Transcriptional Dynamics of NRF2 Overexpression and KEAP1-NRF2 Inhibitors in Human Cell Line and Primary Lung Cells †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation of Commercial Cells
2.2. mRNA Transfection and Compound 7 Treatment
2.3. Antioxidant Response Element (ARE) Luciferase Assay
2.4. Western Blot
2.5. RT-qPCR (BEAS-2B and HPF)
2.6. Human Cytokine Measurement (IL-8)
2.7. HBEC Next-Generation Sequencing (NGS) Study Sample Generation
2.8. NGS Study-Library Preparation, Sequencing, and Data Clean-Up
2.9. Differential Gene Expression Determination in NGS Study
2.10. Alveolar Macrophages
3. Results
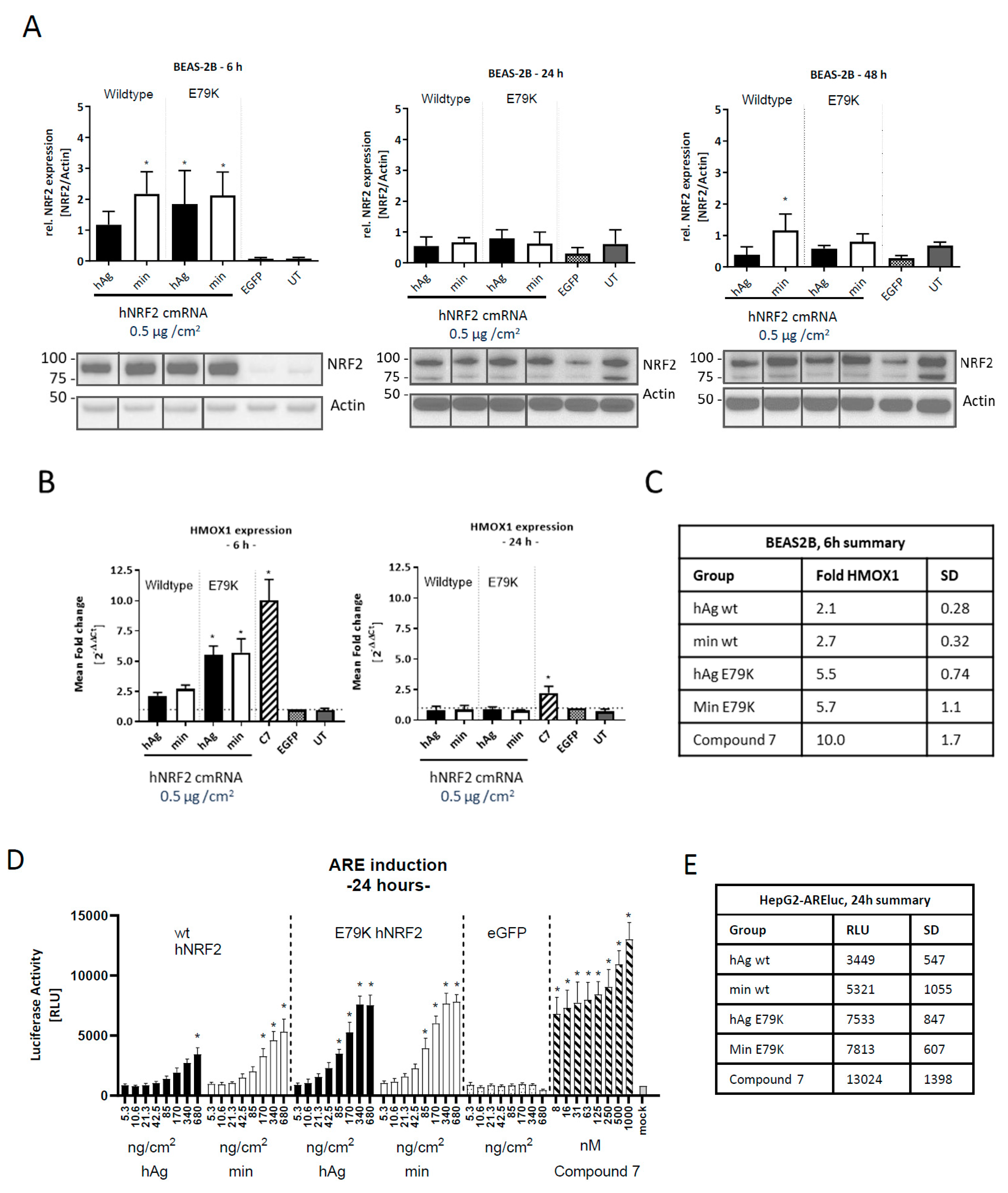
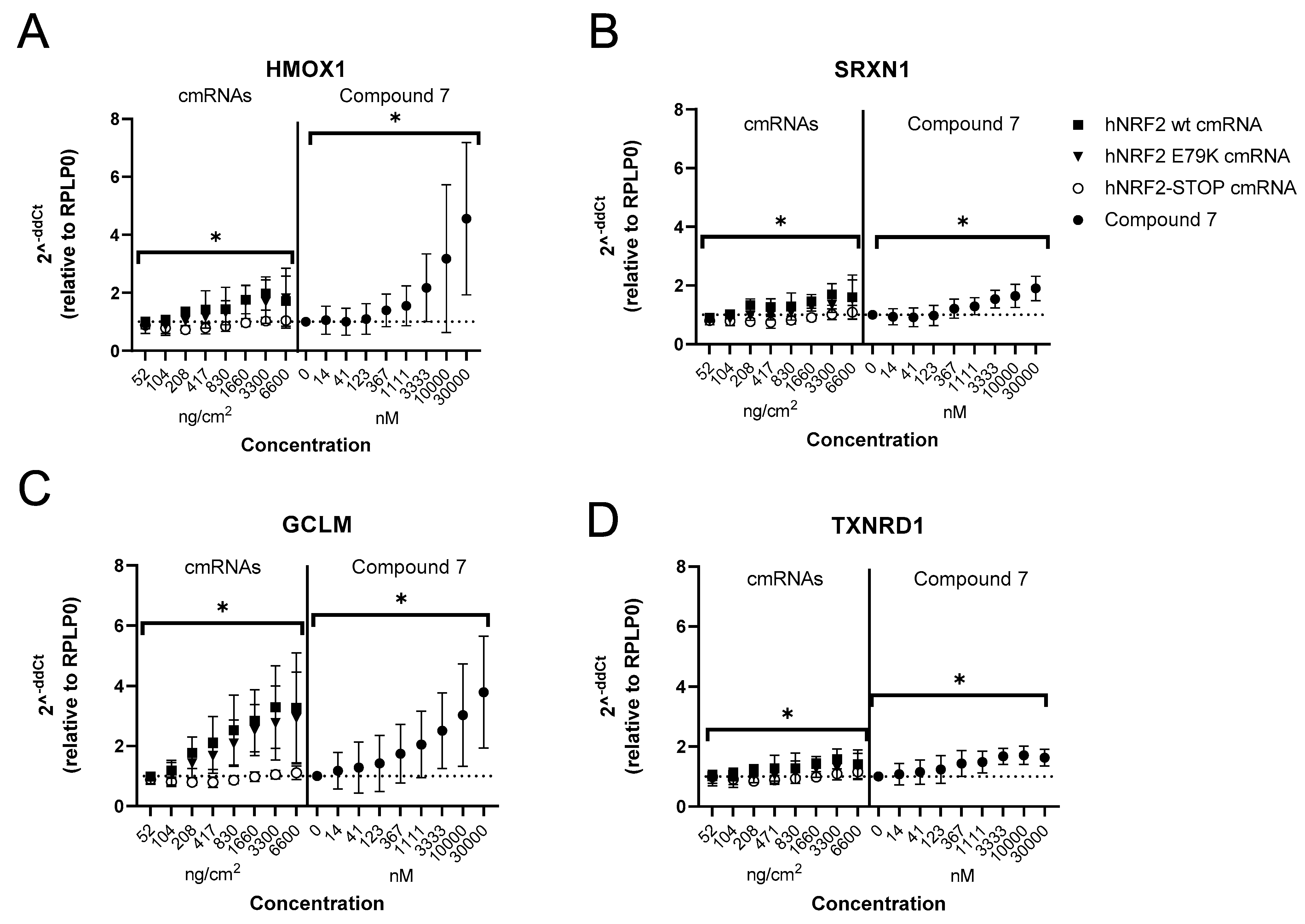
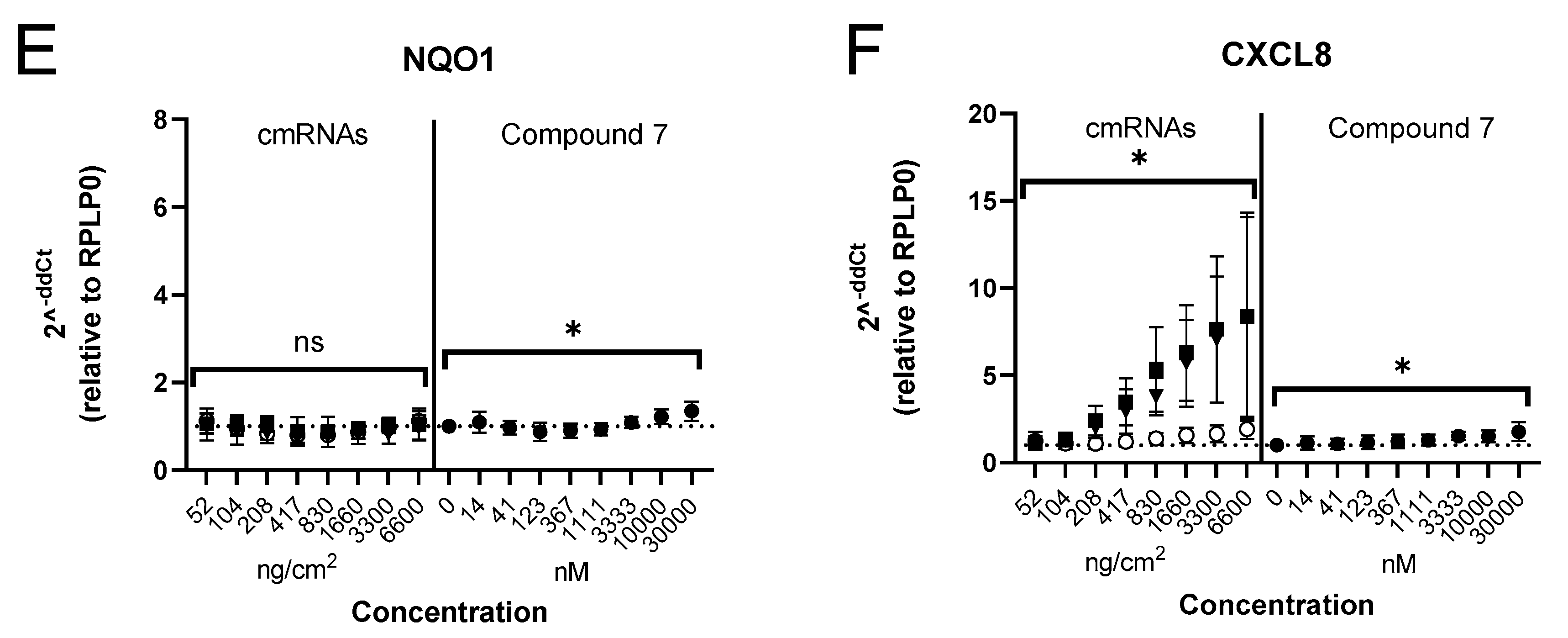
3.1. Expression of Functional NRF2 in Human Cell Lines
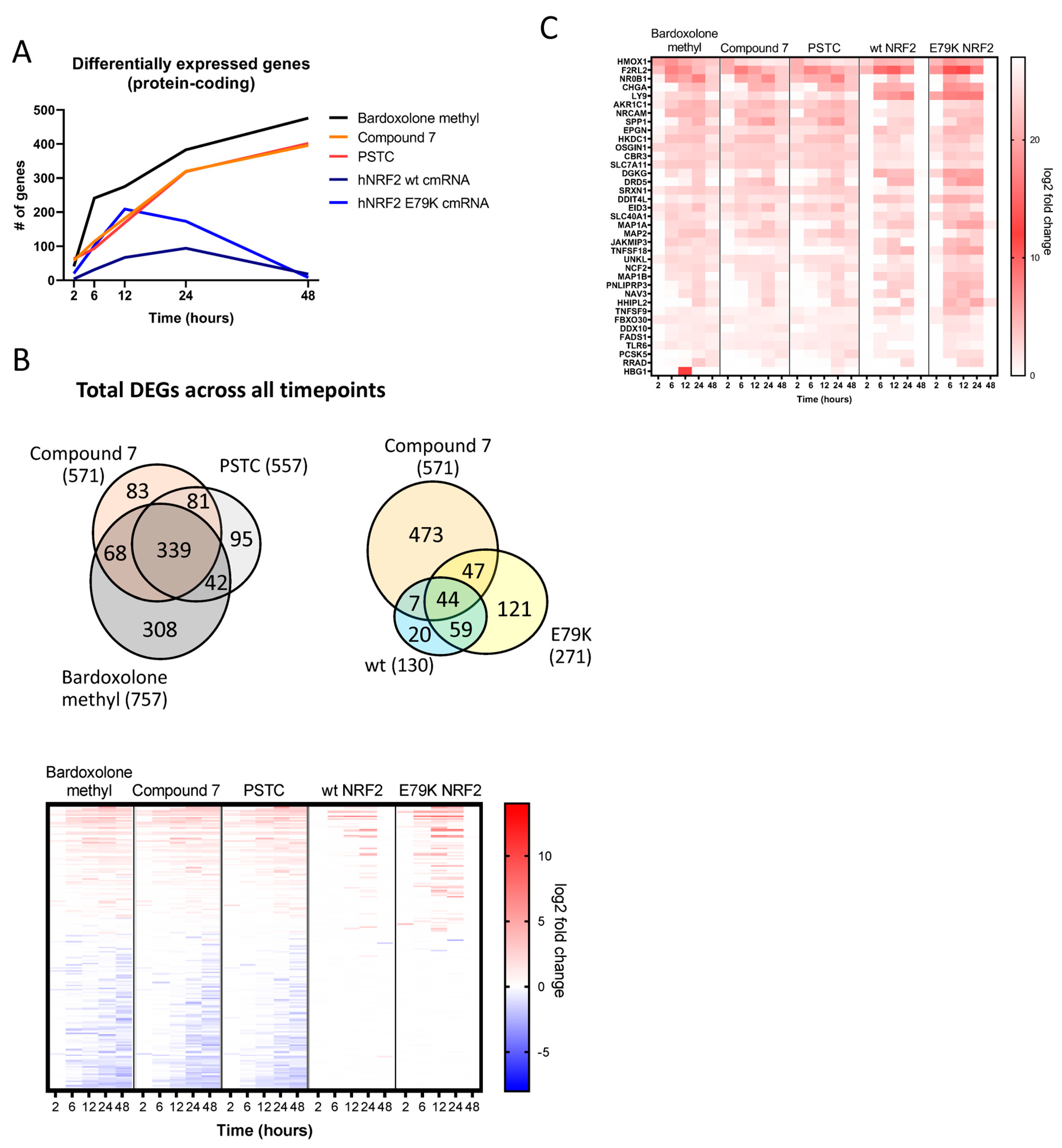
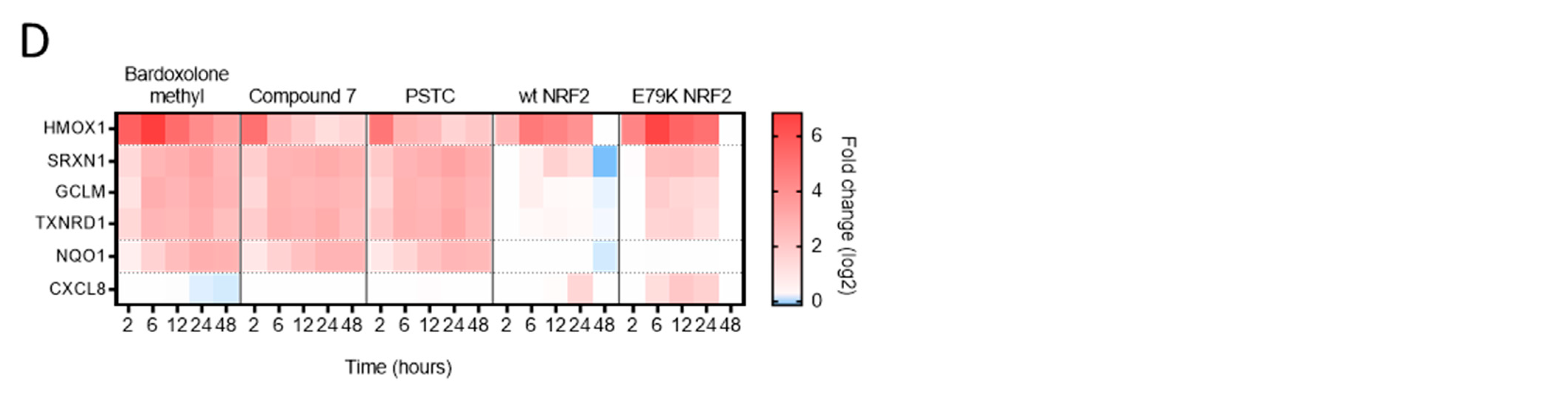
3.2. Identification of an NRF2 Gene Signature in Primary Human Bronchial Epithelial Cells
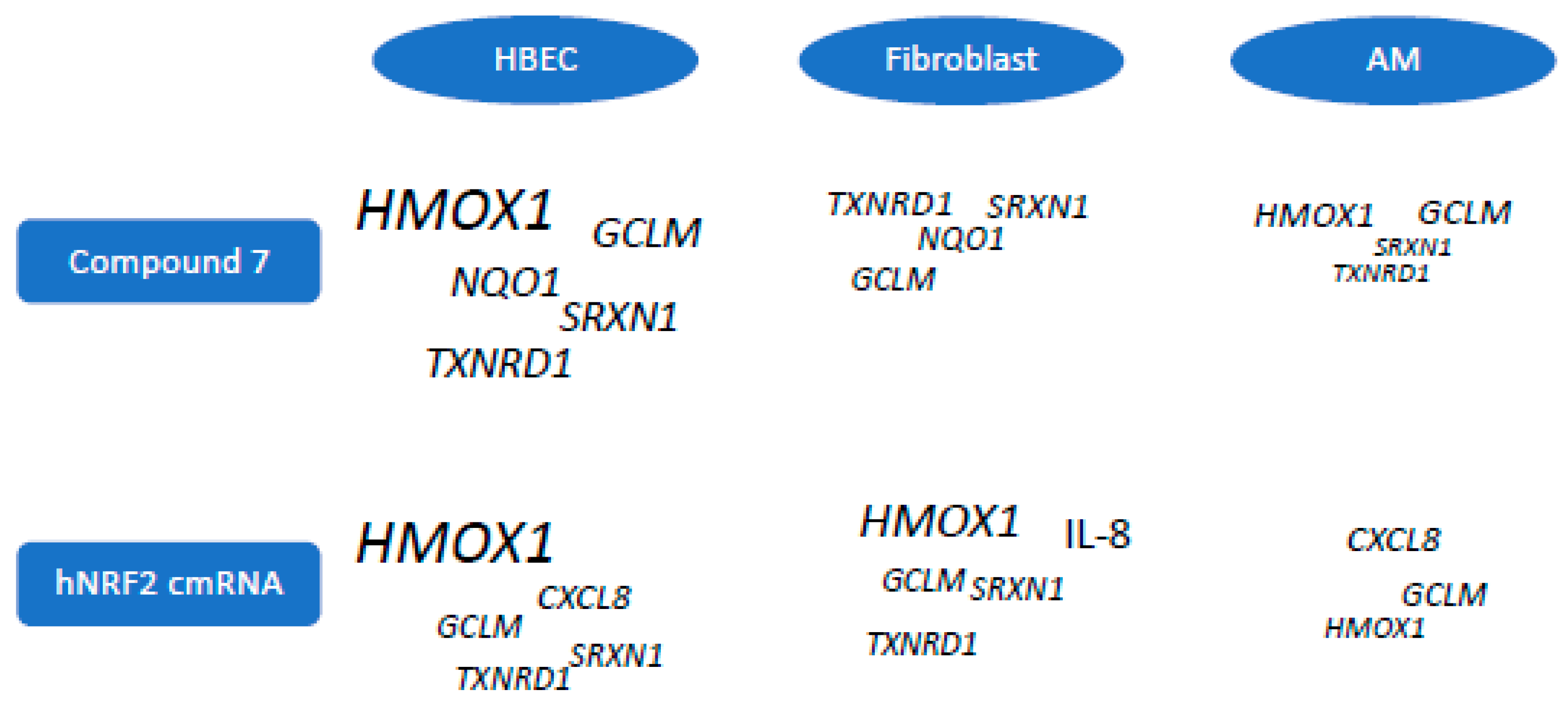
3.3. Selection of Gene Signature for Additional Studies
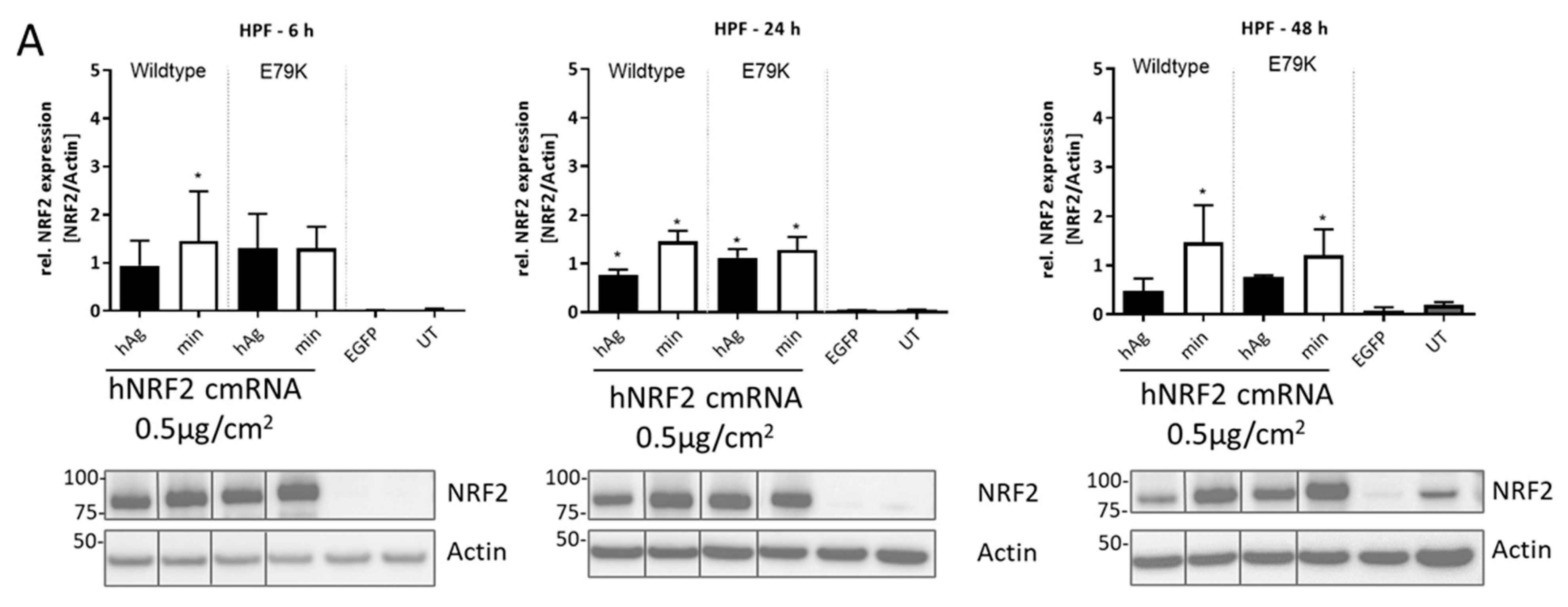
3.4. Expression of Functional NRF2 in Primary Human Lung Fibroblasts
3.5. Expression of Functional NRF2 in Primary Human Alveolar Macrophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, P.J. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020, 33, 101544. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Van’t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying oxidative stress by F(2)-isoprostane levels across human diseases: A meta-analysis. Redox Biol. 2017, 12, 582–599. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Pajares, M.; Cuadrado, A.; Rojo, A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017, 11, 543–553. [Google Scholar] [CrossRef]
- Li, D.; Sun, D.; Zhu, Y. Expression of nuclear factor erythroid-2-related factor 2, broad complex-tramtrack-bric a brac and Cap‘n’collar homology 1 and gamma-glutamic acid cysteine synthase in peripheral blood of patients with chronic obstructive pulmonary disease and its clinical significance. Exp. Ther. Med. 2021, 21, 516. [Google Scholar]
- Li, J.; Baker, J.; Higham, A.; Shah, R.; Montero-Fernandez, A.; Murray, C.; Cooper, N.; Lucas, C.; Fox, C.; Singh, D.; et al. COPD lung studies of Nrf2 expression and the effects of Nrf2 activators. Inflammopharmacology 2022, 30, 1431–1443. [Google Scholar] [CrossRef]
- Goven, D.; Boutten, A.; Leçon-Malas, V.; Marchal-Sommé, J.; Amara, N.; Crestani, B.; Fournier, M.; Lesèche, G.; Soler, P.; Boczkowski, J.; et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 2008, 63, 916–924. [Google Scholar] [CrossRef]
- Fratta Pasini, A.M.; Ferrari, M.; Stranieri, C.; Vallerio, P.; Mozzini, C.; Garbin, U.; Zambon, G.; Cominacini, L. Nrf2 expression is increased in peripheral blood mononuclear cells derived from mild-moderate ex-smoker COPD patients with persistent oxidative stress. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1733–1743. [Google Scholar] [CrossRef]
- Fratta Pasini, A.M.; Stranieri, C.; Ferrari, M.; Garbin, U.; Cazzoletti, L.; Mozzini, C.; Spelta, F.; Peserico, D.; Cominacini, L. Oxidative stress and Nrf2 expression in peripheral blood mononuclear cells derived from COPD patients: An observational longitudinal study. Respir. Res. 2020, 21, 37. [Google Scholar] [CrossRef]
- Betsuyaku, T.; Fuke, S.; Inomata, T.; Kaga, K.; Morikawa, T.; Odajima, N.; Adair-Kirk, T.; Nishimura, M. Bronchiolar epithelial catalase is diminished in smokers with mild COPD. Eur. Respir. J. 2013, 42, 42–53. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, C.; Hua, D.X.; Li, M.D.; Lv, B.B.; Fu, L.; Zhao, H. Serum 8-Hydroxy-2′-deoxyguanosine Predicts Severity and Prognosis of Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Lung 2022, 200, 31–39. [Google Scholar] [CrossRef]
- Nadeem, A.; Raj, H.G.; Chhabra, S.K. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation 2005, 29, 23–32. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; Jiang, Y.; Lu, G.; Huang, X.; Guan, K. Local and systemic oxidative stress status in chronic obstructive pulmonary disease patients. Can Respir. J. 2013, 20, 35–41. [Google Scholar] [CrossRef]
- Davies, T.G.; Wixted, W.E.; Coyle, J.E.; Griffiths-Jones, C.; Hearn, K.; McMenamin, R.; Norton, D.; Rich, S.J.; Richardson, C.; Saxty, G.; et al. Monoacidic Inhibitors of the Kelch-like ECH-Associated Protein 1: Nuclear Factor Erythroid 2-Related Factor 2 (KEAP1:NRF2) Protein-Protein Interaction with High Cell Potency Identified by Fragment-Based Discovery. J. Med. Chem. 2016, 59, 3991–4006. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Fuchs, R.J.; Malhotra, D.; Scollick, C.; Traore, K.; Bream, J.H.; Trush, M.A.; Liby, K.T.; Sporn, M.B.; Kensler, T.W.; et al. Preclinical evaluation of targeting the Nrf2 pathway by triterpenoids (CDDO-Im and CDDO-Me) for protection from LPS-induced inflammatory response and reactive oxygen species in human peripheral blood mononuclear cells and neutrophils. Antioxid. Redox Signal. 2007, 9, 1963–1970. [Google Scholar] [CrossRef]
- Yates, M.S.; Tauchi, M.; Katsuoka, F.; Flanders, K.C.; Liby, K.T.; Honda, T.; Gribble, G.W.; Johnson, D.A.; Johnson, J.A.; Burton, N.C.; et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007, 6, 154–162. [Google Scholar] [CrossRef]
- Yonchuk, J.G.; Foley, J.P.; Bolognese, B.J.; Logan, G.; Wixted, W.E.; Kou, J.P.; Chalupowicz, D.G.; Feldser, H.G.; Sanchez, Y.; Nie, H.; et al. Characterization of the Potent, Selective Nrf2 Activator, 3-(Pyridin-3-Ylsulfonyl)-5-(Trifluoromethyl)-2H-Chromen-2-One, in Cellular and In Vivo Models of Pulmonary Oxidative Stress. J. Pharmacol. Exp. Ther. 2017, 363, 114–125. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free. Radic Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Z.; Zhou, Y.; Xiao, Q.; Wang, H.; Peng, Y. Emerging Substrate Proteins of Kelch-like ECH Associated Protein 1 (Keap1) and Potential Challenges for the Development of Small-Molecule Inhibitors of the Keap1-Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Protein-Protein Interaction. J. Med. Chem. 2020, 63, 7986–8002. [Google Scholar] [CrossRef]
- Karikó, K.; Kuo, A.; Barnathan, E. Overexpression of urokinase receptor in mammalian cells following administration of the in vitro transcribed encoding mRNA. Gene Ther. 1999, 6, 1092–1100. [Google Scholar] [CrossRef]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar] [PubMed]
- Gustafsson, C.; Govindarajan, S.; Minshull, J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004, 22, 346–353. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Shibata, T.; Ohta, T.; Tong, K.I.; Kokubu, A.; Odogawa, R.; Tsuta, K.; Asamura, H.; Yamamoto, M.; Hirohashi, S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl. Acad. Sci. USA 2008, 105, 13568–13573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Song, H.; Chen, H.Z.; Rovin, B.H. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur. J. Immunol. 2005, 35, 3258–3567. [Google Scholar] [CrossRef]
- Katsuoka, F.; Motohashi, H.; Ishii, T.; Aburatani, H.; Engel, J.D.; Yamamoto, M.G. Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol. Cell Biol. 2005, 25, 8044–8051. [Google Scholar] [CrossRef]
- Asfaha, S.; Dubeykovskiy, A.N.; Tomita, H.; Yang, X.; Stokes, S.; Shibata, W.; Friedman, R.A.; Ariyama, H.; Dubeykovskaya, Z.A.; Muthupalani, S.; et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013, 144, 155–166. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]



| Plate Format | RNA [µg]/Well | Transfection Reagent [µL]/Well | Lipoplexes/Well |
|---|---|---|---|
| 96-well | 2 µg–2.7 ng (1:3 dilution series in 5 µL Opti-MEM) | 0.3 µL in 5 µL Opti-MEM | 10 µL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamblet, C.; Björhall, K.; Busch, S.; Gehrmann, U.; Öberg, L.; Kubisch-Dohmen, R.; Haas, S.; Aneja, M.K.; Geiger, J.; Rudolph, C.; et al. Transcriptional Dynamics of NRF2 Overexpression and KEAP1-NRF2 Inhibitors in Human Cell Line and Primary Lung Cells. Antioxidants 2024, 13, 924. https://doi.org/10.3390/antiox13080924
Hamblet C, Björhall K, Busch S, Gehrmann U, Öberg L, Kubisch-Dohmen R, Haas S, Aneja MK, Geiger J, Rudolph C, et al. Transcriptional Dynamics of NRF2 Overexpression and KEAP1-NRF2 Inhibitors in Human Cell Line and Primary Lung Cells. Antioxidants. 2024; 13(8):924. https://doi.org/10.3390/antiox13080924
Chicago/Turabian StyleHamblet, Corinne, Karin Björhall, Susann Busch, Ulf Gehrmann, Lisa Öberg, Rebekka Kubisch-Dohmen, Sonja Haas, Manish K. Aneja, Johannes Geiger, Carsten Rudolph, and et al. 2024. "Transcriptional Dynamics of NRF2 Overexpression and KEAP1-NRF2 Inhibitors in Human Cell Line and Primary Lung Cells" Antioxidants 13, no. 8: 924. https://doi.org/10.3390/antiox13080924





