Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Sample
2.2. Laboratory Analysis
2.2.1. Phenolic Compound Evaluation
2.2.2. Identification and Quantification of Melatonin
2.2.3. Total Antioxidant Capacity
2.3. Statistical Tests
3. Results and Discussion
3.1. Effect of Different Yeasts on Wine Bioactive Compounds
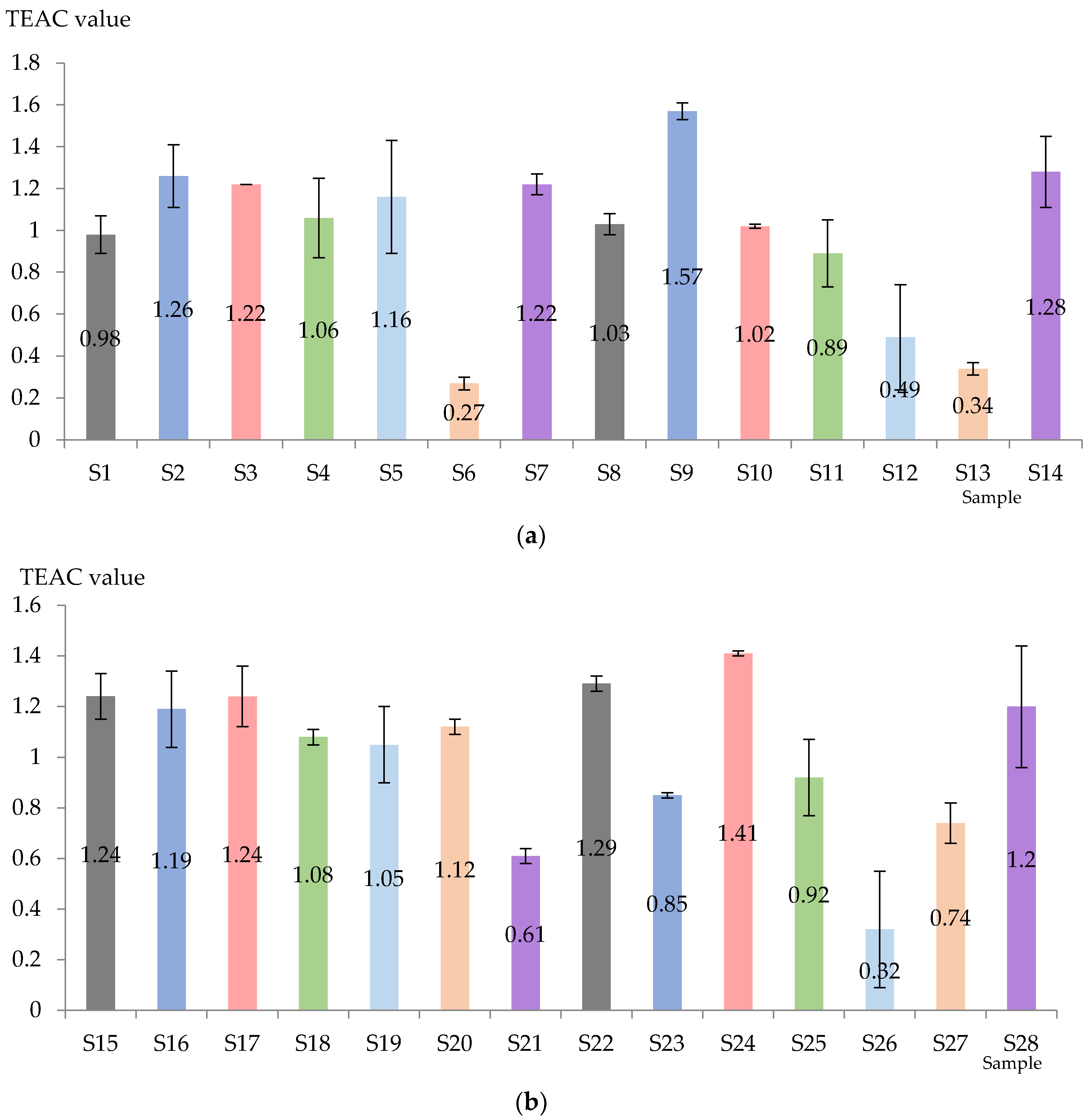
3.2. Total Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alugoju, P.; Kumaree, K.K.; Prasansuklab, A.; Tencomnao, T. Melatonin as a vital metabolite in medicinal and food plants. In Advancement of Melatonin Research in Plants; Roychoudhury, A., Ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 67–83. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, temperature, and light on the stability of melatonin in aqueous solutions and fruit juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef]
- Vázquez, J.; González, B.; Sempere, V.; Mas, A.; Torija, M.; Beltran, G. Melatonin Reduces Oxidative Stress Damage Induced by Hydrogen Peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1066. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Shi, T.; Song, S.; Zhang, Z.W.; Fang, Y.L. Melatonin in grapes and grape-related foodstuffs. Food Chem. 2017, 231, 185–191. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Wu, T.; Zhai, R.; Yang, C.; Wang, Z.; Fengwang, M.; Lingfei, X. Effects of melatonin treatment of postharvest pear fruit on aromatic volatile biosynthesis. Molecules 2019, 24, 4233. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in wine and beer: Beneficial effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef] [PubMed]
- Boban, D.; Dželalija, A.M.; Jurić, D.; Benzon, B.; Ključević, N.; Boban, Z.; Mudnić, I.; Grković, I. Differential effects of white wine and ethanol consumption on survival of rats after a myocardial infarction. Appl. Sci. 2023, 13, 1450. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.L.; Ion, M. Different extraction approaches for the analysis of melatonin from Cabernet Sauvignon and Feteasca neagra wines using a validated HPLC-FL method. Molecules 2023, 28, 2768. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Albu, C.; Radu, G.L.; Alecu, A.; Brinduse, E. The influence of melatonin treatment in the vinification of Feteasca neagra and Cabernet Sauvignon wines on the profile of polyphenolic compounds and antioxidant activity. Antioxidants 2023, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Marrero, S.C.; Martínez-Rodríguez, A.; Maestre Pérez, S.E.; Moya, S.P. New trends and applications in fermented beverages. In The Sciences of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston: UK; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 31–66. [Google Scholar]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin. Food Chem. 2011, 130, 797–813. [Google Scholar] [CrossRef]
- Thapa, N.; Tamang, J.P. Functionality and therapeutic values of fermented foods. In Health Benefits of Fermented Foods and Beverages; Tamang, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Tamang, J.P.; Thapa, N.; Tamang, B.; Rai, A.; Chettri, R. Microorganisms in fermented foods and beverages. In Health Benefits of Fermented Foods and Beverages; Tamang, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Gomez, F.J.V.; Raba, J.; Cerutti, S.; Silva, F. Monitoring melatonin and its isomer in Vitis vinifera cv Malbec by UHPLC-MS/MS from grape to bottle. J. Pineal Res. 2012, 52, 349–355. [Google Scholar] [CrossRef]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and derived L-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Albu, C.; Radu, L.E.; Radu, G.L. Assessment of melatonin and its precursors content by a HPLC-MS/MS method from different Romanian wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, Á.; Troncoso, A.M.; Cantos-Villar, E.; García-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Niculaua, M.; Gheldiu, A.; Cotea, V.V.; Vlase, L. Evaluation of the nutritional quality of some fruits grown in Romania. In Recent Advances in Technology Research and Education (Inter-Academia 2023); Ono, Y., Kondoh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 122–139. [Google Scholar] [CrossRef]
- Schrire, Z.M.; Phillips, C.L.; Chapman, J.L.; Duffy, S.L.; Wong, G.; D’Rozario, A.L.; Comas, M.; Raisin, I.; Saini, B.; Gordon, C.J.; et al. Safety of higher doses of melatonin in adults: A systematic review and meta-analysis. J. Pineal Res. 2021, 72, e12782. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Calvo, S.; Bisquert, R.; Guillamón, J.M. Melatonin in yeast and fermented beverages: Analytical tools for detection physiological role and biosynthesis. Melatonin Res. 2020, 3, 144–160. [Google Scholar] [CrossRef]
- Scutarașu, E.C.; Luchian, C.E.; Vlase, L.; Colibaba, L.C.; Gheldiu, A.M.; Cotea, V.V. Evolution of phenolic profile of white wines treated with enzymes. Food Chem. 2021, 340, 127910. [Google Scholar] [CrossRef]
- Vlase, L.; Kiss, B.; Leucuta, S.E.; Gocan, S. A Rapid Method for Determination of resveratrol in wines by HPLC-MS. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2105–2121. [Google Scholar] [CrossRef]
- Vlase, A.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium species: From optimization of the extraction process to evaluation of biological properties. Antioxidants 2022, 12, 91. [Google Scholar] [CrossRef]
- Pérez-Navarro, J.; Izquierdo-Cañas, P.; Mena-Morales, A.; Chacón-Vozmediano, J.; Martínez-Gascueña, J.; García-Romero, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S. Comprehensive chemical and sensory assessment of wines made from white grapes of Vitis vinifera cultivars Albillo Dorado and Montonera del Casar: A comparative study with Airén. Foods 2020, 9, 1282. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Onache, P.A.; Florea, A.; Geana, E.; Ciucure, C.T.; Ionete, R.E.; Sumedrea, D.I.; Tița, O. Assessment of bioactive phenolic compounds in musts and the corresponding wines of white and red grape varieties. Appl. Sci. 2023, 13, 5722. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2011, 44, 3134–3148. [Google Scholar] [CrossRef]
- Rentzsch, M.; Wilkens, A.; Winterhalter, P. Non-flavonoid phenolic compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer Science+Business Media: Berlin, Germany, 2009; pp. 509–527. [Google Scholar]
- Merkytè, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic compounds as markers of wine quality and authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Silkolya, L. Authenticity tests of hite wines from the Apold depression. Manag. Sustain. Dev. 2014, 6, 55. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Carle, R. Evolution of polyphenols during vinification and wine storage. FPSB 2009, 3, 46–59. [Google Scholar]
- Morcillo-Parra, M.Á.; González, B.; Beltran, G.; Mas, A.; Torija, M. Melatonin and glycolytic protein interactions are related to yeast fermentative capacity. Food Microbiol. 2020, 87, 103398. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Vigentini, I.; Lo Faro, A.F.; De Nisi, P.; Foschino, R.; Tirelli, A.; Orioli, M.; Iriti, M. Assessment of tryptophan, tryptophan ethylester, and melatonin derivatives in red wine by SPE-HPLC-FL and SPE-HPLC-MS methods. Foods 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antioxidant capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Sunyer-Figueres, M.; Mas, A.; Beltran, G.; Torija, M. Protective effects of melatonin on Saccharomyces cerevisiae under ethanol stress. Antioxidants 2021, 10, 1735. [Google Scholar] [CrossRef]
- Valera, M.J.; Morcillo-Parra, M.Á.; Zagórska, I.; Mas, A.; Beltran, G.; Torija, M. Effects of melatonin and tryptophol addition on fermentations carried out by Saccharomyces cerevisiae and non-Saccharomyces yeast species under different nitrogen conditions. Int. J. Food Microbiol. 2019, 289, 174–181. [Google Scholar] [CrossRef]
- Fernández-González, M.; Úbeda, J.F.; Cordero-Otero, R.R.; Thanvanthri, G.V.; Briones, A.I. Engineering of an oenological Saccharomyces cerevisiae strain with pectinolytic activity and its effect on wine. Int. J. Food Microbiol. 2005, 102, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Masino, F.; Montevecchi, G.; Arfelli, G.; Antonelli, A. Evaluation of the combined effects of enzymatic treatment and aging on lees on the aroma of wine from Bombino bianco grapes. J. Agric. Food Chem. 2008, 56, 9495–9501. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin minimizes the impact of oxidative stress induced by hydrogen peroxide in Saccharomyces and non-conventional yeast. Front. Microbiol. 2018, 9, 1933. [Google Scholar] [CrossRef] [PubMed]
- Olszowy-Tomczyk, M.; Dawidowicz, A.L.; Jóźwik-Dolęba, M. Are mutual interactions between antioxidants the only factors responsible for antagonistic antioxidant effect of their mixtures? Additive and antagonistic antioxidant effects in mixtures of gallic, ferulic and caffeic acids. Eur. Food Res. Technol. 2019, 245, 1473–1485. [Google Scholar] [CrossRef]

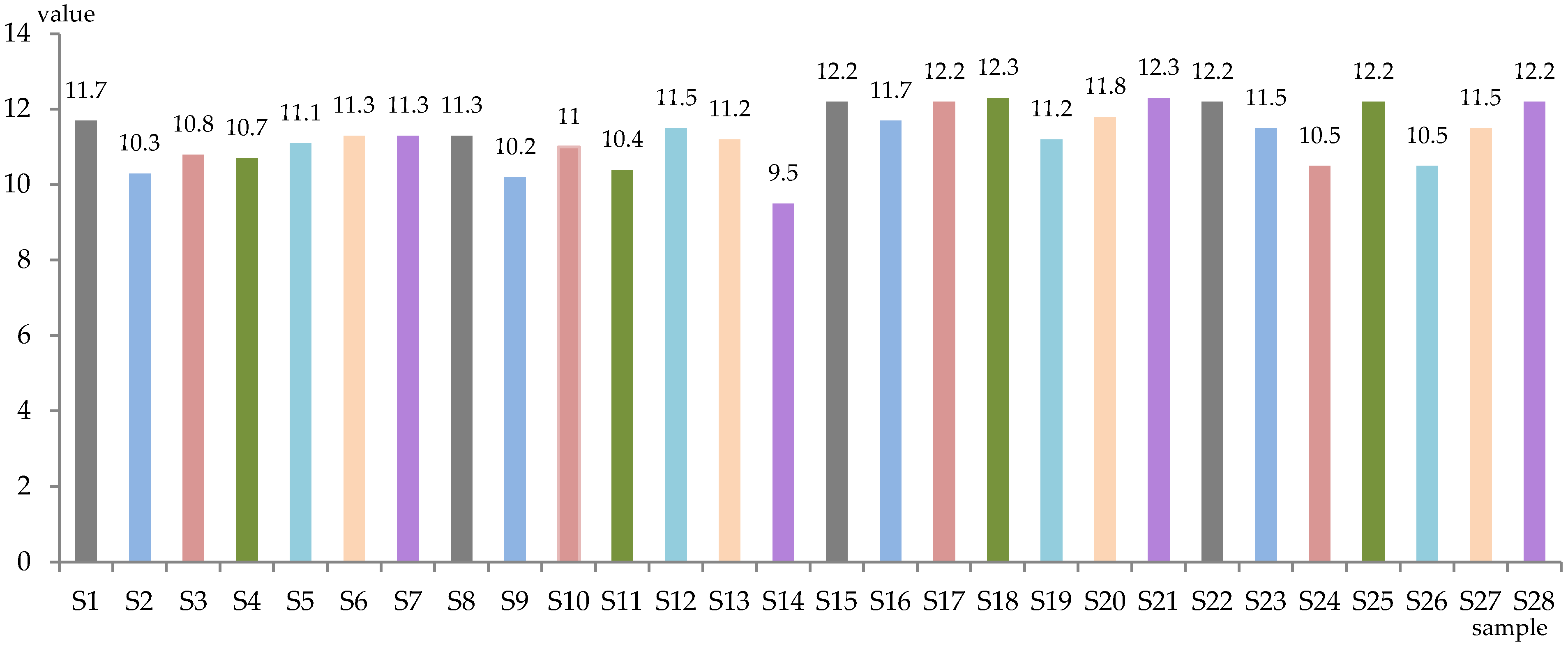

| Aligoté+ Fetească Albă | Sauvignon Blanc | Bâtonnage Treatment | Inoculated Yeasts | Yeasts’ Alcohol Tolerance * |
|---|---|---|---|---|
| S1 | S15 | Control sample, no bâtonnage, no exogenous yeasts | ||
| S2 | S16 | No bâtonnage | Lachancea thermotolerans | 10% |
| S3 | S17 | Saccharomyces cerevisiae | 13% | |
| S4 | S18 | Torulaspora delbrueckii | 11% | |
| S5 | S19 | Pichia kluyveri | 4–5% | |
| S6 | S20 | Saccharomyces cerevisiae + Kluyveromyces thermotolerans | 16% | |
| S7 | S21 | Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae | 15% | |
| S8 | S22 | Control sample, with bâtonnage, no exogenous yeasts | ||
| S9 | S23 | With bâtonnage products | Lachancea thermotolerans | 10% |
| S10 | S24 | Saccharomyces cerevisiae | 13% | |
| S11 | S25 | Torulaspora delbrueckii | 11% | |
| S12 | S26 | Pichia kluyveri | 4–5% | |
| S13 | S27 | Saccharomyces cerevisiae + Kluyveromyces thermotolerans | 16% | |
| S14 | S28 | Kluyveromyces thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae | 15% | |
| Phenolic Compounds | Retention Time (min.) | Specific Ions |
|---|---|---|
| trans-resveratrol | 1.5 | 229 > 134.9 |
| cis-resveratrol | 2.3 | 229 > 134.9 |
| epicatechin | 9.0 | 289 |
| catechin | 6.0 | 289 |
| gallic acid | 1.5 | 169 |
| protocatechuic acid | 2.8 | 153 |
| caftaric acid | 3.54 | 311 > 148.6, 178.6 |
| caffeic acid | 5.60 | 179.4 > 134.7 |
| p-coumaric acid | 9.48 | 163 > 118.7 |
| ferulic acid | 12.8 | 193.2 > 133.7, 148.7, 177.6 |
| Sample | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit Measure | µg/mL | µg/L | |||||||||
| S1 | 0.38 ± 0.08 | 0.68 ± 0.02 | 0.47 ± 0.01 | 0.34 ± 0.04 | 1.83 ± 0.04 | 0.32 ± 0.01 | 19.54 ± 0.00 | 1.51 ± 0.45 | 0.61 ± 0.24 | 0.18 ± 0.12 | 0.22 ± 0.10 |
| S2 | 0.51 ± 0.05 | 0.61 ± 0.10 | 0.48 ± 0.05 | 0.33 ± 0.00 | 1.45 ± 0.01 | 0.46 ± 0.03 | 16.65 ± 0.10 | 1.75 ± 0.12 | 0.97 ± 0.08 | 0.35 ± 0.05 | 0.32 ± 0.02 |
| S3 | 0.39 ± 0.02 | 0.64 ± 0.02 | 0.70 ± 0.25 | 0.53 ± 0.05 | 1.62 ± 0.01 | 0.36 ± 0.00 | 19.90 ± 0.05 | 1.64 ± 0.11 | 0.64 ± 0.05 | 0.20 ± 0.02 | 0.70 ± 0.05 |
| S4 | 0.30 ± 0.01 | 0.61 ± 0.05 | 0.74 ± 0.01 | 0.49 ± 0.15 | 1.65 ± 0.08 | 0.26 ± 0.01 | 16.14 ± 0.15 | 1.51 ± 0.10 | 0.55 ± 0.00 | 0.18 ± 0.10 | 0.03 ± 0.00 |
| S5 | 0.40 ± 0.05 | 0.59 ± 0.10 | 0.67 ± 0.02 | 0.48 ± 0.01 | 1.74 ± 0.00 | 0.32 ± 0.01 | 17.13 ± 0.04 | 1.79 ± 0.04 | 0.58 ± 0.15 | 0.18 ± 0.12 | 0.81 ± 0.03 |
| S6 | 0.28 ± 0.01 | 0.47 ± 0.08 | 0.81 ± 0.00 | 0.60 ± 0.00 | 1.81 ± 0.02 | 0.33 ± 0.04 | 17.68 ± 0.00 | 1.52 ± 0.05 | 0.61 ± 0.30 | 0.18 ± 0.04 | 0.45 ± 0.01 |
| S7 | 0.24 ± 0.10 | 0.58 ± 0.00 | 0.94 ± 0.05 | 0.66 ± 0.02 | 1.82 ± 0.00 | 0.45 ± 0.00 | 18.59 ± 0.12 | 1.57 ± 0.10 | 0.62 ± 0.08 | 0.17 ± 0.15 | 0.24 ± 0.02 |
| p-values | 0.001 | 0.042 | 0.000 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.389 | 0.094 | 0.312 | <0.0001 |
| S8 | 0.11 ± 0.04 | 0.48 ± 0.01 | 0.73 ± 0.01 | 0.52 ± 0.00 | 1.89 ± 0.04 | 0.42 ± 0.00 | 2.97 ± 0.03 | 8.99 ± 0.11 | 1.51 ± 0.12 | 0.63 ± 0.00 | 0.65 ± 0.00 |
| S9 | 0.32 ± 0.06 | 0.44 ± 0.02 | 0.66 ± 0.04 | 0.51 ± 0.08 | 2.35 ± 0.00 | 0.42 ± 0.02 | 3.09 ± 0.06 | 10.08 ± 0.08 | 1.70 ± 0.01 | 0.81 ± 0.02 | 0.50 ± 0.01 |
| S10 | 0.26 ± 0.03 | 0.50 ± 0.04 | 0.75 ± 0.00 | 0.60 ± 0.00 | 1.93 ± 0.20 | 0.40 ± 0.04 | 2.49 ± 0.01 | 10.27 ± 0.04 | 1.79 ± 0.10 | 0.84 ± 0.15 | 0.87 ± 0.01 |
| S11 | 0.26 ± 0.20 | 0.46 ± 0.05 | 0.85 ± 0.10 | 0.56 ± 0.05 | 1.89 ± 0.10 | 0.45 ± 0.01 | 3.28 ± 0.00 | 9.31 ± 0.12 | 1.67 ± 0.05 | 0.71 ± 0.08 | 0.35 ± 0.03 |
| S12 | 0.34 ± 0.10 | 0.45 ± 0.00 | 0.72 ± 0.01 | 0.50 ± 0.04 | 2.13 ± 0.02 | 0.47 ± 0.05 | 2.77 ± 0.03 | 10.12 ± 0.04 | 1.73 ± 0.02 | 0.68 ± 0.12 | 1.10 ± 0.15 |
| S13 | 0.22 ± 0.00 | 0.47 ± 0.04 | 0.82 ± 0.01 | 0.64 ± 0.00 | 2.20 ± 0.05 | 0.65 ± 0.00 | 2.77 ± 0.04 | 10.32 ± 0.01 | 1.79 ± 0.12 | 0.87 ± 0.00 | 1.58 ± 0.10 |
| S14 | 0.18 ± 0.12 | 0.36 ± 0.11 | 0.79 ± 0.08 | 0.62 ± 0.05 | 1.92 ± 0.01 | 0.56 ± 0.02 | 2.85 ± 0.04 | 9.40 ± 0.11 | 1.64 ± 0.35 | 0.73 ± 0.01 | 0.62 ± 0.05 |
| p-values | 0.153 | 0.092 | 0.007 | 0.005 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.356 | 0.019 | <0.0001 |
| Sample | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit Measure | µg/mL | µg/L | |||||||||
| S15 | 0.50 ± 0.07 | 0.89 ± 0.02 | 0.20 ± 0.04 | 0.14 ± 0.04 | 20.62 ± 0.01 | 0.37 ± 0.03 | 3.96 ± 0.00 | 3.42 ± 0.12 | 0.31 ± 0.15 | 0.21 ± 0.05 | 7.81 ± 0.15 |
| S16 | 0.48 ± 0.02 | 0.91 ± 0.08 | 0.22 ± 0.03 | 0.12 ± 0.04 | 18.85 ± 0.02 | 0.42 ± 0.01 | 3.72 ± 0.02 | 3.56 ± 0.15 | 0.31 ± 0.10 | 0.25 ± 0.04 | 0.46 ± 0.05 |
| S17 | 0.50 ± 0.01 | 1.12 ± 0.02 | 0.22 ± 0.04 | 0.16 ± 0.01 | 20.67 ± 0.00 | 0.39 ± 0.00 | 3.68 ± 0.05 | 3.69 ± 0.00 | 0.58 ± 0.08 | 0.35 ± 0.20 | 0.69 ± 0.03 |
| S18 | 0.53 ± 0.20 | 0.77 ± 0.0 | 0.26 ± 0.02 | 0.15 ± 0.00 | 19.69 ± 0.03 | 0.53 ± 0.03 | 3.64 ± 0.04 | 3.62 ± 0.02 | 0.40 ± 0.15 | 0.30 ± 0.05 | 0.40 ± 0.10 |
| S19 | 0.37 ± 0.05 | 0.88 ± 0.00 | 0.22 ± 0.01 | 0.17 ± 0.04 | 19.78 ± 0.02 | 0.35 ± 0.04 | 3.44 ± 0.04 | 3.62 ± 0.08 | 0.34 ± 0.05 | 0.20 ± 0.15 | 0.65 ± 0.08 |
| S20 | 0.27 ± 0.02 | 0.66 ± 0.02 | 0.27 ± 0.02 | 0.17 ± 0.04 | 20.64 ± 0.01 | 0.41 ± 0.03 | 3.99 ± 0.10 | 3.51 ± 0.01 | 0.58 ± 0.02 | 0.38 ± 0.10 | 2.71 ± 0.15 |
| S21 | 0.36 ± 0.04 | 0.93 ± 0.05 | 0.26 ± 0.01 | 0.17 ± 0.03 | 20.70 ± 0.20 | 0.35 ± 0.00 | 4.11 ± 0.20 | 3.47 ± 0.05 | 0.55 ± 0.15 | 0.43 ± 0.20 | 0.41 ± 0.05 |
| p-values | 0.016 | <0.0001 | 0.042 | 0.448 | <0.0001 | <0.0001 | <0.0001 | 0.014 | 0.017 | 0.296 | <0.0001 |
| S22 | 1.01 ± 0.05 | 1.39 ± 0.03 | 0.16 ± 0.05 | 0.14 ± 0.02 | 21.11 ± 0.03 | 0.64 ± 0.02 | 0.24 ± 0.04 | 5.93 ± 0.20 | 2.00 ± 0.08 | 0.76 ± 0.05 | 0.37 ± 0.15 |
| S23 | 0.99 ± 0.00 | 1.79 ± 0.00 | 0.16 ± 0.04 | 0.14 ± 0.01 | 22.96 ± 0.08 | 0.61 ± 0.01 | 0.51 ± 0.05 | 6.67 ± 0.04 | 2.30 ± 0.10 | 0.96 ± 0.04 | 0.21 ± 0.02 |
| S24 | 0.43 ± 0.01 | 1.72 ± 0.03 | 0.16 ± 0.03 | 0.11 ± 0.00 | 20.93 ± 0.20 | 0.34 ± 0.01 | 0.47 ± 0.18 | 6.17 ± 0.01 | 2.72 ± 0.05 | 0.94 ± 0.00 | 0.26 ± 0.03 |
| S25 | 0.80 ± 0.03 | 1.36 ± 0.04 | 0.21 ± 0.01 | 0.12 ± 0.01 | 21.21 ± 0.03 | 0.47 ± 0.02 | 0.28 ± 0.04 | 5.80 ± 0.10 | 2.06 ± 0.02 | 0.91 ± 0.15 | 0.22 ± 0.00 |
| S26 | 0.67 ± 0.04 | 1.29 ± 0.05 | 0.17 ± 0.00 | 0.13 ± 0.04 | 20.70 ± 0.03 | 0.54 ± 0.03 | 0.24 ± 0.22 | 5.56 ± 0.08 | 1.97 ± 0.15 | 0.74 ± 0.04 | 0.25 ± 0.05 |
| S27 | 0.77 ± 0.00 | 1.30 ± 0.00 | 0.28 ± 0.01 | 0.18 ± 0.0 | 20.65 ± 0.08 | 0.60 ± 0.01 | 0.00 ± 0.00 | 6.02 ± 0.05 | 1.45 ± 0.04 | 0.81 ± 0.00 | 0.28 ± 0.08 |
| S28 | 1.21 ± 0.01 | 1.32 ± 0.00 | 0.22 ± 0.05 | 0.16 ± 0.02 | 21.22 ± 0.05 | 0.56 ± 0.03 | 0.28 ± 0.05 | 6.24 ± 0.02 | 2.51 ± 0.08 | 0.94 ± 0.01 | 0.52 ± 0.04 |
| p-values | <0.0001 | <0.0001 | 0.004 | 0.016 | <0.0001 | <0.0001 | 0.015 | <0.0001 | <0.0001 | 0.002 | <0.0001 |
| Source | Beta Coefficient | Standard Error | t | Pr > |t| | Lower Bound (95%) | Upper Bound (95%) | Value | Standard Error | t | Pr > |t| | Lower Bound (95%) | Upper Bound (95%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without bâtonnage | With bâtonnage | |||||||||||
| C1 | −5.593 | 4.276 | −1.308 | 0.223 ° | −15.266 | 4.081 | 0.697 | 0.611 | 1.142 | 0.283 ° | −0.684 | 2.078 |
| C2 | 3.961 | 0.619 | 6.396 | 0.000 *** | 2.560 | 5.362 | −5.568 | 3.293 | −1.691 | 0.125 ° | −13.017 | 1.881 |
| C3 | 0.110 | 0.481 | 0.228 | 0.824 ° | −0.978 | 1.197 | −2.791 | 1.653 | −1.688 | 0.126 ° | −6.531 | 0.949 |
| C4 | −2.121 | 2.063 | −1.028 | 0.331 ° | −6.787 | 2.546 | 7.070 | 1.675 | 4.222 | 0.002 * | 3.282 | 10.859 |
| C5 | −1.744 | 0.579 | −3.011 | 0.015 * | −3.054 | −0.434 | 1.573 | 0.792 | 1.987 | 0.078 . | −0.218 | 3.364 |
| C6 | 2.584 | 0.771 | 3.352 | 0.008 ** | 0.840 | 4.328 | −6.185 | 3.654 | −1.692 | 0.125 ° | −14.452 | 2.082 |
| C7 | −0.002 | 0.049 | −0.033 | 0.975 ° | −0.112 | 0.109 | 0.178 | 0.374 | 0.476 | 0.645 ° | −0.668 | 1.025 |
| C8 | 1.449 | 0.555 | 2.612 | 0.028 * | 0.194 | 2.704 | −1.100 | 0.396 | −2.781 | 0.021 * | −1.995 | −0.205 |
| C9 | −2.062 | 0.599 | −3.443 | 0.007 ** | −3.416 | −0.707 | 1.937 | 0.863 | 2.245 | 0.051 . | −0.015 | 3.889 |
| C10 | 3.458 | 2.819 | 1.227 | 0.251 ° | −2.919 | 9.835 | 1.054 | 0.790 | 1.334 | 0.215 ° | −0.733 | 2.842 |
| C11 | 362.824 | 431.340 | 0.841 | 0.422 ° | −612.934 | 1338.583 | 271.168 | 675.415 | 0.401 | 0.697 ° | −1256.727 | 1799.062 |
| Source | Beta Coefficient | Standard Error | t | Pr > |t| | Lower Bound (95%) | Upper Bound (95%) | Value | Standard Error | t | Pr > |t| | Lower Bound (95%) | Upper Bound (95%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without bâtonnage | With bâtonnage | |||||||||||
| C1 | −0.856 | 0.157 | −5.442 | 0.000 *** | −1.212 | −0.500 | 5.372 | 1.082 | 4.963 | 0.001 *** | 2.924 | 7.820 |
| C2 | 0.652 | 0.200 | 3.265 | 0.010 ** | 0.200 | 1.103 | 10.197 | 2.431 | 4.194 | 0.002 ** | 4.697 | 15.697 |
| C3 | −0.460 | 0.137 | −3.357 | 0.008 ** | −0.770 | −0.150 | 7.216 | 2.710 | 2.663 | 0.026 * | 1.086 | 13.346 |
| C4 | −0.092 | 0.161 | −0.570 | 0.582 ° | −0.457 | 0.273 | 5.486 | 4.511 | 1.216 | 0.255 ° | −4.719 | 15.690 |
| C5 | −1.027 | 0.169 | −6.079 | 0.000 *** | −1.410 | −0.645 | −0.849 | 0.177 | −4.808 | 0.001 *** | −1.249 | −0.450 |
| C6 | 0.836 | 0.200 | 4.170 | 0.002 ** | 0.382 | 1.289 | −4.380 | 1.699 | −2.578 | 0.030 * | −8.224 | −0.536 |
| C7 | −1.061 | 0.217 | −4.884 | 0.001 *** | −1.552 | −0.569 | −0.899 | 0.962 | −0.934 | 0.374 ° | −3.077 | 1.278 |
| C8 | −0.185 | 0.329 | −0.562 | 0.588 ° | −0.929 | 0.559 | −3.338 | 1.248 | −2.674 | 0.025 * | −6.161 | −0.514 |
| C9 | 1.331 | 0.294 | 4.533 | 0.001 ** | 0.667 | 1.995 | −0.275 | 0.455 | −0.603 | 0.561 ° | −1.304 | 0.755 |
| C10 | 0.398 | 0.126 | 3.162 | 0.012 * | 0.113 | 0.683 | −0.523 | 0.785 | −0.666 | 0.522 ° | −2.298 | 1.252 |
| C11 | 1.643 | 0.122 | 13.462 | <0.0001 *** | 1.367 | 1.920 | 792.310 | 489.546 | 1.618 | 0.140 ° | −315.120 | 1899.741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutarașu, E.C.; Niță, R.G.; Vlase, L.; Zamfir, C.I.; Cioroiu, B.I.; Colibaba, L.C.; Muntean, D.; Luchian, C.E.; Vlase, A.M.; Cotea, V. Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation. Antioxidants 2024, 13, 916. https://doi.org/10.3390/antiox13080916
Scutarașu EC, Niță RG, Vlase L, Zamfir CI, Cioroiu BI, Colibaba LC, Muntean D, Luchian CE, Vlase AM, Cotea V. Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation. Antioxidants. 2024; 13(8):916. https://doi.org/10.3390/antiox13080916
Chicago/Turabian StyleScutarașu, Elena Cristina, Răzvan George Niță, Laurian Vlase, Cătălin Ioan Zamfir, Bogdan Ionel Cioroiu, Lucia Cintia Colibaba, Dana Muntean, Camelia Elena Luchian, Ana Maria Vlase, and Valeriu Cotea. 2024. "Maximizing Wine Antioxidants: Yeast’s Contribution to Melatonin Formation" Antioxidants 13, no. 8: 916. https://doi.org/10.3390/antiox13080916







