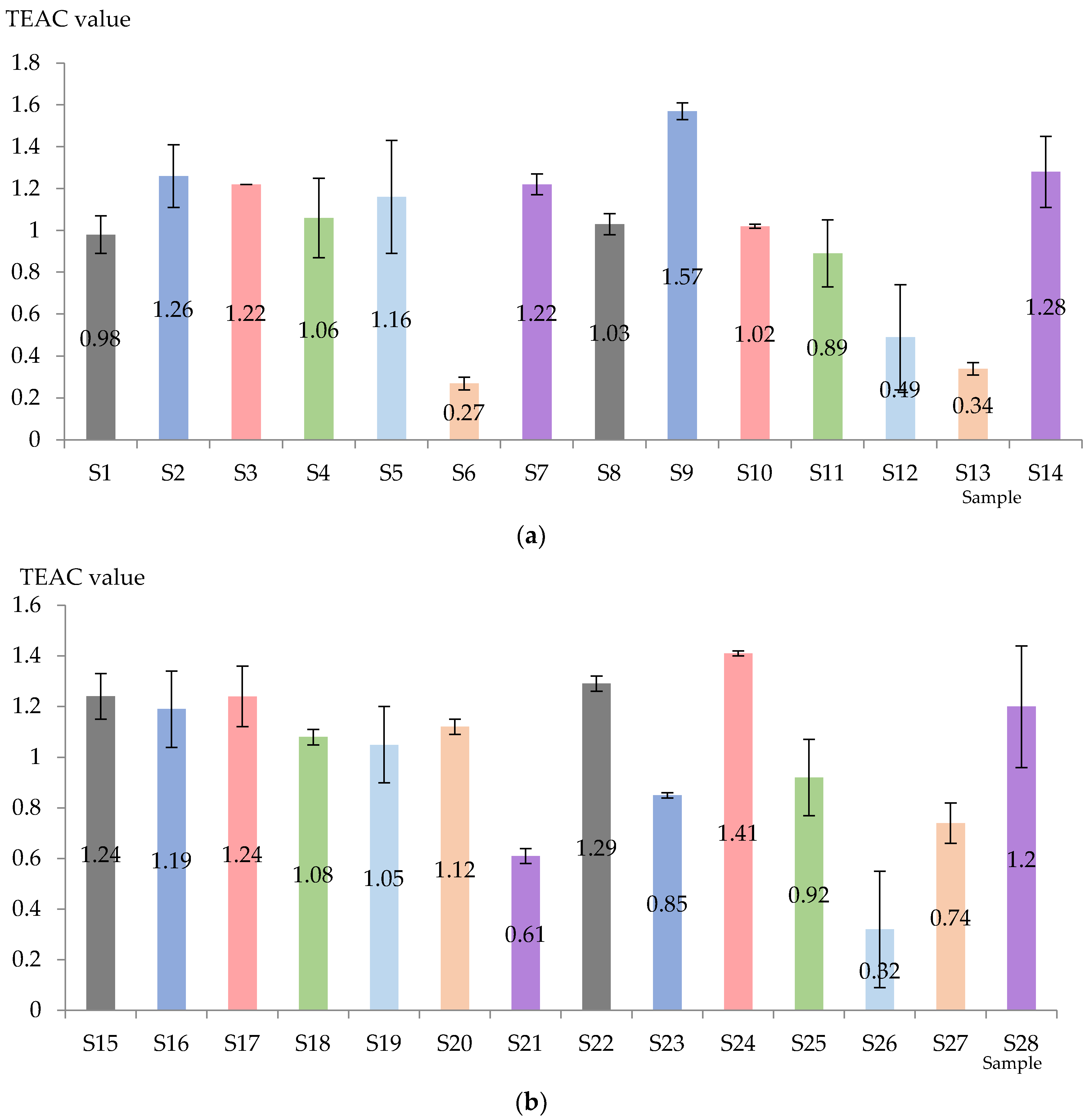
The TEAC value was obviously influenced by the applied technology and the variability of the variety (Figure 4). For Aligoté + Fetească albă (without bâtonnage) the highest value was recorded in the case of S2 (Lachancea thermotolerans yeast), followed by S7 (Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts), S3 (Saccharomyces cerevisiae yeast), and the lowest value was obtained with S6 (Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts). When bâtonnage was applied, the order was as follows: S9 (Lachancea thermotolerans yeast) > S14 (Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts + bâtonnage products) > S8 (control sample, with bâtonnage, no exogenous yeasts) > S10 (Saccharomyces cerevisiae yeast + bâtonnage products) > S11 (Torulaspora delbrueckii yeast + bâtonnage products) > S2 (Lachancea thermotolerans yeast) > S13 Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products). It can be seen that Lachancea thermotolerans yeast was the most effective in increasing the TEAC value, in contrast to the mixture Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts.
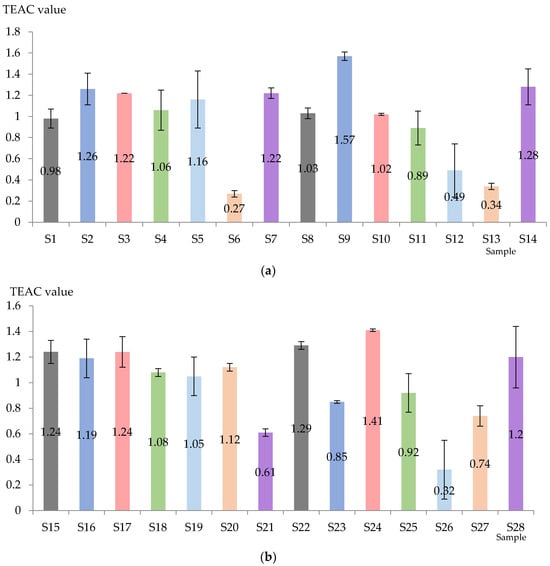
Figure 4.
Total antioxidant activity of Aligote + Fetească albă (a) and Sauvignon blanc (b) wines (mmol Trolox equivalent/L). S1–S14—Aligoté + Fetească albă: S1—control sample, no bâtonnage, no exogenous yeasts; S2—Lachancea thermotolerans yeast; S3—Saccharomyces cerevisiae yeast; S4—Torulaspora delbrueckii yeast; S5—Pichia kluyveri yeast; S6—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts; S7—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts; S8—control sample, with bâtonnage, no exogenous yeasts; S9—Lachancea thermotolerans yeast + bâtonnage products; S10—Saccharomyces cerevisiae yeast + bâtonnage products; S11—Torulaspora delbrueckii yeast + bâtonnage products; S12—Pichia kluyveri yeast + bâtonnage products; S13—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products; S14—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts + bâtonnage products. S15–S28—Sauvignon blanc: S15—control sample, no bâtonnage, no exogenous yeasts; S16—Lachancea thermotolerans yeast; S17—Saccharomyces cerevisiae yeast; S18—Torulaspora delbrueckii yeast; S19—Pichia kluyveri yeast; S20—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts; S21—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts; S22—control sample, with bâtonnage, no exogenous yeasts; S23—Lachancea thermotolerans yeast + bâtonnage products; S24—Saccharomyces cerevisiae yeast + bâtonnage products; S25—Torulaspora delbrueckii yeast + bâtonnage products; S26—Pichia kluyveri yeast + bâtonnage products; S27—Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts + bâtonnage products; S28—Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts + bâtonnage products. The results are significantly influenced by the applied technology when p-value is less than 0.05.
Sauvignon blanc wines are generally characterized by higher TEAC values. In the samples without bâtonnage, the highest antioxidant activity was suggested for S15 (control sample, no bâtonnage, no exogenous yeasts), followed by S17 (Saccharomyces cerevisiae yeast) and S8 (control sample, with bâtonnage, no exogenous yeasts), and the lowest value was found in S21 (Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts). On the other hand, S24 (Saccharomyces cerevisiae yeast) and S22 (control sample, with bâtonnage, no exogenous yeasts) were highlighted as having the highest TEAC values, while S27 (Saccharomyces cerevisiae + Kluyveromyces thermotolerans yeasts) and S26 recorded the lowest (Pichia kluyveri yeast + bâtonnage products). It was observed that samples without bâtonnage generally had a higher TEAC value. Favorable results were also obtained by the yeasts inoculated in S2 (Lachancea thermotolerans) and S3 (Saccharomyces cerevisiae). The higher value of TEAC could suggest a higher antioxidant activity, thus indicating better oxidative stability, improved nutritional value and even potential health benefits.
The results obtained from linear regression (Table 5 and Table 6) indicate that over 90% of the variability of the dependent variables (TEAC) is explained by the explanatory variable (bioactive compounds) for each groups of samples (91% for Aligoté + Fetească albă without bâtonnage, 94% for Aligoté + Fetească albă with bâtonnage, 94% for Sauvignon blanc without bâtonnage, 98% for Sauvignon blanc with bâtonnage). This fact suggests that the analyzed compounds are generally the main antioxidants in white wines, at least those analyzed in this study.

Table 5.
The contribution of each bioactive compound on total antioxidant capacity of Aligoté + Fetească albă wines.

Table 6.
The contribution of each bioactive compound on total antioxidant capacity of Sauvignon blanc wines.
In the case of Aligoté + Fetească albă samples, compounds such as trans-resveratrol, catechin, gallic acid, p-coumaric acid, and caftaric acid showed a negative influence on the TEAC value, suggesting a lower antioxidant activity. On the other hand, melatonin followed by cis-resveratrol, ferulic acid, protocatechuic acid, caffeic and epicatechin, presented a positive contribution to the increase in the TEAC value, indicating a greater antioxidant activity. For the second category, wines with bâtonnage, cis-resveratrol, protocatechuic acid, caffeic acid showed a negative impact on the TEAC value, while melatonin > catechin > p-coumaric > gallic acid > ferulic acid > trans-resveratrol > caftaric acid showed a positive contribution on the TEAC value.
In Sauvignon blanc wines, the TEAC value was negatively influenced by the concentration of compounds such as gallic, protocatechuic, caftaric, caffeic, p-coumaric, and ferulic acids. Also, the higher positive contribution was evident for melatonin, followed by cis-resveratrol, epicatechin, catechin, and trans-resveratrol. After the administration of bâtonnage products, melatonin exhibited the greatest influence, followed by p-coumaric, protocatechuic, cis-resveratrol and ferulic acids.
Therefore, the hypothesis is confirmed that although it is found in very small proportions in wines, melatonin may have made the largest contribution to antioxidant activity in the analyzed samples. Similar results have been reported previously. According to Sunyer-Figueres [35], melatonin exhibits direct antioxidant action (by eliminating reactive oxygen species) and indirect (by decreasing oxidized glutathione and activating genes involved in the response to oxidative stress such as catalase, glutathione, glutaredoxin and thioredoxin). Also, the authors postulated that melatonin confers protection against ethanol stress. Melatonin may act synergistically with other wine antioxidants, resulting in an increased cytoprotective impact against oxidative stress [8,9]. The results are in accordance with Vasquez et al. [39], confirming that melatonin manifests important anti-scavenging action on Saccharomyces cerevisiae yeast but, in the present study, the results showed a better efficiency when Saccharomyces cerevisiae yeast was inoculated in combination with non-Saccharomyces species.
There are numerous chemical, environmental, and methodological elements that can interact with phenolic acids in wine and may influence their contribution to total antioxidant activity. These interactions can also have positive or negative oxidative effects. Therefore, phenolic acids can form complexes with other wine components, such as proteins, metals, or other phenolic compounds. Consequently, phenolic acids have the ability to form complexes with other elements found in wine, including proteins, metals, and other phenolic compounds. These complexes have the potential to modify the phenolic acids’ availability or reactivity, which might impact their antioxidant efficacy. These complexes may occasionally promote oxidation processes as opposed to inhibiting them. Phenolic acids’ antioxidant activity can change depending on the pH and external factors like temperature and oxygen exposure. For instance, phenolic acids’ ionization state and reactivity can change in response to pH changes, which might impact their capacity to scavenge free radicals and contribute hydrogen atoms or electrons. The assessment of total antioxidant activity may also be impacted by the analysis technique adopted [40]. This might be due to side reactions such as the formation of coupling adducts with ABTS+ by different phenolic acids or a pro-oxidation reaction. Variations in the reported effects of phenolic acids may result from various assays that capture different features of antioxidant capacity or are more sensitive to particular types of antioxidants [40].
The presented results show that the antioxidant action of melatonin is dependent on various factors, such as the variability of the variety, the chemical composition and the applied technology. These variations may have an impact on the interactions of melatonin in each grape variety. It is important to explore more about the distinct qualities of each wine variety, their individual compositions, and the ways in which melatonin interacts with those components to explain the variations in its contribution to antioxidant activity that have been found.
4. Conclusions
The findings reported in this article indicate that the production of health-promoting compounds depends not only on a strain-specific property of the yeasts in the environment, but also on the varietal characteristics of the grape. Choosing the appropriate strain of primary yeasts is an effective way to enrich wines with health-promoting compounds other than the well-known and much-studied phenolic compounds. Melatonin and phenolic compounds play a significant role in defining the antioxidant activity of wines. The analyzed wine samples displayed different variations in the concentration of bioactive compounds, depending on the type of inoculated yeasts. For Aligoté + Fetească albă samples, caftaric acid was predominant in samples where Saccharomyces cerevisiae yeast was inoculated. The administration of bâtonnage products generated better efficiency from the Torulaspora delbrueckii yeast for this compound. Melatonin was found in higher amounts in samples where Pichia kluyveri yeast was inoculated, while the use of bâtonnage products led to increased levels in the Saccharomyces cerevisiae + Kluyveromyces thermotolerans mix. The blend of Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts generated the highest concentrations of gallic acid in Sauvignon blanc wines. For this category of samples, melatonin was predominant in the control sample, where no treatment was applied, while bâtonnage addition generated an increased content for this compound in samples where Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae yeasts were added. The administered bâtonnage products increased the efficiency of Lachancea thermotolerans yeasts in obtaining higher values for cis-resveratrol, caftaric, caffeic and ferulic acids. In general, samples with higher melatonin values also showed important concentrations of phenolic compounds. It can thus be concluded that the antioxidant properties of melatonin contribute to the stability of phenolic compounds, helping to maintain their concentration and biological activity in wine. Although found in low concentrations in wine, the increase in antioxidant capacity is significantly dependent on the value of this compound. The contribution of yeasts to the production of melatonin is still poorly investigated, so future research is needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13080916/s1, Table S1: Student’s t-test for Fetească regală+Aligoté wines; Table S2: Student’s t-test for Sauvignon blanc wines; Table S3: t-test between samples without and with bâtonnage.
Author Contributions
Conceptualization, R.G.N. and C.I.Z.; methodology, E.C.S. and R.G.N.; software, E.C.S.; validation, B.I.C., L.V. and A.M.V.; formal analysis, B.I.C. and L.V.; investigation, A.M.V., D.M. and L.V.; resources, V.C. and C.I.Z.; data curation, C.E.L.; writing—original draft preparation, E.C.S.; writing—review and editing, E.C.S. and C.E.L.; visualization, E.C.S. and L.C.C.; supervision, V.C.; project administration, C.E.L.; funding acquisition, V.C. and E.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Ion Ionescu de la Brad” Iași University of Life Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alugoju, P.; Kumaree, K.K.; Prasansuklab, A.; Tencomnao, T. Melatonin as a vital metabolite in medicinal and food plants. In Advancement of Melatonin Research in Plants; Roychoudhury, A., Ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 67–83. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef]
- Vázquez, J.; González, B.; Sempere, V.; Mas, A.; Torija, M.; Beltran, G. Melatonin Reduces Oxidative Stress Damage Induced by Hydrogen Peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1066. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Shi, T.; Song, S.; Zhang, Z.W.; Fang, Y.L. Melatonin in grapes and grape-related foodstuffs. Food Chem. 2017, 231, 185–191. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Wu, T.; Zhai, R.; Yang, C.; Wang, Z.; Fengwang, M.; Lingfei, X. Effects of melatonin treatment of postharvest pear fruit on aromatic volatile biosynthesis. Molecules 2019, 24, 4233. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in wine and beer: Beneficial effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Boban, D.; Dželalija, A.M.; Jurić, D.; Benzon, B.; Ključević, N.; Boban, Z.; Mudnić, I.; Grković, I. Differential effects of white wine and ethanol consumption on survival of rats after a myocardial infarction. Appl. Sci. 2023, 13, 1450. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.L.; Ion, M. Different extraction approaches for the analysis of melatonin from Cabernet Sauvignon and Feteasca neagra wines using a validated HPLC-FL method. Molecules 2023, 28, 2768. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.L.; Alecu, A.; Brinduse, E. The influence of melatonin treatment in the vinification of Feteasca neagra and Cabernet Sauvignon wines on the profile of polyphenolic compounds and antioxidant activity. Antioxidants 2023, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Marrero, S.C.; Martínez-Rodríguez, A.; Maestre Pérez, S.E.; Moya, S.P. New trends and applications in fermented beverages. In The Sciences of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston: UK; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 31–66. [Google Scholar]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin. Food Chem. 2011, 130, 797–813. [Google Scholar] [CrossRef]
- Thapa, N.; Tamang, J.P. Functionality and therapeutic values of fermented foods. In Health Benefits of Fermented Foods and Beverages; Tamang, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Tamang, J.P.; Thapa, N.; Tamang, B.; Rai, A.; Chettri, R. Microorganisms in fermented foods and beverages. In Health Benefits of Fermented Foods and Beverages; Tamang, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gomez, F.J.V.; Raba, J.; Cerutti, S.; Silva, F. Monitoring melatonin and its isomer in Vitis vinifera cv Malbec by UHPLC-MS/MS from grape to bottle. J. Pineal Res. 2012, 52, 349–355. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and derived L-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of melatonin and its precursors content by a HPLC-MS/MS method from different Romanian wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, Á.; Troncoso, A.M.; Cantos-Villar, E.; García-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Niculaua, M.; Gheldiu, A.; Cotea, V.V.; Vlase, L. Evaluation of the nutritional quality of some fruits grown in Romania. In Recent Advances in Technology Research and Education (Inter-Academia 2023); Ono, Y., Kondoh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 122–139. [Google Scholar] [CrossRef]
- Schrire, Z.M.; Phillips, C.L.; Chapman, J.L.; Duffy, S.L.; Wong, G.; D’Rozario, A.L.; Comas, M.; Raisin, I.; Saini, B.; Gordon, C.J.; et al. Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 2021, 72, e12782. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Calvo, S.; Bisquert, R.; Guillamón, J.M. Melatonin in yeast and fermented beverages: Analytical tools for detection physiological role and biosynthesis. Melatonin Res. 2020, 3, 144–160. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Vlase, L.; Colibaba, L.C.; Gheldiu, A.M.; Cotea, V.V. Evolution of phenolic profile of white wines treated with enzymes. Food Chem. 2021, 340, 127910. [Google Scholar] [CrossRef]
- Vlase, L.; Kiss, B.; Leucuta, S.E.; Gocan, S. A Rapid Method for Determination of resveratrol in wines by HPLC-MS. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2105–2121. [Google Scholar] [CrossRef]
- Vlase, A.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium species: From optimization of the extraction process to evaluation of biological properties. Antioxidants 2022, 12, 91. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.; Mena-Morales, A.; Chacón-Vozmediano, J.; Martínez-Gascueña, J.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Comprehensive chemical and sensory assessment of wines made from white grapes of Vitis vinifera cultivars Albillo Dorado and Montonera del Casar: A comparative study with Airén. Foods 2020, 9, 1282. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Onache, P.A.; Florea, A.; Geana, E.; Ciucure, C.T.; Ionete, R.E.; Sumedrea, D.I.; Tița, O. Assessment of bioactive phenolic compounds in musts and the corresponding wines of white and red grape varieties. Appl. Sci. 2023, 13, 5722. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2011, 44, 3134–3148. [Google Scholar] [CrossRef]
- Rentzsch, M.; Wilkens, A.; Winterhalter, P. Non-flavonoid phenolic compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer Science+Business Media: Berlin, Germany, 2009; pp. 509–527. [Google Scholar]
- Merkytè, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic compounds as markers of wine quality and authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Silkolya, L. Authenticity tests of hite wines from the Apold depression. Manag. Sustain. Dev. 2014, 6, 55. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Carle, R. Evolution of polyphenols during vinification and wine storage. FPSB 2009, 3, 46–59. [Google Scholar]
- Morcillo-Parra, M.Á.; González, B.; Beltran, G.; Mas, A.; Torija, M. Melatonin and glycolytic protein interactions are related to yeast fermentative capacity. Food Microbiol. 2020, 87, 103398. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of tryptophan, tryptophan ethylester, and melatonin derivatives in red wine by SPE-HPLC-FL and SPE-HPLC-MS methods. Foods 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antioxidant capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Sunyer-Figueres, M.; Mas, A.; Beltran, G.; Torija, M. Protective effects of melatonin on Saccharomyces cerevisiae under ethanol stress. Antioxidants 2021, 10, 1735. [Google Scholar] [CrossRef]
- Valera, M.J.; Morcillo-Parra, M.Á.; Zagórska, I.; Mas, A.; Beltran, G.; Torija, M. Effects of melatonin and tryptophol addition on fermentations carried out by Saccharomyces cerevisiae and non-Saccharomyces yeast species under different nitrogen conditions. Int. J. Food Microbiol. 2019, 289, 174–181. [Google Scholar] [CrossRef]
- Fernández-González, M.; Úbeda, J.F.; Cordero-Otero, R.R.; Thanvanthri, G.V.; Briones, A.I. Engineering of an oenological Saccharomyces cerevisiae strain with pectinolytic activity and its effect on wine. Int. J. Food Microbiol. 2005, 102, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Masino, F.; Montevecchi, G.; Arfelli, G.; Antonelli, A. Evaluation of the combined effects of enzymatic treatment and aging on lees on the aroma of wine from Bombino bianco grapes. J. Agric. Food Chem. 2008, 56, 9495–9501. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin minimizes the impact of oxidative stress induced by hydrogen peroxide in Saccharomyces and non-conventional yeast. Front. Microbiol. 2018, 9, 1933. [Google Scholar] [CrossRef] [PubMed]
- Olszowy-Tomczyk, M.; Dawidowicz, A.L.; Jóźwik-Dolęba, M. Are mutual interactions between antioxidants the only factors responsible for antagonistic antioxidant effect of their mixtures? Additive and antagonistic antioxidant effects in mixtures of gallic, ferulic and caffeic acids. Eur. Food Res. Technol. 2019, 245, 1473–1485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).