Bioaffinity Ultrafiltration Combined with HPLC-ESI-qTOF-MS/MS for Screening Potential Bioactive Components from the Stems of Dendrobium fimbriatum and In Silico Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Preparation of D. fimbriatum Extract
2.3. Preparation of Pure Components
2.4. Reverse-Phase HPLC
2.5. Determination of Total Phenolic Content (TPC)
2.6. Determination of Total Flavonoid Content (TFC)
2.7. DPPH Radical Scavenging Activity
2.8. ABTS Radical Scavenging Activity
2.9. Superoxide Radical Scavenging Activity
2.10. Ferric Reducing Antioxidant Power (FRAP)
2.11. α-Glucosidase Inhibitory Activity Assay
2.12. Acetylcholinesterase Inhibitory Assay
2.13. Bioaffinity Ultrafiltration Assay
2.14. Cell Culture
2.15. Nitric Oxide (NO) Inhibition Assay
2.16. Cytotoxicity Assay
2.17. Western Blotting
2.18. Molecular Modeling Docking Study
2.19. Statistical Analysis
3. Results and Discussion
3.1. TPC, TFC, and Yields of Different Solvent Extracts
3.2. DPPH Radical Scavenging Effect
3.3. ABTS Cation Radical Scavenging Effect
3.4. Superoxide Radical Scavenging Effect
3.5. Ferric Reducing Antioxidant Power
3.6. α-Glucosidase Inhibitory Effect
3.7. Acetylcholinesterase (AChE) Inhibitory Activity
3.8. Anti-Inflammatory Activity
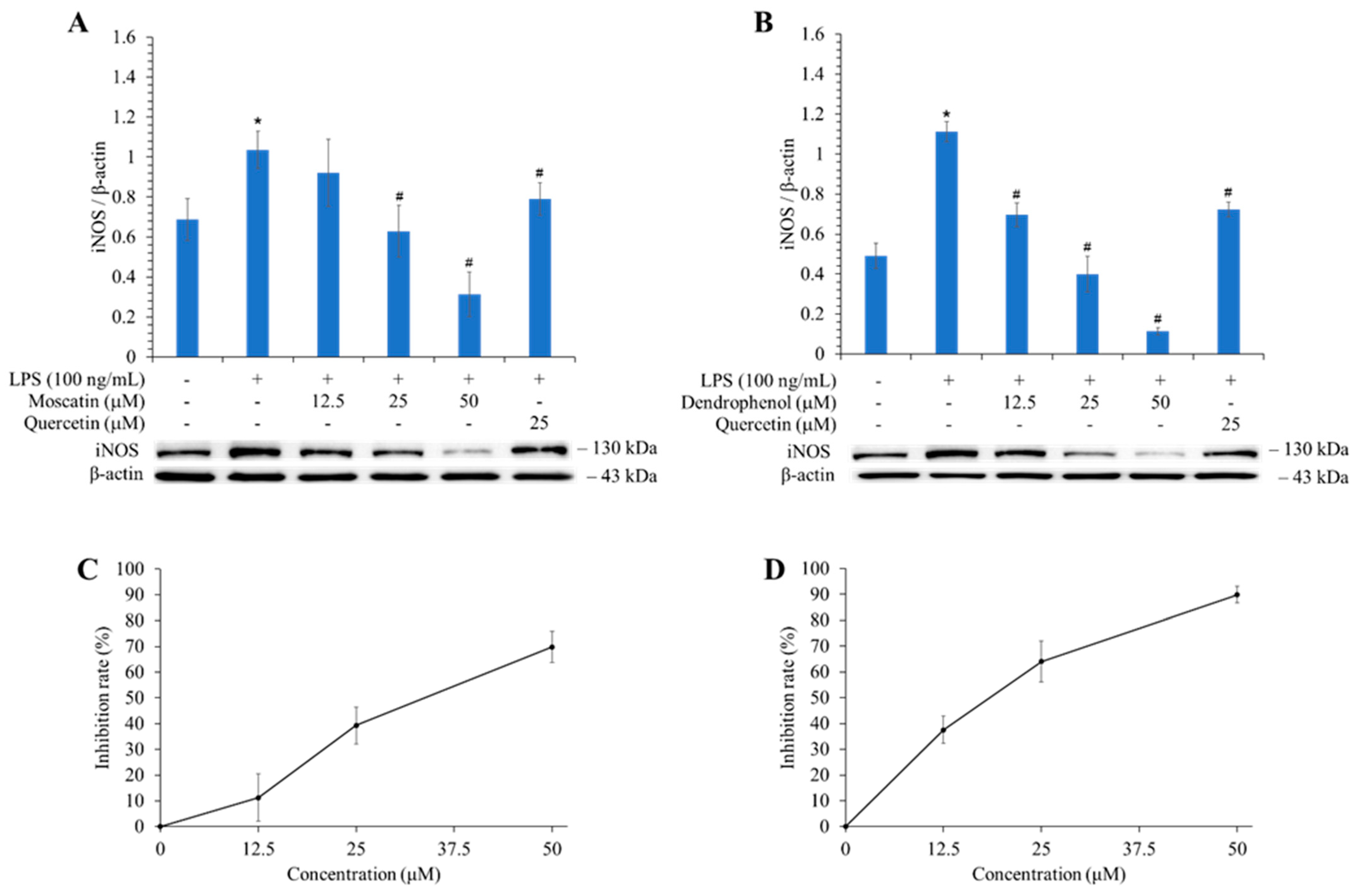
3.9. Effects of Active Components on the Expression of iNOS
3.10. HPLC-ESI-qTOF-MS/MS Analysis
3.11. Bioaffinity Ultrafiltration

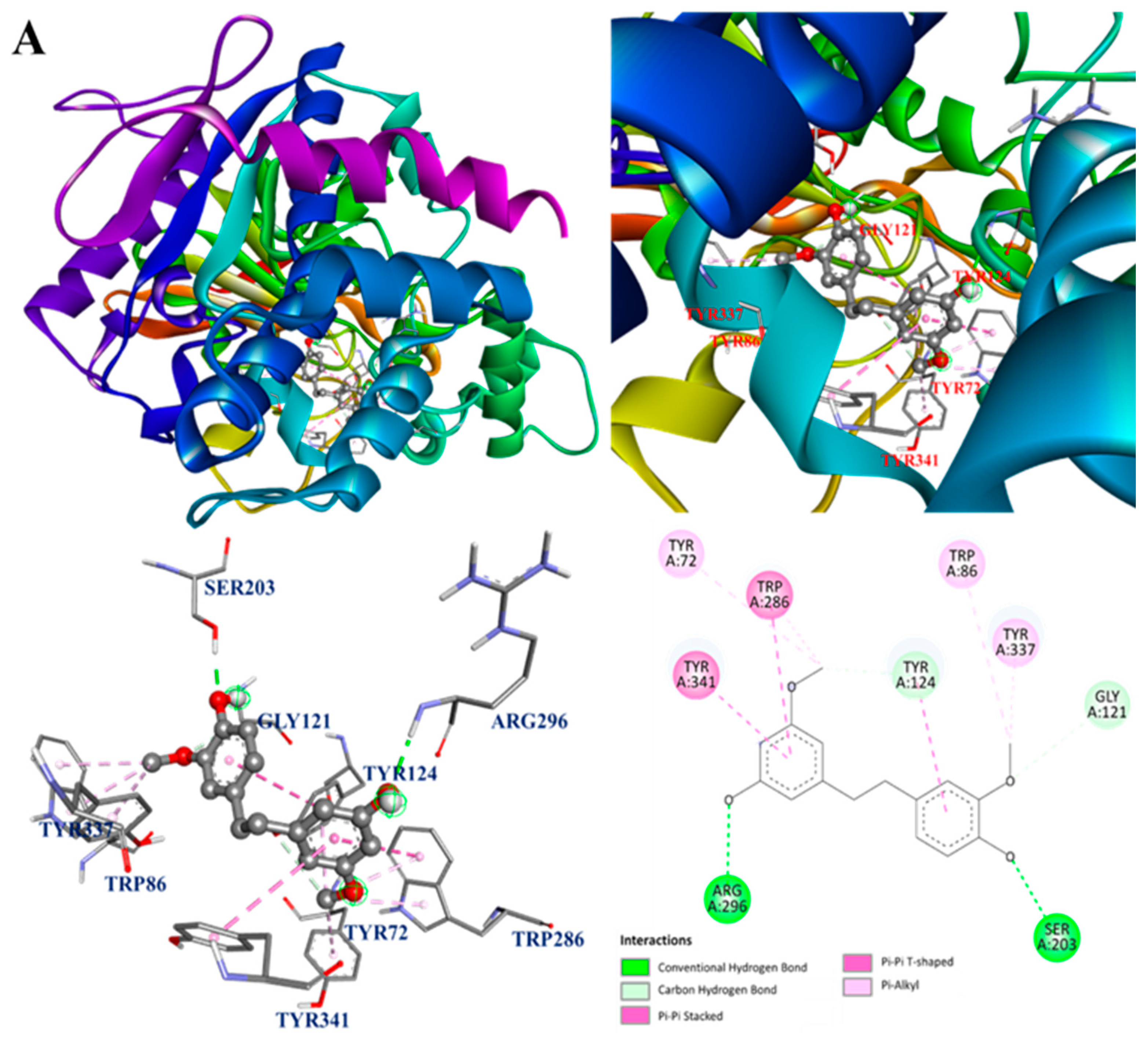
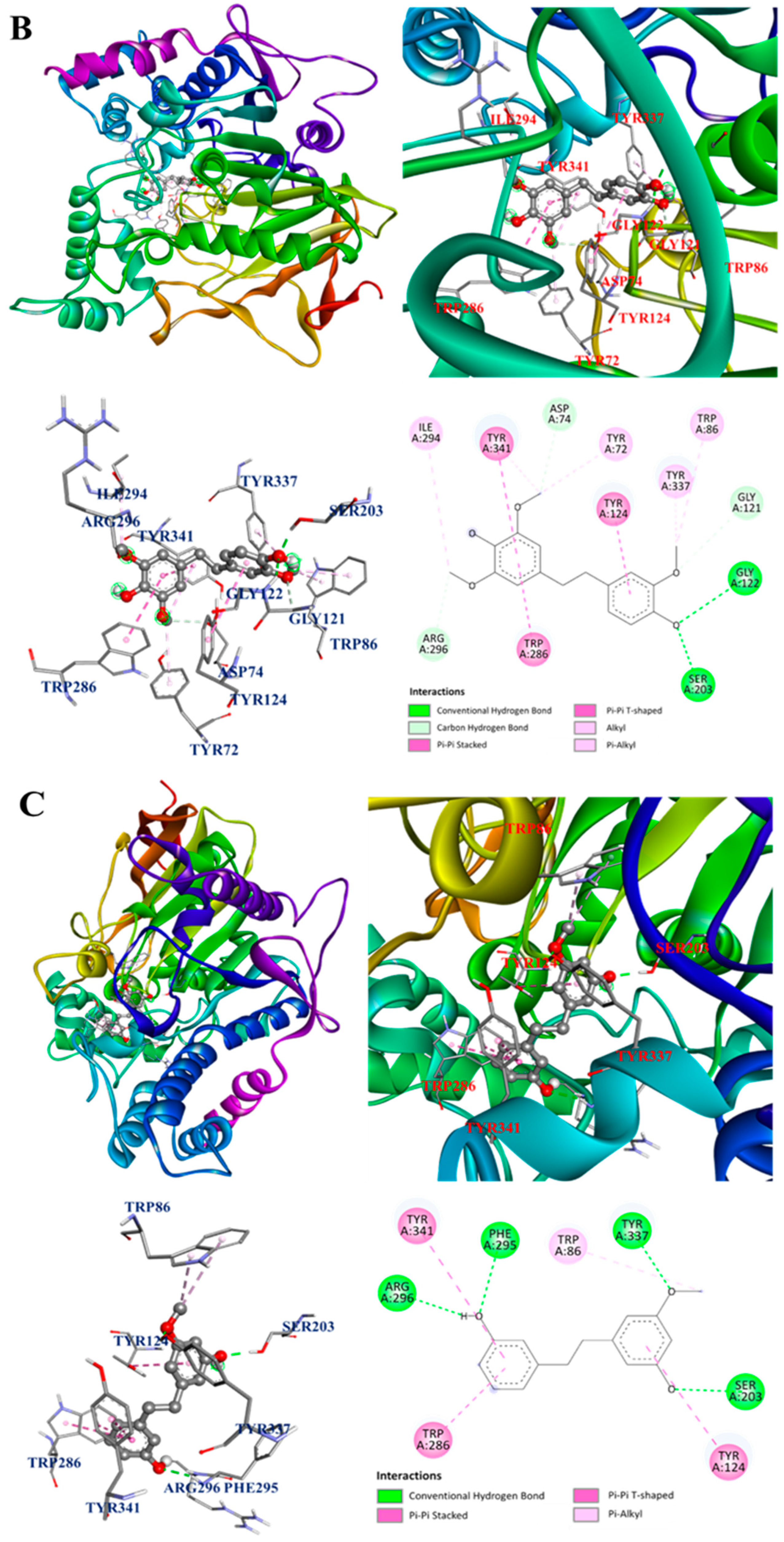
3.12. Molecular Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, W.; Fang, L.; Bao, W.R.; Ma, D.L.; Leung, C.H.; Nie, S.P.; Han, Q.B. Structure characterization and immunomodulating effects of polysaccharides isolated from Dendrobium officinale. J. Agric. Food Chem. 2016, 64, 881–889. [Google Scholar] [CrossRef]
- Xu, J.; Han, Q.B.; Li, S.L.; Chen, X.J.; Wang, X.N.; Zhao, Z.Z.; Chen, H.B. Chemistry, bioactivity, and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. [Google Scholar] [CrossRef]
- Yuan, S.C.; Lekawatana, S.; Amore, T.D.; Chen, F.C.; Chin, S.W.; Vega, D.M.; Wang, Y.T. The Global Orchid Market. In The Orchid Genome; Chen, F.C., Chin, S.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–28. [Google Scholar]
- Wang, Y.H. Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: A review. J. Ethnopharmacol. 2021, 24, 113851. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Luo, M.; Xie, G.Y.; Zeng, W.W.; Wu, Y.X.; Zhu, Y.H.; Yang, X.L.; Guo, A.Y. Systematic Transcriptome and Regulatory Network Analyses Reveal the Hypoglycemic Mechanism of Dendrobium fimbriatum. Mol. Ther. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Xu, F.Q.; Fan, W.W.; Zi, C.T.; Dong, F.W.; Yang, D.; Zhou, J.; Hu, J.M. Four new glycosides from the stems of Dendrobium fimbriatum Hook. Nat. Prod. Res. 2017, 31, 797–801. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, N.; Wang, P.; Liu, J.; Sun, J.; Ye, W.; Fan, B. Flavonoid biosynthesis in four Dendrobium species based on transcriptome sequencing and metabolite analysis. Mol. Biol. Rep. 2022, 49, 2047–2057. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wan, D.L.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Structural characteristics and immunostimulatory activities of a new polysaccharide from Dendrobium fimbriatum Hook. Food Funct. 2021, 12, 3057–3068. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef]
- Sun, J.; Liu, P.F.; Liu, J.N.; Lu, C.; Tong, L.T.; Wang, Y.Q.; LIu, J.M.; Fan, B.; Wang, F.Z. Combined metabolomics and proteomics to reveal beneficial mechanisms of Dendrobium fimbriatum against gastric mucosal injury. Front. Pharmacol. 2022, 30, 948987. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, H.Y.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum polysaccharide ameliorates DSS-induced intestinal mucosal injury by IL-22-regulated intestinal stem cell regeneration. Int. J. Biol. Macromol. 2023, 230, 123199. [Google Scholar] [CrossRef]
- Xiao, F.H.; Guo, Y.J.; Wang, S.Y.; Wen, G.S.; Yang, S.C.; Duan, C.L. Investigations on the planting regions of main medicinal Dendrobium species in yunnan and preliminary evaluation on their suitability. J. Yunnan Agric. Univ. 2008, 23, 498–505. [Google Scholar]
- Tao, Y.; Cai, H.; Li, W.; Cai, B. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale. Anal. Bioanal. Chem. 2015, 407, 6081–6093. [Google Scholar] [CrossRef]
- Li, S.; Tang, Y.; Liu, C.; Zhang, Y. Development of a method to screen and isolate potential α-glucosidase inhibitors from Panax japonicus C.A. Meyer by ultrafiltration, liquid chromatography, and counter-current chromatography. J. Sep. Sci. 2015, 38, 2014–2023. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Y.; Fu, X.; Shen, J.; Chen, Z.; Wang, Q.; Bi, X.; Liu, W.; Li, L.; Liang, G.; et al. Multiple ligand detection and affinity measurement by ultrafiltration and mass spectrometry analysis applied to fragment mixture screening. Anal. Chim. Acta 2015, 30, 98–106. [Google Scholar] [CrossRef]
- Chen, G.; Huang, B.X.; Guo, M. Current advances in screening for bioactive components from medicinal plants by affinity ultrafiltration mass spectrometry. Phytochem. Anal. 2018, 29, 375–386. [Google Scholar] [CrossRef]
- Andrews, G.R. Promoting health and function in an ageing population. Br. Med. J. 2001, 322, 728–729. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendîa, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 16, 1162. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Lee, H.S.; Kwon, Y.J.; Seo, E.B.; Kim, S.K.; Lee, H.; Lee, J.T.; Chang, P.S.; Choi, Y.J.; Lee, S.H.; Ye, S.K. Anti-inflammatory effects of Allium cepa L. peel extracts via inhibition of JAK-STAT pathway in LPS-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 5, 116851. [Google Scholar] [CrossRef]
- Nicoletti, M. Nutraceuticals and botanicals: Overview and perspectives. Int. J. Food Sci. Nutr. 2012, 63, 2–6. [Google Scholar] [CrossRef]
- Zhu, A.L.; Hao, J.W.; Liu, L.; Wang, Q.; Chen, N.D.; Wang, G.L.; Liu, X.Q.; Li, Q.; Xu, H.M.; Yang, W.H. Simultaneous quantification of 11 phenolic compounds and consistency evaluation in four Dendrobium species used as ingredients of the traditional Chinese medicine Shihu. Front. Nutr. 2021, 4, 771078. [Google Scholar] [CrossRef]
- Chu, Y.C.; Yang, C.S.; Cheng, M.J.; Fu, S.L.; Chen, J.J. Comparison of various solvent extracts and major bioactive components from unsalt-fried and salt-fried rhizomes of Anemarrhena asphodeloides for antioxidant, anti-α-glucosidase, and anti-acetylcholinesterase activities. Antioxidants 2022, 11, 385. [Google Scholar] [CrossRef]
- Liang, J.H.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Wang, I.C.; Chen, J.J. Bioactive components from Ampelopsis japonica with antioxidant, anti-α-glucosidase, and anti-acetylcholinesterase activities. Antioxidants 2022, 11, 1228. [Google Scholar] [CrossRef]
- Hsu, J.H.; Yang, C.S.; Chen, J.J. Antioxidant, anti-α-glucosidase, anti-tyrosinase, and anti-inflammatory activities of bioactive components from Morus alba. Antioxidants 2022, 11, 2222. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Meng, L.; Yang, Y.; Gao, Y.; Sun, J. Rapid screening and characterization of acetylcholinesterase inhibitors from yinhuang oral liquid using ultrafiltration-liquid chromatography-electrospray ionization tandem mass spectrometry. Pharmacogn. Mag. 2018, 14, 248–252. [Google Scholar]
- Guo, Y.; Fu, R.; Qian, Y.; Zhou, Z.; Liu, H.; Qi, J.; Zhang, B.; Yu, B. Comprehensive screening and identification of natural inducible nitric oxide synthase inhibitors from Radix Ophiopogonis by off-line multi-hyphenated analyses. J. Chromatogr. A 2019, 10, 55–63. [Google Scholar] [CrossRef]
- Maccallini, C.; Di Matteo, M.; Ammazzalorso, A.; D’Angelo, A.; De Filippis, B.; Di Silvestre, S.; Fantacuzzi, M.; Giampietro, L.; Pandolfi, A.; Amoroso, R. Reversed-phase high-performance liquid chromatography method with fluorescence detection to screen nitric oxide synthases inhibitors. J. Sep. Sci. 2014, 37, 1380–1385. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Zhang, Y.; Yang, M.; Chen, J.; Guo, M. Potential hypoglycemic, hypolipidemic, and anti-inflammatory bioactive components in Nelumbo nucifera leaves explored by bioaffinity ultrafiltration with multiple targets. Food Chem. 2022, 375, 131856. [Google Scholar] [CrossRef]
- Chen, I.S.; Chen, J.J.; Duh, C.Y.; Tsai, I.L. Cytotoxic lignans from formosan Hernandia nymphaeifolia. Phytochemistry 1997, 45, 991–996. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Okhlopkova, Z.M.; Zulfugarov, I.S. Chemical Composition and Antioxidant Activity of Tánara Ótó (Dracocephalum palmatum Stephan), a Medicinal Plant Used by the North-Yakutian Nomads. Molecules 2013, 18, 14105–14121. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.M.; Mittal, A.; Sharma, M.; Bharatam, P.V. Design of Benzene-1,2-diamines as selective inducible nitric oxide synthase inhibitors: A combined de novo design and docking analysis. J. Mol. Model. 2008, 14, 215–224. [Google Scholar] [CrossRef] [PubMed]

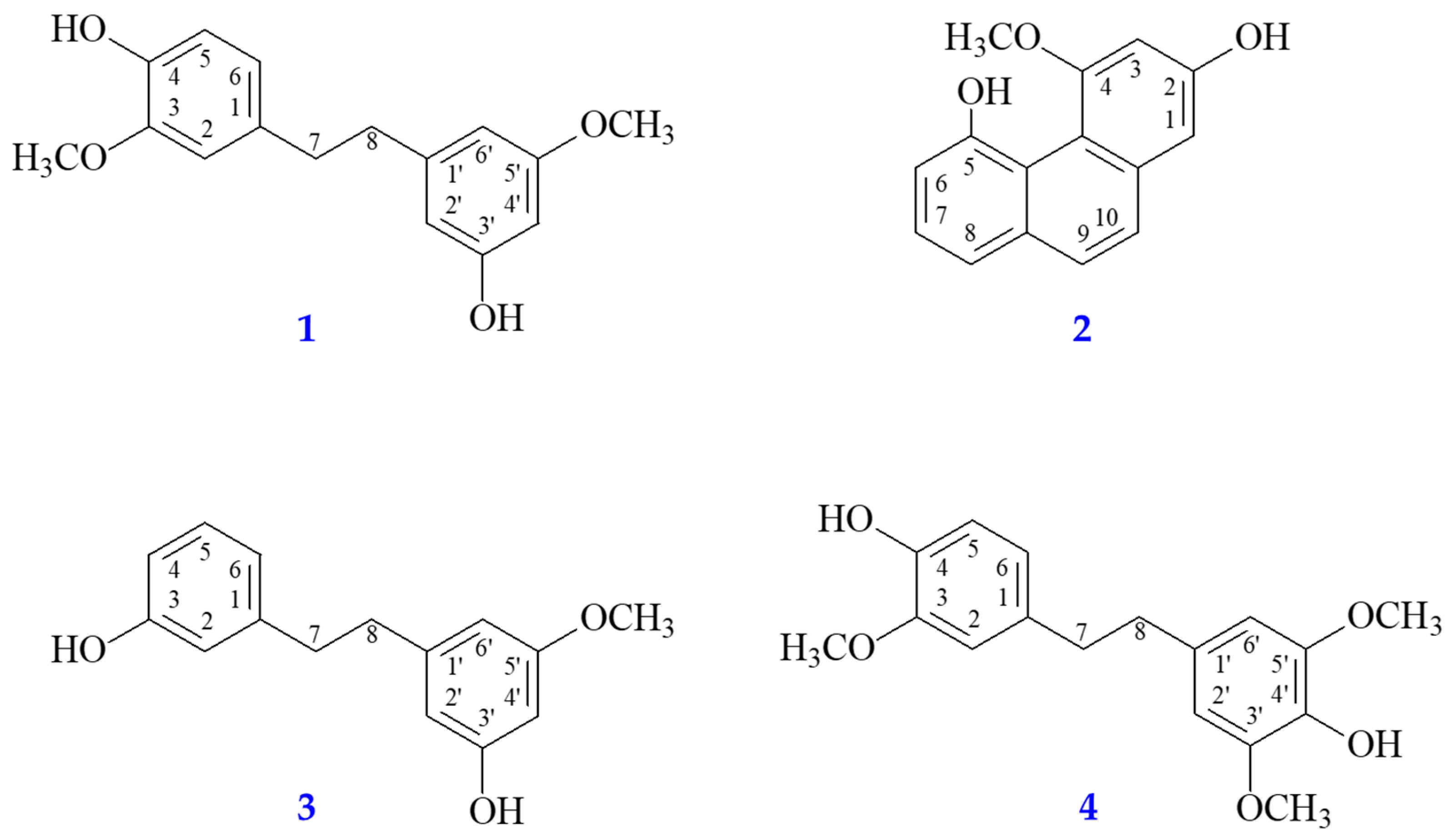



| Extracting Solvents | TPC (mg/g) A (GAE) | TFC (mg/g) B (QE) | Yields (%) C |
|---|---|---|---|
| n-Hexane | 1.67 ± 0.27 | 51.53 ± 1.88 | 0.30 ± 0.08 |
| Dichloromethane | 18.17 ± 2.48 | 49.69 ± 1.96 | 0.74 ± 0.05 |
| Ethyl acetate | 33.92 ± 0.75 | 44.05 ± 0.65 | 0.70 ± 0.04 |
| Acetone | 34.48 ± 1.50 | 34.20 ± 3.43 | 1.10 ± 0.03 |
| Methanol | 5.88 ± 0.16 | <1.00 | 7.46 ± 0.12 |
| Ethanol | 9.91 ± 0.34 | 3.46 ± 0.26 | 14.11 ± 0.37 |
| Water | 3.43 ± 0.09 | <1.00 | 11.40 ± 0.28 |
| Water 100 °C | 5.54 ± 0.24 | <1.00 | 19.70 ± 1.64 |
| Extracting Solvents | SC50 (μg/mL) A | TE (mM/g) C | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| n-Hexane | >400 | >400 | >400 | 10.78 ± 0.69 c |
| Dichloromethane | 88.44 ± 13.19 b | 108.47 ± 9.03 b | >400 | 562.26 ± 14.21 c |
| Ethyl acetate | 53.08 ± 8.37 b | 57.62 ± 5.38 b | >400 | 882.70 ± 7.26 c |
| Acetone | 55.89 ± 7.68 b | 59.78 ± 5.26 b | >400 | 861.19 ± 11.31 a |
| Methanol | >400 | >400 | >400 | 162.15 ± 7.59 c |
| Ethanol | 374.61 ± 27.50 c | >400 | >400 | 273.21 ± 7.98 c |
| Water | >400 | >400 | >400 | 85.29 ± 3.04 c |
| Water 100 °C | >400 | >400 | >400 | 133.19 ± 2.73 c |
| BHT B | 36.64 ± 2.22 | 46.83 ± 0.44 | - | 4296.98 ± 48.40 |
| Cynaroside B | - | - | 112.76 ± 5.84 | - |
| Compounds | SC50 (μM) A | TE (mM/g) C | ||
|---|---|---|---|---|
| DPPH | ABTS | Superoxide | FRAP | |
| Gigantol (1) | 35.18 ± 1.34 c | 12.25 ± 0.54 c | >400 | 1483.20 ± 63.49 c |
| Moscatin (2) | 26.66 ± 1.63 c | 10.34 ± 1.92 c | >400 | 1396.40 ± 57.03 c |
| Batatasin III (3) | >400 | 30.14 ± 1.70 b | >400 | 559.67 ± 51.49 c |
| Dendrophenol (4) | 11.64 ± 0.77 c | 7.64 ± 0.63 c | >400 | 1686.53 ± 16.61 c |
| BHT B | 90.77 ± 5.65 | 53.72 ± 1.48 | - | 4355.95 ± 99.23 |
| Cynaroside B | - | - | 50.56 ± 2.62 | - |
| Extracting Solvents | IC50 (μg/mL) A | |
|---|---|---|
| α-Glucosidase | AChE | |
| n-Hexane | 91.43 ± 2.87 c | 13.77 ± 4.09 c |
| Dichloromethane | 294.09 ± 8.61 | 31.89 ± 6.89 b |
| Ethyl acetate | 62.02 ± 0.21 c | 11.59 ± 1.52 b |
| Acetone | 51.62 ± 0.84 c | 92.05 ± 5.58 a |
| Methanol | >400 | 80.71 ± 8.44 |
| Ethanol | >400 | 51.96 ± 4.92 a |
| Water | >400 | 133.53 ± 8.04 b |
| Water 100 °C | >400 | 75.87 ± 7.91 |
| Acarbose B | 316.30 ± 11.15 | - |
| Chlorogenic acid B | - | 75.99 ± 6.21 |
| Compounds | IC50 (μM) A | |
|---|---|---|
| α-Glucosidase | AChE | |
| Gigantol (1) | 469.72 ± 28.51 | 176.05 ± 19.08 b |
| Moscatin (2) | 478.46 ± 33.43 | 161.86 ± 16.45 b |
| Batatasin III (3) | 723.21 ± 53.54 c | 258.29 ± 18.52 |
| Dendrophenol (4) | 608.37 ± 48.16 c | 165.19 ± 13.25 c |
| Acarbose B | 490.00 ± 17.23 | - |
| Chlorogenic acid B | - | 236.24 ± 15.85 |
| Extracting Solvents | NO Inhibition IC50 (μg/mL) A |
|---|---|
| n-Hexane | 9.19 ± 0.49 b |
| Dichloromethane | 20.60 ± 1.46 b |
| Ethyl acetate | 18.56 ± 1.54 b |
| Acetone | 21.67 ± 1.05 c |
| Methanol | 66.48 ± 1.42 c |
| Ethanol | 22.55 ± 1.28 b |
| Water | 33.49 ± 1.04 c |
| Water 100 °C | 25.24 ± 0.24 c |
| Quercetin B | 6.98 ± 0.43 |
| Compounds | NO Inhibition IC50 (μM) A |
|---|---|
| Gigantol (1) | 63.71 ± 11.41 a |
| Moscatin (2) | 23.46 ± 3.16 |
| Batatasin III (3) | 51.32 ± 5.51 a |
| Dendrophenol (4) | 14.31 ± 3.17 a |
| Quercetin B | 23.09 ± 1.43 |
| No. | RT a (min) | [M + H]+ | MS/MS (m/z) | Mw | Compounds | AChE b | iNOS c | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EF (%) d | BE (kcal/mol) e | Active Amino Acid Residues | EF (%) d | BE (kcal/mol) e | Active Amino Acid Residues | ||||||
| 1 | 14.2 | 229.08 | 151.07 137.06 123.08 | C14H12O3 | 2,4,7-Trihydroxy-9,10-dihydrophenanthrene | 1.32 | – | – | 0.91 | – | – |
| 2 | 14.5 | 231.10 | 137.06 121.07 107.05 | C14H14O3 | Dihydroresveratrol | 0.08 | – | – | 0.62 | – | – |
| 3 | 17.1 | 243.10 | 153.06 129.06 116.06 | C15H14O3 | Coelonin | 2.00 | – | – | 5.09 | – | – |
| 4 | 18.4 | 271.10 | 193.06 181.06 165.07 | C16H14O4 | Nudol | 29.56 | – | – | 24.39 | – | – |
| 5 | 19.7 | 275.13 | 137.06 122.04 103.05 | C16H18O4 | Gigantol | 54.60 | −8.6 | Arg296, Gly121, Ser203, Trp86, Trp286, Tyr72, Tyr124, Tyr337, Tyr341 | 44.84 | −7.9 | Ala191, Cys194, Phe363, Trp188, Trp366 |
| 6 | 20.0 | 245.12 | 151.08 121.06 105.04 | C15H16O3 | Batatasin III | 31.87 | −8.5 | Arg296, Phe295, Ser203, Trp86, Trp286, Tyr124, Tyr337, Tyr341 | 27.18 | −8.2 | Asn364, Cys194, Leu203, Phe363, Pro344, Trp188, Val346 |
| 7 | 20.4 | 305.14 | 181.09 151.08 137.06 | C17H20O5 | Dendrophenol | 59.48 | −8.0 | Arg296, Asp74, Gly121, Gly122, Ile294, Ser203, Trp86, Trp286, Tyr72, Tyr124, Tyr337, Tyr341 | 65.11 | −7.8 | Ala191, Cys194, Gly196, Met368, Phe363, Trp188, Trp366, Tyr483 |
| 8 | 22.7 | 241.09 | 181.06 152.06 127.05 | C15H12O3 | Moscatin | 66.31 | −8.6 | Ser293, Trp286, Tyr72 | 88.99 | −9.1 | Ala191, Cys194, Leu203, Phe363, Trp188 |
| 9 | 23.0 | 319.15 | 137.06 122.04 107.05 | C18H22O5 | Erianin | 0.23 | – | – | 1.72 | – | – |
| 10 | 23.8 | 261.11 | 129.07 128.06 115.05 | C15H16O4 | Tristin | 5.83 | – | – | 0.95 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-H.; Chuang, W.-C.; Lee, M.-C.; Fan, Y.-H.; Huang, N.-K.; Chen, J.-J. Bioaffinity Ultrafiltration Combined with HPLC-ESI-qTOF-MS/MS for Screening Potential Bioactive Components from the Stems of Dendrobium fimbriatum and In Silico Analysis. Antioxidants 2024, 13, 918. https://doi.org/10.3390/antiox13080918
Hsieh Y-H, Chuang W-C, Lee M-C, Fan Y-H, Huang N-K, Chen J-J. Bioaffinity Ultrafiltration Combined with HPLC-ESI-qTOF-MS/MS for Screening Potential Bioactive Components from the Stems of Dendrobium fimbriatum and In Silico Analysis. Antioxidants. 2024; 13(8):918. https://doi.org/10.3390/antiox13080918
Chicago/Turabian StyleHsieh, Yu-Hui, Wu-Chang Chuang, Ming-Chung Lee, Yu-Hsin Fan, Nai-Kuei Huang, and Jih-Jung Chen. 2024. "Bioaffinity Ultrafiltration Combined with HPLC-ESI-qTOF-MS/MS for Screening Potential Bioactive Components from the Stems of Dendrobium fimbriatum and In Silico Analysis" Antioxidants 13, no. 8: 918. https://doi.org/10.3390/antiox13080918







