Pterostilbene, a Resveratrol Derivative, Improves Ovary Function by Upregulating Antioxidant Defenses in the Aging Chickens via Increased SIRT1/Nrf2 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Plasma Analysis
2.3. Biochemical Analysis
2.4. Sample Collection
2.5. SWFs Culture and Treatment of Chemicals
2.5.1. Experiment 1 Impact of PTS on D-Gal Triggered Aging in SWFs
2.5.2. Experiment 2 Treatment with Inhibitors
2.6. Morphological Observation
2.6.1. Hematoxylin and Eosin (H&E) Staining
2.6.2. Oil Red O Staining
2.6.3. TUNEL Staining
2.6.4. BrdU Staining
2.7. RNA Extraction and qRT-PCR
2.8. Analysis Using Western Blot Technique
2.9. Statistical Analysis
3. Results
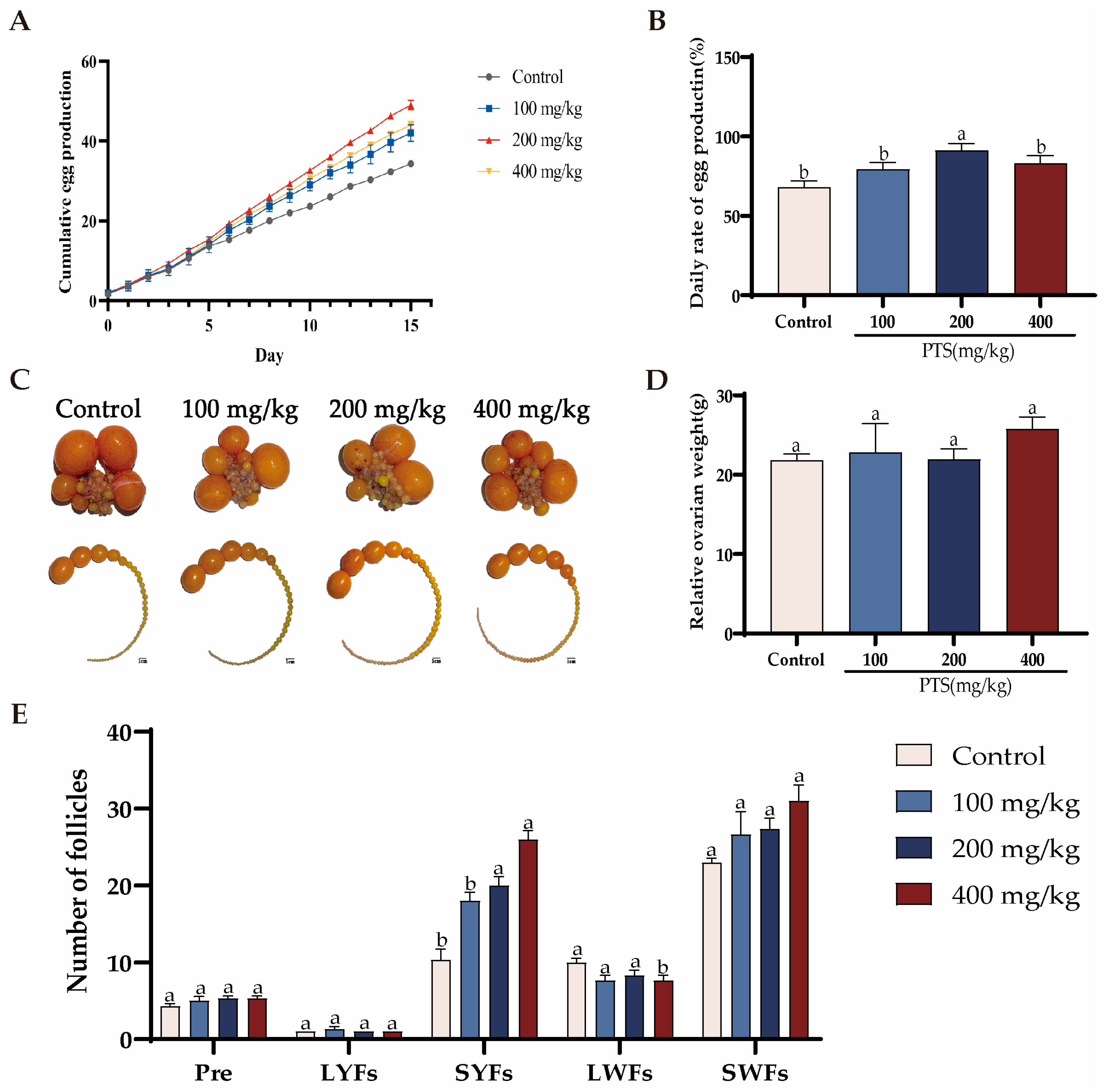
3.1. Effects of PTS Supplementation on Laying Performance and Serum in Aged Hens
3.1.1. Effects of PTS Supplementation on Egg Production and Follicle Development
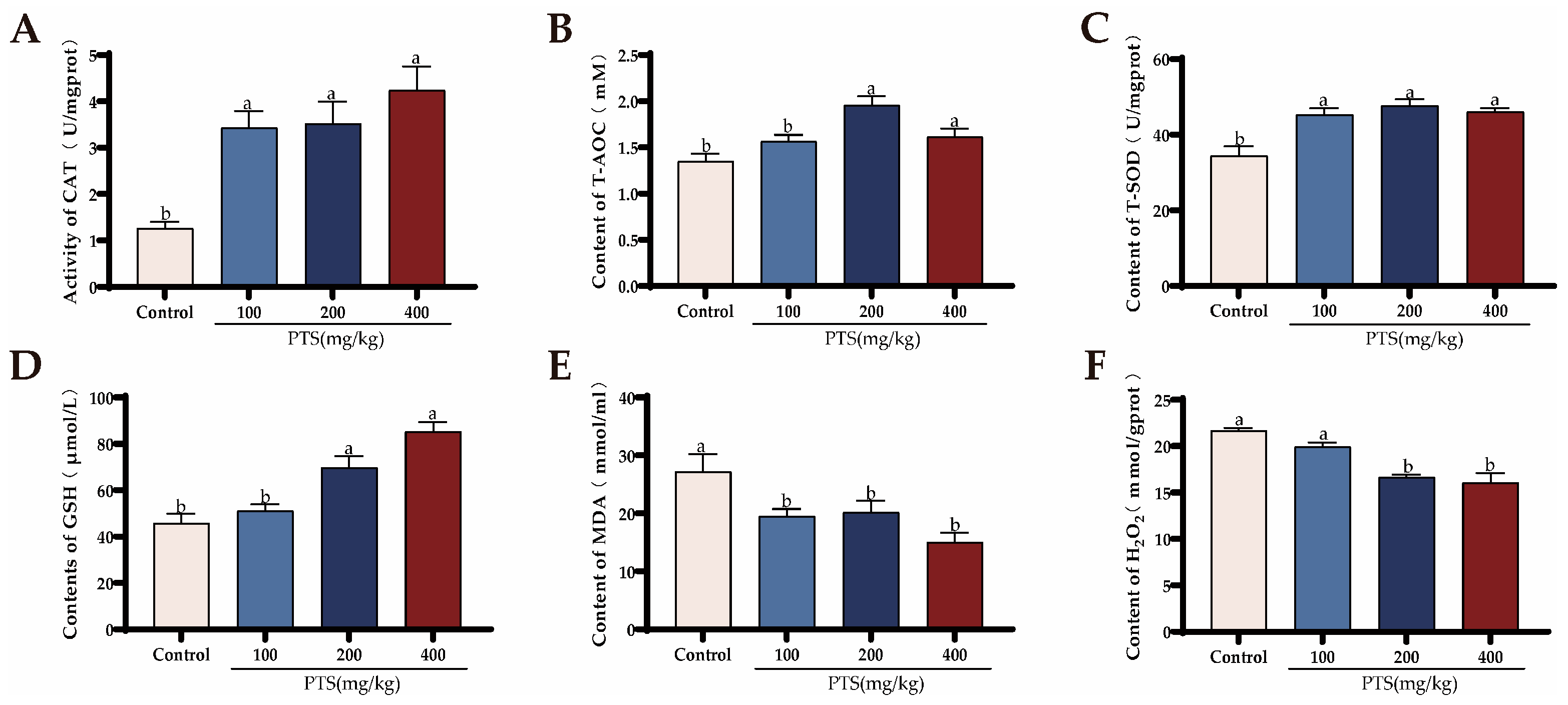
3.1.2. Effects of PTS Supplementation on Serum Antioxidant Capacity and Biochemical Parameters
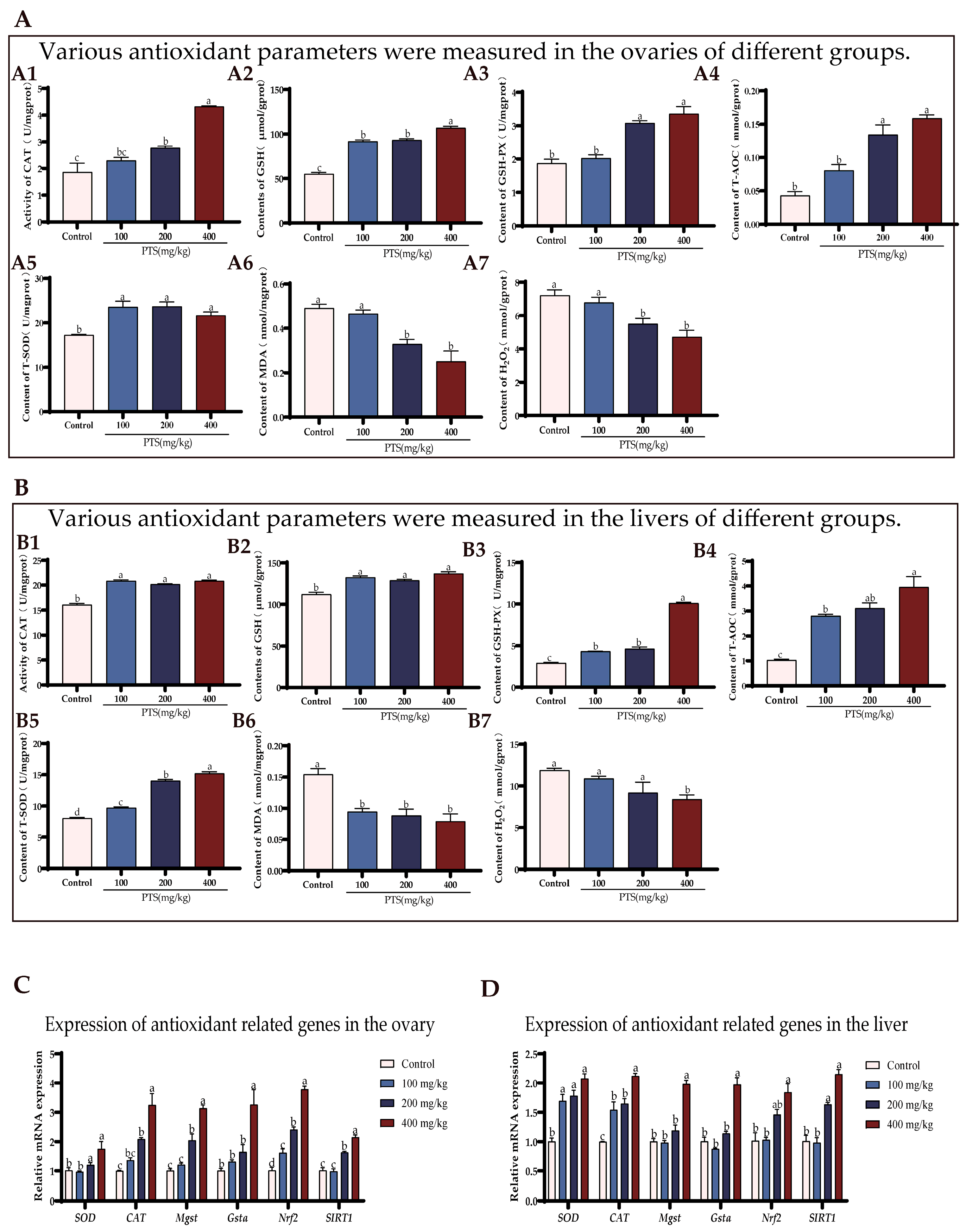
3.2. PTS Supplementation Demonstrates Antioxidative Effects on Ovary and Liver In Vivo
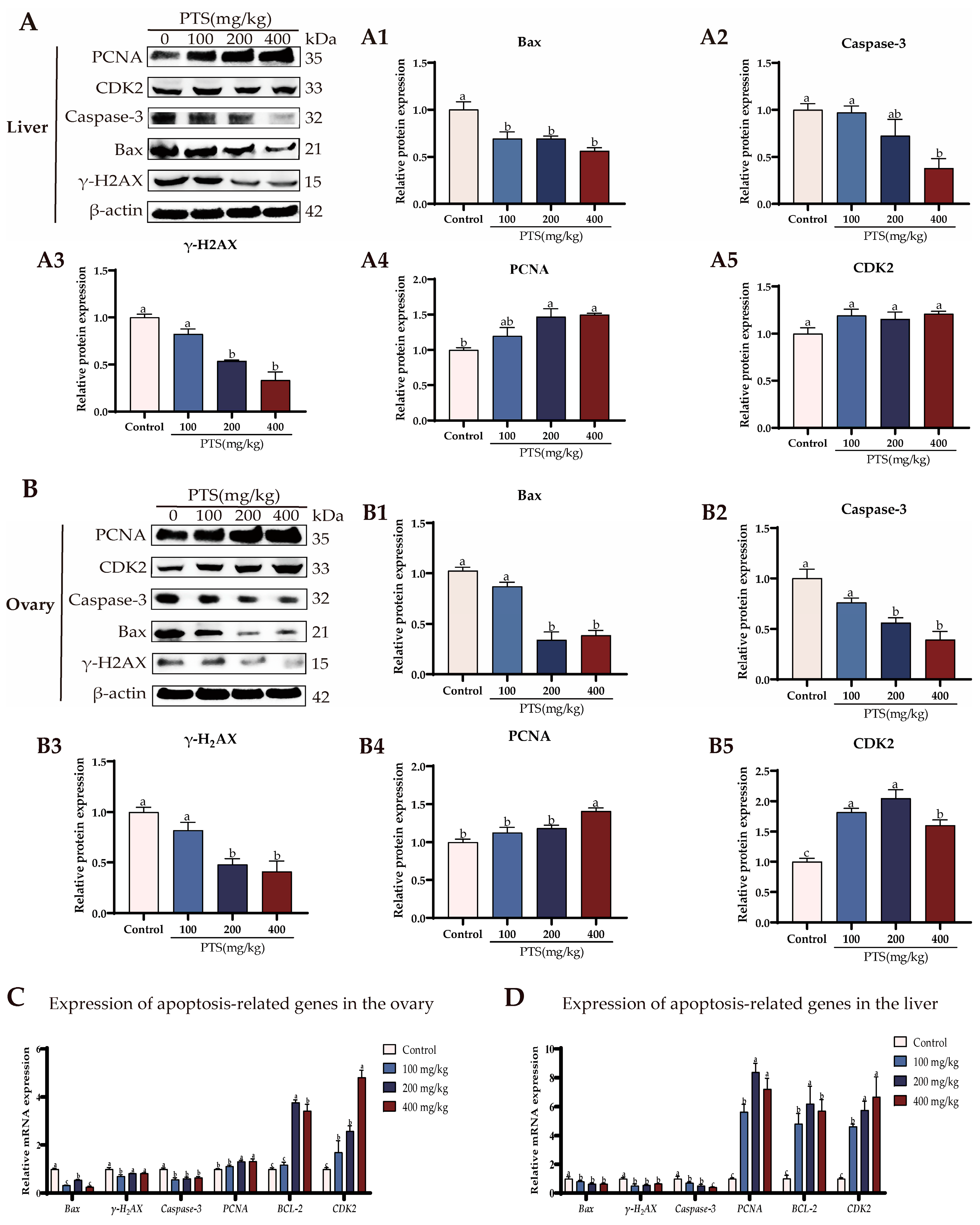
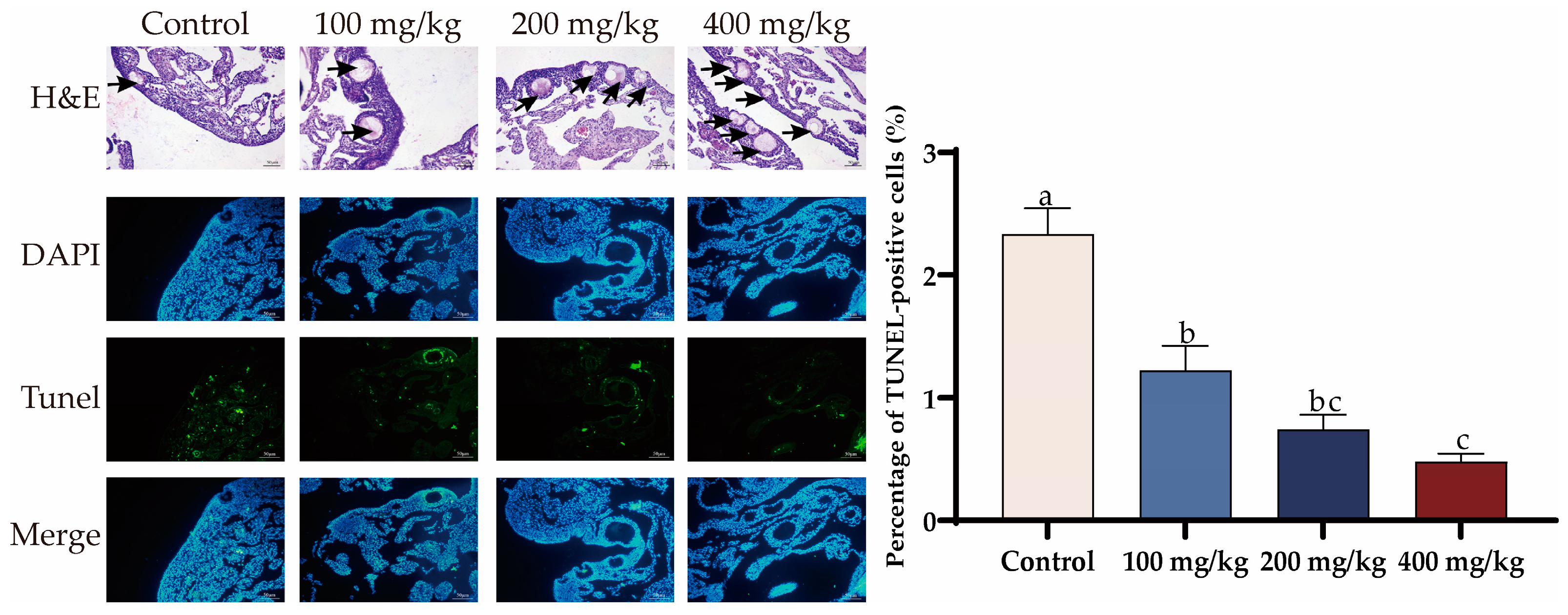
3.3. PTS Supplementation Demonstrates Anti-Apoptotic Effects on Ovary and Liver In Vivo
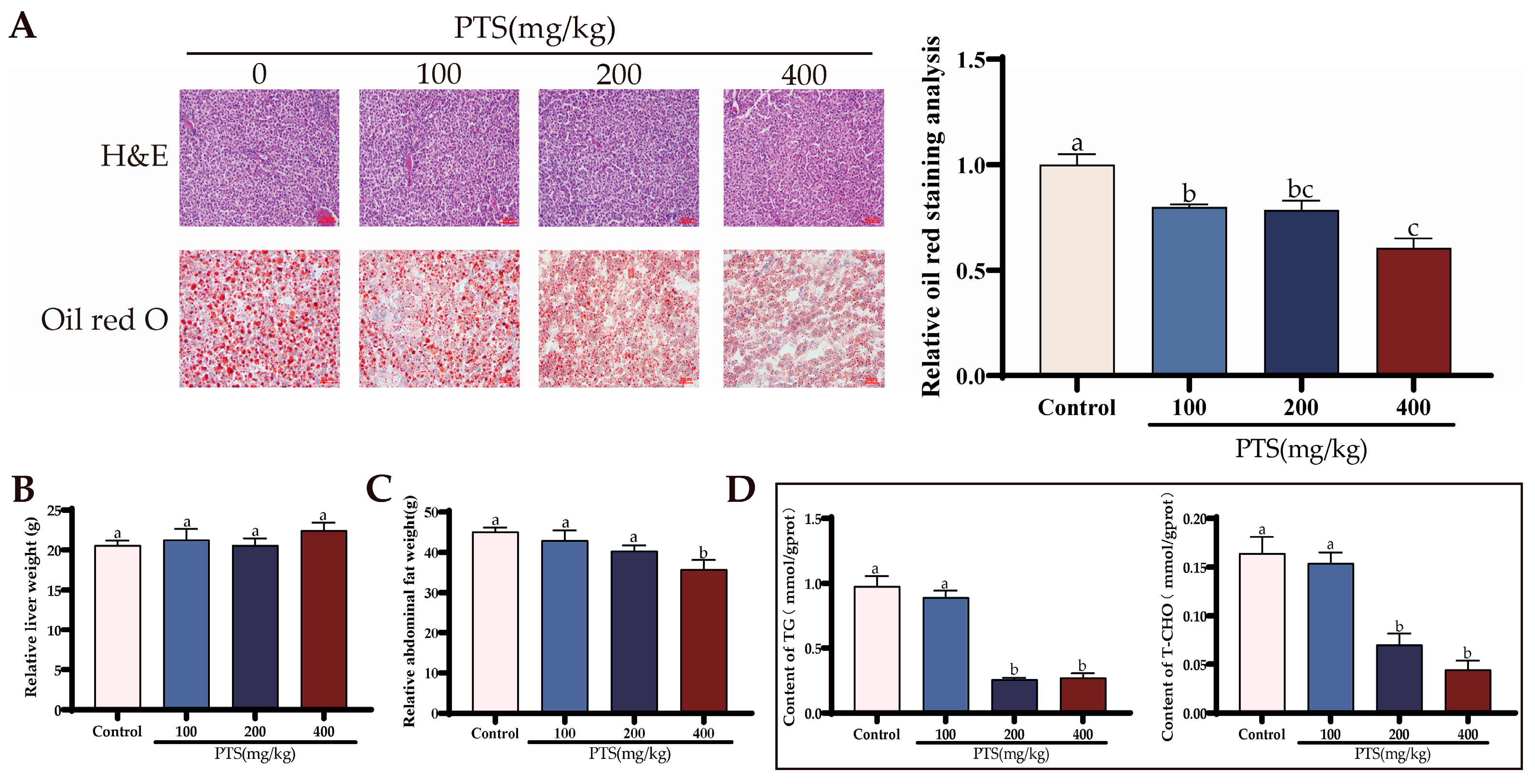
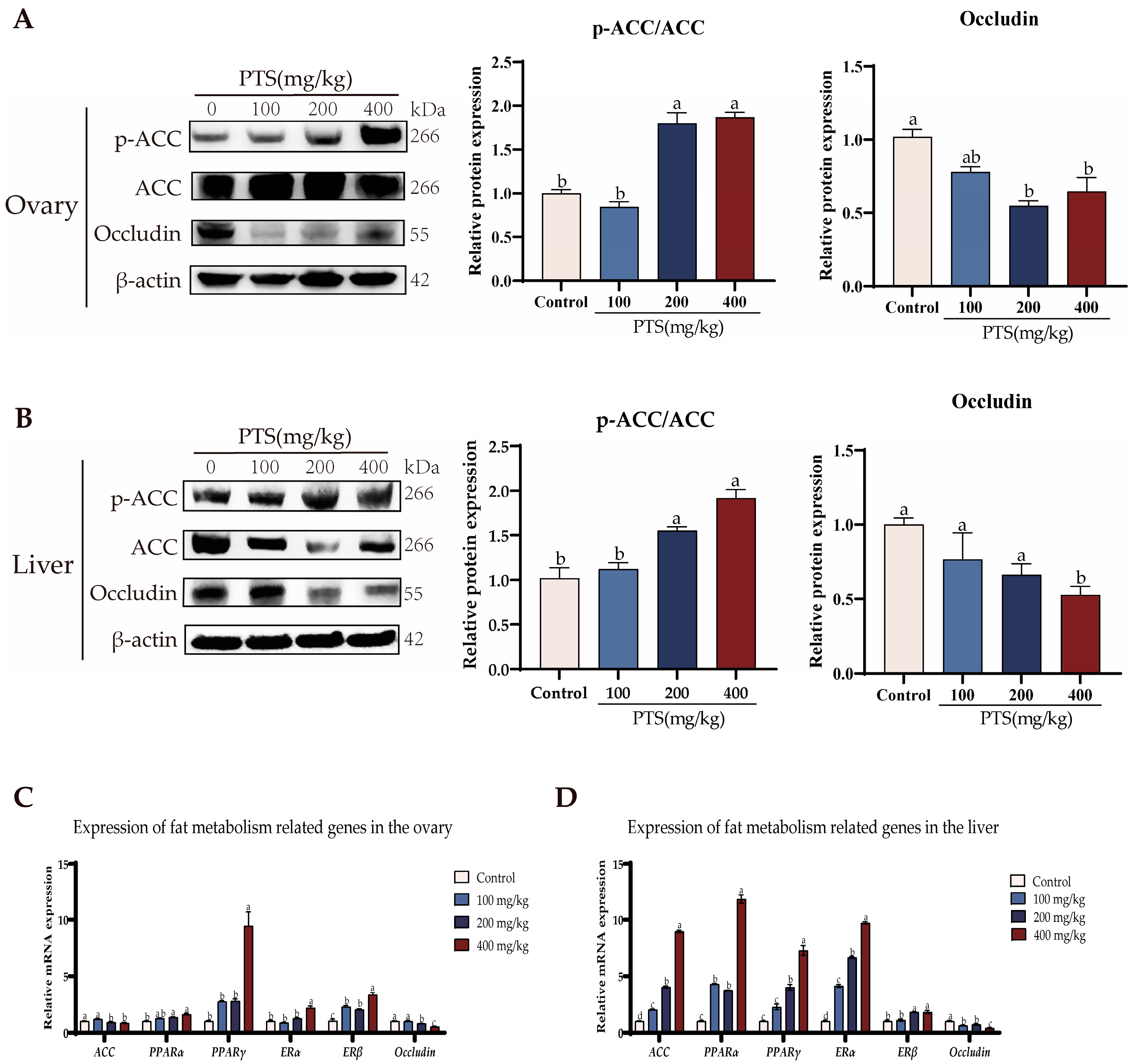
3.4. PTS Supplementation Demonstrates Fat Metabolism Effects on Ovary and Liver In Vivo
3.5. PTS Exhibits Anti-Apoptotic and Anti-Oxidative Effects on D-gal-Induced Aged SWFs In Vitro
3.5.1. PTS Exhibits Anti-Apoptotic Effects on D-gal-Induced Aged SWFs In Vitro
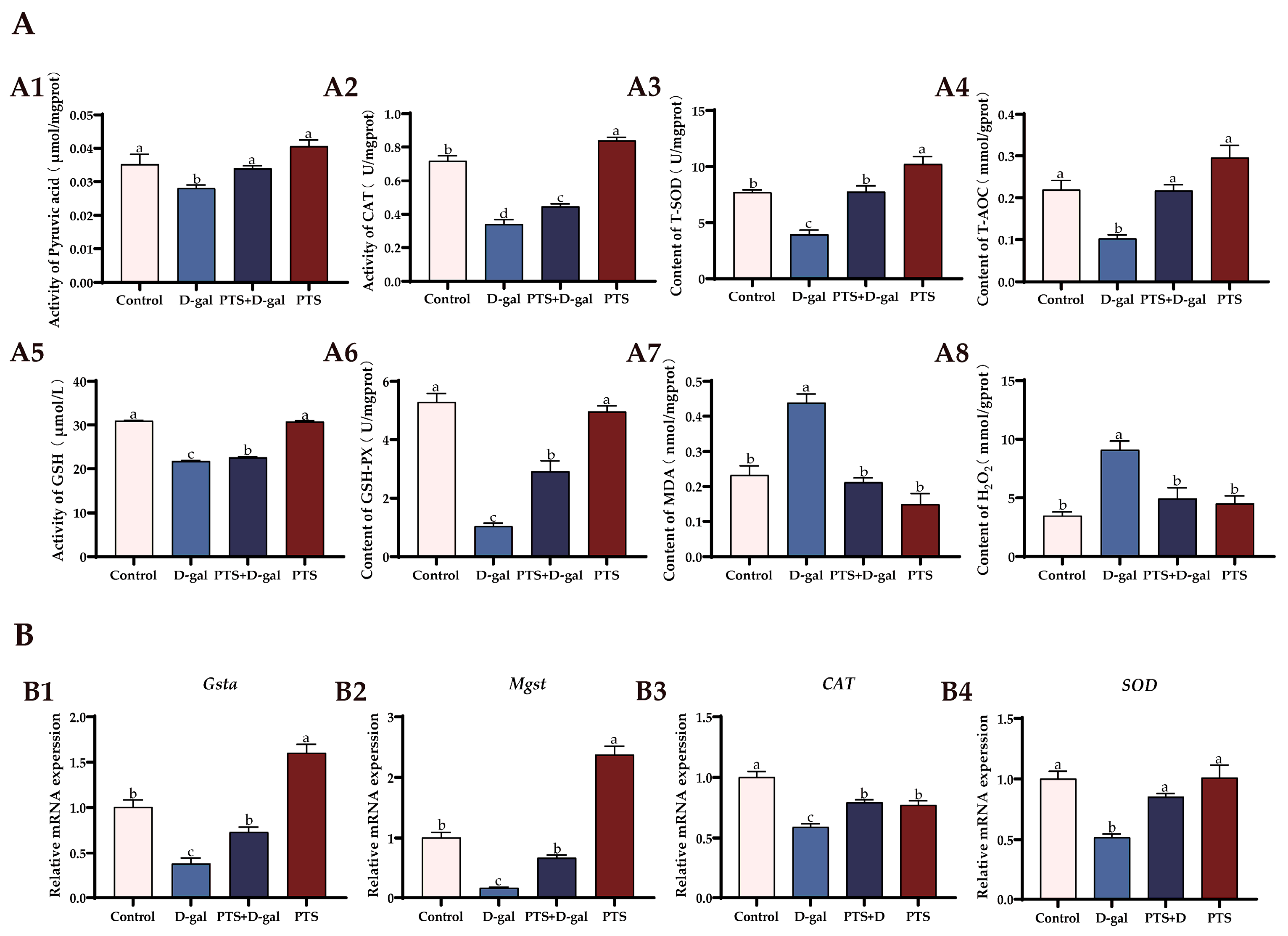
3.5.2. PTS Exhibits Anti-Oxidative Stress Effects on D-gal-Induced Aged SWFs In Vitro
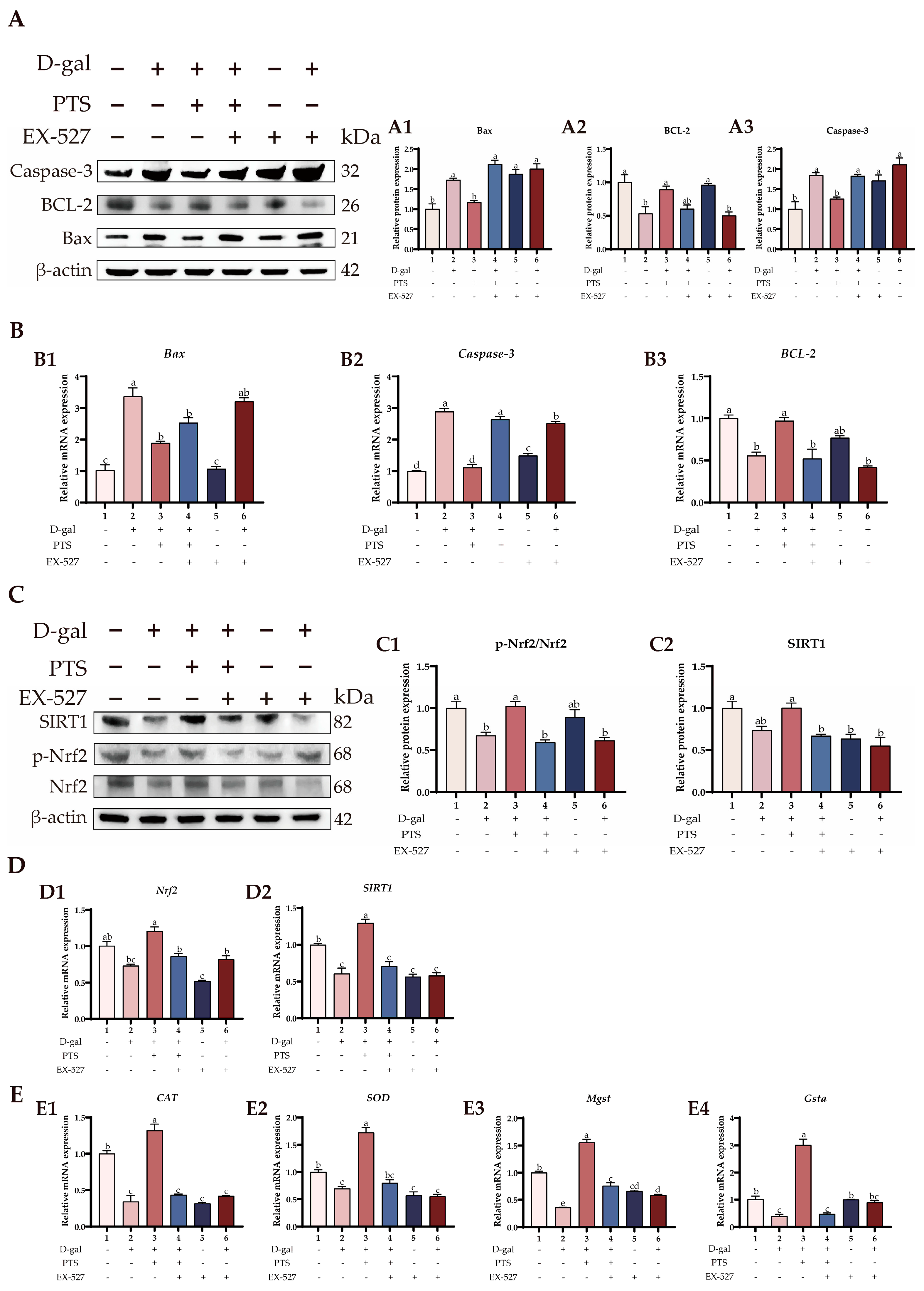
3.6. PTS Protected D-gal-Induced Aged SWFs from Oxidative Stress and Apoptosis via Increased SIRT1/Nrf2 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsueh, A.J.W.; Kawamura, K.; Cheng, Y.; Fauser, B.C.J.M. Intraovarian Control of Early Folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Li, H.; Mu, H.; Zeng, L.; Cai, S.; Su, P.; Li, H.; Zhang, L.; Xiang, W. miR-484 Mediates Oxidative Stress-Induced Ovarian Dysfunction and Promotes Granulosa Cell Apoptosis via SESN2 Downregulation. Redox. Biol. 2023, 62, 102684. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; Le Cessie, S.; Noordam, R.; Van Heemst, D. Ageing, Age-Related Diseases and Oxidative Stress: What to Do Next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ning, C.; Zhang, J.; Wang, Y.; Tang, Q.; Kui, H.; Wang, T.; He, M.; Jin, L.; Li, J.; et al. Dynamic Transcriptome and Chromatin Architecture in Granulosa Cells during Chicken Folliculogenesis. Nat. Commun. 2022, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, H.; Tang, C.; Zhao, Q.; Zhang, J. Dietary Supplementation with Astaxanthin Alleviates Ovarian Aging in Aged Laying Hens by Enhancing Antioxidant Capacity and Increasing Reproductive Hormones. Poult. Sci. 2023, 102, 102258. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.B.; Perry, M.M.; Waddington, D.; Hardie, M.A. Role of Atresia in Establishing the Follicular Hierarchy in the Ovary of the Domestic Hen (Gallus Domesticus). J. Reprod. Fertil. 1983, 69, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hou, Y.; Li, H.; Wu, H.; Hu, J.; Lu, Y.; Liu, X. Endoplasmic Reticulum Stress Is Involved in Small White Follicular Atresia in Chicken Ovaries. Theriogenology 2022, 184, 140–152. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Gong, H.; Celi, P.; Zhuo, Y.; Ding, X.; Bai, S.; Zeng, Q.; Yin, H.; Xu, S.; et al. The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxidants 2021, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, X.; Zhang, S.; Guo, C.; Li, J.; Mi, Y.; Zhang, C. Lycopene Ameliorates Oxidative Stress in the Aging Chicken Ovary via Activation of Nrf2/HO-1 Pathway. Aging 2018, 10, 2016–2036. [Google Scholar] [CrossRef]
- Kakimoto, P.A.; Kowaltowski, A.J. Effects of High Fat Diets on Rodent Liver Bioenergetics and Oxidative Imbalance. Redox. Biol. 2016, 8, 216–225. [Google Scholar] [CrossRef]
- Van Den Beld, A.W.; Kaufman, J.-M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; Van Der Lely, A.J. The Physiology of Endocrine Systems with Ageing. Lancet. Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Carbone, M.C.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-Dependent Changes in the Expression of Superoxide Dismutases and Catalase Are Associated with Ultrastructural Modifications in Human Granulosa Cells. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fang, L.; Lu, Z.; Xiong, J.; Wu, M.; Shi, L.; Luo, A.; Wang, S. Are Sirtuins Markers of Ovarian Aging? Gene 2016, 575, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in Gamete Biology and Reproductive Physiology: Emerging Roles and Therapeutic Potential in Female and Male Infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Huang, Y.; Wu, M.; Li, Y.; Li, Y.; Zhu, X.; Wu, M. Role of the Nrf2 Signaling Pathway in Ovarian Aging: Potential Mechanism and Protective Strategies. Int. J. Mol. Sci. 2023, 24, 13327. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Khan, H.; Majumdar, A. Crosstalk between Sirtuins and Nrf2: SIRT1 Activators as Emerging Treatment for Diabetic Neuropathy. Metab. Brain Dis. 2022, 37, 2181–2195. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef]
- Ding, X.; Cai, C.; Jia, R.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; Zhang, K.; Wang, J. Dietary Resveratrol Improved Production Performance, Egg Quality, and Intestinal Health of Laying Hens under Oxidative Stress. Poult. Sci. 2022, 101, 101886. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, A.; Wu, Y.; Mi, Y.; Zhang, C. Protective Effect of Grape Seed Proanthocyanidins on Oxidative Damage of Chicken Follicular Granulosa Cells by Inhibiting FoxO1-Mediated Autophagy. Front. Cell Dev. Biol. 2022, 10, 762228. [Google Scholar] [CrossRef]
- Beghelli, D.; Zallocco, L.; Barbalace, M.C.; Paglia, S.; Strocchi, S.; Cirilli, I.; Marzano, V.; Putignani, L.; Lupidi, G.; Hrelia, S.; et al. Pterostilbene Promotes Mean Lifespan in Both Male and Female Drosophila Melanogaster Modulating Different Proteins in the Two Sexes. Oxid. Med. Cell. Longev. 2022, 2022, 1744408. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.D.; Ghaskadbi, S.S. Protective Effect of Pterostilbene against Free Radical Mediated Oxidative Damage. BMC Complement. Altern. Med. 2013, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Lin, C. Effect of Resveratrol and Pterostilbene on Aging and Longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zou, T.; Yang, X.; Zhang, Z.; Cao, P.; Han, J.; Duan, Y.; Ruan, B.-F.; Li, Q.-S. Discovery of Novel Pterostilbene Derivatives That Might Treat Sepsis by Attenuating Oxidative Stress and Inflammation through Modulation of MAPKs/NF-κB Signaling Pathways. Antioxidants 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, Oral Bioavailability, and Metabolic Profile of Resveratrol and Its Dimethylether Analog, Pterostilbene, in Rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and Its Derivative Pterostilbene Attenuate Oxidative Stress-Induced Intestinal Injury by Improving Mitochondrial Redox Homeostasis and Function via SIRT1 Signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Q.L.; Li, Z.H.; Ji, R.; Wang, J.Y.; Cao, M.L.; Mu, X.F.; Zhang, Y.; Guo, D.Y.; Yang, J. Pterostilbene Ameliorates Oxidative Damage and Ferroptosis in Human Ovarian Granulosa Cells by Regulating the Nrf2/HO-1 Pathway. Arch. Biochem. Biophys. 2023, 738, 109561. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yan, Z.; Ma, W.; Qian, Y.; Zou, X.; Cui, Y.; Liu, J.; Meng, Y. Peroxiredoxin 4 Protects against Ovarian Ageing by Ameliorating D-Galactose-Induced Oxidative Damage in Mice. Cell Death Dis. 2020, 11, 1053. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, Z.; Liang, W.; Yang, Z.; Du, Z.; Gong, S. D-Galactose-Induced Accelerated Aging Model on Auditory Cortical Neurons by Regulating Oxidative Stress and Apoptosis in Vitro. J. Nutr. Health Aging 2022, 26, 13–22. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, J.; Zhou, S.; Mi, Y.; Tan, X.; Zhang, C. Enhancing Effect of FSH on Follicular Development through Yolk Formation and Deposition in the Low-Yield Laying Chickens. Theriogenology 2020, 157, 418–430. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Lin, X.; Zeng, W.; Mi, Y.; Zhang, C. Effect of Dietary N-Carbamylglutamate on Development of Ovarian Follicles via Enhanced Angiogenesis in the Chicken. Poult. Sci. 2020, 99, 578–589. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Chen, Y.; Li, Y.; Jia, P.; Ji, S.; Zhou, Y.; Wang, T. Dietary Pterostilbene Supplementation Attenuates Intestinal Damage and Immunological Stress of Broiler Chickens Challenged with Lipopolysaccharide. J. Anim. Sci. 2020, 98, skz373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Li, Y.; Wang, T. Pterostilbene Confers Protection against Diquat-Induced Intestinal Damage with Potential Regulation of Redox Status and Ferroptosis in Broiler Chickens. Oxid. Med. Cell. Longev. 2023, 2023, 8258354. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, X.; Ma, Y.; Mi, Y.; Zeng, W.; Li, J.; Zhang, C. Coherent Apoptotic and Autophagic Activities Involved in Regression of Chicken Postovulatory Follicles. Aging 2018, 10, 819–832. [Google Scholar] [CrossRef]
- Van Der Pol, A.; Van Gilst, W.H.; Voors, A.A.; Van Der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Luo, H.; Chiang, H.-H.; Louw, M.; Susanto, A.; Chen, D. Nutrient Sensing and the Oxidative Stress Response. Trends Endocrinol. Metab. 2017, 28, 449–460. [Google Scholar] [CrossRef]
- Yang, B.; Huang, S.; Zhao, G.; Ma, Q. Dietary Supplementation of Porcine Bile Acids Improves Laying Performance, Serum Lipid Metabolism and Cecal Microbiota in Late-Phase Laying Hens. Anim. Nutr. 2022, 11, 283–292. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful Aging Mediated by Inhibition of Oxidative Stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Amicarelli, F.; Carbone, M.C.; Monteleone, P.; Caserta, D.; Marci, R.; Artini, P.G.; Piomboni, P.; Focarelli, R. Cellular and Molecular Aspects of Ovarian Follicle Ageing. Hum. Reprod. Update 2008, 14, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Luderer, U. Oxidative Damage Increases and Antioxidant Gene Expression Decreases with Aging in the Mouse Ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Li, J.; Yu, Y.; Zhang, W.; Song, M.; Liu, Z.; Min, Z.; Hu, H.; Jing, Y.; et al. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell 2020, 180, 585–600.e19. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Sun, L.; Zhang, Z.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Zhang, M. Alpha-Lipoic Acid Supplementation Reverses the Declining Quality of Oocytes Exposed to Cyclophosphamide. Food Chem. Toxicol. 2023, 181, 114090. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Milton-Laskíbar, I.; Aguirre, L.; Fernández-Quintela, A.; Xiao, J.; Portillo, M.P. Effects of Pterostilbene on Diabetes, Liver Steatosis and Serum Lipids. Curr. Med. Chem. 2020, 28, 238–252. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Tu, F.; Cao, J.; Hou, X.; Chen, Y.; Yan, J. Effects of Resveratrol and Its Derivative Pterostilbene on Hepatic Injury and Immunological Stress of Weaned Piglets Challenged with Lipopolysaccharide. J. Anim. Sci. 2022, 100, skac339. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Aguirre, L.; Crujeiras, A.B.; Irles, E.; Segues, N.; Bujanda, L.; Portillo, M.P. Comparative Effects of Pterostilbene and Its Parent Compound Resveratrol on Oxidative Stress and Inflammation in Steatohepatitis Induced by High-Fat High-Fructose Feeding. Antioxidants 2020, 9, 1042. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, X.; Chai, X.; Zhou, X.; Wang, Y.; Liu, M.; Feng, X. Pterostilbene on the Immune Response, Antioxidant Function, and Jejunal Structure of Broilers. Animals 2024, 14, 1851. [Google Scholar] [CrossRef]
- Rudin, C.M.; Thompson, C.B. APOPTOSIS AND DISEASE: Regulation and Clinical Relevance of Programmed Cell Death. Annu. Rev. Med. 1997, 48, 267–281. [Google Scholar] [CrossRef]
- Dong, J.; Guo, C.; Yang, Z.; Wu, Y.; Zhang, C. Follicle-Stimulating Hormone Alleviates Ovarian Aging by Modulating Mitophagy- and Glycophagy-Based Energy Metabolism in Hens. Cells 2022, 11, 3270. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures, and Advances in Therapy. Sig. Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Li, J.; Shang, L.; Zhou, F.; Wang, S.; Liu, N.; Zhou, M.; Lin, Q.; Zhang, M.; Cai, Y.; Chen, G.; et al. Herba Patriniae and Its Component Isovitexin Show Anti-Colorectal Cancer Effects by Inducing Apoptosis and Cell-Cycle Arrest via P53 Activation. Biomed. Pharmacother. 2023, 168, 115690. [Google Scholar] [CrossRef]
- Stringer, J.M.; Alesi, L.R.; Winship, A.L.; Hutt, K.J. Beyond Apoptosis: Evidence of Other Regulated Cell Death Pathways in the Ovary throughout Development and Life. Hum. Reprod. Update 2023, 29, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, T.; Czabotar, P.E. BAX, BAK, and BOK: A Coming of Age for the BCL-2 Family Effector Proteins. Cold Spring Harb. Perspect. Biol. 2020, 12, a036319. [Google Scholar] [CrossRef] [PubMed]
- Unnisa, A.; Greig, N.H.; Kamal, M.A. Inhibition of Caspase 3 and Caspase 9 Mediated Apoptosis: A MultimodalTherapeutic Target in Traumatic Brain Injury. Curr. Neuropharmacol. 2023, 21, 1001–1012. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Bai, J.; Wang, X.; Yuan, Q.; Mi, Y.; Zhang, C. Bioactive Lignan Honokiol Alleviates Ovarian Oxidative Stress in Aging Laying Chickens by Regulating SIRT3/AMPK Pathway. Antioxidants 2024, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gong, Y.; Sun, H.; Yang, C.; Tang, Y.; Yin, L.; Zhang, D.; Wang, Y.; Yu, C.; Liu, Y. Lipid Deposition and Progesterone Synthesis Are Increased by miR-181b-5p through RAP1B/ERK1/2 Pathway in Chicken Granulosa Cells. J. Agric. Food Chem. 2023, 71, 12910–12924. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, D.; Pogrmic-Majkic, K.; Fa, S.; Stanic, B.; Jasnic, J.; Andric, N. Bisphenol A Decreases Progesterone Synthesis by Disrupting Cholesterol Homeostasis in Rat Granulosa Cells. Mol. Cell. Endocrinol. 2018, 461, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, A.; Jung, D.; Kim, J.-H.; Lee, H.; Jung, M. Gomisin N Alleviates Ethanol-Induced Liver Injury through Ameliorating Lipid Metabolism and Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2601. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, Y.; Zhou, S.; Bao, T.; Mi, Y.; Zeng, W.; Li, J.; Zhang, C. Metformin Prevents Follicular Atresia in Aging Laying Chickens through Activation of PI3K/AKT and Calcium Signaling Pathways. Oxid. Med. Cell. Longev. 2020, 2020, 3648040. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.S.V.; Ghoshal, S.; DePeralta, D.K.; Moaven, O.; Wei, L.; Masia, R.; Erstad, D.J.; Fujiwara, N.; Leong, V.; Houde, V.P.; et al. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metab. 2019, 29, 174–182.e5. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Hou, Y.; Huang, L.; Hu, J.; Lu, Y.; Liu, X. Differences in Egg Yolk Precursor Formation of Guangxi Ma Chickens with Dissimilar Laying Rate at the Same or Various Ages. Theriogenology 2022, 184, 13–25. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; Li, H.; Hou, Y.; Hu, J.; Huang, L.; Lu, Y.; Liu, X. Hepatic Glycerolipid Metabolism Is Critical to the Egg Laying Rate of Guangxi Ma Chickens. Gene 2022, 830, 146500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xu, S.; Zhang, J.; Xiang, S.; Hu, Y. Rosmarinic Acid Mitigates Intestinal Inflammation and Oxidative Stress in Bullfrogs (Lithobates Catesbeiana) Fed High Soybean Meal Diets. Fish Shellfish Immunol. 2024, 150, 109655. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Yao, J.; Zhou, S.; Ma, Y.; Dong, J.; Zhang, C.; Mi, Y. Naringin Prevents Follicular Atresia by Inhibiting Oxidative Stress in the Aging Chicken. Poult. Sci. 2022, 101, 101891. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, X.; Chen, H.; Wang, W.; Gu, C.; Li, B. Decrease in Ovarian Reserve through the Inhibition of SIRT1-Mediated Oxidative Phosphorylation. Aging 2022, 14, 2335–2347. [Google Scholar] [CrossRef]
- Lee, J.-J.; Ng, S.-C.; Hsu, J.-Y.; Liu, H.; Chen, C.-J.; Huang, C.-Y.; Kuo, W.-W. Galangin Reverses H2O2-Induced Dermal Fibroblast Senescence via SIRT1-PGC-1α/Nrf2 Signaling. Int. J. Mol. Sci. 2022, 23, 1387. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef]


| Genes | Accession No. | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| CDK-2 | NM_001199857.1 | F: TCCGTATCTTCCGCACGTTG R: GCTTGTTGGGATCGTAGTGC | 183 |
| PCNA | NM_204170.2 | F: GGGCGTCAACCTAAACAGCA R: AGCCAACGTATCCGCATTGT | 97 |
| Caspase-3 | NM_204725.1 | F: CAGCTGAAGGCTCCTGGTTT R: GCCACTCTGCGATTTACACG | 98 |
| BCL-2 | NM_205339.2 | F: ATCGTCGCCTTCTTCGAGTT R: ATCCCATCCTCCGTTGTCCT | 150 |
| Bax | XM_015290060.2 | F: GGATGACAGGAAAGTACGGCA R: TCACCAGGAAGACAGCGTAT | 173 |
| γ-H2AX | BM488821.1 | F: AACAAGAAGACGCGCATCAT R: GTAGCACGGCCTGAATGTT | 143 |
| Mgst | NM_001135550.1 | F: GGCATTTGCCAACCCAGAAG R: CAAGGTCATTCAGGTGGCCT | 116 |
| Gsta | NM_204818.2 | F: GCAGAGCCATCCTCAGCTAC R: CCTTTGCCTCAGGTGGAGAG | 150 |
| Cat | NM_001031215.2 | F: TCAGGAGATGTGCAGCGTTT R: TCTTACACAGCCTTTGGCGT | 109 |
| SOD | NM_205064.1 | F: GGCAATGTGACTGCAAAGGG R: CCCCTCTACCCAGGTCATCA | 133 |
| Nrf2 | NM_001030756.1 | F: CTGCTAGTGGATGGCGAGAC R: CTCCGAGTTCTCCCCGAAAG | 132 |
| SIRT1 | NM_001004767.2 | F: CCCCGCAGCCCGATAAC R: ATACGTGGTCTTGGGGTCCA | 127 |
| ERα | NM_205183.2 | F: CAGGCCTGCCGACTAAGAAA R: TTCATCATTTCGCCGCCTCT | 86 |
| ERβ | NM_204794.2 | F: ACCCCATTCAGTGTCAATCAGG R: AGCCAATACCAGCAGGTGAG | 163 |
| ACC | NM_205505.1 | F: GTCTGCTCAACTGCGACCG R: CAAAGCGACTTCCTCTGGTCAGT | 102 |
| PPARα | NM_001001464.1 | F: TAGTAAGCTCTCAGAAACTTT R: GAAACAGAAGCCGCTTTCCA | 157 |
| PPARγ | NM_001001460.1 | F: GCTGTGAAGTTCAACGCACT R: CCTGGGCGATCTCCACTTAG | 87 |
| Occludin | NM_205128.1 | F: CTCTGGGAAGGGCTGAGGT R: GCCTTCCCAAAAAGCCCTGA | 170 |
| β-actin | NM_205518 | F: ACACCCACACCCCTGTGATGAA R: TGCTGCTGACACCTTCACCATT | 136 |
| Item | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | p-Value |
|---|---|---|---|---|---|
| Egg weight (g) | 59.30 ± 0.493 | 61.38 ± 2.311 | 58.98 ± 1.984 | 59.85 ± 1.154 | 0.742 |
| Shell strength (ksf) | 3.75 ± 0.169 | 3.50 ± 0.166 | 4.09 ± 0.303 | 3.54 ± 0.160 | 0.204 |
| Haugh Unit | 76.77 ± 6.250 b | 83.15 ± 4.204 ab | 92.72 ± 1.882 ab | 93.82 ± 2.814 a | 0.024 |
| Shell thickness (mm) | 0.37 ± 0.011 | 0.37 ± 0.008 | 0.37 ± 0.009 | 0.36 ± 0.011 | 0.561 |
| Egg yolk index | 5.97 ± 0.170 b | 6.58 ± 0.128 a | 6.62 ± 0.120 a | 6.58 ± 0.178 a | 0.017 |
| Item | Control | 100 mg/kg | 200 mg/kg | 400 mg/kg | p-Value |
|---|---|---|---|---|---|
| ALT | 10.60 ± 1.430 | 10.89 ± 1.376 | 10.11 ± 0.695 | 7.87 ± 0.476 | 0.213 |
| AST | 269.50 ± 3.863 a | 266.00 ± 5.092 a | 259.70 ± 3.058 a | 237.20 ± 6.501 b | 0.0005 |
| GLU | 12.05 ± 0.090 | 12.26 ± 0.170 | 12.26 ± 0.169 | 11.91 ± 0.353 | 0.618 |
| HDL | 0.77 ± 0.147 | 0.71 ± 0.078 | 0.66 ± 0.053 | 0.52 ± 0.033 | 0.252 |
| LDL | 2.14 ± 0.237 a | 1.76 ± 0.212 ab | 1.63 ± 0.190 ab | 1.29 ± 0.079 b | 0.032 |
| TC | 10.08 ± 0.126 a | 5.37 ± 0.631 b | 5.33 ± 0.652 b | 3.84 ± 0.170 b | <0.0001 |
| TG | 24.06 ± 0.607 a | 22.50 ± 0.179 b | 22.53 ± 0.304 b | 19.10 ± 0.327 c | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yuan, Q.; Xiao, Y.; Cai, X.; Yang, Z.; Zeng, W.; Mi, Y.; Zhang, C. Pterostilbene, a Resveratrol Derivative, Improves Ovary Function by Upregulating Antioxidant Defenses in the Aging Chickens via Increased SIRT1/Nrf2 Expression. Antioxidants 2024, 13, 935. https://doi.org/10.3390/antiox13080935
Wang X, Yuan Q, Xiao Y, Cai X, Yang Z, Zeng W, Mi Y, Zhang C. Pterostilbene, a Resveratrol Derivative, Improves Ovary Function by Upregulating Antioxidant Defenses in the Aging Chickens via Increased SIRT1/Nrf2 Expression. Antioxidants. 2024; 13(8):935. https://doi.org/10.3390/antiox13080935
Chicago/Turabian StyleWang, Xinyu, Qiongyu Yuan, Yingyu Xiao, Xiangyu Cai, Zhaoyu Yang, Weidong Zeng, Yuling Mi, and Caiqiao Zhang. 2024. "Pterostilbene, a Resveratrol Derivative, Improves Ovary Function by Upregulating Antioxidant Defenses in the Aging Chickens via Increased SIRT1/Nrf2 Expression" Antioxidants 13, no. 8: 935. https://doi.org/10.3390/antiox13080935







