Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review
Abstract
:1. Introduction
2. Anaphylaxis, Oxidative Stress and Mitochondria
2.1. Definition, Epidemiology, Pathophysiology, and Clinical Presentations of Anaphylaxis
2.1.1. Definition of Anaphylaxis
2.1.2. Epidemiology of Anaphylaxis
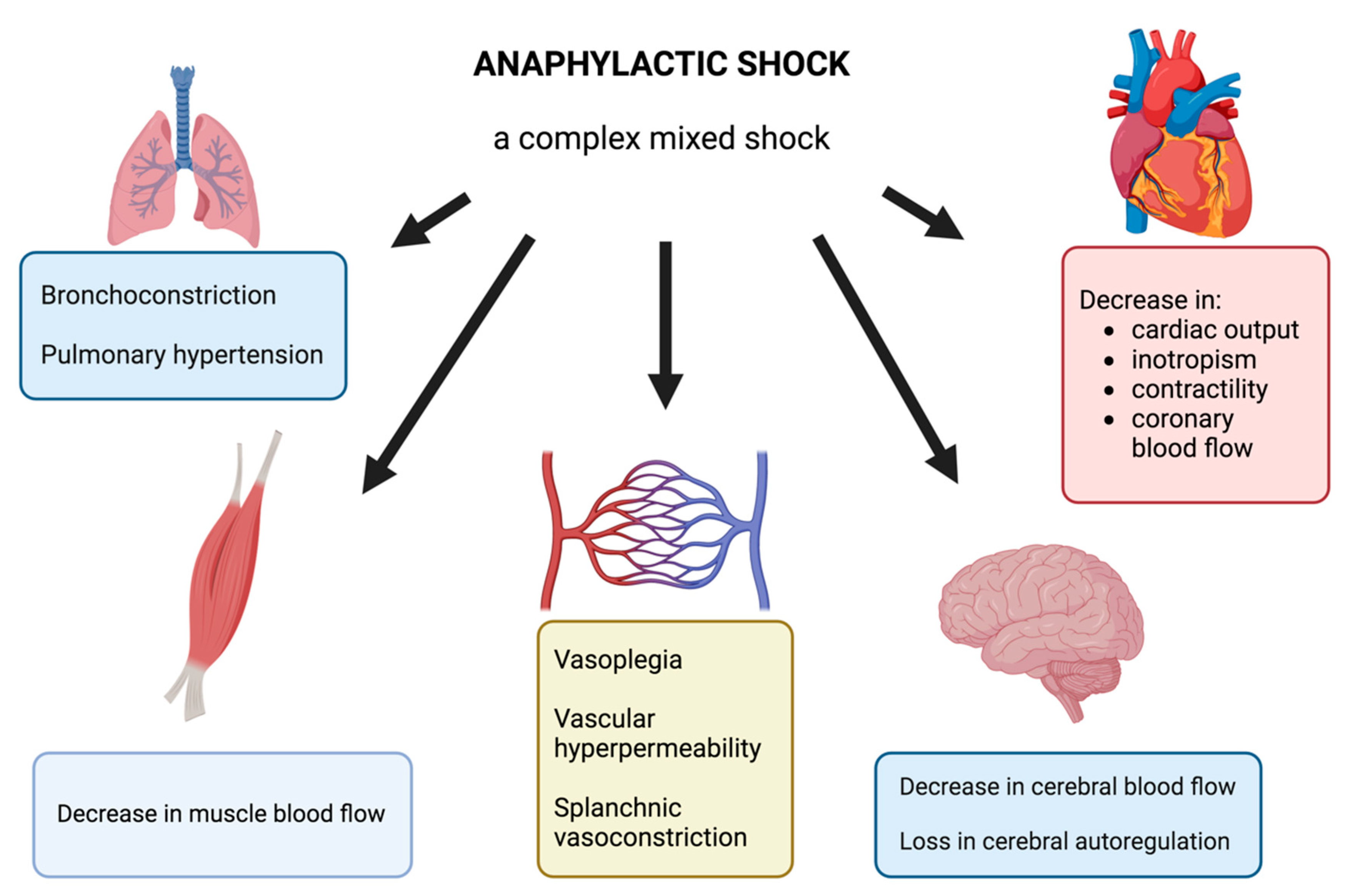
2.1.3. Anaphylaxis: A Complex Immune System Underlying Multiple Cellular Pathways
2.1.4. Clinical Presentation of Anaphylaxis
2.2. Oxidative Stress and Mitochondria
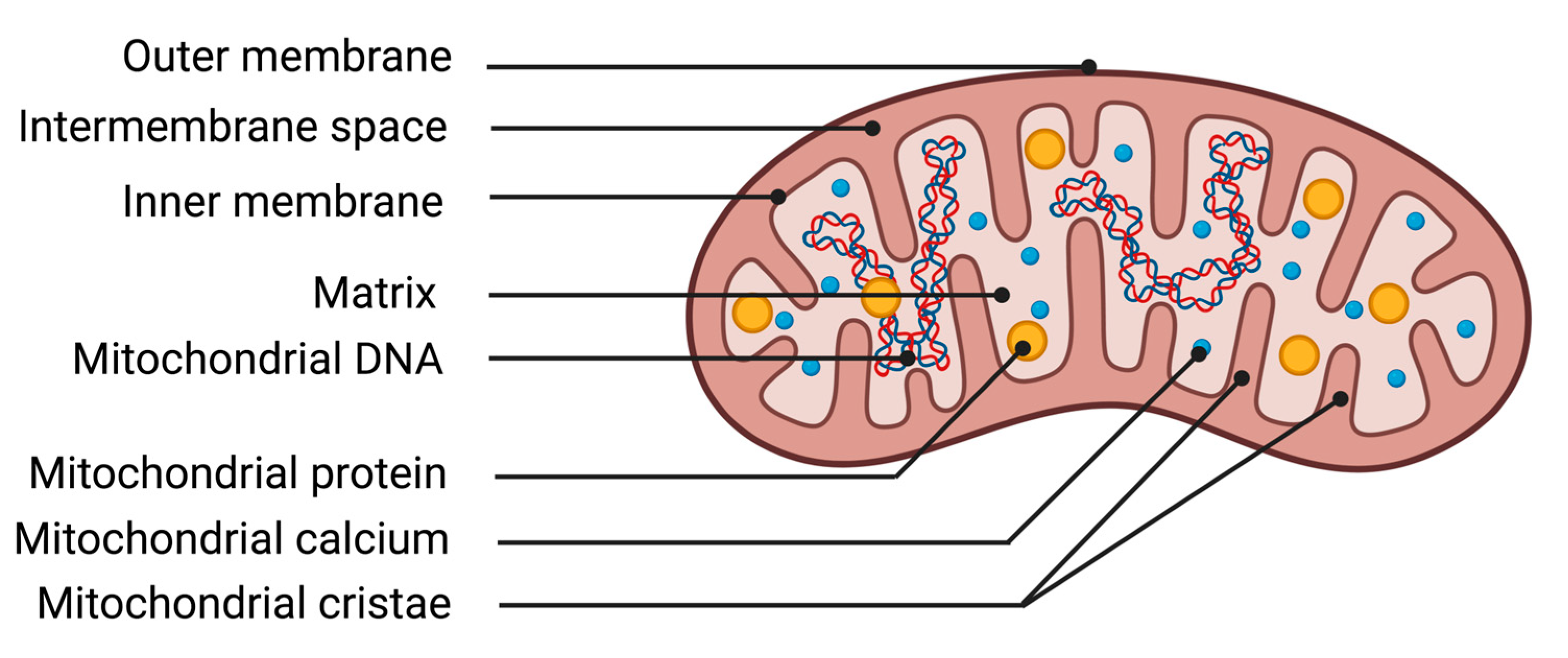
2.2.1. Description of Mitochondria
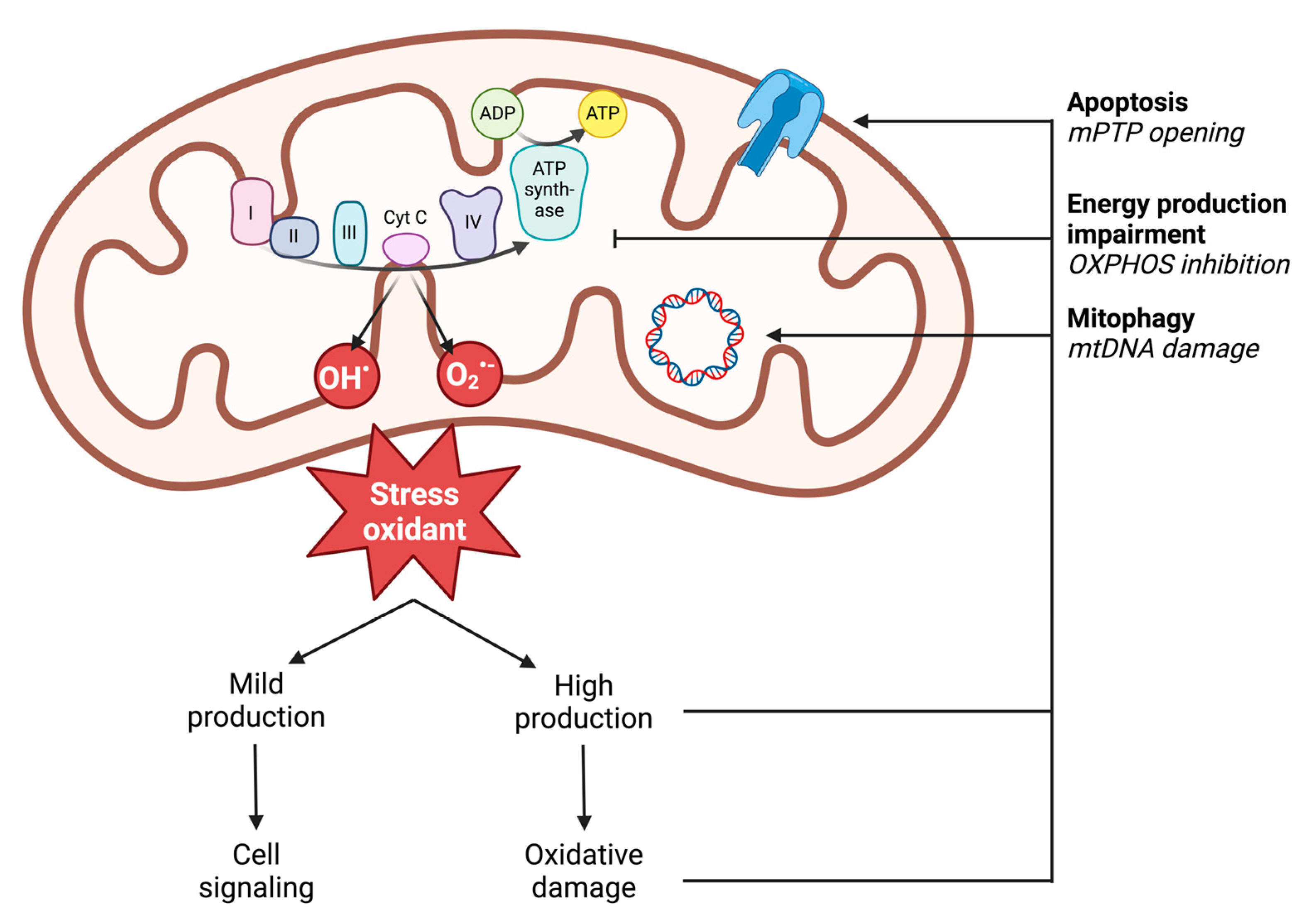
2.2.2. Definition of Oxidative Stress
2.2.3. ROS Production
- Primary free radicals resulting directly from the reduction of oxygen. Example: superoxide anion O2•−, hydroxyl radical OH•.
- Secondary free radicals formed by the reaction of primary free radicals with cellular biochemical compounds. Example: reaction of superoxide anion O2•− with nitric oxide to form peroxynitrite ONOO−.
- Active oxygen species: these molecules do not possess unpaired electrons but have a strong oxidizing power as they can generate free radicals. Example: hydrogen peroxide H2O2.
2.2.4. Deleterious Effects of ROS: Oxidative Damage
3. Methods of the Systematic Review of the Literature
3.1. Selection Criteria
3.2. Research Strategy and Data Collection Process
4. Results
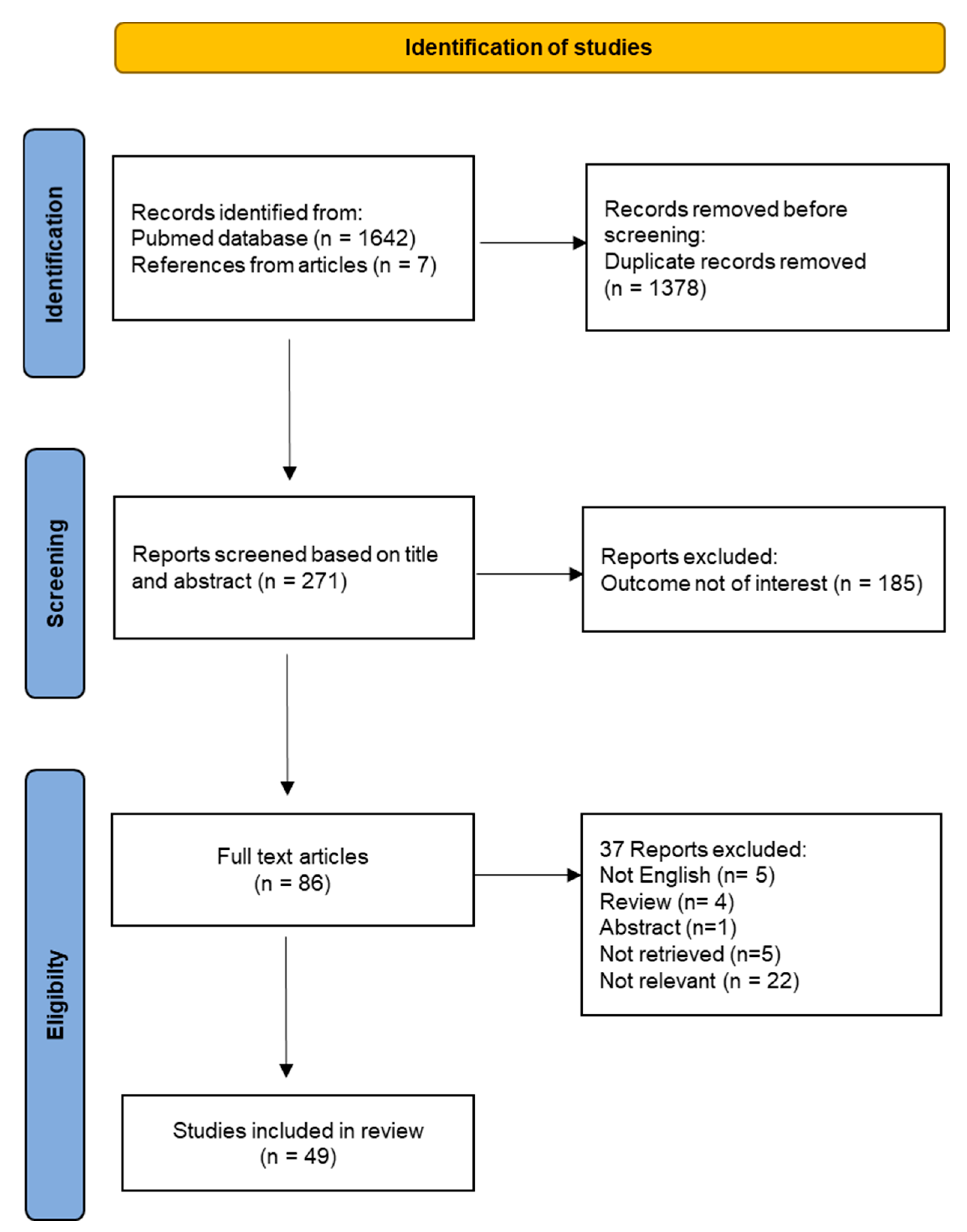
4.1. Study Selection
4.2. Study Characteristics
4.3. Mitochondrial Function and Mast Cell Degranulation
5. Discussion
5.1. Experimental Studies in Animal Models
5.2. Cellular Studies
5.2.1. Relation between Mast Cell Degranulation and Mitochondrial Function
5.2.2. Relation between Mast Cell Degranulation and Oxidative Stress
5.2.3. Mitochondrial Transcription Pathways
5.2.4. Role of Mitochondria on Ca2+ Flux during Mast Cell Degranulation
5.2.5. Cellular Consequences of Anaphylaxis-Associated Oxidative Stress
5.2.6. Mitochondrial Morphology and Dynamics
6. Targeting Mitochondria and Oxidative Stress in Anaphylaxis
7. Conclusions and Future Perspectives
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerci, P.; Tacquard, C.; Chenard, L.; Millard, D.; Soufir, L.; Malinovsky, J.-M.; Garot, M.; Lalot, J.-M.; Besch, G.; Louis, G.; et al. Epidemiology and Outcome of Patients Admitted to Intensive Care after Anaphylaxis in France: A Retrospective Multicentre Study. Br. J. Anaesth. 2020, 125, 1025–1033. [Google Scholar] [CrossRef]
- Panesar, S.S.; Javad, S.; de Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The Epidemiology of Anaphylaxis in Europe: A Systematic Review. Allergy 2013, 68, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The Pathophysiology of Anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Jouan-Hureaux, V.; Franck, P.; Menu, P.; de Talance, N.; Zannad, F.; Laxenaire, M.C.; Longrois, D.; Mertes, P.M. Anaphylactic Shock: A Form of Distributive Shock without Inhibition of Oxygen Consumption. Anesthesiology 2005, 103, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Oulehri, W.; Collange, O.; Tacquard, C.; Bellou, A.; Graff, J.; Charles, A.-L.; Geny, B.; Mertes, P.-M. Impaired Myocardial Mitochondrial Function in an Experimental Model of Anaphylactic Shock. Biology 2022, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Muñoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F.; Bock, S.A.; Branum, A.; Brown, S.G.A.; Camargo, C.A.; Cydulka, R.; Galli, S.J.; et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J. Allergy Clin. Immunol. 2006, 117, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilò, M.B.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.M.; Lieberman, P.; Lockey, R.F.; Muraro, A.; Roberts, G.; et al. International Consensus on (ICON) Anaphylaxis. World Allergy Organ. J. 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.; Camargo, C.A.; Bohlke, K.; Jick, H.; Miller, R.L.; Sheikh, A.; Simons, F.E.R. Epidemiology of Anaphylaxis: Findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann. Allergy Asthma Immunol. 2006, 97, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Dribin, T.E.; Motosue, M.S.; Campbell, R.L. Overview of Allergy and Anaphylaxis. Emerg. Med. Clin. N. Am. 2022, 40, 435–451. [Google Scholar] [CrossRef]
- Liew, W.K.; Williamson, E.; Tang, M.L.K. Anaphylaxis Fatalities and Admissions in Australia. J. Allergy Clin. Immunol. 2009, 123, 434–442. [Google Scholar] [CrossRef]
- Banerji, A.; Rudders, S.; Clark, S.; Wei, W.; Long, A.A.; Camargo, C.A. Retrospective Study of Drug-Induced Anaphylaxis Treated in the Emergency Department or Hospital: Patient Characteristics, Management, and 1-Year Follow-Up. J. Allergy Clin. Immunol. Pract. 2014, 2, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Wei, W.; Rudders, S.A.; Camargo, C.A. Risk Factors for Severe Anaphylaxis in Patients Receiving Anaphylaxis Treatment in US Emergency Departments and Hospitals. J. Allergy Clin. Immunol. 2014, 134, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal Anaphylaxis: Mortality Rate and Risk Factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Clarke, R.C.; Mertes, P.-M.; Platt, P.R.; Sabato, V.; Sadleir, P.H.M. Molecular Mechanisms and Pathophysiology of Perioperative Hypersensitivity and Anaphylaxis: A Narrative Review. Br. J. Anaesth. 2019, 123, e38–e49. [Google Scholar] [CrossRef] [PubMed]
- Grevers, G.; Röcken, M. Atlas de Poche D’allergologie; Flammarion: Paris, France, 2002. [Google Scholar]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, Mast Cells, Basophils, and Eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Novey, H.S.; Pahl, M.; Haydik, I.; Vaziri, N.D. Immunologic Studies of Anaphylaxis to Iron Dextran in Patients on Renal Dialysis. Ann. Allergy 1994, 72, 224–228. [Google Scholar] [PubMed]
- Umeda, Y.; Fukumoto, Y.; Miyauchi, T.; Imaizumi, M.; Shimabukuro, K.; Mori, Y.; Takemura, H. Anaphylactic shock related to aprotinin induced by anti-aprotinin immunoglobulin G antibody alone; report of a case. Kyobu Geka 2007, 60, 69–71. [Google Scholar] [PubMed]
- Baldo, B.A.; Pham, N.H. Histamine-Releasing and Allergenic Properties of Opioid Analgesic Drugs: Resolving the Two. Anaesth. Intensive Care 2012, 40, 216–235. [Google Scholar] [CrossRef]
- Krithika, N.; Pramod, S.N.; Mahesh, P.A.; Venkatesh, Y.P. Banana Lectin (BanLec) Induces Non-Specific Activation of Basophils and Mast Cells in Atopic Subjects. Eur. Ann. Allergy Clin. Immunol. 2018, 50, 243–253. [Google Scholar] [CrossRef]
- Mertes, P.M.; Laxenaire, M.C. Allergy and Anaphylaxis in Anaesthesia. Minerva Anestesiol. 2004, 70, 285–291. [Google Scholar]
- Withington, D.E.; Patrick, J.A.; Reynolds, F. Histamine Release by Morphine and Diamorphine in Man. Anaesthesia 1993, 48, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Sahai, J.; Healy, D.P.; Shelton, M.J.; Miller, J.S.; Ruberg, S.J.; Polk, R. Comparison of Vancomycin- and Teicoplanin-Induced Histamine Release and “Red Man Syndrome”. Antimicrob. Agents Chemother. 1990, 34, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.B. Histamine Release by Neuromuscular Blocking Agents. Br. J. Anaesth. 1996, 76, 471. [Google Scholar] [CrossRef] [PubMed]
- Mertes, P.M.; Aimone-Gastin, I.; Guéant-Rodriguez, R.M.; Mouton-Faivre, C.; Audibert, G.; O’Brien, J.; Frendt, D.; Brezeanu, M.; Bouaziz, H.; Guéant, J.L. Hypersensitivity Reactions to Neuromuscular Blocking Agents. Curr. Pharm. Des. 2008, 14, 2809–2825. [Google Scholar] [CrossRef] [PubMed]
- Sauder, R.A.; Hirshman, C.A. Protamine-Induced Histamine Release in Human Skin Mast Cells. Anesthesiology 1990, 73, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Guilarte, M.; Sala-Cunill, A.; Luengo, O.; Labrador-Horrillo, M.; Cardona, V. The Mast Cell, Contact, and Coagulation System Connection in Anaphylaxis. Front. Immunol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-Related G Protein–Coupled Receptor X2 on Mast Cell–Mediated Host Defense, Pseudoallergic Drug Reactions, and Chronic Inflammatory Diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef]
- Elst, J.; Maurer, M.; Sabato, V.; Faber, M.A.; Bridts, C.H.; Mertens, C.; Van Houdt, M.; Van Gasse, A.L.; van der Poorten, M.-L.M.; De Puysseleyr, L.P.; et al. Novel Insights on MRGPRX2-Mediated Hypersensitivity to Neuromuscular Blocking Agents And Fluoroquinolones. Front. Immunol. 2021, 12, 668962. [Google Scholar] [CrossRef]
- Nuñez-Borque, E.; Fernandez-Bravo, S.; Yuste-Montalvo, A.; Esteban, V. Pathophysiological, Cellular, and Molecular Events of the Vascular System in Anaphylaxis. Front. Immunol. 2022, 13, 836222. [Google Scholar] [CrossRef]
- Bilò, M.B.; Martini, M.; Tontini, C.; Mohamed, O.E.; Krishna, M.T. Idiopathic Anaphylaxis. Clin. Exp. Allergy 2019, 49, 942–952. [Google Scholar] [CrossRef]
- Nguyen, S.M.T.; Rupprecht, C.P.; Haque, A.; Pattanaik, D.; Yusin, J.; Krishnaswamy, G. Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond. Int. J. Mol. Sci. 2021, 22, 7785. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, S.; Xiao, Z.; Pan, Q.; Wang, X.; Shen, K.; Wang, S.; Yang, L.; Guo, F.; Liu, H.-F.; et al. The Development and Improvement of Immunodeficient Mice and Humanized Immune System Mouse Models. Front. Immunol. 2022, 13, 1007579. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D. Anaphylaxis: Lessons from Mouse Models. J. Allergy Clin. Immunol. 2007, 120, 506–515; quiz 516–517. [Google Scholar] [CrossRef] [PubMed]
- Hosken, N.A.; Shibuya, K.; Heath, A.W.; Murphy, K.M.; O’Garra, A. The Effect of Antigen Dose on CD4+ T Helper Cell Phenotype Development in a T Cell Receptor-Alpha Beta-Transgenic Model. J. Exp. Med. 1995, 182, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Khodoun, M.V.; Strait, R. Human IgE-Independent Systemic Anaphylaxis. J. Allergy Clin. Immunol. 2016, 137, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Epp, A.; Fanny, M.E.; Miller, S.J.; Stranks, A.J.; Teague, J.E.; Clark, R.A.; van de Rijn, M.; Oettgen, H.C. Tissue-Specific Expression of the Low-Affinity IgG Receptor, FcγRIIb, on Human Mast Cells. Front. Immunol. 2018, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Savic, L. Perioperative Anaphylaxis: Pathophysiology, Clinical Presentation and Management. BJA Educ. 2019, 19, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.H.; Dewachter, P.; Hepner, D.L.; Mertes, P.M.; Voltolini, S.; Clarke, R.; Cooke, P.; Garcez, T.; Guttormsen, A.B.; Ebo, D.G.; et al. Management of Suspected Immediate Perioperative Allergic Reactions: An International Overview and Consensus Recommendations. Br. J. Anaesth. 2019, 123, e50–e64. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Fernandez Rivas, M.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Sanchez Borges, M.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Davidson, J.; Zheng, F.; Tajima, K.; Barthel, G.; Alb, I.; Tabarna, A.; Thornton, S.N.; Lambert, M.; Longrois, D.; Audibert, G.; et al. Anaphylactic Shock Decreases Cerebral Blood Flow More than What Would Be Expected from Severe Arterial Hypotension. Shock 2012, 38, 429–435. [Google Scholar] [CrossRef]
- Zhang, W.; Shibamoto, T.; Tanida, M.; Wang, M.; Sun, L.; Kurata, Y. Rat Hepatic and Splanchnic Vascular Responses to Anaphylactic Shock, Compared with Hemorrhagic or Vasodilator-Induced Shock. In Vivo 2013, 27, 485–493. [Google Scholar] [PubMed]
- Wang, M.; Shibamoto, T.; Tanida, M.; Kuda, Y.; Kurata, Y. Mouse Anaphylactic Shock Is Caused by Reduced Cardiac Output, but Not by Systemic Vasodilatation or Pulmonary Vasoconstriction, via PAF and Histamine. Life Sci. 2014, 116, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Pasdois, P.; Parker, J.E.; Griffiths, E.J.; Halestrap, A.P. The Role of Oxidized Cytochrome c in Regulating Mitochondrial Reactive Oxygen Species Production and Its Perturbation in Ischaemia. Biochem. J. 2011, 436, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial Fusion and Fission: The Fine-Tune Balance for Cellular Homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef] [PubMed]
- Hom, J.; Sheu, S.-S. Morphological Dynamics of Mitochondria--a Special Emphasis on Cardiac Muscle Cells. J. Mol. Cell Cardiol. 2009, 46, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Santel, A.; Fuller, M.T. Control of Mitochondrial Morphology by a Human Mitofusin. J. Cell Sci. 2001, 114, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Kim, W. Mitochondrial Dynamics in the Heart as a Novel Therapeutic Target for Cardioprotection. Chonnam Med. J. 2013, 49, 101–107. [Google Scholar] [CrossRef]
- Yoon, Y.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. The Mitochondrial Protein hFis1 Regulates Mitochondrial Fission in Mammalian Cells through an Interaction with the Dynamin-like Protein DLP1. Mol. Cell Biol. 2003, 23, 5409–5420. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein Degradation and Protection against Misfolded or Damaged Proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The Role of Autophagy in Cardiomyocytes in the Basal State and in Response to Hemodynamic Stress. Nat. Med. 2007, 13, 619–624. [Google Scholar] [CrossRef]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct Roles of Autophagy in the Heart during Ischemia and Reperfusion: Roles of AMP-Activated Protein Kinase and Beclin 1 in Mediating Autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. Enhancing Macroautophagy Protects against Ischemia/Reperfusion Injury in Cardiac Myocytes. J. Biol. Chem. 2006, 281, 29776–29787. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, H.; Takemura, G.; Maruyama, R.; Goto, K.; Tsujimoto, A.; Ogino, A.; Li, L.; Kawamura, I.; Takeyama, T.; Kawaguchi, T.; et al. Functional Significance and Morphological Characterization of Starvation-Induced Autophagy in the Adult Heart. Am. J. Pathol. 2009, 174, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Shanmughapriya, S.; Langford, D.; Natarajaseenivasan, K. Inter and Intracellular Mitochondrial Trafficking in Health and Disease. Ageing Res. Rev. 2020, 62, 101128. [Google Scholar] [CrossRef] [PubMed]
- Favier, A. Intérêt conceptuel et expérimental dans la compréhension des mécanismes des maladies et potentiel thérapeutique. L’actualité Chim. 2003, 270, 108–115. [Google Scholar]
- Migdal, C.; Serres, M. Espèces Réactives de l’oxygène et Stress Oxydant. Med. Sci. 2011, 27, 405–412. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial Generation of Superoxide and Hydrogen Peroxide as the Source of Mitochondrial Redox Signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Paradis, S.; Charles, A.-L.; Meyer, A.; Lejay, A.; Scholey, J.W.; Chakfé, N.; Zoll, J.; Geny, B. Chronology of Mitochondrial and Cellular Events during Skeletal Muscle Ischemia-Reperfusion. Am. J. Physiol.-Cell Physiol. 2016, 310, C968–C982. [Google Scholar] [CrossRef]
- Charles, A.-L.; Guilbert, A.-S.; Guillot, M.; Talha, S.; Lejay, A.; Meyer, A.; Kindo, M.; Wolff, V.; Bouitbir, J.; Zoll, J.; et al. Muscles Susceptibility to Ischemia-Reperfusion Injuries Depends on Fiber Type Specific Antioxidant Level. Front. Physiol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Goncalves, R.L.S.; Hey-Mogensen, M.; Yadava, N.; Bunik, V.I.; Brand, M.D. The 2-Oxoacid Dehydrogenase Complexes in Mitochondria Can Produce Superoxide/Hydrogen Peroxide at Much Higher Rates than Complex I. J. Biol. Chem. 2014, 289, 8312–8325. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Carrière, A.; Galinier, A.; Fernandez, Y.; Carmona, M.-C.; Pénicaud, L.; Casteilla, L. Les Espèces Actives de l’oxygène: Le Yin et Le Yang de La Mitochondrie. Med. Sci. 2006, 22, 47–53. [Google Scholar] [CrossRef]
- Catalá, A.; Díaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Ait-Aissa, K.; Blaszak, S.C.; Beutner, G.; Tsaih, S.-W.; Morgan, G.; Santos, J.H.; Flister, M.J.; Joyce, D.L.; Camara, A.K.S.; Gutterman, D.D.; et al. Mitochondrial Oxidative Phosphorylation Defect in the Heart of Subjects with Coronary Artery Disease. Sci. Rep. 2019, 9, 7623. [Google Scholar] [CrossRef]
- Garcia, Y.J.; Rodríguez-Malaver, A.J.; Peñaloza, N. Lipid Peroxidation Measurement by Thiobarbituric Acid Assay in Rat Cerebellar Slices. J. Neurosci. Methods 2005, 144, 127–135. [Google Scholar] [CrossRef]
- Tomita, K.; Takashi, Y.; Ouchi, Y.; Kuwahara, Y.; Igarashi, K.; Nagasawa, T.; Nabika, H.; Kurimasa, A.; Fukumoto, M.; Nishitani, Y.; et al. Lipid Peroxidation Increases Hydrogen Peroxide Permeability Leading to Cell Death in Cancer Cell Lines That Lack mtDNA. Cancer Sci. 2019, 110, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J.; Hartmann, M.; Rehling, P. The Role of Mitochondrial Cardiolipin in Heart Function and Its Implication in Cardiac Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 810–821. [Google Scholar] [CrossRef]
- Anderson, E.J.; Katunga, L.A.; Willis, M.S. Mitochondria as a Source and Target of Lipid Peroxidation Products in Healthy and Diseased Heart. Clin. Exp. Pharmacol. Physiol. 2012, 39, 179–193. [Google Scholar] [CrossRef]
- PRISMA Statement. 1999. Available online: https://www.Prisma-Statement.org/ (accessed on 26 May 2024).
- Ovary, Z. Passive Cutaneous Anaphylaxis in the Mouse. J. Immunol. 1958, 81, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, T.; Pfeiffenberger, M.; Frischbutter, S.; Krauß, P.-L.; Chen, Y.; Maurer, M.; Buttgereit, F.; Gaber, T. Inhibition of Complex I of the Respiratory Chain, but Not Complex III, Attenuates Degranulation and Cytokine Secretion in Human Skin Mast Cells. Int. J. Mol. Sci. 2022, 23, 11591. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yoshimaru, T.; Inoue, T.; Ra, C. Mitochondrial Ca2+ Flux Is a Critical Determinant of the Ca2+ Dependence of Mast Cell Degranulation. J. Leukoc. Biol. 2006, 79, 508–518. [Google Scholar] [CrossRef]
- Zhang, B.; Weng, Z.; Sismanopoulos, N.; Asadi, S.; Therianou, A.; Alysandratos, K.-D.; Angelidou, A.; Shirihai, O.; Theoharides, T.C. Mitochondria Distinguish Granule-Stored from de Novo Synthesized Tumor Necrosis Factor Secretion in Human Mast Cells. Int. Arch. Allergy Immunol. 2012, 159, 23–32. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Anderson, C.C.; Fudge, D.H.; Roede, J.R.; Brown, J.M. Metabolic Consequences of IgE- and Non-IgE-Mediated Mast Cell Degranulation. J. Immunol. 2021, 207, 2637–2648. [Google Scholar] [CrossRef]
- Takekawa, M.; Furuno, T.; Hirashima, N.; Nakanishi, M. Mitochondria Take up Ca2+ in Two Steps Dependently on Store-Operated Ca2+ Entry in Mast Cells. Biol. Pharm. Bull. 2012, 35, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Chodaczek, G.; Bacsi, A.; Dharajiya, N.; Sur, S.; Hazra, T.K.; Boldogh, I. Ragweed Pollen-Mediated IgE-Independent Release of Biogenic Amines from Mast Cells via Induction of Mitochondrial Dysfunction. Mol. Immunol. 2009, 46, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Suzuki, Y.; Ra, C. Epigallocatechin-3-Gallate Inhibits Mast Cell Degranulation, Leukotriene C4 Secretion, and Calcium Influx via Mitochondrial Calcium Dysfunction. Free Radic. Biol. Med. 2010, 49, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Sangroula, S.; Baez Vasquez, A.Y.; Raut, P.; Obeng, B.; Shim, J.K.; Bagley, G.D.; West, B.E.; Burnell, J.E.; Kinney, M.S.; Potts, C.M.; et al. Triclosan Disrupts Immune Cell Function by Depressing Ca2+ Influx Following Acidification of the Cytoplasm. Toxicol. Appl. Pharmacol. 2020, 405, 115205. [Google Scholar] [CrossRef]
- Erlich, T.H.; Yagil, Z.; Kay, G.; Peretz, A.; Migalovich-Sheikhet, H.; Tshori, S.; Nechushtan, H.; Levi-Schaffer, F.; Saada, A.; Razin, E. Mitochondrial STAT3 Plays a Major Role in IgE-Antigen-Mediated Mast Cell Exocytosis. J. Allergy Clin. Immunol. 2014, 134, 460–469. [Google Scholar] [CrossRef]
- Weatherly, L.M.; Shim, J.; Hashmi, H.N.; Kennedy, R.H.; Hess, S.T.; Gosse, J.A. Antimicrobial Agent Triclosan Is a Proton Ionophore Uncoupler of Mitochondria in Living Rat and Human Mast Cells and in Primary Human Keratinocytes. J. Appl. Toxicol. 2016, 36, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, L.M.; Nelson, A.J.; Shim, J.; Riitano, A.M.; Gerson, E.D.; Hart, A.J.; de Juan-Sanz, J.; Ryan, T.A.; Sher, R.; Hess, S.T.; et al. Antimicrobial Agent Triclosan Disrupts Mitochondrial Structure, Revealed by Super-Resolution Microscopy, and Inhibits Mast Cell Signaling via Calcium Modulation. Toxicol. Appl. Pharmacol. 2018, 349, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Sharkia, I.; Hadad Erlich, T.; Landolina, N.; Assayag, M.; Motzik, A.; Rachmin, I.; Kay, G.; Porat, Z.; Tshori, S.; Berkman, N.; et al. Pyruvate Dehydrogenase Has a Major Role in Mast Cell Function, and Its Activity Is Regulated by Mitochondrial Microphthalmia Transcription Factor. J. Allergy Clin. Immunol. 2017, 140, 204–214.e8. [Google Scholar] [CrossRef] [PubMed]
- Oulehri, W.; Persello, A.; Blangy-Letheule, A.; Tacquard, C.; Rozec, B.; Charles, A.-L.; Geny, B.; Lauzier, B.; Mertes, P.M.; Collange, O. NButGT Reinforces the Beneficial Effects of Epinephrine on Cardiac Mitochondrial Respiration, Lactatemia and Cardiac Output in Experimental Anaphylactic Shock. Int. J. Mol. Sci. 2024, 25, 3316. [Google Scholar] [CrossRef] [PubMed]
- Bellou, A.; Sennoun, N.; Aburawi, E.H.; Jayaraj, R.L.; Alper, S.L.; Alfaki, I.A.; Yasin, J.; Sekar, S.; Shafiuallah, M.; Al-Salam, S.; et al. Combined Treatment with KV Channel Inhibitor 4-Aminopyridine and Either γ-Cystathionine Lyase Inhibitor β-Cyanoalanine or Epinephrine Restores Blood Pressure, and Improves Survival in the Wistar Rat Model of Anaphylactic Shock. Biology 2022, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Kloek, J.; Mortaz, E.; Van Ark, I.; Bloksma, N.; Garssen, J.; Nijkamp, F.P.; Folkerts, G. A Whey-Based Glutathione-Enhancing Diet Decreases Allergen-Induced Airway Contraction in a Guinea-Pig Model of Asthma. Br. J. Nutr. 2011, 105, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Paparo, L.; Aitoro, R.; Fierro, C.; Varchetta, M.; Nocerino, R.; Mollica, M.; Berni Canani, R. Hepatic Mitochondrial Dysfunction and Immune Response in a Murine Model of Peanut Allergy. Nutrients 2018, 10, 744. [Google Scholar] [CrossRef]
- Milicic, V.; Zivkovic, V.; Jeremic, N.; Arsenijevic, N.; Djuric, D.; Jakovljevic, V.L. Coronary Flow and Oxidative Stress during Local Anaphylactic Reaction in Isolated Mice Heart: The Role of Nitric Oxide (NO). Mol. Cell Biochem. 2016, 412, 221–227. [Google Scholar] [CrossRef]
- Rosic, M.; Parodi, O.; Jakovljevic, V.; Colic, M.; Zivkovic, V.; Jokovic, V.; Pantovic, S. Glucagon Effects on 3H-Histamine Uptake by the Isolated Guinea-Pig Heart during Anaphylaxis. BioMed Res. Int. 2014, 2014, 782709. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Ultrastructural Changes in Heart Muscle Associated with Anaphylaxis in the Guinea Pig. Tohoku J. Exp. Med. 1972, 106, 109–123. [Google Scholar] [CrossRef]
- Dimlich, R.V.; Cardell, R.R. Morphometric Analysis of Hepatocytes from Rats Subjected to Compound 48/80-Induced Anaphylactic Shock. Tissue Cell 1985, 17, 293–308. [Google Scholar] [CrossRef]
- Tajima, K.; Zheng, F.; Collange, O.; Barthel, G.; Thornton, S.N.; Longrois, D.; Levy, B.; Audibert, G.; Malinovsky, J.M.; Mertes, P.M. Time to Achieve Target Mean Arterial Pressure during Resuscitation from Experimental Anaphylactic Shock in an Animal Model. A Comparison of Adrenaline Alone or in Combination with Different Volume Expanders. Anaesth. Intensive Care 2013, 41, 765–773. [Google Scholar] [CrossRef]
- Brooks, A.C.; Whelan, C.J.; Purcell, W.M. Reactive Oxygen Species Generation and Histamine Release by Activated Mast Cells: Modulation by Nitric Oxide Synthase Inhibition. Br. J. Pharmacol. 1999, 128, 585–590. [Google Scholar] [CrossRef]
- Swindle, E.J.; Metcalfe, D.D.; Coleman, J.W. Rodent and Human Mast Cells Produce Functionally Significant Intracellular Reactive Oxygen Species but Not Nitric Oxide. J. Biol. Chem. 2004, 279, 48751–48759. [Google Scholar] [CrossRef] [PubMed]
- Collaco, C.R.; Hochman, D.J.; Goldblum, R.M.; Brooks, E.G. Effect of Sodium Sulfite on Mast Cell Degranulation and Oxidant Stress. Ann. Allergy Asthma Immunol. 2006, 96, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tung, H.-Y.; Tsai, Y.-M.; Hsu, S.-C.; Chang, H.-W.; Kawasaki, H.; Tseng, H.-C.; Plunkett, B.; Gao, P.; Hung, C.-H.; et al. Aryl Hydrocarbon Receptor Controls Murine Mast Cell Homeostasis. Blood 2013, 121, 3195–3204. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Suzuki, Y.; Matsui, T.; Yamashita, K.; Ochiai, T.; Yamaki, M.; Shimizu, K. Blockade of Superoxide Generation Prevents High-Affinity Immunoglobulin E Receptor-Mediated Release of Allergic Mediators by Rat Mast Cell Line and Human Basophils. Clin. Exp. Allergy 2002, 32, 612–618. [Google Scholar] [CrossRef]
- Yasui, Y.; Sasao, E.; Sakata, M.; Matsui, N.; Fukuishi, N.; Akagi, R.; Akagi, M. Upregulation of Heme Oxygenase-1 by Degranulation in Rat Basophilic Leukemia Cells. Biol. Pharm. Bull. 2007, 30, 443–446. [Google Scholar] [CrossRef]
- Inoue, T.; Suzuki, Y.; Yoshimaru, T.; Ra, C. Reactive Oxygen Species Produced Up- or Downstream of Calcium Influx Regulate Proinflammatory Mediator Release from Mast Cells: Role of NADPH Oxidase and Mitochondria. Biochim. Biophys. Acta 2008, 1783, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yoshimaru, T.; Matsui, T.; Inoue, T.; Niide, O.; Nunomura, S.; Ra, C. Fc Epsilon RI Signaling of Mast Cells Activates Intracellular Production of Hydrogen Peroxide: Role in the Regulation of Calcium Signals. J. Immunol. 2003, 171, 6119–6127. [Google Scholar] [CrossRef]
- Itoh, T.; Fujita, Y.; Ito, M.; Masuda, A.; Ohno, K.; Ichihara, M.; Kojima, T.; Nozawa, Y.; Ito, M. Molecular Hydrogen Suppresses FcepsilonRI-Mediated Signal Transduction and Prevents Degranulation of Mast Cells. Biochem. Biophys. Res. Commun. 2009, 389, 651–656. [Google Scholar] [CrossRef]
- Tagen, M.; Elorza, A.; Kempuraj, D.; Boucher, W.; Kepley, C.L.; Shirihai, O.S.; Theoharides, T.C. Mitochondrial Uncoupling Protein 2 Inhibits Mast Cell Activation and Reduces Histamine Content. J. Immunol. 2009, 183, 6313–6319. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, N.; Mao, M.; Zhou, Y.; Wu, Y.; Li, J.; Zhang, W.; Peng, C.; Chen, X.; Li, J. Fine Particulate Matter (PM2.5) Promotes IgE-Mediated Mast Cell Activation through ROS/Gadd45b/JNK Axis. J. Dermatol. Sci. 2021, 102, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hochman, D.J.; Collaco, C.R.; Brooks, E.G. Acrolein Induction of Oxidative Stress and Degranulation in Mast Cells. Environ. Toxicol. 2014, 29, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Erlich, T.H.; Sharkia, I.; Landolina, N.; Assayag, M.; Goldberger, O.; Berkman, N.; Levi-Schaffer, F.; Razin, E. Modulation of Allergic Responses by Mitochondrial STAT3 Inhibitors. Allergy 2018, 73, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Pavlyuchenkova, A.N.; Chelombitko, M.A.; Fedorov, A.V.; Kuznetsova, M.K.; Zinovkin, R.A.; Razin, E. The Distinct Effects of the Mitochondria-Targeted STAT3 Inhibitors Mitocur-1 and Mitocur-3 on Mast Cell and Mitochondrial Functions. Int. J. Mol. Sci. 2023, 24, 1471. [Google Scholar] [CrossRef]
- Bilotta, S.; Paruchuru, L.B.; Feilhauer, K.; Köninger, J.; Lorentz, A. Resveratrol Is a Natural Inhibitor of Human Intestinal Mast Cell Activation and Phosphorylation of Mitochondrial ERK1/2 and STAT3. Int. J. Mol. Sci. 2021, 22, 7640. [Google Scholar] [CrossRef]
- Paruchuru, L.B.; Govindaraj, S.; Razin, E. The Critical Role Played by Mitochondrial MITF Serine 73 Phosphorylation in Immunologically Activated Mast Cells. Cells 2022, 11, 589. [Google Scholar] [CrossRef]
- Guo, Y.; Ollé, L.; Proaño-Pérez, E.; Aparicio, C.; Guerrero, M.; Muñoz-Cano, R.; Martín, M. MRGPRX2 Signaling Involves the Lysyl-tRNA Synthetase and MITF Pathway. Front. Immunol. 2023, 14, 1154108. [Google Scholar] [CrossRef] [PubMed]
- Furuno, T.; Shinkai, N.; Inoh, Y.; Nakanishi, M. Impaired Expression of the Mitochondrial Calcium Uniporter Suppresses Mast Cell Degranulation. Mol. Cell Biochem. 2015, 410, 215–221. [Google Scholar] [CrossRef]
- Cuong, D.V.; Kim, H.K.; Marquez, J.; Kim, N.; Ko, K.S.; Rhee, B.D.; Han, J. Mitochondrial Calcium Uniporter Inhibition Attenuates Mouse Bone Marrow-Derived Mast Cell Degranulation Induced by Beta-1,3-Glucan. Korean J. Physiol. Pharmacol. 2016, 20, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yoshimaru, T.; Inoue, T.; Nunomura, S.; Ra, C. The High-Affinity Immunoglobulin E Receptor (FcepsilonRI) Regulates Mitochondrial Calcium Uptake and a Dihydropyridine Receptor-Mediated Calcium Influx in Mast Cells: Role of the FcepsilonRIbeta Chain Immunoreceptor Tyrosine-Based Activation Motif. Biochem. Pharmacol. 2008, 75, 1492–1503. [Google Scholar] [CrossRef]
- Qi, R.; Kang, Y.; Li, X.; Zhang, X.; Han, Y.; Cai, R.; Gao, Y.; Qi, Y. Forsythiasides-Rich Extract From Forsythiae Fructus Inhibits Mast Cell Degranulation by Enhancing Mitochondrial Ca2+ Uptake. Front. Pharmacol. 2021, 12, 696729. [Google Scholar] [CrossRef]
- Obeng, B.; Potts, C.M.; West, B.E.; Burnell, J.E.; Fleming, P.J.; Shim, J.K.; Kinney, M.S.; Ledue, E.L.; Sangroula, S.; Baez Vazquez, A.Y.; et al. Pharmaceutical Agent Cetylpyridinium Chloride Inhibits Immune Mast Cell Function by Interfering with Calcium Mobilization. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Chiang, D.M.-L.; Lin, Z.-J.; Hsieh, C.-C.; Yin, G.-C.; Weng, I.-C.; Guttmann, P.; Werner, S.; Henzler, K.; Schneider, G.; et al. Nanoimaging Granule Dynamics and Subcellular Structures in Activated Mast Cells Using Soft X-ray Tomography. Sci. Rep. 2016, 6, 34879. [Google Scholar] [CrossRef]
- Zhang, B.; Alysandratos, K.-D.; Angelidou, A.; Asadi, S.; Sismanopoulos, N.; Delivanis, D.-A.; Weng, Z.; Miniati, A.; Vasiadi, M.; Katsarou-Katsari, A.; et al. Human Mast Cell Degranulation and Preformed TNF Secretion Require Mitochondrial Translocation to Exocytosis Sites: Relevance to Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1522–1531.e8. [Google Scholar] [CrossRef]
- Zhang, B.; Asadi, S.; Weng, Z.; Sismanopoulos, N.; Theoharides, T.C. Stimulated Human Mast Cells Secrete Mitochondrial Components That Have Autocrine and Paracrine Inflammatory Actions. PLoS ONE 2012, 7, e49767. [Google Scholar] [CrossRef]
- Maneechote, C.; Palee, S.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Inhibition of Mitochondrial Fission Attenuates Cardiac Ischemia-Reperfusion Injury in Pre-Diabetic Rats. Biochem. Pharmacol. 2020, 182, 114295. [Google Scholar] [CrossRef] [PubMed]
- Paradis, S.; Charles, A.-L.; Giannini, M.; Meyer, A.; Lejay, A.; Talha, S.; Laverny, G.; Charloux, A.; Geny, B. Targeting Mitochondrial Dynamics during Lower-Limb Ischemia Reperfusion in Young and Old Mice: Effect of Mitochondrial Fission Inhibitor-1 (mDivi-1). Int. J. Mol. Sci. 2024, 25, 4025. [Google Scholar] [CrossRef]
- Navinés-Ferrer, A.; Ainsua-Enrich, E.; Serrano-Candelas, E.; Proaño-Pérez, E.; Muñoz-Cano, R.; Gastaminza, G.; Olivera, A.; Martin, M. MYO1F Regulates IgE and MRGPRX2-Dependent Mast Cell Exocytosis. J. Immunol. 2021, 206, 2277–2289. [Google Scholar] [CrossRef]
- Pavlyuchenkova, A.N.; Zinovkin, R.A.; Makievskaya, C.I.; Galkin, I.I.; Chelombitko, M.A. Mitochondria-Targeted Triphenylphosphonium-Based Compounds Inhibit FcεRI-Dependent Degranulation of Mast Cells by Preventing Mitochondrial Dysfunction through Erk1/2. Life Sci. 2022, 288, 120174. [Google Scholar] [CrossRef] [PubMed]
- Maestraggi, Q.; Lebas, B.; Clere-Jehl, R.; Ludes, P.-O.; Chamaraux-Tran, T.-N.; Schneider, F.; Diemunsch, P.; Geny, B.; Pottecher, J. Skeletal Muscle and Lymphocyte Mitochondrial Dysfunctions in Septic Shock Trigger ICU-Acquired Weakness and Sepsis-Induced Immunoparalysis. BioMed Res. Int. 2017, 2017, 7897325. [Google Scholar] [CrossRef] [PubMed]
- Riou, M.; Enache, I.; Sauer, F.; Charles, A.-L.; Geny, B. Targeting Mitochondrial Metabolic Dysfunction in Pulmonary Hypertension: Toward New Therapeutic Approaches? Int. J. Mol. Sci. 2023, 24, 9572. [Google Scholar] [CrossRef] [PubMed]
- McLeod, J.J.A.; Caslin, H.L.; Spence, A.J.; Kolawole, E.M.; Qayum, A.A.; Paranjape, A.; Taruselli, M.; Haque, T.T.; Kiwanuka, K.N.; Elford, H.L.; et al. Didox (3,4-Dihydroxybenzohydroxamic Acid) Suppresses IgE-Mediated Mast Cell Activation through Attenuation of NFκB and AP-1 Transcription. Cell Immunol. 2017, 322, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A.; Averina, O.A.; Vasilyeva, T.V.; Pletiushkina, O.Y.; Popova, E.N.; Fedorov, A.V.; Chernyak, B.V.; Shishkina, V.S.; Ilinskaya, O.P. Mitochondria-Targeted Antioxidant SkQ1 (10-(6’-Plastoquinonyl)Decyltriphenylphosphonium Bromide) Inhibits Mast Cell Degranulation in Vivo and in Vitro. Biochemistry 2017, 82, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Zeng, H.-L.; Shang, J.; Wang, X.; Xu, L.; Fang, M.; Zeng, F.; Yang, Q. Inhibitory Effect of Rosmarinic Acid on IgE-Trigged Mast Cell Degranulation in Vitro and in Vivo. Mol. Biol. Rep. 2024, 51, 194. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, G.; Ricci, F.; Gallina, S.; Di Baldassarre, A.; Ghinassi, B. Mitochondrial Dysfunction and Heart Disease: Critical Appraisal of an Overlooked Association. Int. J. Mol. Sci. 2021, 22, 614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Heinemann, N.; Rademacher, F.; Darvin, M.E.; Raab, C.; Keck, C.M.; Vollert, H.; Fluhr, J.W.; Gläser, R.; Harder, J.; et al. Skin Care Product Rich in Antioxidants and Anti-Inflammatory Natural Compounds Reduces Itching and Inflammation in the Skin of Atopic Dermatitis Patients. Antioxidants 2022, 11, 1071. [Google Scholar] [CrossRef]
- Ardizzone, A.; Repici, A.; Capra, A.P.; De Gaetano, F.; Bova, V.; Casili, G.; Campolo, M.; Esposito, E. Efficacy of the Radical Scavenger, Tempol, to Reduce Inflammation and Oxidative Stress in a Murine Model of Atopic Dermatitis. Antioxidants 2023, 12, 1278. [Google Scholar] [CrossRef]
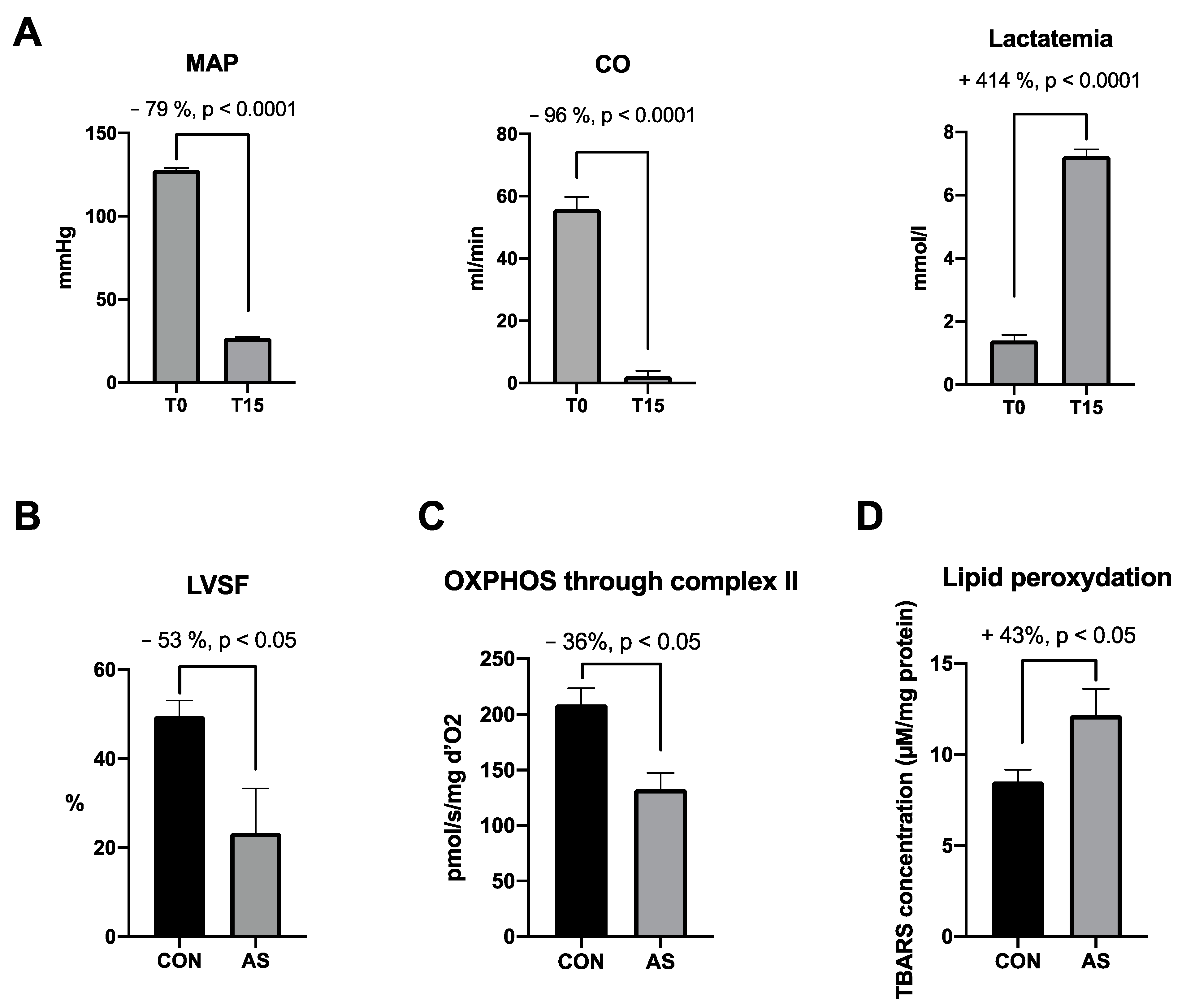
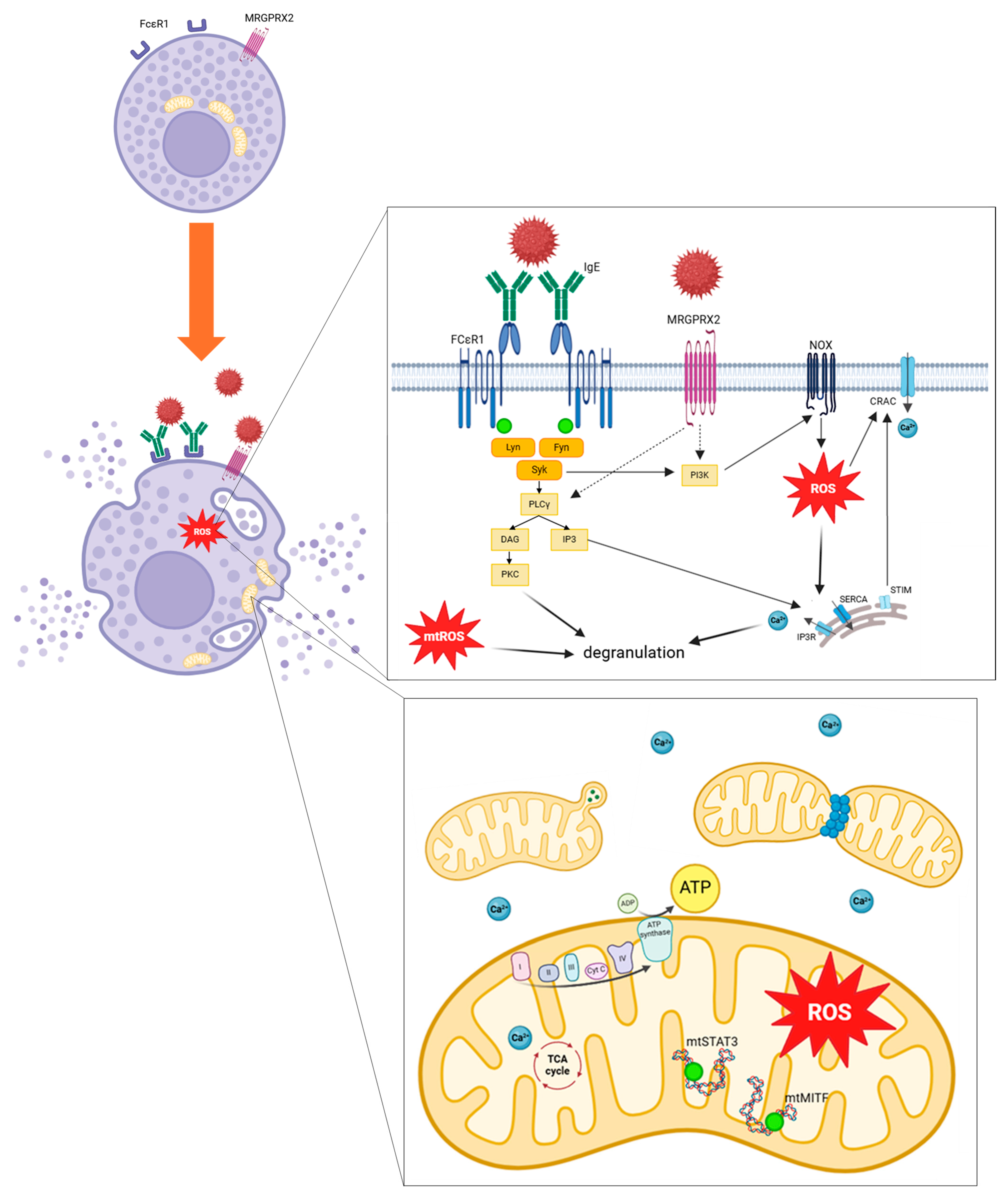
| Models | Hemodynamic Signs | Oxidative Stress Antioxidant System | Oxidative Damage | Mitochondria | Publications |
|---|---|---|---|---|---|
| IgE-mediated Guinea pigs cardiomyocytes | Tachycardia at first, then bradycardia with conduction disorders | NA | NA | Shrunken or disrupted mitochondria with close cristae and dense matrix | Suzuki et al., 1972 [92] |
| 48/80-induced AS Rats Hepatocytes | NA | NA | NA | Significant decrease in mitochondrial granules | Dimlich et al., 1985 [93] |
| IgG-mediated Guinea pigs Lung | Anaphylactic airway contraction | Increased or decreased lung GSH level is associated with higher or lower airway hyperreactivity | Increase in lipid peroxidation in pulmonary tract (TBARs) | NA | Kloek et al., 2011 [88] |
| IgE-mediated Guinea pigs Isolated heart | NA | Increase in superoxide anion radical O2•− and in H2O2 | NA | NA | Rosic et al., 2014 [91] |
| IgE-mediated Mice Isolated heart | Coronary flow decrease | Cardiac decrease in NO production and no change in O2•− within 2 min after OVA challenge | Cardiac lipid peroxidation (TBARs) decrease within 2 min after OVA challenge | NA | Milicic et al., 2016 [90] |
| IgE-mediated Mice Hepatocytes | Body temperature decrease | Increase in H2O2 production Aconitase inactivation Increase in SOD activity | NA | OXPHOS impairment through complexes I and II | Trinchese et al., 2018 [89] |
| IgE-mediated Brown Norway rats Cardiomyocytes | Severe and rapid decrease in MAP | Trend towards increase in peroxynitrite Increase in SOD activity | Increase in lipid peroxidation (TBARs) | Mitochondrial respiration impairment through complexes I and II | Oulehri et al., 2022 [5] |
| IgE-mediated Wistar rats Plasma | Severe decrease in MAP | Increase in SOD plasmatic level No difference in catalase activity | No change in plasmatic lipid peroxidation (TBARs) level | NA | Bellou et al., 2022 [87] |
| IgE-mediated Brown Norway rats Cardiomyocytes | Severe and rapid decrease in MAP, CO and cardiac contractility | NA | NA | Early (15 min) OXPHOS impairment through complex II Delayed (60 min) impairment through complexes I and II | Oulehri et al., 2024 [86] |
| Cells | Mast Cells Activation | Assessment of Mast Cell Degranulation | Oxidative Stress | Lipid Peroxidation | Publications |
|---|---|---|---|---|---|
| RBL-2H3 cells, HL | IgE | histamine release | ROS production in stimulated cells | NA | Yoshimaru T et al., 2002 [97] |
| RBL-2H3 cells, mice BMMCs | IgE | β-hexosaminidase release | ROS production in stimulated mast cells is mediated by PI3K and NADPH activity; PLCγ is ROS dependent | NA | Suzuki Y et al., 2003 [98] |
| RBL-2H3 cells | non-IgE | β-hexosaminidase, serotonin and histamine release | Production of ROS during degranulation; tetramethylthiourea and NAC blocked ROS generation and degranulation | NA | Collaco CR et al., 2006 [99] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | Production of ROS during degranulation | NA | Yasui Y et al., 2007 [100] |
| mice BMMCs, RBL-2H3 cells | IgE and non-IgE | β-hexosaminidase release | IgE stimulation increased ROS production via NADPH oxidase, but to a lesser extent following non-IgE activation | NA | Inoue, T., et al., 2008 [101] |
| RBL-2H3 cells | IgE and non-IgE | β-hexosaminidase and histamine release, β-hexosaminidase activity | complex I and II inhibitors decreased mtROS generation after stimulation; complex III inhibitor increased H2O2 secretion; NAC decreased spontaneous and mtROS-induced degranulation; decrease in mtROS or ROSIC levels reduced degranulation; mtROS regulated PKC activity | NA | Chodaczek G et al., 2009 [79] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | Hydrogen attenuated NADPH oxidase activity, decreased antigen-induced production of H2O2 and O2− | NA | Itoh et al., 2009 [102] |
| BMMCs, FSMCs, LAD2 cells | IgE and non-IgE | histamine release | UCP2 knockdown enhanced ROS production, SOD-mimetic treatment reduced degranulation | NA | Tagen M et al., 2009 [103] |
| RBL-2H3 cells, mice BMMCs | IgE | β-hexosaminidase release | EGCG enhanced antigen-induced ROS production | EGCG induced cardiolipin oxidation | Inoue T et al., 2010 [80] |
| mice BMMCs, HCMCs | IgE | β-hexosaminidase release | Increase in [ROS]IC levels after stimulation; ROSIC production depends on [Ca2+]IC | NA | Zhou Y et al., 2013 [104] |
| RBL-2H3 cells | IgE and non-IgE | serotonin release | Acrolein increased ROSIC in dose-dependent manner; NAC reduced ROS production and degranulation | NA | Hochman DJ et al., 2014 [105] |
| mice BMMCs, MPMCs | IgE | CD63 MFI and cytokine release | Didox decreased oxidative stress, enhanced catalase and SOD1 expression | NA | McLeod JJA et al., 2017 [106] |
| MPMCs, RBL-2H3 cells | IgE and non-IgE | β-hexosaminidase activity | SkQ1 inhibited degranulation | NA | Chelombitko MA et al., 2017 [107] |
| PHK, NIH-3T3 mouse fibroblast, RBL-2H3 cells | IgE | NA | ROS production in stimulated cells; TCS mildly increased ROS production of stimulated cells | NA | Weatherly LM et al., 2018 [84] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | PM2.5 increased ROS levels and degranulation | NA | Wang Y et al., 2021 [108] |
| RBL 2H3 cells | IgE | β-hexosaminidase release | SkQ1 decreased degranulation but did not change ROS production | NA | Pavlyuchenkova AN et al., 2022 [109] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | Mitocur 1 and 3 increased ROS levels | Mitocur 1 and 3 increased cardiolipin peroxidation | Pavlyuchenkova AN et al., 2023 [110] |
| RBL-2H3 cells | IgE | β-hexosaminidase and histamine release | Rosmarinic downregulated ROS overproduction during degranulation | NA | Jia B et al., 2024 [111] |
| Cells | Mast Cells Activation | Assessment of Mast Cell Degranulation | Mitochondrial Respiration | Mitochondrial Transcription Factors and mtDNA | Calcium Flux | Mitochondrial Dynamic and Morphology | Publications |
|---|---|---|---|---|---|---|---|
| RBL-2H3 cells, mice BMMCs | IgE | β-hexosaminidase release | ROT had no inhibitory effect on IgE-induced ROS production | NA | inhibition of IgE-mediated ROS production reduced Ca2+ influx | NA | Suzuki Y et al., 2003 [98] |
| RBL-2H3 cells, mice BMMCs | IgE and non-IgE | β-hexosaminidase release | ROT, AMA, OMY and FCCP decreased IgE degranulation; ROT and FCCP reduced non-IgE degranulation | NA | IgE stimulation induced [Ca2+]m drop | NA | Suzuki et al., 2006 [75] |
| RBL-2H3 cells, mice BMMCs | IgE and non-IgE | NA | NA | NA | increase in [Ca2+]m in activated cells; mitochondria take up Ca2+ via MCU | NA | Suzuki Y, 2008 [112] |
| mice BMMCs, RBL-2H3 cells | IgE and non-IgE | β-hexosaminidase release | NA | NA | ROS production depends on [Ca2+]m | NA | Inoue, T., et al., 2008 [101] |
| RBL-2H3 cells | IgE and non-IgE | β-hexosaminidase and histamine release, β-hexosaminidase activity | complex I and II inhibitors inhibited degranulation; complex III inhibitor enhanced degranulation | NA | NA | NA | Chodaczek G et al., 2009 [79] |
| BMMCs, FSMCs, LAD2 cells | IgE and non-IgE | histamine release | UCP2 knockdown enhanced degranulation | NA | NA | NA | Tagen M et al., 2009 [103] |
| RBL-2H3 cells, mice BMMCs | IgE | β-hexosaminidase release | high concentration FCCP suppressed degranulation | NA | EGCG induced mtCa2+ release | NA | Inoue T et al., 2010 [80] |
| hCBMCs, LAD2 cells, hSKM from control and patients with AD | IgE and non-IgE | β-hexosaminidase release | NA | NA | Drp1 activation depends on [Ca2+]IC increase | mitochondrial translocation near cell surface; Drp1 inhibitor inhibits mitochondrial translocation and degranulation | Zhang B et al., 2011 [113] |
| LAD2 cells, hCBMCs | IgE and non-IgE | β-hexosaminidase release | OMY inhibited TNF secretion | NA | NA | transient mitochondrial translocation near cell surface | Zhang B et al., 2012 [76] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | ROT and AMA inhibited degranulation | NA | ROT and AMA reduced the [Ca2+]m uptake after stimulation | NA | Takekawa M et al., 2012 [78] |
| LAD2 cells, hCBMCs | IgE and non-IgE | β-hexosaminidase and histamine release | NA | NA | NA | mitochondrial translocation near cell surface | Zhang B et al., 2012 [114] |
| mice BMMCs, HCMCs | IgE | β-hexosaminidase release | NA | NA | increase in [Ca2+]IC in activated cells | NA | Zhou Y et al., 2013 [104] |
| RBL-2H3 cells, mice BMMCs, hCBMCs | IgE | β-hexosaminidase release | major part of energy for degranulation derived from mitochondrial ATP | STAT3 inhibition abolished degranulation; mtSTAT3 phosphorylation in activated cells | NA | NA | Erlich et al., 2014 [82] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | NA | NA | MCU needed for mast cell degranulation | NA | Furuno T et al., 2015 [115] |
| PHK, HMC, NIH-3T3 mice fibroblasts, RBL-2H3 cells | IgE and non-IgE | β-hexosaminidase release | TCS inhibited ATP production and degranulation | NA | NA | NA | Weatherly LM et al., 2016 [83] |
| mice BMMCs | non-IgE | tryptase release | NA | NA | stimulation increased both [Ca2+]IC and [Ca2+]m; MCU blocker reduced degranulation | NA | Cuong DV et al., 2016 [116] |
| RBL-2H3 cells | IgE | cryofluorescence and soft X-ray tomography to determine location of granule | NA | NA | NA | translocation of mitochondria to cell surface; morphological changes of mitochondria | Chen HY et al., 2016 [117] |
| RBL-2H3 cells, BMMCs, hCBMCs | IgE | β-hexosaminidase release | PDH inhibition reduced mitochondrial ATP levels in a dose-dependent manner and degranulation | PDH dephosphorylation and detachment from MITF in activated cells | NA | NA | Sharkia I et al., 2017 [85] |
| mice BMMCs, MPMCs | IgE | CD63 MFI and cytokine release | Didox suppressed cytokine release and CD63 MFI | NA | NA | NA | McLeod JJA et al., 2017 [106] |
| PHK, NIH-3T3 mouse fibroblast, RBL-2H3 cells | IgE | NA | TCS decreased ATP production | NA | TCS disrupts mitochondrial Ca2+ buffering capacity | TCS inhibits mitochondrial translocation in stimulated cells | Weatherly LM et al., 2018 [84] |
| RBL-2H3 cells, BMMC, hCBMCs | IgE | β hexosaminidase release and IL-6 release | Mitocur 1 and 3 reduced mitochondrial ATP levels in a dose-dependent manner and reduced OCR | Mitocur 1 and 3 reduced degranulation | NA | NA | Erlich TH et al., 2018 [118] |
| RBL-2H3 cells, Jurkat T cells | IgE | β hexosaminidase release | NA | NA | TCS inhibited degranulation by inhibiting CRAC channel | NA | Sangroula S et al., 2020 [81] |
| LAD2 cells | IgE and non-IgE | β-hexosaminidase release | NA | NA | NA | MYOF1 knockdown decreased degranulation | Navinés-Ferrer A et al., 2021 [119] |
| RBL-2H3, LAD2 cells, MPMCs | IgE and non-IgE | β-hexosaminidase release | NA | NA | Forsythiae fractus decreased [Ca2+]IC by enhancing [Ca2+]m and reduced degranulation | NA | Qi R et al., 2021 [120] |
| human mast cells from intestinal tissue | IgE | β-hexosaminidase release | NA | Resveratrol inhibited mtSTAT3 phosphorylation and reduced degranulation | NA | NA | Bilotta S et al., 2021 [121] |
| mice BMMCs | IgE and non-IgE | β-hexosaminidase release | OMY decreased AgNP-induced degranulation but not IgE- nor C48/80-induced degranulation | NA | NA | NA | Mendoza et al., 2021 [77] |
| RBL 2H3 cells | IgE | β-hexosaminidase release | NA | NA | NA | SkQ1 inhibited Erk1/2-dependent mitochondrial fragmentation | Pavlyuchenkova AN et al., 2022 [109] |
| RBL-2H3 cells, mice BMMCs | IgE | β-hexosaminidase release | increased OXPHOS activity in stimulated cells | phosphorylated MITF in activated cells | NA | NA | Paruchuru LB et al., 2022 [122] |
| hSKM | IgE | β-hexosaminidase release | increased OCR in activated cells; no effect of inhibition of complex 3 on degranulation; inhibition of complex I decreased degranulation | NA | NA | NA | Buttgereit T et al., 2022 [74] |
| RBL-2H3 cells | IgE | β-hexosaminidase release | NA | Mitocur 1 and 3 reduced STAT3 phosphorylation in activated cells | NA | Mitocur 1 and 3 caused mitochondrial fragmentation and swelling | Pavlyuchenkova AN et al., 2023 [110] |
| RBL-2H3 cells | non-IgE | β-hexosaminidase release | NA | NA | CC inhibits degranulation by decreasing Ca2+ efflux from RE, reduces [Ca2+]m and [Ca2+]IC | NA | Obeng B et al., 2023 [123] |
| LAD2 cells | non-IgE | β-hexosaminidase release | NA | MRGPRX2 activation increased MITF phosphorylation and activity; MITF silencing reduced degranulation | NA | NA | Guo Y et al., 2023 [124] |
| RBL-2H3 cells | IgE | β-hexosaminidase and histamine release | NA | Rosmarinic down-regulated mRNA expression of genes implicated in the oxidative stress signaling pathway (NQO1, Nrf2, HO-1) of degranulated cells | Rosmarinic inhibited flux of [Ca2+]IC in stimulated mast cells | NA | Jia B et al., 2024 [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotin, A.; Oulehri, W.; Charles, A.-L.; Tacquard, C.; Collange, O.; Mertes, P.-M.; Geny, B. Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review. Antioxidants 2024, 13, 920. https://doi.org/10.3390/antiox13080920
Piotin A, Oulehri W, Charles A-L, Tacquard C, Collange O, Mertes P-M, Geny B. Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review. Antioxidants. 2024; 13(8):920. https://doi.org/10.3390/antiox13080920
Chicago/Turabian StylePiotin, Anays, Walid Oulehri, Anne-Laure Charles, Charles Tacquard, Olivier Collange, Paul-Michel Mertes, and Bernard Geny. 2024. "Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review" Antioxidants 13, no. 8: 920. https://doi.org/10.3390/antiox13080920
APA StylePiotin, A., Oulehri, W., Charles, A.-L., Tacquard, C., Collange, O., Mertes, P.-M., & Geny, B. (2024). Oxidative Stress and Mitochondria Are Involved in Anaphylaxis and Mast Cell Degranulation: A Systematic Review. Antioxidants, 13(8), 920. https://doi.org/10.3390/antiox13080920






