Synthesis, Cytotoxicity and Antioxidant Activity Evaluation of Some Thiazolyl–Catechol Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Consumables and Instruments
2.1.1. Chemicals and Consumables
2.1.2. Instruments
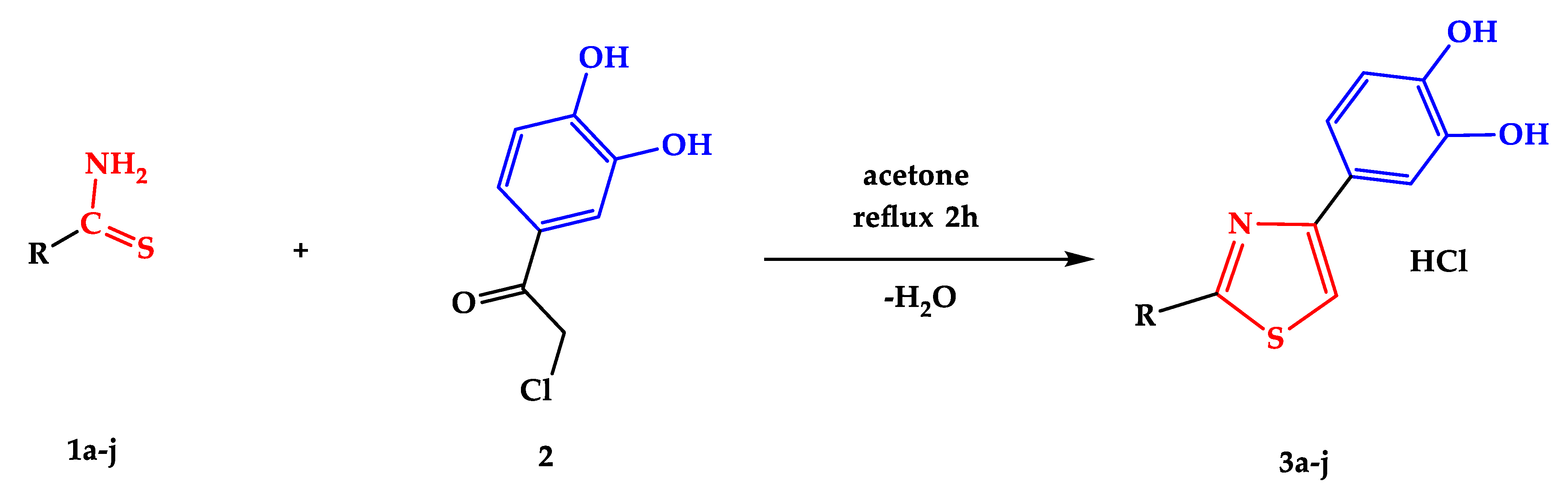
2.1.3. Synthesis Protocol and Characterization of Final Compounds
2.2. Antiradical, Electron Transfer, and Ferrous Ion Chelation Assays
2.2.1. Antiradical Assays
2.2.2. Electron Transfer Assays
2.2.3. Ferrous Ion Chelation Assay
2.3. In Silico Studies
2.3.1. Theoretical Quantum Calculations
2.3.2. Molecular Properties with Influence on the Pharmacokinetics
2.4. Cytotoxicity Studies
3. Results
3.1. Chemical Synthesis of the Compounds
3.2. Antiradical, Electron Transfer, and Ferrous Ion Chelation Assays
3.2.1. Antiradical Assays
3.2.2. Electron Transfer Assays
3.2.3. Ferrous Ion Chelation Assay
3.3. In Silico Studies
3.3.1. Theoretical Quantum Calculations
3.3.2. Molecular Properties with Influence on the Pharmacokinetics of Compounds
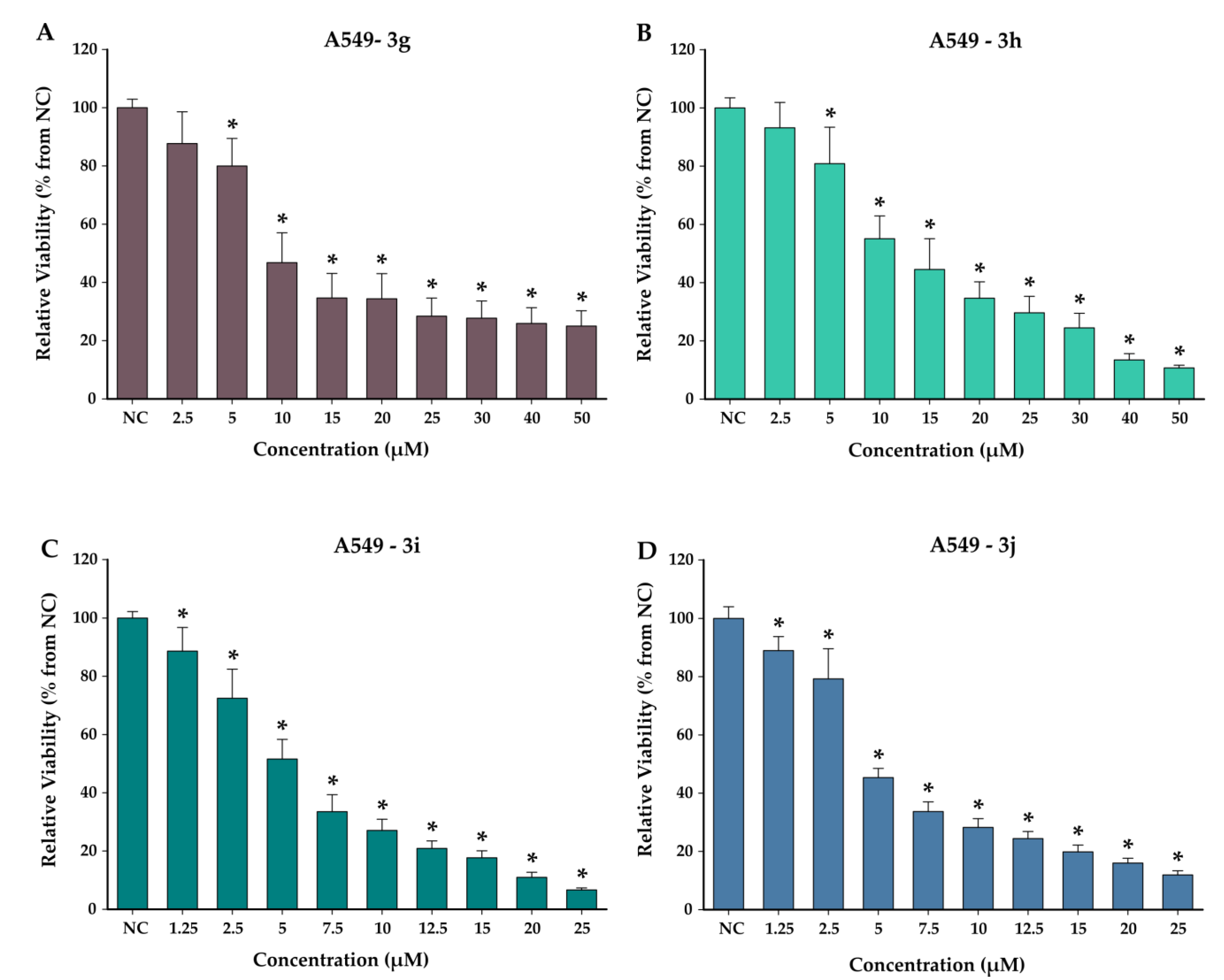
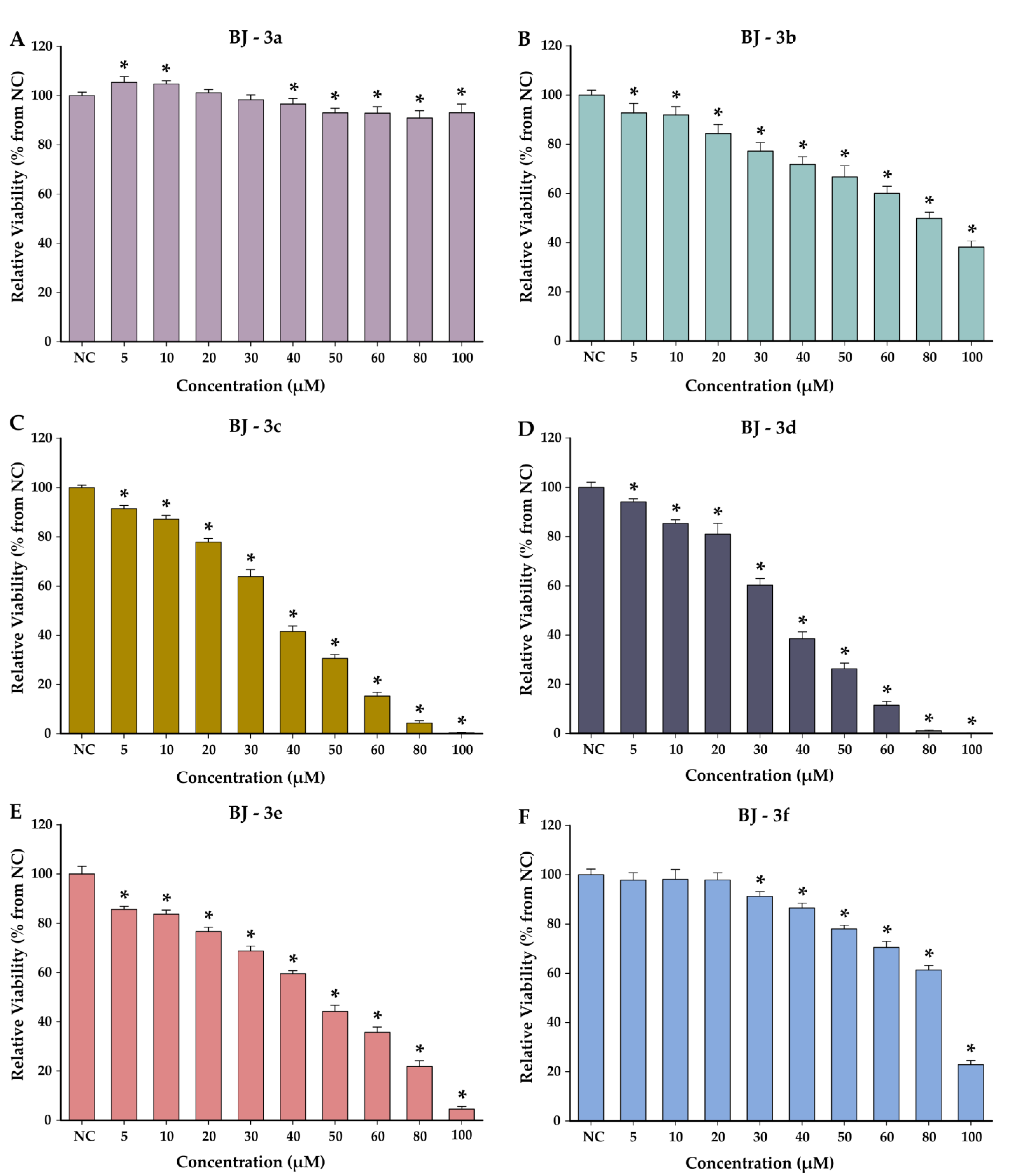
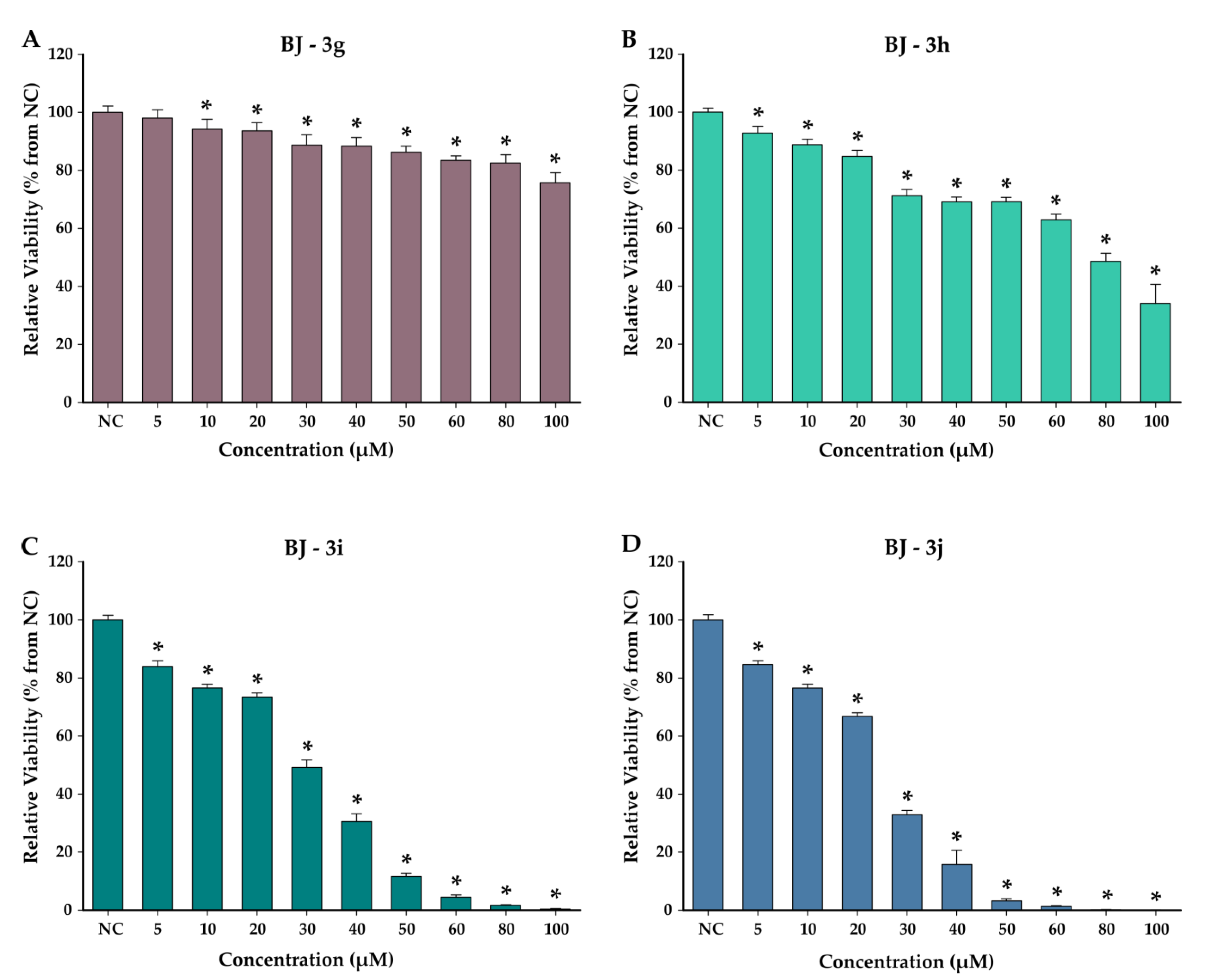
3.4. Cytotoxicity Studies
4. Discussion
4.1. Chemical Synthesis of the Compounds
4.2. Antiradical, Electron Transfer, and Ferrous Ion Chelation Assays
4.2.1. Antiradical Assays
4.2.2. Electron Transfer Assays
4.2.3. Ferrous Ion Chelation Assay
4.3. In Silico Studies
4.3.1. Theoretical Quantum Calculations
4.3.2. Molecular Properties with Influence on the Pharmacokinetics of Compounds
4.4. Cytotoxicity Studies on Normal and Cancerous Cell Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 17–19. [Google Scholar] [CrossRef]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole Ring—A Biologically Active Scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Malhotra, D.; Singh, K.; Chadha, R.; Bedi, P.M.S. Thiazole derivatives in medicinal chemistry: Recent advancements in synthetic strategies, structure activity relationship and pharmacological outcomes. J. Mol. Struct. 2022, 1266, 133479. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015, 366, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Marc, G.; Stana, A.; Franchini, A.H.; Vodnar, D.C.; Barta, G.; Tertiş, M.; Şanta, I.; Cristea, C.; Pîrnău, A.; Ciorîţă, A.; et al. Phenolic Thiazoles with Antioxidant and Antiradical Activity. Synthesis, In Vitro Evaluation, Toxicity, Electrochemical Behavior, Quantum Studies and Antimicrobial Screening. Antioxidants 2021, 10, 1707. [Google Scholar] [CrossRef] [PubMed]
- Araniciu, C.; Palage, M.; Oniga, S.; Pirnau, A.; Verité, P.; Oniga, O. Synthesis and characterization of some novel 5,2-and 4,2-bisthiazoles derivatives. Rev. Chim. 2013, 64, 1067–1071. [Google Scholar]
- Araniciu, C.; Pârvu, A.E.; Tiperciuc, B.; Palage, M.; Oniga, S.; Verité, P.; Oniga, O. Synthesis and evaluation of the anti-inflammatory activity of some 2-(Trimethoxyphenyl)-4-R1-5-R2-Thiazoles. Dig. J. Nanomater. Biostruct. 2013, 8, 699–709. [Google Scholar]
- Araniciu, C.; Pârvu, A.E.; Palage, M.D.; Oniga, S.D.; Benedec, D.; Oniga, I.; Oniga, O. The effect of some 4,2 and 5,2 bisthiazole derivatives on nitro-oxidative stress and phagocytosis in acute experimental inflammation. Molecules 2014, 19, 9240–9256. [Google Scholar] [CrossRef]
- Ali, S.H.; Sayed, A.R. Review of the synthesis and biological activity of thiazoles. Synth. Commun. 2021, 51, 670–700. [Google Scholar] [CrossRef]
- Narayana, B.; Ashalatha, B.V.; Vijaya Raj, K.K.; Suchetha Kumari, N. Synthesis of some new 4-{2-[(aryl)amino]-1,3-thiazol-4-yl}benzene-1,2-diols as possible antibacterial and antifungal agents. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 1381–1389. [Google Scholar] [CrossRef]
- Wang, W.L.; Chai, S.C.; Huang, M.; He, H.Z.; Hurley, T.D.; Ye, Q.Z. Discovery of inhibitors of Escherichia coli methionine aminopeptidase with the Fe(II)-form selectivity and antibacterial activity. J. Med. Chem. 2008, 51, 6110–6120. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A. Thiazole Derivates and Methods of Using the Same. WO Patent 2023192595A1, 5 October 2023. [Google Scholar]
- Chihiro, M.; Komatsu, H.; Michiaki, T.; Yoichi, Y. Superoxide Radical Inhibitors. U.S. Patent 6080764A, 27 June 2000. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bedlovicová, Z.; Strapác, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. Ferulic, Sinapic, 3,4-Dimethoxycinnamic Acid and Indomethacin Derivatives with Antioxidant, Anti-Inflammatory and Hypolipidemic Functionality. Antioxidants 2023, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Baig, H.; Ahmed, D.; Zara, S.; Aujla, M.I.; Asghar, M.N. In Vitro Evaluation of Antioxidant Properties of Different Solvent Extracts of Rumex acetosella Leaves. Orient. J. Chem. 2011, 27, 1509–1516. [Google Scholar]
- Ramadan, S.K.; Elrazaz, E.Z.; Abouzid, K.A.M.; El-Naggar, A.M. Design, synthesis and in silico studies of new quinazolinone derivatives as antitumor PARP-1 inhibitors. RSC Adv. 2020, 10, 29475–29492. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 15–27. [Google Scholar]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.; Bektaşoğlu, B.; Berker, K.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Bhagat, M.; Gupta, S.; Singh, J.; Koul, A. Iron (FeII) Chelation, Ferric Reducing Antioxidant Power, and Immune Modulating Potential of Arisaema jacquemontii (Himalayan Cobra Lily). BioMed Res. Int. 2014, 2014, 179865. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Şendil, K.; Şengül, E.; Gültekin, M.S.; Taslimi, P.; Gulçin, İ.; Supuran, C.T. The synthesis of some β-lactams and investigation of their metal-chelating activity, carbonic anhydrase and acetylcholinesterase inhibition profiles. J. Enzyme Inhib. Med. Chem. 2016, 31, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid. Based Complement. Altern. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef] [PubMed]
- Hossen, J.; Pal, T.K.; Hasan, T. Theoretical investigations on the antioxidant potential of 2,4,5-trihydroxybutyrophenone in different solvents: A DFT approach. Results Chem. 2022, 4, 100515. [Google Scholar] [CrossRef]
- Rodríguez, S.A.; Baumgartner, M.T. Theoretical study of the reaction mechanism of a series of 4-hydroxycoumarins against the DPPH radical. Chem. Phys. Lett. 2014, 601, 116–123. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Giacomelli, C.; Miranda, F.d.S.; Gonçalves, N.S.; Spinelli, A. Antioxidant activity of phenolic and related compounds: A density functional theory study on the O–H bond dissociation enthalpy. Redox Rep. 2004, 9, 263–269. [Google Scholar] [CrossRef]
- Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Molaei, S.; Tehrani, A.D.; Shamlouei, H. Antioxidant activates of new carbohydrate based gallate derivatives: A DFT study. J. Mol. Liq. 2023, 377, 121506. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Topală, T.L.; Fizeşan, I.; Petru, A.; Castiñeiras, A.; Bodoki, A.E.; Oprean, L.S.; Escolano, M.; Alzuet-Piña, G. Evaluation of DNA and BSA-Binding, Nuclease Activity, and Anticancer Properties of New Cu(II) and Ni(II) Complexes with Quinoline-Derived Sulfonamides. Inorganics 2024, 12, 158. [Google Scholar] [CrossRef]
- Șandor, A.; Fizeșan, I.; Ionuț, I.; Marc, G.; Moldovan, C.; Oniga, I.; Pîrnău, A.; Vlase, L.; Petru, A.-E.; Macasoi, I.; et al. Discovery of A Novel Series of Quinazoline–Thiazole Hybrids as Potential Antiproliferative and Anti-Angiogenic Agents. Biomolecules 2024, 14, 218. [Google Scholar] [CrossRef]
- ESMO Lung & Chest Tumours. Available online: https://interactiveguidelines.esmo.org/esmo-web-app/toc/index.php?subjectAreaID=1&loadPdf=1 (accessed on 25 July 2024).
- El-Naggar, A.M.; Zidan, A.; Elkaeed, E.B.; Taghour, M.S.; Badawi, W.A. Design, synthesis and docking studies of new hydrazinyl-thiazole derivatives as anticancer and antimicrobial agents. J. Saudi Chem. Soc. 2022, 26, 101488. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mohamed, N.K.; Aly, A.A.; Ramadan, M.; Gomaa, H.A.M.; Abdel-Aziz, A.T.; Youssif, B.G.M.; Bräse, S.; Fuhr, O. Synthesis and Antiproliferative Potential of Thiazole and 4-Thiazolidinone Containing Motifs as Dual Inhibitors of EGFR and BRAFV600E. Molecules 2023, 28, 7951. [Google Scholar] [CrossRef]
- Opitz, S.; Smrke, S.; Goodman, B.; Keller, M.; Schenker, S.; Yeretzian, C. Antioxidant Generation during Coffee Roasting: A Comparison and Interpretation from Three Complementary Assays. Foods 2014, 3, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Sredoja Tisma, V.; Zarkovic, N. Short Overview of Some Assays for the Measurement of Antioxidant Activity of Natural Products and Their Relevance in Dermatology. Molecules 2021, 26, 5301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, D.; Liu, C.; Tang, S.; Wang, S.; Wang, Y.; Yang, Y. Enrichment, analysis, identification and mechanism of antioxidant components in Toona sinensis. Chin. J. Anal. Chem. 2023, 51, 100198. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Hou, B.; Zhang, P.; Wang, Q.; Zhang, B.-L.; Huang, Y.-W.; Wang, Y.; Xiang, Z.-M.; Zi, C.-T.; et al. Synthesis, antioxidant activity, and density functional theory study of catechin derivatives. RSC Adv. 2017, 7, 54136–54141. [Google Scholar] [CrossRef]


| Compound | R | Compound | R |
|---|---|---|---|
| 1a, 3a |  | 1f, 3f | 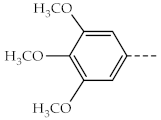 |
| 1b, 3b | 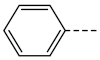 | 1g, 3g | 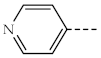 |
| 1c, 3c | 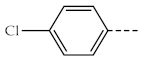 | 1h, 3h | 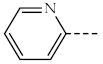 |
| 1d, 3d | 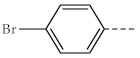 | 1i, 3i |  |
| 1e, 3e | 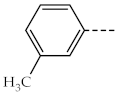 | 1j, 3j | 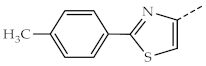 |
| Compound | DPPH• | ABTS•+ |
|---|---|---|
| 3a | 51.31 | 12.01 |
| 3b | 50.19 | 14.03 |
| 3c | 47.83 | 15.74 |
| 3d | 48.34 | 12.62 |
| 3e | 46.76 | 13.88 |
| 3f | 54.61 | 13.36 |
| 3g | 36.69 | 7.59 |
| 3h | 40.30 | 13.49 |
| 3i | 45.86 | 14.08 |
| 3j | 49.59 | 17.66 |
| Ascorbic acid | 53.49 | NT |
| Trolox | 38.10 | 15.87 |
| Compound | TAC | RP | FRAP | CUPRAC | |
|---|---|---|---|---|---|
| Eq Ascorbic Acid | Eq Ascorbic Acid | Eq Trolox | Eq Trolox | Eq Trolox | |
| 3a | 0.38 | 1.73 | 1.44 | 0.95 | 3.17 |
| 3b | 1.00 | 1.60 | 1.33 | 1.07 | 2.27 |
| 3c | 0.83 | 1.47 | 1.22 | 1.10 | 2.38 |
| 3d | 0.71 | 1.63 | 1.35 | 1.09 | 2.62 |
| 3e | 0.91 | 1.64 | 1.37 | 1.07 | 3.13 |
| 3f | 1.22 | 1.75 | 1.46 | 1.06 | 3.05 |
| 3g | 0.64 | 2.04 | 1.69 | 1.63 | 3.60 |
| 3h | 1.34 | 2.06 | 1.71 | 1.03 | 2.13 |
| 3i | 0.51 | 1.63 | 1.35 | 1.14 | 1.84 |
| 3j | 0.36 | 1.81 | 1.50 | 1.06 | 1.61 |
| Compound | 2 µM | 1.5 µM | 1 µM | 0.5 µM | 0.25 µM |
|---|---|---|---|---|---|
| 3a | - | - | - | - | - |
| 3b | - | - | - | - | - |
| 3c | - | - | - | - | - |
| 3d | - | - | - | - | - |
| 3e | - | - | - | - | - |
| 3f | - | - | - | - | - |
| 3g | - | - | - | - | - |
| 3h | - | - | - | - | - |
| 3i | - | - | - | - | - |
| 3j | - | - | - | - | - |
| EDTA–Na2 | 96.20 | 86.52 | 73.41 | 55.24 | 47.21 |
| Vacuum | Non-Polar | Water | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOMO | LUMO | Gap | η | µ | HOMO | LUMO | Gap | η | µ | HOMO | LUMO | Gap | η | µ | |
| 3a | −5.31 | −0.84 | 4.47 | 2.24 | −3.08 | −5.40 | −0.88 | 4.52 | 2.26 | −3.14 | −5.42 | −0.89 | 4.53 | 2.27 | −3.16 |
| 3b | −5.32 | −1.42 | 3.90 | 1.95 | −3.37 | −5.40 | −1.50 | 3.90 | 1.95 | −3.45 | −5.42 | −1.51 | 3.91 | 1.96 | −3.47 |
| 3c | −5.43 | −1.65 | 3.78 | 1.89 | −3.54 | −5.44 | −1.65 | 3.79 | 1.90 | −3.55 | −5.44 | −1.65 | 3.79 | 1.90 | −3.55 |
| 3d | −5.44 | −1.68 | 3.76 | 1.88 | −3.56 | −5.44 | −1.68 | 3.76 | 1.88 | −3.56 | −5.45 | −1.68 | 3.77 | 1.89 | −3.57 |
| 3e | −5.29 | −1.39 | 3.90 | 1.86 | −3.44 | −5.37 | −1.47 | 3.90 | 1.95 | −3.42 | −5.54 | −1.60 | 3.94 | 1.97 | −3.57 |
| 3f | −5.27 | −1.35 | 3.92 | 1.96 | −3.31 | −5.37 | −1.49 | 3.88 | 1.94 | −3.43 | −5.39 | −1.51 | 3.88 | 1.94 | −3.45 |
| 3g | −5.53 | −1.87 | 3.66 | 1.83 | −3.70 | −5.50 | −1.89 | 3.61 | 1.81 | −3.70 | −5.50 | −1.90 | 3.60 | 1.80 | −3.70 |
| 3h | −5.31 | −1.61 | 3.70 | 1.85 | −3.46 | −5.39 | −1.68 | 3.71 | 1.86 | −3.54 | −5.57 | −1.78 | 3.79 | 1.90 | −3.68 |
| 3i | −5.32 | −1.64 | 3.68 | 1.84 | −3.48 | −5.41 | −1.70 | 3.71 | 1.86 | −3.56 | −5.56 | −1.83 | 3.73 | 1.87 | −3.70 |
| 3j | −5.29 | −1.58 | 3.71 | 1.95 | −3.34 | −5.40 | −1.67 | 3.73 | 1.87 | −3.54 | −5.43 | −1.69 | 3.74 | 1.87 | −3.56 |
| Compound | Vacuum | Non-Polar | Water | |||
|---|---|---|---|---|---|---|
| para | meta | para | meta | para | meta | |
| 3a | 66.51 | 68.76 | 67.37 | 70.01 | 69.87 | 72.62 |
| 3b | 66.72 | 68.94 | 67.75 | 70.25 | 70.26 | 72.83 |
| 3c | 67.05 | 69.09 | 67.85 | 70.28 | 70.34 | 72.82 |
| 3d | 67.05 | 69.12 | 67.96 | 70.37 | 70.47 | 72.97 |
| 3e | 66.63 | 68.82 | 66.07 | 68.32 | 70.17 | 72.75 |
| 3f | 66.69 | 68.93 | 67.71 | 70.19 | 70.25 | 72.81 |
| 3g | 67.34 | 69.33 | 68.30 | 70.54 | 70.81 | 73.12 |
| 3h | 66.72 | 68.67 | 66.18 | 68.24 | 70.26 | 72.71 |
| 3i | 66.90 | 68.96 | 66.33 | 68.57 | 70.42 | 72.92 |
| 3j | 66.83 | 68.90 | 67.86 | 70.22 | 70.39 | 72.83 |
| Compound | MW | Rotatable Bonds | HBA | HBD | TPSA (Å2) | MLogP | Water Solubility (µg/mL) | Lipinski Violations |
|---|---|---|---|---|---|---|---|---|
| 3a | 207.25 | 1 | 3 | 2 | 81.59 | 0.61 | 1.69 | 0 |
| 3b | 269.32 | 2 | 3 | 2 | 81.59 | 2.01 | 1.38 | 0 |
| 3c | 303.76 | 2 | 3 | 2 | 81.59 | 2.52 | 4.19 | 0 |
| 3d | 348.21 | 2 | 3 | 2 | 81.59 | 2.65 | 2.30 | 0 |
| 3e | 283.34 | 2 | 3 | 2 | 81.59 | 2.25 | 7.63 | 0 |
| 3f | 359.40 | 5 | 6 | 2 | 109.28 | 1.05 | 1.32 | 0 |
| 3g | 270.31 | 2 | 4 | 2 | 94.48 | 0.90 | 6.47 | 0 |
| 3h | 270.31 | 2 | 4 | 2 | 94.48 | 0.90 | 6.19 | 0 |
| 3i | 352.43 | 3 | 4 | 2 | 122.72 | 1.97 | 2.18 | 0 |
| 3j | 366.46 | 3 | 4 | 2 | 122.72 | 2.20 | 1.16 | 0 |
| Compound | IC50 (µM) (±SD) | Selectivity Index | |
|---|---|---|---|
| A549 | BJ | ||
| 3a | 31.53 (±6.71) | >100 | >3.17 |
| 3b | 8.91 (±0.62) | 78.61 (±9.52) | 8.82 |
| 3c | 8.16 (±0.46) | 35.83 (±2.92) | 4.39 |
| 3d | 6.08 (±1.22) | 34.44 (±2.50) | 5.66 |
| 3e | 6.46 (±1.16) | 46.77 (±5.52) | 7.23 |
| 3f | 8.76 (±1.48) | 82.61 (±3.68) | 9.43 |
| 3g | 7.34 (±1.02) | >100 | >13.62 |
| 3h | 13.13 (±1.16) | 76.72 (±9.92) | 5.84 |
| 3i | 5.18 (±0.70) | 29.39 (±2.08) | 5.67 |
| 3j | 4.28 (±0.37) | 24.25 (±1.60) | 5.67 |
| Gefitinib | 15.93 (±0.50) | 25.50 (±0.51) | 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornea, A.C.; Marc, G.; Ionuț, I.; Moldovan, C.; Fizeșan, I.; Petru, A.-E.; Creștin, I.-V.; Pîrnău, A.; Vlase, L.; Oniga, O. Synthesis, Cytotoxicity and Antioxidant Activity Evaluation of Some Thiazolyl–Catechol Compounds. Antioxidants 2024, 13, 937. https://doi.org/10.3390/antiox13080937
Cornea AC, Marc G, Ionuț I, Moldovan C, Fizeșan I, Petru A-E, Creștin I-V, Pîrnău A, Vlase L, Oniga O. Synthesis, Cytotoxicity and Antioxidant Activity Evaluation of Some Thiazolyl–Catechol Compounds. Antioxidants. 2024; 13(8):937. https://doi.org/10.3390/antiox13080937
Chicago/Turabian StyleCornea, Alexandra Cătălina, Gabriel Marc, Ioana Ionuț, Cristina Moldovan, Ionel Fizeșan, Andreea-Elena Petru, Ionuț-Valentin Creștin, Adrian Pîrnău, Laurian Vlase, and Ovidiu Oniga. 2024. "Synthesis, Cytotoxicity and Antioxidant Activity Evaluation of Some Thiazolyl–Catechol Compounds" Antioxidants 13, no. 8: 937. https://doi.org/10.3390/antiox13080937
APA StyleCornea, A. C., Marc, G., Ionuț, I., Moldovan, C., Fizeșan, I., Petru, A.-E., Creștin, I.-V., Pîrnău, A., Vlase, L., & Oniga, O. (2024). Synthesis, Cytotoxicity and Antioxidant Activity Evaluation of Some Thiazolyl–Catechol Compounds. Antioxidants, 13(8), 937. https://doi.org/10.3390/antiox13080937










