Exploring the Transformative Potential of Functionalized Mesoporous Silica in Enhancing Antioxidant Activity: A Comprehensive Review
Abstract
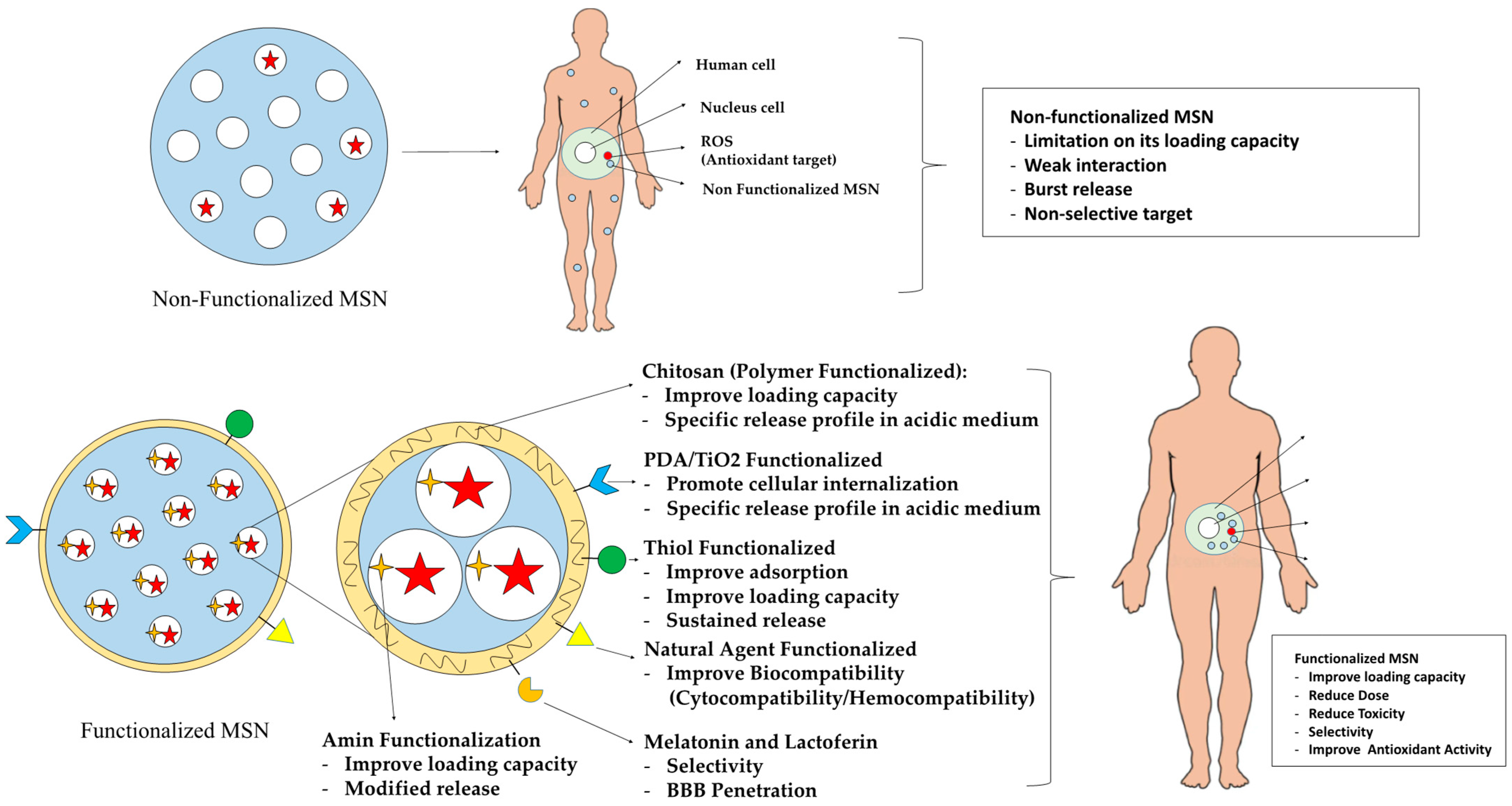
:1. Introduction
2. Methodology
3. Functionalized Mesoporous Silica: An Overview
3.1. Definition and Characteristics of Mesoporous Silica
3.2. Functionalized Mesoporous Silica Nanoparticles
3.3. Techniques for Functionalization
3.3.1. Co-Condensation
3.3.2. Post-Synthesis Grafting
3.3.3. Click Chemistry
3.3.4. Electrostatic Assembly
3.4. Brief Review of Previous Studies Involving Mesoporous Silica in Pharmaceutical Applications
3.4.1. Drug Delivery
3.4.2. Bioimaging
3.4.3. Biosensing
4. Antioxidant Activity of Active Pharmaceutical Ingredients (API): Fundamental Concepts
4.1. Importance of Antioxidants in Pharmaceuticals
- AH: Antioxidant species;
- R*: Free radical species;
- A*: Signifies the radical form of the antioxidant subsequent to electron donation
- RH: Stable product subsequent to antioxidant-mediated free radical neutralization.
4.2. Types of Antioxidants Used in Pharmaceuticals
4.3. Physicochemical Properties
4.4. Optimizing Antioxidant Activity of APIs
4.5. Antioxidant Effectiveness IC50 Values
4.6. Current Challenges and Strategies to Maximize Antioxidant Activity of APIs
4.7. Strategies to Overcome Challenges
5. Case Studies and Experimental Findings
5.1. Presentation of Key Findings Regarding the Impact of Functionalized Mesoporous Silica on API Antioxidant Activity
5.1.1. Significantly Improve Loading Capacity
5.1.2. Provide Controlled Release Behavior
5.1.3. Provide Targeted Drug Delivery System
5.1.4. Improve Biocompatibility and Safety Profile
5.1.5. Improve Penetration
6. Author Perspectives
7. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J.; et al. Effects of Different Extraction Methods on Contents, Profiles, and Antioxidant Abilities of Free and Bound Phenolics of Sargassum Polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef]
- Budiman, A.; Rusdin, A.; Aulifa, D.L. Current Techniques of Water Solubility Improvement for Antioxidant Compounds and Their Correlation with Its Activity: Molecular Pharmaceutics. Antioxidants 2023, 12, 378. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Y.P.; Liu, T.P.; Chien, F.C.; Chou, C.M.; Chen, C.T.; Mou, C.Y. Approach to Deliver Two Antioxidant Enzymes with Mesoporous Silica Nanoparticles into Cells. ACS Appl. Mater. Interfaces 2016, 8, 17944–17954. [Google Scholar] [CrossRef]
- Arriagada, F.; Correa, O.; Günther, G.; Nonell, S.; Mura, F.; Olea-Azar, C.; Morales, J. Morin Flavonoid Adsorbed on Mesoporous Silica, a Novel Antioxidant Nanomaterial. PLoS ONE 2016, 11, e0164507. [Google Scholar] [CrossRef]
- Lau, M.; Giri, K.; Garcia-Bennett, A.E. Antioxidant Properties of Probucol Released from Mesoporous Silica. Eur. J. Pharm. Sci. 2019, 138, 105038. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, H.; Liu, C.G.; Kanubaddi, K.R.; Lee, C.H.; Wang, S.B.; Cui, W.; Santos, H.A.; Lin, K.L.; Chen, A.Z. Metal Species–Encapsulated Mesoporous Silica Nanoparticles: Current Advancements and Latest Breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- De Juan, F.; Ruiz-Hitzky, E. Selective Functionalization of Mesoporous Silica. Adv. Mater. 2000, 12, 430–432. [Google Scholar] [CrossRef]
- Brühwiler, D. Postsynthetic Functionalization of Mesoporous Silica. Nanoscale 2010, 2, 887–892. [Google Scholar] [CrossRef]
- Budiman, A.; Aulifa, D.L. A Comparative Study of the Pharmaceutical Properties between Amorphous Drugs Loaded-Mesoporous Silica and Pure Amorphous Drugs Prepared by Solvent Evaporation. Pharmaceuticals 2022, 15, 730. [Google Scholar] [CrossRef]
- Meka, A.K.; Gopalakrishna, A.; Iriarte-Mesa, C.; Rewatkar, P.; Qu, Z.; Wu, X.; Cao, Y.; Prasadam, I.; Janjua, T.I.; Kleitz, F.; et al. Influence of Pore Size and Surface Functionalization of Mesoporous Silica Nanoparticles on the Solubility and Antioxidant Activity of Confined Coenzyme Q10. Mol. Pharm. 2023, 20, 2966–2977. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.; Aulifa, D.L. Encapsulation of Drug into Mesoporous Silica by Solvent Evaporation: A Comparative Study of Drug Characterization in Mesoporous Silica with Various Molecular Weights. Heliyon 2021, 7, e08627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, M.; Liu, A.; Zhai, G. Advances in Functionalized Mesoporous Silica Nanoparticles for Tumor Targeted Drug Delivery and Theranostics. Curr. Pharm. Des. 2017, 23, 3367–3382. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Luan, Y.; Yang, X.; Jiang, Y.; Zhao, L.; Zong, Y.; Li, Z. Functionalized Mesoporous Silica Particles for Application in Drug Delivery System. Mini Rev. Med. Chem. 2012, 12, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.S.; Nakhjiri, A.T.; Khakzad, M.J.; Rezayat, S.M.; Ebrahimnejad, P.; Heydarinasab, A.; Akbarzadeh, A.; Marjani, A. Mesoporous Silica Nanoparticles as a Versatile Nanocarrier for Cancer Treatment: A Review. J. Mol. Liq. 2021, 328, 115417. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous Silica Nanoparticles: Synthesis, Classification, Drug Loading, Pharmacokinetics, Biocompatibility, and Application in Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.; Aulifa, D.L. Characterization of Drugs with Good Glass Formers in Loaded-Mesoporous Silica and Its Theoretical Value Relevance with Mesopores Surface and Pore-Filling Capacity. Pharmaceuticals 2022, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Balas, F.; Manzano, M.; Vallet-Regí, M. Novel Method to Synthesize Ordered Mesoporous Silica with High Surface Areas. Solid State Sci. 2008, 10, 408–415. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Shi, Y.; Miller, M.L.; Di Pasqua, A.J. Biocompatibility of Mesoporous Silica Nanoparticles? Comments Inorg. Chem. 2016, 36, 2265–2284. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Zhang, H. Synthesis, Functionalization, and Applications of Morphology-Controllable Silica-Based Nanostructures: A Review. Prog. Solid State Chem. 2016, 44, 1–19. [Google Scholar] [CrossRef]
- Bao, W.; Ma, H.; Wang, N.; He, Z. PH-Responsive Mesoporous Silica Drug Delivery System for Targeted Cancer Chemotherapy. Mater. Technol. 2021, 36, 308–316. [Google Scholar] [CrossRef]
- Lohiya, G.; Katti, D.S. Carboxylated Chitosan-Mediated Improved Efficacy of Mesoporous Silica Nanoparticle-Based Targeted Drug Delivery System for Breast Cancer Therapy. Carbohydr. Polym. 2022, 277, 118822. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and Functionalization of a Mesoporous Silica Nanoparticle Based on the Sol-Gel Process and Applications in Controlled Release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; De Lange Davies, C.; Sahlgren, C.; et al. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef]
- Cha, B.G.; Kim, J. Functional Mesoporous Silica Nanoparticles for Bio-Imaging Applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1515. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional Mesoporous Silica Nanoparticles for Biomedical Applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent Advances in Functionalization of Mesoporous Silica. J. Nanosci. Nanotechnol. 2005, 5, 347–371. [Google Scholar] [CrossRef]
- Estevão, B.M.; Miletto, I.; Hioka, N.; Marchese, L.; Gianotti, E. Mesoporous Silica Nanoparticles Functionalized with Amino Groups for Biomedical Applications. ChemistryOpen 2021, 10, 1251–1259. [Google Scholar] [CrossRef]
- Fapojuwo, D.P.; Akinnawo, C.A.; Oseghale, C.O.; Meijboom, R. Tailoring the Surface Wettability of Mesoporous Silica for Selective Hydrogenation of Cinnamaldehyde to Hydrocinnamaldehyde in a Pickering Emulsion System. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130231. [Google Scholar] [CrossRef]
- Ribeiro, J.d.O.N.; da Silva, D.G.; Vasconcelos, D.C.L.; Vasconcelos, W.L. Influence of Functionalization Method on Isomorphic Substitution and Formation of Extra-Framework Oxides in Silica for Water Vapor Adsorption. Adsorption 2022, 28, 67–83. [Google Scholar] [CrossRef]
- Firmansyah, A.; Nugrahani, I.; Wirasutisna, K.R.; Ibrahim, S. Formation of Boron—Silica Based Mesoporous and Studies of Its Adsorption Ability for Curcuminoids. Biointerface Res. Appl. Chem. 2020, 10, 4977–4981. [Google Scholar] [CrossRef]
- Shan, B.Q.; Xing, J.L.; Yang, T.Q.; Peng, B.; Hao, P.; Zong, Y.X.; Chen, X.Q.; Xue, Q.S.; Zhang, K.; Wu, P. One-Pot Co-Condensation Strategy for Dendritic Mesoporous Organosilica Nanospheres with Fine Size and Morphology Control. CrystEngComm 2019, 21, 4030–4035. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A.; Nebhani, L.; Hazra, C.K. Sustainable Sulfonic Acid Functionalized Tubular Shape Mesoporous Silica as a Heterogeneous Catalyst for Selective Unsymmetrical Friedel-Crafts Alkylation in One Pot. JACS Au 2023, 3, 3400–3411. [Google Scholar] [CrossRef]
- Lee, Y.; Ochi, M.; Matsuyama, T.; Ida, J. Preparation of Mesoporous Silica with Monomodal and Bimodal Pore Structure Using Co-Condensation Method and Its Application for CO2 Separation. Bull. Mater. Sci. 2022, 45, 187. [Google Scholar] [CrossRef]
- Rahmat, N.; Sadon, N.; Yusof, M.A. Thermogravimetric Analysis (TGA) Profile at Different Calcination Conditions for Synthesis of PTES-SBA-15. Am. J. Appl. Sci. 2017, 14, 938–944. [Google Scholar] [CrossRef]
- Yuan, X.; Sperger, D.; Munson, E.J. Investigating Miscibility and Molecular Mobility of Nifedipine-PVP Amorphous Solid Dispersions Using Solid-State NMR Spectroscopy. Mol. Pharm. 2014, 11, 329–337. [Google Scholar] [CrossRef]
- Von Baeckmann, C.; Guillet-Nicolas, R.; Renfer, D.; Kählig, H.; Kleitz, F. A Toolbox for the Synthesis of Multifunctionalized Mesoporous Silica Nanoparticles for Biomedical Applications. ACS Omega 2018, 3, 17496–17510. [Google Scholar] [CrossRef]
- Kobayashi, T.; Pruski, M. Spatial Distribution of Silica-Bound Catalytic Organic Functional Groups Can Now Be Revealed by Conventional and DNP-Enhanced Solid-State NMR Methods. ACS Catal. 2019, 9, 7238–7249. [Google Scholar] [CrossRef]
- Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics 2022, 10, 87. [Google Scholar] [CrossRef]
- Moitra, N.; Trens, P.; Raehm, L.; Durand, J.O.; Cattoën, X.; Chi Man, M.W. Facile Route to Functionalized Mesoporous Silica Nanoparticles by Click Chemistry. J. Mater. Chem. 2011, 21, 13476–13482. [Google Scholar] [CrossRef]
- Vilà, N.; Walcarius, A. Bis(Terpyridine) Iron(II) Functionalized Vertically-Oriented Nanostructured Silica Films: Toward Electrochromic Materials. Front. Chem. 2020, 8, 830. [Google Scholar] [CrossRef]
- Tan, L.; Deng, F.; Luo, X.; Pan, X.; Zhang, L.; Marina, M.L.; Jiang, Z. Glycosyl Imprinted Mesoporous Microspheres for the Determination of Glycopeptide Antibiotics Using Ultra-High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Chromatogr. A 2021, 1659, 462630. [Google Scholar] [CrossRef]
- Antochshuk, V.; Jaroniec, M. Functionalized Mesoporous Materials Obtained via Interfacial Reactions in Self-Assembled Silica-Surfactant Systems. Chem. Mater. 2000, 12, 2496–2501. [Google Scholar] [CrossRef]
- Yokoi, T.; Tatsumi, T. Synthesis of Mesoporous Silica Materials by Using Anionic Surfactants as Template. J. Japan Pet. Inst. 2007, 50, 299–311. [Google Scholar] [CrossRef]
- Zhang, M.; Zou, Y.; Zhou, X.; Yan, F.; Ding, Z. Vertically-Ordered Mesoporous Silica Films for Electrochemical Detection of Hg(II) Ion in Pharmaceuticals and Soil Samples. Front. Chem. 2022, 10, 952936. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhai, J.; Xiao, Q.; Zhao, S.; Li, C. Polysaccharide/Mesoporous Silica Nanoparticle-Based Drug Delivery Systems: A Review. Int. J. Biol. Macromol. 2021, 193, 457–473. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous Silica Nanoparticles in Target Drug Delivery System: A Review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Isroni, M.; Sagita, F.; Culsum, N.T.U.; Kadja, G.T.M. Molecule Gated Mesoporous Silica for On-Command Drug Delivery: A Review. Results Chem. 2023, 6, 101053. [Google Scholar] [CrossRef]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse Gatekeepers for Mesoporous Silica Nanoparticle Based Drug Delivery Systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Sangvanich, T.; Reda, M.; Gu, S.; Bejan, D.; Yantasee, W. Lyophilization and Stability of Antibody-Conjugated Mesoporous Silica Nanoparticle with Cationic Polymer and PEG for SiRNA Delivery. Int. J. Nanomed. 2018, 13, 4015–4027. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef]
- Sreejith, S.; Ma, X.; Zhao, Y. Graphene Oxide Wrapping on Squaraine-Loaded Mesoporous Silica Nanoparticles for Bioimaging. J. Am. Chem. Soc. 2012, 134, 17346–17349. [Google Scholar] [CrossRef]
- Yuan, D.; Ellis, C.M.; Davis, J.J. Mesoporous Silica Nanoparticles in Bioimaging. Materials 2020, 13, 3795. [Google Scholar] [CrossRef]
- Ezquerro, C.; López, I.P.; Serrano, E.; Alfaro-Arnedo, E.; Lalinde, E.; Larráyoz, I.M.; Pichel, J.G.; García-Martínez, J.; Berenguer, J.R. Highly Emissive Hybrid Mesoporous Organometallo-Silica Nanoparticles for Bioimaging. Mater. Adv. 2022, 3, 3582–3592. [Google Scholar] [CrossRef]
- Xie, M.; Shi, H.; Ma, K.; Shen, H.; Li, B.; Shen, S.; Wang, X.; Jin, Y. Hybrid Nanoparticles for Drug Delivery and Bioimaging: Mesoporous Silica Nanoparticles Functionalized with Carboxyl Groups and a near-Infrared Fluorescent Dye. J. Colloid Interface Sci. 2013, 395, 306–314. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, H. Core-Shell-Type Magnetic Mesoporous Silica Nanocomposites for Bioimaging and Therapeutic Agent Delivery. Adv. Mater. 2015, 27, 576–585. [Google Scholar] [CrossRef]
- Shirani, M.P.; Ensafi, A.A.; Rezaei, B.; Amirghofran, Z. Folic Acid and Carbon Dots-Capped Mesoporous Silica for PH-Responsive Targeted Drug Delivery and Bioimaging. J. Iran. Chem. Soc. 2023, 20, 2257–2268. [Google Scholar] [CrossRef]
- Kumar, H.; Pani, B.; Kumar, J.; Kumar, P. In Vitro and Bioimaging Studies of Mesoporous Silica Nanocomposites Encapsulated Iron-Oxide and Loaded Doxorubicin Drug (DOX/IO@Silica) as Magnetically Guided Drug Delivery System. Curr. Pharm. Biotechnol. 2022, 24, 1297–1306. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Pillai, S.S.; Yukawa, H.; Onoshima, D.; Biju, V.; Baba, Y. Quantum Dot-Peptide Nanoassembly on Mesoporous Silica Nanoparticle for Biosensing. Nano Hybrids Compos. 2018, 19, 55–72. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; Tang, D. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem. 2017, 89, 5152–5160. [Google Scholar] [CrossRef]
- Xing, C.; Deng, J.; Fu, W.; Li, J.; Xu, L.; Sun, R.; Wang, D.; Li, C.; Liang, K.; Gao, M.; et al. Interfacially Super-Assembled Benzimidazole Derivative-Based Mesoporous Silica Nanoprobe for Sensitive Copper (II) Detection and Biosensing in Living Cells. Chem.—A Eur. J. 2022, 28, e202103642. [Google Scholar] [CrossRef]
- Aghayan, M.; Mahmoudi, A.; Sazegar, M.R.; Jahanafarin, A.; Nazari, O.; Hamidi, P.; Poorhasan, Z.; Sadat Shafaei, B. The Development of a Novel Copper-Loaded Mesoporous Silica Nanoparticle as a Peroxidase Mimetic for Colorimetric Biosensing and Its Application in H2O2 and GSH Assay. Anal. Sci. 2023, 39, 1257–1267. [Google Scholar] [CrossRef]
- Kumar, S. The Importance of Antioxidant and Their Role in Pharmaceutical Science—A Review. Asian J. Res. Chem. Pharm. Sci. 2014, 1, 27–44. [Google Scholar]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D.; Peralta, D.R. Role of Microbes as Natural Antioxidants of Pharmaceutical Importance. In The Metabolic Syndrome: Dietary Supplements and Food Ingredients; Keservani, R.K., Ed.; Apple Academic Press: Boca Raton, FL, USA, 2023; pp. 19–46. [Google Scholar]
- Petrucci, R.; Bortolami, M.; Di Matteo, P.; Curulli, A. Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances. Nanomaterials 2022, 12, 959. [Google Scholar] [CrossRef]
- Jernigan, C.M.; Fite, C.H.; Vereecken, L.; Berkelhammer, M.B.; Rollins, A.W.; Rickly, P.S.; Novelli, A.; Taraborrelli, D.; Holmes, C.D.; Bertram, T.H. Efficient Production of Carbonyl Sulfide in the Low-NOx Oxidation of Dimethyl Sulfide. Geophys. Res. Lett. 2022, 49, e2021GL096838. [Google Scholar] [CrossRef]
- Harraz, D.M.; Weng, S.; Surendranath, Y. Electrochemically Quantifying Oxygen Reduction Selectivity in Nonaqueous Electrolytes. ACS Catal. 2023, 13, 1462–1469. [Google Scholar] [CrossRef]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Pacheco-Quito, E.M.; Ruiz-Caro, R.; Veiga, M.D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs 2020, 18, 583. [Google Scholar] [CrossRef]
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of Bioactive Compounds Extracted from Olive Industry Wastes: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef]
- Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.M.F.; Rietjens, I.M.C.M. The Influence of PH on Antioxidant Properties and the Mechanism of Antioxidant Action of Hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Doğan, A.; Özdemir, S.; Yalçin, M.S.; Sari, H.; Nural, Y. Naphthoquinone-Thiazole Hybrids Bearing Adamantane: Synthesis, Antimicrobial, DNA Cleavage, Antioxidant Activity, Acid Dissociation Constant, and Drug-Likeness. J. Res. Pharm. 2021, 25, 292–304. [Google Scholar] [CrossRef]
- Ahmad, M.M. Recent Trends in Chemical Modification and Antioxidant Activities of Plants-Based Polysaccharides: A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Mazzone, G.; Russo, N.; Toscano, M. Antioxidant Properties Comparative Study of Natural Hydroxycinnamic Acids and Structurally Modified Derivatives: Computational Insights. Comput. Theor. Chem. 2016, 1077, 39–47. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects about in Vitro Evaluation of Antioxidant Properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Tiwari, A.K. Imbalance in Antioxidant Defence and Human Diseases: Multiple Approach of Natural Antioxidants Therapy. Curr. Sci. 2001, 81, 1179–1187. [Google Scholar]
- Lihi, N.; Balogh, Z.; Diószegi, R.; Forgács, A.; Moldován, K.; May, N.V.; Herman, P.; Fábián, I.; Kalmár, J. Functionalizing Aerogels with Tetraazamacrocyclic Copper(II) Complexes: Nanoenzymes with Superoxide Dismutase Activity. Appl. Surf. Sci. 2023, 611, 155622. [Google Scholar] [CrossRef]
- Estirado, S.; Díaz-García, D.; Fernández-Delgado, E.; Viñuelas-Zahínos, E.; Gómez-Ruiz, S.; Prashar, S.; Rodríguez, A.B.; Luna-Giles, F.; Pariente, J.A.; Espino, J. Melatonin Derivative-Conjugated Formulations of Pd(II) and Pt(II) Thiazoline Complexes on Mesoporous Silica to Enhance Cytotoxicity and Apoptosis against HeLa Cells. Pharmaceutics 2024, 16, 92. [Google Scholar] [CrossRef]
- Szewczyk, A.; Brzezińska-Rojek, J.; Ośko, J.; Majda, D.; Prokopowicz, M.; Grembecka, M. Antioxidant-Loaded Mesoporous Silica—An Evaluation of the Physicochemical Properties. Antioxidants 2022, 11, 1417. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.-P.; Cui, X.; Fu, Y.; Li, G.L.; Wang, W. Surface-Modified Mesoporous Silica Nanorods for the Highly Aging Resistance Rubber through Controlled Release of Antioxidant. Polym. Adv. Technol. 2021, 32, 3384–3391. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Bajenaru, L.; Berger, D.; Mitran, R.-A.; Deaconu, M.; Lincu, D.; Guzun, A.S.; Matei, C.; Moisescu, M.G.; Negreanu-Pirjol, T. Effect of Nanoconfinement of Polyphenolic Extract from Grape Pomace into Functionalized Mesoporous Silica on Its Biocompatibility and Radical Scavenging Activity. Antioxidants 2020, 9, 696. [Google Scholar] [CrossRef]
- Blázquez-Blázquez, E.; Barranco-García, R.; Díez-Rodríguez, T.M.; Posadas, P.; Pérez, E.; Cerrada, M.L. Improvement of Thermal Protection in Recycled Polyolefins through Hybrid Mesoporous Silica–Antioxidant Particles. Recycling 2024, 9, 3. [Google Scholar] [CrossRef]
- Leccese, S.; Onfroy, T.; Wilson, A.; Kirilovsky, D.; Casale, S.; Guira, S.; Selmane, M.; Jolivalt, C.; Mezzetti, A. Immobilization of Orange Carotenoid Protein on Mesoporous Silica SBA-15 for the Development of Photoactivable Nanodevices. Microporous Mesoporous Mater. 2022, 340, 112007. [Google Scholar] [CrossRef]
- Kumari, R.; Narvi, S.S.; Dutta, P.K. Thiol Modified Chitosan-Silica Nanohybrid for Antibacterial, Antioxidant and Drug Delivery Application. J. Indian Chem. Soc. 2021, 98, 100108. [Google Scholar] [CrossRef]
- Fatemi, M.; Meshkini, A.; Matin, M.M. A Dual Catalytic Functionalized Hollow Mesoporous Silica-Based Nanocarrier Coated with Bacteria-Derived Exopolysaccharides for Targeted Delivery of Irinotecan to Colorectal Cancer Cells. Int. J. Biol. Macromol. 2024, 259, 129179. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wen, S.; Li, Y.; An, J.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Novel Lactoferrin-Functionalized Manganese-Doped Silica Hollow Mesoporous Nanoparticles Loaded with Resveratrol for the Treatment of Ischemic Stroke. Mater. Today Adv. 2022, 15, 100262. [Google Scholar] [CrossRef]
- Xu, J.; You, L.; Zhao, Z. Synthesize of the Chitosan-TPP Coated Betanin-Quaternary Ammonium-Functionalized Mesoporous Silica Nanoparticles and Mechanism for Inhibition of Advanced Glycation End Products Formation. Food Chem. 2023, 407, 135110. [Google Scholar] [CrossRef] [PubMed]
- Purikova, O.; Tkachenko, I.; Šmíd, B.; Veltruská, K.; Dinhová, T.N.; Vorokhta, M.; Kopecký, V.; Hanyková, L.; Ju, X. Free-Blockage Mesoporous Silica Nanoparticles Loaded with Cerium Oxide as ROS-Responsive and ROS-Scavenging Nanomedicine. Adv. Funct. Mater. 2022, 32, 2208316. [Google Scholar] [CrossRef]
- Kumari, R.; Narvi, S.S.; Dutta, P.K. Synthesis of Chitosan Succinate-g-Amine Functionalized Mesoporous Silica: Inorganic-Organic Nanohybrid for Antibacterial Assessment, Antioxidant Activity and PH-Controlled Drug Delivery. Int. J. Biol. Macromol. 2023, 234, 123763. [Google Scholar] [CrossRef] [PubMed]
- Ioniţă, S.; Lincu, D.; Mitran, R.-A.; Ziko, L.; Sedky, N.K.; Deaconu, M.; Brezoiu, A.-M.; Matei, C.; Berger, D. Resveratrol Encapsulation and Release from Pristine and Functionalized Mesoporous Silica Carriers. Pharmaceutics 2022, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Srivastava, R.; Singh, Y. Cationized Silica Ceria Nanocomposites to Target Biofilms in Chronic Wounds. Biomater. Adv. 2022, 138, 212939. [Google Scholar] [CrossRef]
- Sayadi, K.; Rahdar, A.; Hajinezhad, M.R.; Nikazar, S.; Susan, M.A.B.H. Atorvastatin-Loaded SBA-16 Nanostructures: Synthesis, Physical Characterization, and Biochemical Alterations in Hyperlipidemic Rats. J. Mol. Struct. 2020, 1202, 127296. [Google Scholar] [CrossRef]
- Kerry, R.G.; Singh, K.R.; Mahari, S.; Jena, A.B.; Panigrahi, B.; Pradhan, K.C.; Pal, S.; Kisan, B.; Dandapat, J.; Singh, J.; et al. Bioactive Potential of Morin Loaded Mesoporous Silica Nanoparticles: A Nobel and Efficient Antioxidant, Antidiabetic and Biocompatible Abilities in In-Silico, In-Vitro, and In-Vivo Models. OpenNano 2023, 10, 100126. [Google Scholar] [CrossRef]
- Rasool, N.; Negi, D.; Singh, Y. Thiol-Functionalized, Antioxidant, and Osteogenic Mesoporous Silica Nanoparticles for Osteoporosis. ACS Biomater. Sci. Eng. 2023, 9, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Wallace, W.T.; Sambi, J.; Rogers, D.T.; Littleton, J.M.; Rankin, S.E.; Knutson, B.L. Nanoharvesting of Bioactive Materials from Living Plant Cultures Using Engineered Silica Nanoparticles. Mater. Sci. Eng. C 2020, 106, 110190. [Google Scholar] [CrossRef] [PubMed]
- Sumithaa, C.; Gajda-Morszewski, P.; Ishaniya, W.; Khamrang, T.; Velusamy, M.; Bhuvanesh, N.; Brindell, M.; Mazuryk, O.; Ganeshpandian, M. Design of an Anticancer Organoruthenium Complex as the Guest and Polydiacetylene-Coated Fluorogenic Nanocarrier as the Host: Engineering Nanocarrier Using Ene-Yne Conjugation for Sustained Guest Release, Enhanced Anticancer Activity and Reduced In Vivo to. Dalt. Trans. 2023, 53, 966–985. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, L.; Ganji, F.; Dezfoulian, F. Amine-Functionalized and Non-Functionalized Mesoporous Silica Nanoparticles as the Delivery System for Tertiary Butylhydroquinone (TBHQ). J. Porous Mater. 2022, 29, 1113–1122. [Google Scholar] [CrossRef]
- Javdani, H.; Etemad, L.; Moshiri, M.; Zarban, A.; Hanafi-Bojd, M.Y. Effect of Tannic Acid-Templated Mesoporous Silica Nanoparticles on Iron-Induced Oxidative Stress and Liver Toxicity in Rats. Toxicol. Rep. 2021, 8, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, M.; Abolmaali, S.S.; Tamaddon, A.M.; Abedanzadeh, M.; Abedi, M. One-Pot Synthesis of Poly(Alkyl Methacrylate)-Functionalized Mesoporous Silica Hybrid Nanocomposites for Microencapsulation of Poorly Soluble Phytochemicals. Colloids Interface Sci. Commun. 2020, 37, 100298. [Google Scholar] [CrossRef]
- Xu, J.; Lai, H.; You, L.; Zhao, Z. Improvement of the Stability and Anti-AGEs Ability of Betanin through Its Encapsulation by Chitosan-TPP Coated Quaternary Ammonium-Functionalized Mesoporous Silica Nanoparticles. Int. J. Biol. Macromol. 2022, 222, 1388–1399. [Google Scholar] [CrossRef]
- Adhikari, J.; Dasgupta, S.; Barui, A.; Ghosh, M.; Saha, P. Functionalized Mesoporous SiO2-CaO-Na2O-P2O5 Based Nanometric Glass-Ceramic Particles with Enhanced Dispersibility and Bioactivity. J. Sol-Gel Sci. Technol. 2023, 106, 757–774. [Google Scholar] [CrossRef]
- Jermy, B.R.; Almohazey, D.; Alamoudi, W.A.; Palanivel, R.M.; AlSudairi, N.; Dafalla, H.; Almofleh, A.A.; Alfareed, T.M.; Ravinayagam, V. Synergistic Action of Curcumin and Cisplatin on Spinel Ferrite/Hierarchical MCM-41 Nanocomposite against MCF-7, HeLa and HCT 116 Cancer Cell Line. Cancer Nanotechnol. 2021, 12, 33. [Google Scholar] [CrossRef]
- Muñoz-Pina, S.; Duch-Calabuig, A.; Ruiz De Assín David, E.; Ros-Lis, J.V.; Amorós, P.; Argüelles, Á.; Andrés, A. Bioactive Compounds and Enzymatic Browning Inhibition in Cloudy Apple Juice by a New Magnetic UVM-7-SH Mesoporous Material. Food Res. Int. 2022, 162, 112073. [Google Scholar] [CrossRef] [PubMed]

| Type | Pore Size | Structure | Properties | Limitations |
|---|---|---|---|---|
| MCM-41 (Mobil Crystalline Material-41) | 2–6 nm | Ordered hexagonal arrangement of uniform pores | High surface area and well-defined pore structure; ideal for controlled drug delivery or separation processes involving small molecules | Small pore size limits capacity for larger molecules like therapeutic proteins or bioimaging agents |
| SBA-15 (Santa Barbara Amorphous-15) | 5–30 nm | Ordered, large-pore, mesoporous silica with hexagonal arrangement | Larger pore size accommodates larger cargo molecules; suitable for drug delivery applications involving proteins, bioimaging agents, or gene delivery vectors | Amorphous nature can lead to slightly less uniform pore size distribution compared to MCM-41 |
| MCM-48 | 2–30 nm | Ordered cubic arrangement of interconnected pores | Unique cubic pore structure provides a more intricate network for cargo loading and release; advantageous for controlled release applications or incorporating multiple functionalities within the MSN framework | Slightly more challenging to synthesize compared to hexagonal structures of MCM-41 and SBA-15 |
| Other Mesoporous Silica Types | Various | Diverse pore sizes and structures | Examples include FDU-12 (lamellar pore structure for catalysis and controlled release) and TUD-1 (unique 3D network of interconnected pores for high surface area and efficient mass transport) | N/A |
| No | Antioxidants | Antioxidant Acitivty | Solubility in Water |
|---|---|---|---|
| 1 | Alpha Mangostin | 66.63 ± 34.65 µg/mL | 2.03 × 10−4 mg in 1 L at 25 °C |
| 2 | α-tocopherol | 0.059/0.01 mM | Insoluble in waterr |
| 3 | Ascorbic acid | 8.9 ± 0.1 µg/mL | Soluble in water |
| 4 | Buthyl Hydroxy Toluene (BHT) | 0.020 ± 0.001 µg/mL | Insoluble in water |
| 5 | Buthyl Hydroxy Anisole | 0.035 ± 0.007 µg/mL | Insoluble in water |
| 6 | β-carotene | 24.99 µg/mL | 0.0006 g in 1 L at 25 °C |
| 7 | Curcumin | 32.86 µM | 3.12 mg in 1 L at 25 °C |
| 8 | Catechin | 170.3 ± 2.0 µg/mL | 0.45 mg in 1 mL at 25 °C |
| 9 | Quercetin | 19.3 µ/mL | 60 mg in 1 L |
| 10 | Lycopene | 57.93 µ/mL | Insoluble in water |
| 11 | Lutein | 35 µ/mL | Insoluble in water |
| 12 | Tertbutyl hydroquinone (TBHQ) | 14.9 ± 0.81 mM | Practically insoluble in |
| 13 | Ferulic Acid | 56.4 ± 4.6 µ/mL | 0.78 g in 1 L |
| 14 | Myricitrin | 32.7 ± 0.9 µ/mL | Practically insoluble in water |
| 15 | Ethyl Gallate | 35.3 ± 4.0 µ/mL | Sparingly soluble in aqueous buffers |
| 16 | Resveratrol | 0.49 ± 0.03 mM | 3 mg in 100 mL |
| 17 | Rutin | 2.77 ± 0.09 mM | 12.5 mg in 100 mL |
| 18 | Kaempferol | 0.82 ± 0.04 mM | 0.18 g in 1 L |
| 19 | Myricetin | 3.66 ± 0.30 mM | Very insoluble (<5 μg in 1 mL) in pure water |
| 20 | Isobavachalcone | 250.8 µ/mL | Insoluble in water |
| IC50 µg/mL | Interpretation |
|---|---|
| <50 | Very strong antioxidant activity |
| 50–100 | Strong antioxidant activity |
| 101–150 | Moderate antioxidant activity |
| 150–250 | Weak antioxidant activity |
| IC50 > 250 | Very weak or negligible antioxidant activity |
| No | Drug Payload | Aims of Study | Functionalized Mesoporous Silica | Impact of Functionalized and Antioxidant Efficacy Result | References |
|---|---|---|---|---|---|
| 1 | Copper(II) complexes of cyclen and cyclam | Covalently immobilized copper(II) complexes in mesoporous silica aerogels for nanoenzyme applications with enhanced SOD activities. Exploring altered reactivities due to covalent immobilization and nanoporous confinement. | Aerogel polymer functionalized | Improve SOD activity | [86] |
| 2 | Platinum Pt(II) and Palladium Pd(II) | Improve the cytotoxic activity of metal complexes by supporting them on mesoporous silica nanoparticles (MSN) with a melatonin derivative (5 MT). Covalently functionalized systems for increased selectivity and reduced side effects. | Melatonin derivates (5-methoxytryptamine, 5 MT) | Improve selectivity Reduce dose Reduce potential side effect Improve antioxidant effect | [87] |
| 3 | Gallic acid, protocatechuic acid, chlorogenic acid, and 4-hydroxybenzoic acid | Investigate adsorption and desorption of various acids using commercially available mesoporous silica materials. Evaluate antioxidant potential using the Folin–Ciocalteu method. | Amine and Thiol | Improve loading capacity Improve antioxidant effect | [88] |
| 4 | N-(1,3-dimethyl)butyl-N′-phenyl-p-phenylenediamine | Synthesis of thiol-functionalized mesoporous silica nanorods loaded with antioxidant N-(1,3-dimethyl)butyl-N′-phenyl-p-phenylenediamine for anti-aging performance in styrene-butadiene rubber (SBR). | Thiol | Improve loading capacity Controlled release Prevent the bloom release Improve antioxidant effect | [89] |
| 5 | Mamaia grape pomace polyphenolic extract | Assess properties of Mamaia (MM) grape pomace polyphenolic extract loaded onto pristine and functionalized MCM-41 mesoporous silica. Evaluate the antioxidant effect on NIH3T3 cells. | Mercaptopropyl, propyl sulfonic acid, cyanoethyl and propionic acid | Improve release in PBS Improve cytocompatibility Improve RSA Improve antioxidant effect | [90] |
| 6 | Irganox | Incorporation of Irganox 1076 antioxidants into mesoporous MCM-41 silica for improving thermal stability and crystallization | Hybrid particles | Significantly improve Thermal stability Improve antioxidant effect | [91] |
| 7 | Orange Carotenoid Protein (OCP) | Immobilization of photoactive Orange Carotenoid Protein (OCP) on SBA-15 mesoporous silica nanoparticles for potential use as a photochromic material and antioxidant drug delivery. | Amine | Provide controlled release in appropriate condition Improve antioxidant effect | [92] |
| 8 | quercetin | Synthesis of chitosan-silica nanohybrid with quercetin for antibacterial and antioxidant properties. | Chitosan-Thiol | Improve antibacterial effect Improve antioxidant effect Has specific release in acidic medium | [93] |
| 9 | irinotecan | Introduction of multifunctional hollow mesoporous silica-based nanocarrier for targeted delivery of irinotecan to colorectal cancer cells. Incorporation of cerium and iron oxides for oxidative stress-mediated cytotoxicity. | Cerium, Iron oxide, and bacterial-derived exopolysaccharide (EPS) polymer | Greater cytotoxicity in cancer cells Selective delivery to cancerous tissue Induction of antioxidant effects. | [94] |
| 10 | Reserveratol | Design of lactoferrin-functionalized hollow mesoporous silica nanoparticles for delivery of insoluble drug resveratrol. Antioxidant stress, anti-inflammatory, and neuroprotective effects. | Lactoferin | Reduction in inflammation and protection of nerve cells Improved motor function recovery Improve penetration in BBB | [95] |
| 11 | Betanin | Encapsulation of betanin in chitosan-sodium tripolyphosphate coated quaternary ammonium-functionalized mesoporous silica nanoparticles for enhanced inhibition of AGEs formation. | Chitosan-tpp-amine | Higher inhibition rate of AGEs formation pH-dependent drug-releasing behavior Enhanced inhibition of methylglyoxal. | [96] |
| 12 | Cerium | Evaluation of ROS-responsive “nanogate” MSNs loaded with resveratrol for enhanced anticancer effects against lung and breast cancer cells. | methylthiopropyl | Improved anticancer effects against A549 and MDA-MB-231 cells Specific Release in ROS environment | [97] |
| 13 | Curcumin | Synthesis of inorganic-organic nanohybrid using amine-modified MCM-41 and chitosan succinate for controlled drug release, antibacterial activity, and antioxidant properties. | Chitosan-Amine | Controlled drug release with pH-dependent behavior Improve Antibacterial and antioxidant activities | [98] |
| 14 | Polyphenols-Reserveratol | Investigation of MSNs with ROS-responsive “nanogate” as drug delivery platforms for biomedical applications. | aminopropyl, isocyanate, phenyl, mercaptopropyl, and propionic acid | Enhanced anticancer effects against A549 and MDA-MB-231 cells. Specific release in acidic medium | [99] |
| 15 | Cerium oxide | Development of FSC as an antibiotic-free system for treating biofilms. Exhibits antioxidant activity, antibacterial effects, and potential for chronic wound healing applications. | Silica and ceria | Strong Inhibition against S. aureus and E. coli biofilms Hemocompatibility Good Potential for chronic wound healing. Improve safety Improve antioxidant effect | [100] |
| 16 | Atorvastatin | Synthesis of mesoporous SBA-16 silica nanocarrier functionalized with dopamine for in vivo delivery of atorvastatin. | silanizing agent, 3-amino-propyl-triethoxysilane, cyanuric chloride and dopamine | Improve antioxidant enzymes | [101] |
| 17 | Morin | Design a customized Mesoporous silica nanoparticle (MSiNPs) for Morin (MO) adsorption. Evaluate structural, thermal, and biological properties, with a focus on MO adsorption efficiency and biocompatibility. | Silane | Has Nontoxic and biocompatible Improve loading capacity. Reduce dose Improve biocompatibility Improve thermal stability Improve antioxidant efffect | [102] |
| 18 | Gold-Cerium | Develop thiolated, bioactive mesoporous silica nanoparticles (MSN-SH) for bone tissue regeneration. Investigate antioxidant properties, cell adhesion, osteogenic potential, and regenerative nature of MSN-SH. | Thiol | High antioxidant activity, neutralized ROS formed in cells, and provided protection against ROS-induced cell damage Induce cell-proliferative, Has a good osteogenic potential, and Induce regenerative nature | [103] |
| 19 | Polyphenolic flavonoids | Utilize engineered mesoporous silica nanoparticles (MSNPs) for nano harvesting of polyphenolic flavonoids from Solidago nemoralis hairy root cultures. Evaluate intracellular uptake, antioxidant activity, and pharmacological effects of the recovered solutes. | Yitanium dioxide (TiO2) and amines (NH2) | Increased antiradical activity Imrpove antioxidant effect | [104] |
| 20 | Ruthenium (ii) | Evaluate ruthenium(ii) complexes with pyridylimidazo[1,5-a]pyridine ligand on PDA-coated amino-functionalized mesoporous silica nanoparticles (AMSNs) for anticancer effects. Assess release profile, cellular internalization, and zebrafish embryotoxicity. | Amine | Enhanced cytotoxicity, Enhance anticancer potency, and cellular internalization Improve antioxidant effect | [105] |
| 21 | 2-tert-butylhydroquinone (TBHQ) | Encapsulate 2-tert-butylhydroquinone (TBHQ) in mesoporous silica nanoparticles (MSNs) and amine-functionalized MSNs (AP-MSNs). Investigate release profiles, cytotoxicity, and antioxidant effects. | Amine | Has sustained release of TBHQ Increased antioxidant activity Lower cytotoxicity compared to free-TBHQ. Improve antioxidant effect | [106] |
| 22 | Tannic Acid | Investigate amino-functionalized tannic acid-templated mesoporous silica nanoparticles (TA-MS-NH2 NPs) for protection against iron-induced liver toxicity. Assess hepatotoxicity markers, oxidative stress, and acute iron toxicity. | Amine | Protection against iron-induced liver toxicity Improved hepatotoxicity markers and oxidative stress. Improve antioxidant effect | [107] |
| 23 | Silibinin | Develop hybrid mesoporous silica nanocomposites composed of polymerized alkyl methacrylate monomers for enhanced dissolution and antioxidant activity of Silibinin (SBN). Evaluate in-vitro dissolution and antioxidant activity. | alkyl methacrylate monomers | A remarkable increase in in-vitro dissolution and antioxidant activity of SBN Has specific release in acidic gastric medium. | [108] |
| 24 | Betanin | Encapsulate betanin in chitosan-sodium tripolyphosphate coated quaternary ammonium-functionalized mesoporous silica nanoparticles (CS@QAMSNPs). Evaluate morphology, loading capacity, antioxidant stability, and cell viability. | Chitosan-Tpp, and Amine functionalized | Improved stability of betanin Good safety High antioxidant activity. | [109] |
| 25 | Bioactive glass-ceramic (BGC) consisting of SiO2, CaO, Na2O, and P2O5 | Functionalize bioactive glass-ceramic (BGC) particles with dopamine, glutamic acid, and cystamine dihydrochloride. Evaluate antibacterial, antioxidant, bioactivity, and dispersion stability properties for potential use in electrospinning and bioprinting. | Dopamine, glutamic acid, and cystamine dihydrochloride | Significant improve antibacterial, antioxidant, and bioactivity Good dispersion stability in dilute polymer solutions. | [110] |
| 26 | Cisplatin-Curcumin | Impregnate CuFe2O4/mesosilicalite and CuFe2O4/MCM-41 with cisplatin and curcumin for combinational drug therapy. Evaluate cytotoxic efficiency on various cell lines. | Magnetic spinel ferrite | Synergistic effect on reducing cell viability, triggering apoptotic signaling pathway Improve Antioxidant effect | [111] |
| 27 | Flavonoid-Vit C | Functionalize magnetized mesoporous silica material (Fe3O4NPs-UVM-7) with thiol and amine groups. Evaluate effects on enzymatic browning, physicochemical properties, bioactive compounds, and antioxidant capacity of cloudy apple juice. | Amine and Thiol | Reduced enzymatic browning; Maintained physicochemical properties and bioactive compounds Mitigated loss of total polyphenols and antioxidant capacity. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budiman, A.; Rusdin, A.; Wardhana, Y.W.; Puluhulawa, L.E.; Cindana Mo’o, F.R.; Thomas, N.; Gazzali, A.M.; Aulifa, D.L. Exploring the Transformative Potential of Functionalized Mesoporous Silica in Enhancing Antioxidant Activity: A Comprehensive Review. Antioxidants 2024, 13, 936. https://doi.org/10.3390/antiox13080936
Budiman A, Rusdin A, Wardhana YW, Puluhulawa LE, Cindana Mo’o FR, Thomas N, Gazzali AM, Aulifa DL. Exploring the Transformative Potential of Functionalized Mesoporous Silica in Enhancing Antioxidant Activity: A Comprehensive Review. Antioxidants. 2024; 13(8):936. https://doi.org/10.3390/antiox13080936
Chicago/Turabian StyleBudiman, Arif, Agus Rusdin, Yoga Windhu Wardhana, Lisa Efriani Puluhulawa, Faradila Ratu Cindana Mo’o, Nurain Thomas, Amirah Mohd Gazzali, and Diah Lia Aulifa. 2024. "Exploring the Transformative Potential of Functionalized Mesoporous Silica in Enhancing Antioxidant Activity: A Comprehensive Review" Antioxidants 13, no. 8: 936. https://doi.org/10.3390/antiox13080936









