Asparagopsis taxiformis as a Novel Antioxidant Ingredient for Climate-Smart Aquaculture: Antioxidant, Metabolic and Digestive Modulation in Juvenile White Seabream (Diplodus sargus) Exposed to a Marine Heatwave
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
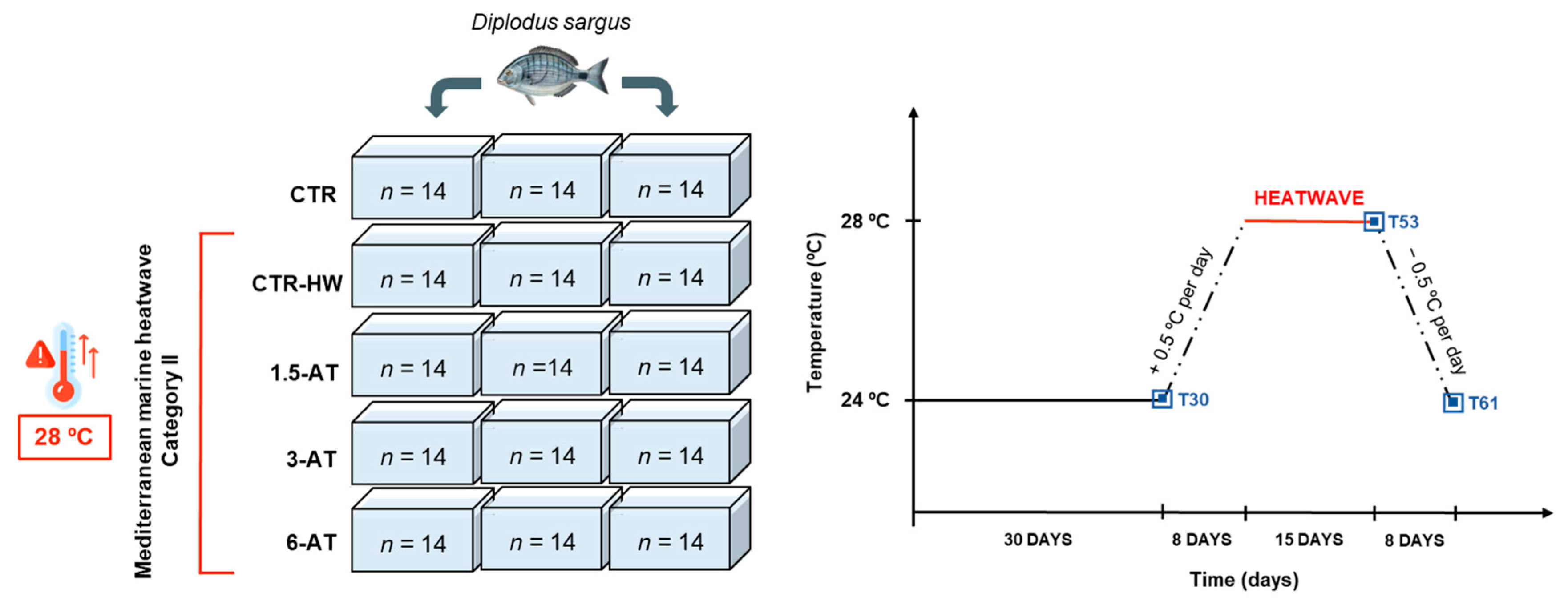
2.2. Feeding Trial and Fish Sampling
2.3. Fish Growth and Feed Efficiency
2.4. Biochemical Analyses
2.4.1. Oxidative Stress
2.4.2. Metabolic Enzymes
2.4.3. Digestive Enzymes
2.5. Histological Analysis
2.6. Data Analysis
3. Results
3.1. Fish Growth and Feed Efficiency
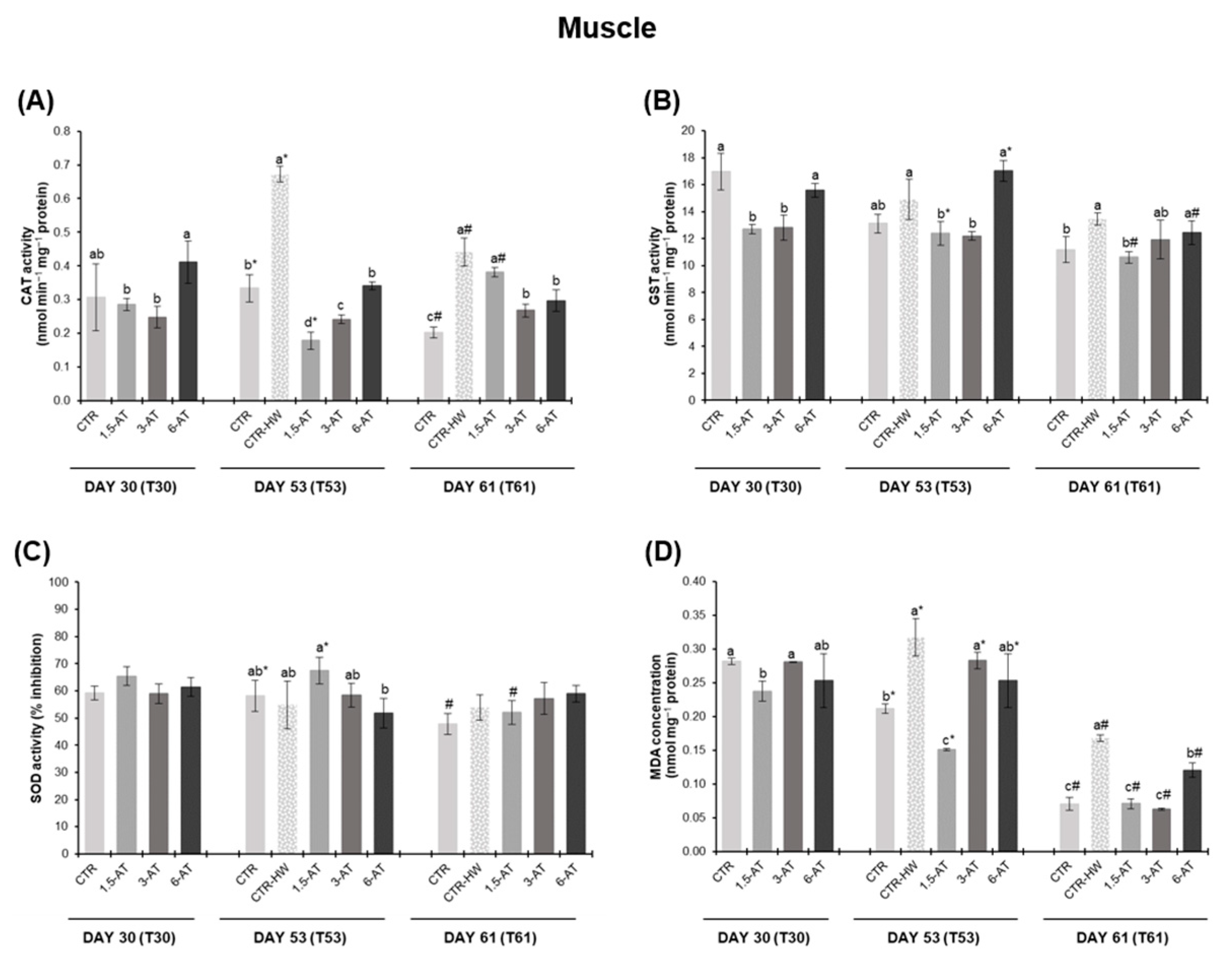
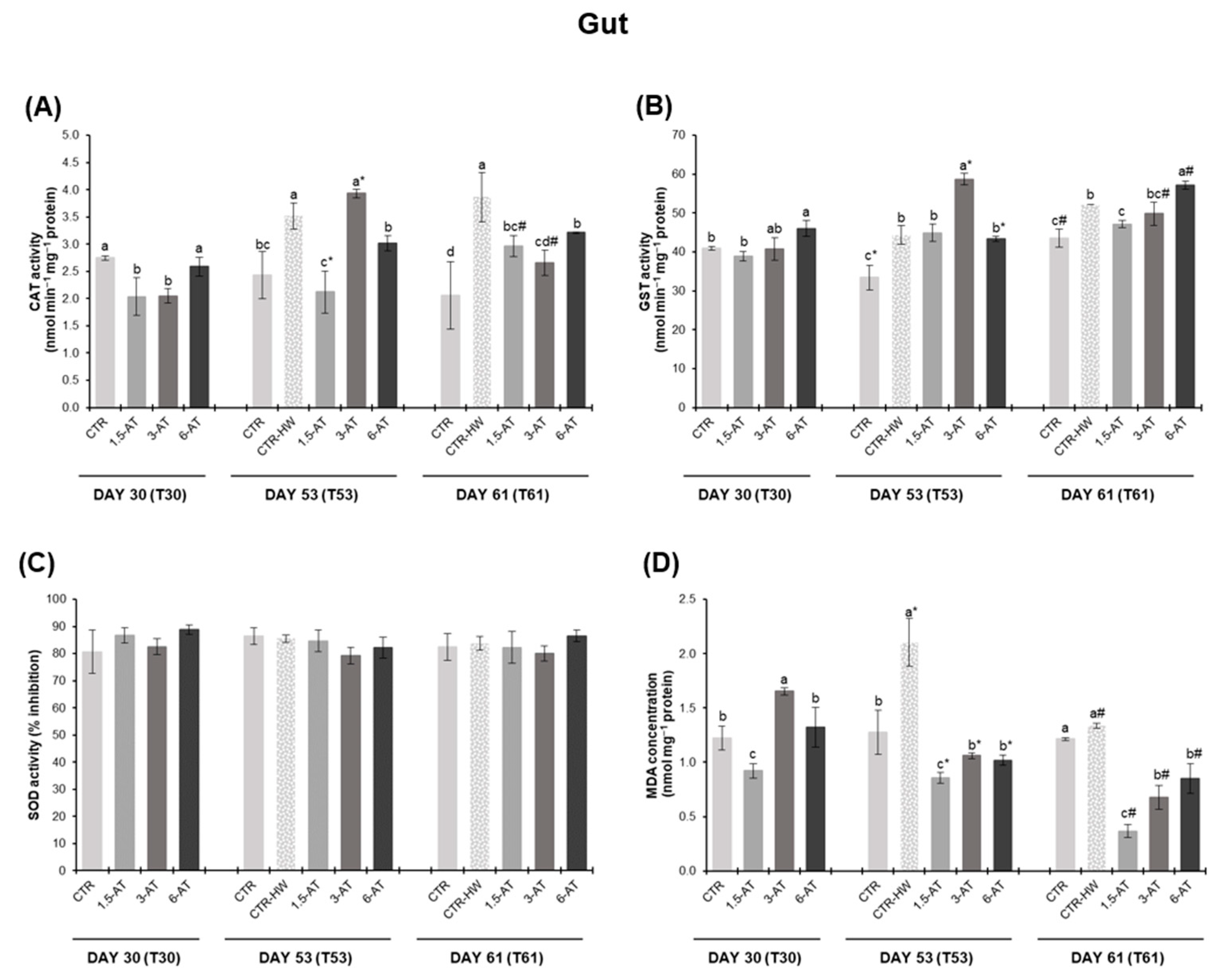
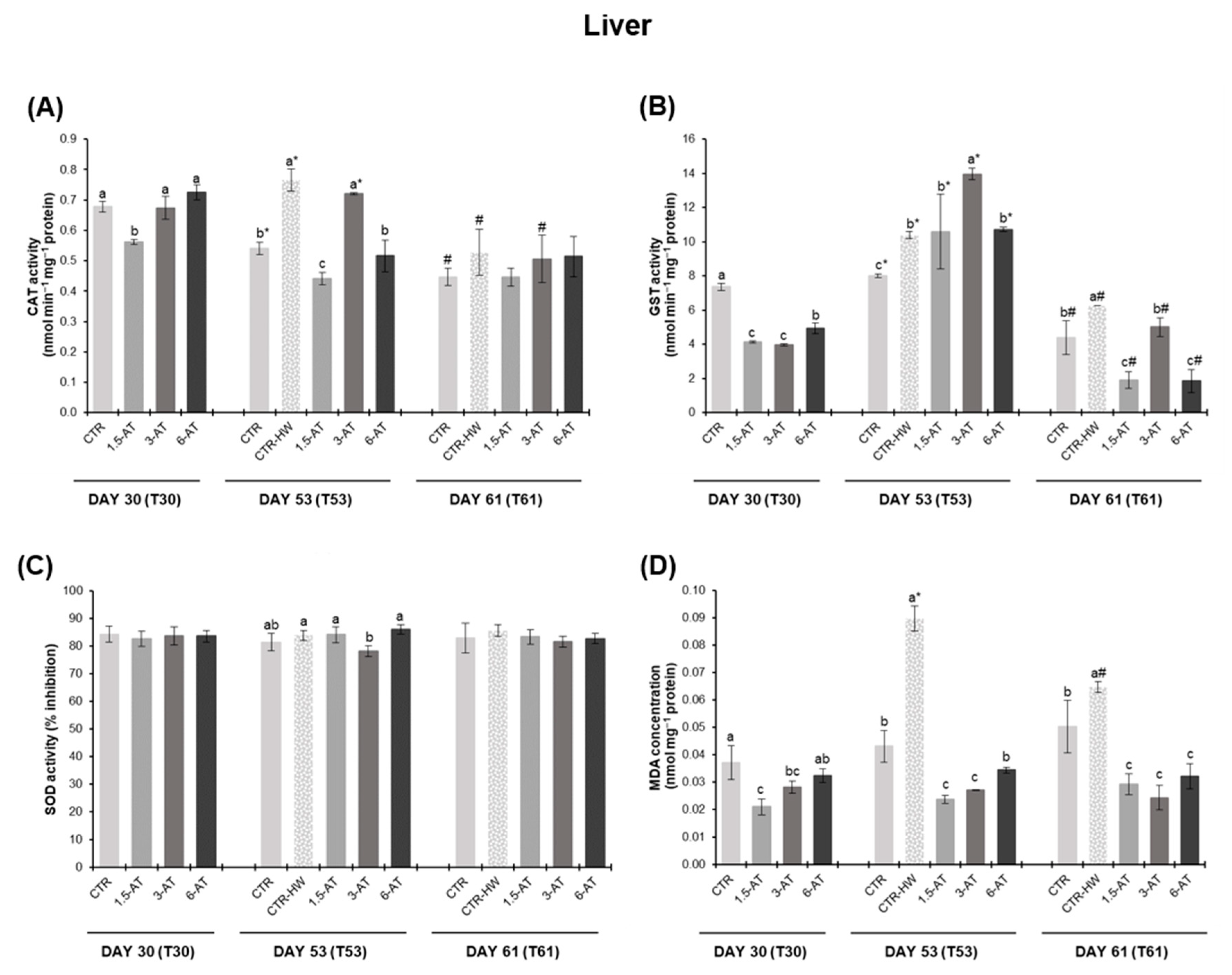
3.2. Oxidative Stress
3.3. Metabolic Responses
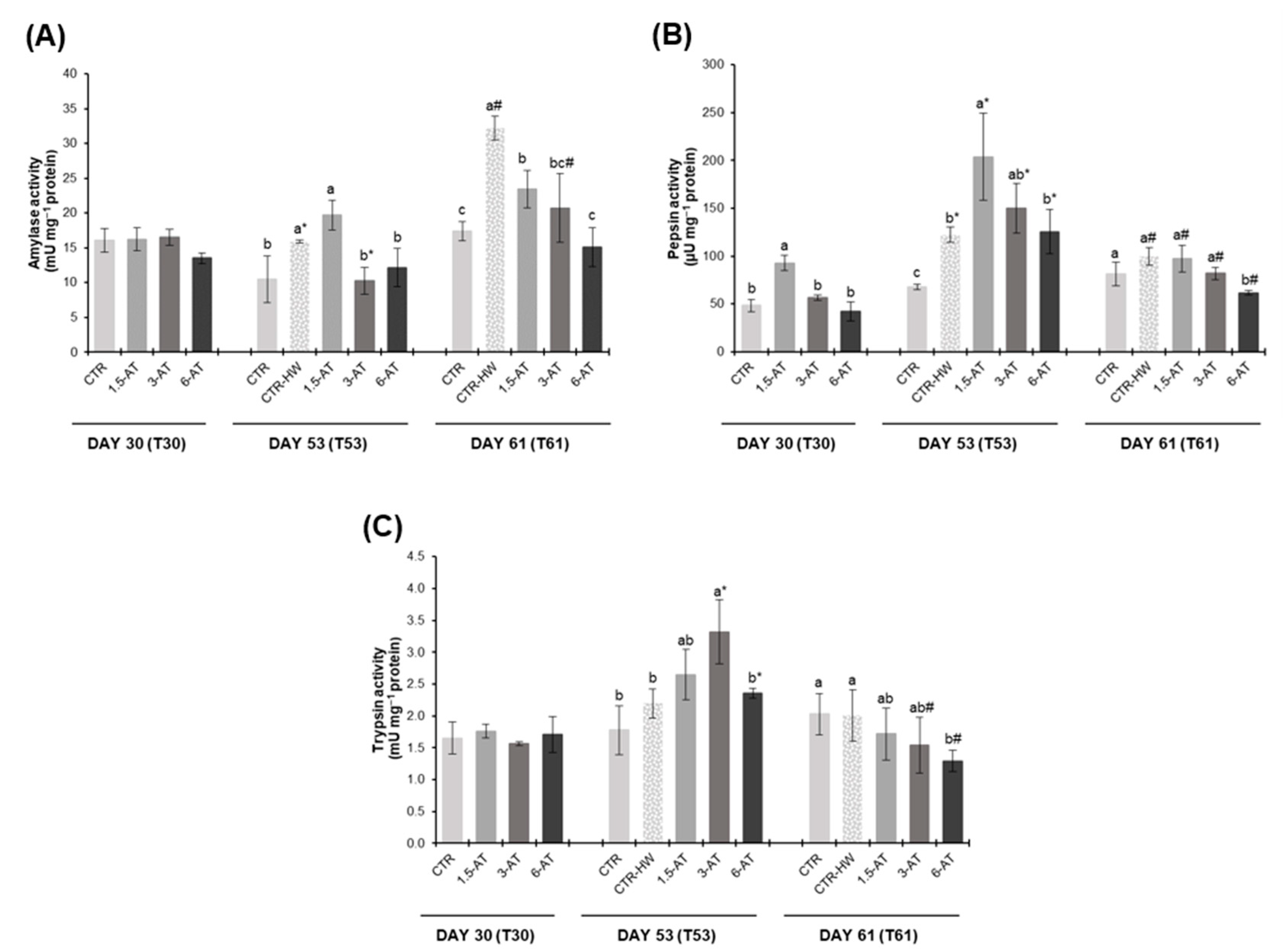
3.4. Digestive Responses
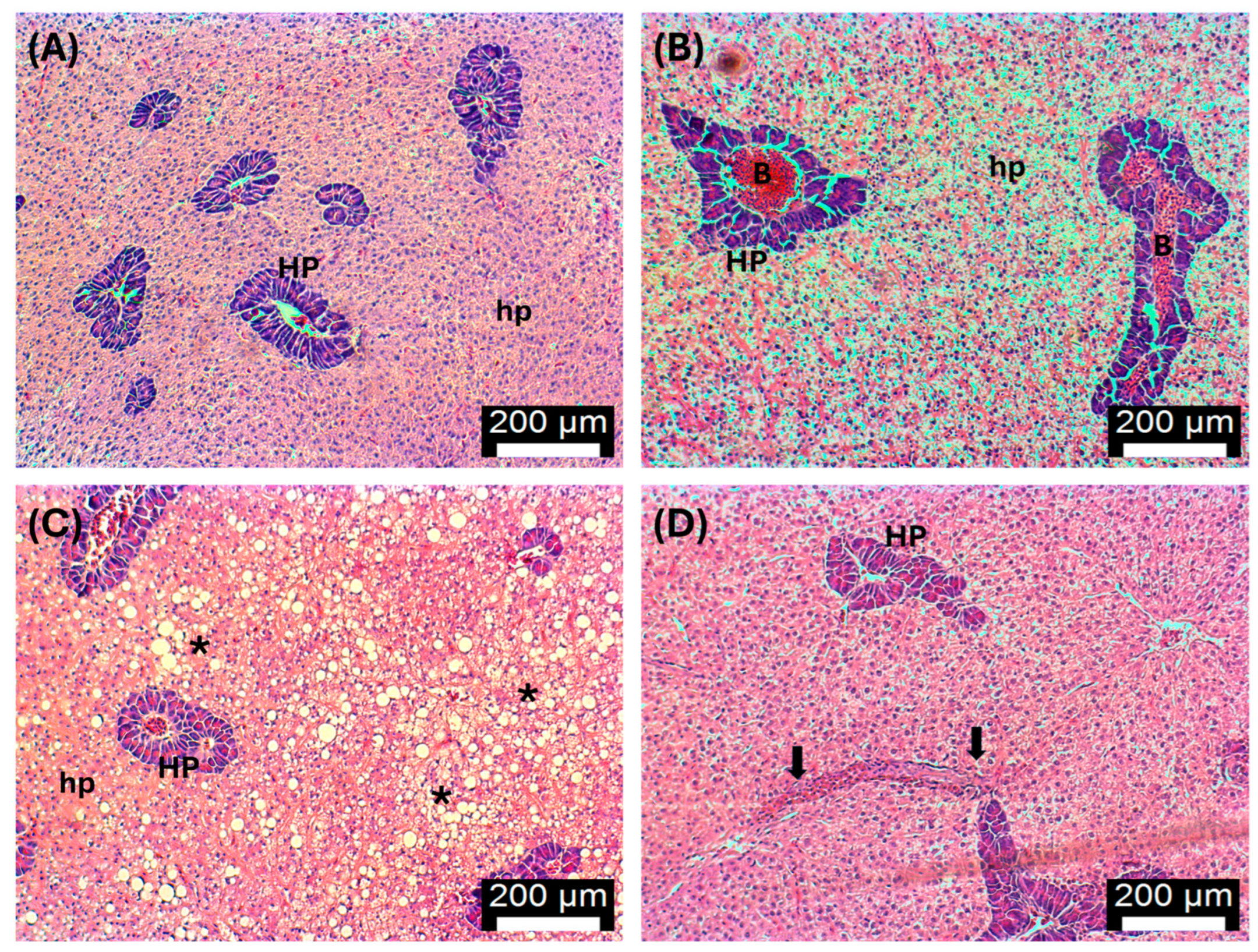
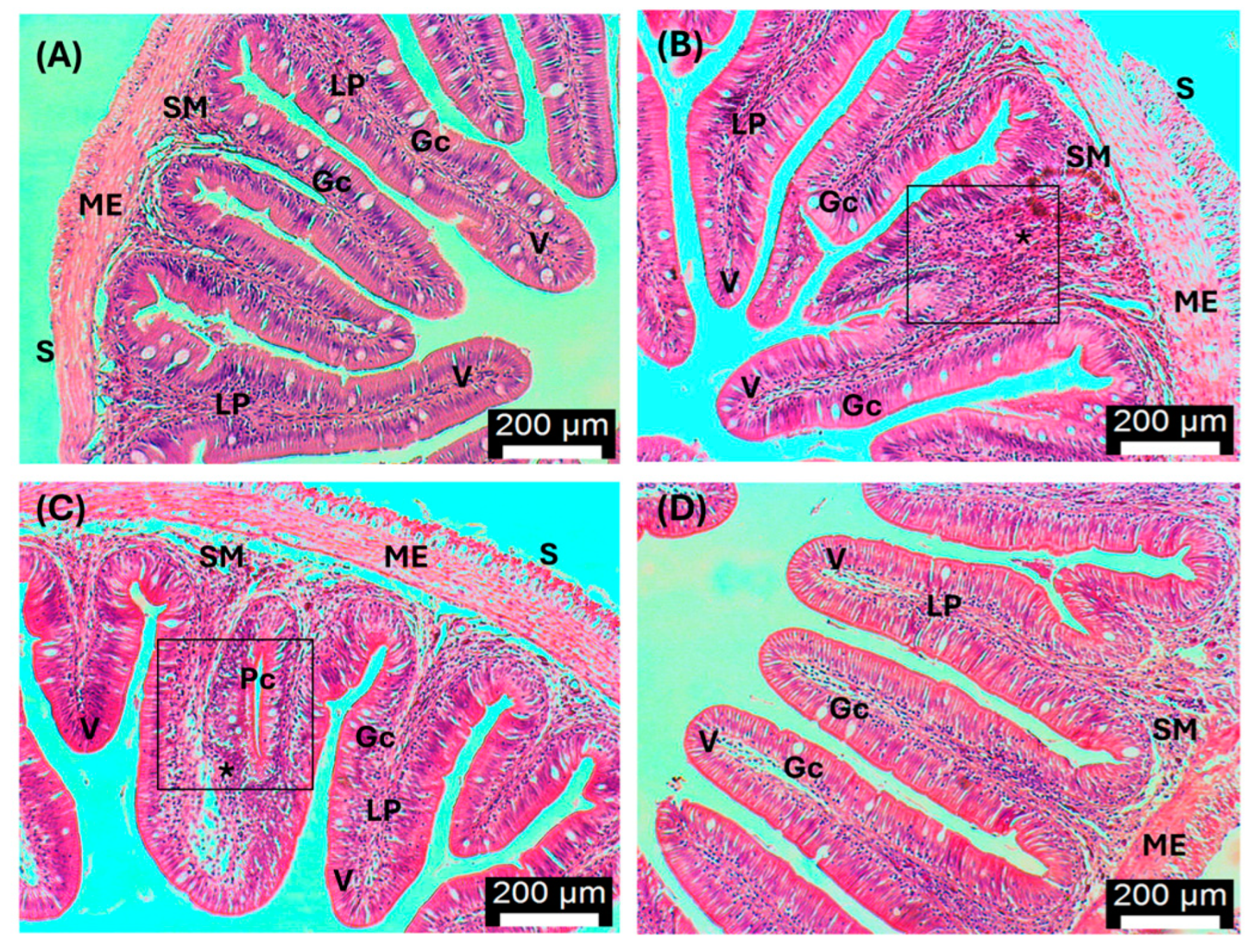
3.5. Histopathology
4. Discussion
4.1. Biofortification with A. taxiformis
4.2. Interactive Effects of MHW and A. taxiformis Biofortification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbosa, A.B. Seasonal and Interannual Variability of Planktonic Microbes in a Mesotidal Coastal Lagoon (Ria Formosa, SE Portugal): Impact of Climatic Changes and Local Human Influences. In Coastal Lagoons: Critical Habitats of Environmental Change; Paerl, H., Kennish, M., Eds.; Marine Science Book Series; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 335–366. [Google Scholar]
- Cascarano, M.C.; Stavrakidis-Zachou, O.; Mladineo, I.; Thompson, K.D.; Papandroulakis, N.; Katharios, P. Mediterranean Aquaculture in a Changing Climate: Temperature Effects on Pathogens and Diseases of Three Farmed Fish Species. Pathogens 2021, 10, 1205. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A Hierarchical Approach to Defining Marine Heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and More Frequent Marine Heatwaves over the Past Century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Frölicher, T.L.; Fischer, E.M.; Gruber, N. Marine Heatwaves under Global Warming. Nature 2018, 560, 360–364. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Burrows, M.T.; Donat, M.G.; Sen Gupta, A.; Alexander, L.V.; Perkins-Kirkpatrick, S.E.; Benthuysen, J.A.; Hobday, A.J.; Holbrook, N.J.; Moore, P.J.; et al. Projected Marine Heatwaves in the 21st Century and the Potential for Ecological Impact. Front. Mar. Sci. 2019, 6, 734. [Google Scholar] [CrossRef]
- Wu, F.; Yang, C.; Wen, H.; Zhang, C.; Jiang, M.; Liu, W.; Tian, J.; Yu, L.; Lu, X. Improving Low-temperature Stress Tolerance of Tilapia, Oreochromis niloticus: A Functional Analysis of Astragalus membranaceus. J. World Aquac. Soc. 2019, 50, 749–762. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Bögner, M.; Meyer, A.; Thiele, R.; James Slater, M. Metabolic and Molecular Stress Responses of European Seabass, Dicentrarchus labrax at Low and High Temperature Extremes. Ecol. Indic. 2020, 112, 106118. [Google Scholar] [CrossRef]
- Islam, M.J.; Slater, M.J.; Bögner, M.; Zeytin, S.; Kunzmann, A. Extreme Ambient Temperature Effects in European Seabass, Dicentrarchus labrax: Growth Performance and Hemato-Biochemical Parameters. Aquaculture 2020, 522, 735093. [Google Scholar] [CrossRef]
- Tejpal, C.S.; Sumitha, E.B.; Pal, A.K.; Shivananda Murthy, H.; Sahu, N.P.; Siddaiah, G.M. Effect of Dietary Supplementation of L-Tryptophan on Thermal Tolerance and Oxygen Consumption Rate in Cirrhinus mrigala Fingerlings under Varied Stocking Density. J. Therm. Biol. 2014, 41, 59–64. [Google Scholar] [CrossRef]
- Madeira, D.; Vinagre, C.; Diniz, M.S. Are Fish in Hot Water? Effects of Warming on Oxidative Stress Metabolism in the Commercial Species Sparus aurata. Ecol. Indic. 2016, 63, 324–331. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Camacho, C.; Barbosa, V.; Alves, R.; Anacleto, P.; Cunha, S.C.; Fernandes, J.O.; Pousão-Ferreira, P.; Paula, J.R.; Rosa, R.; et al. Bioaccumulation and Ecotoxicological Responses of Juvenile White Seabream (Diplodus sargus) Exposed to Triclosan, Warming and Acidification. Environ. Pollut. 2019, 245, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Marques, A.; Nunes, M.L. Impact of Climate Change in Mediterranean Aquaculture. Rev. Aquac. 2012, 4, 163–177. [Google Scholar] [CrossRef]
- Steiner, K.; Laroche, O.; Walker, S.P.; Symonds, J.E. Effects of Water Temperature on the Gut Microbiome and Physiology of Chinook Salmon (Oncorhynchus tshawytscha) Reared in a Freshwater Recirculating System. Aquaculture 2022, 560, 738529. [Google Scholar] [CrossRef]
- Holbrook, N.J.; Sen Gupta, A.; Oliver, E.C.J.; Hobday, A.J.; Benthuysen, J.A.; Scannell, H.A.; Smale, D.A.; Wernberg, T. Keeping Pace with Marine Heatwaves. Nat. Rev. Earth Environ. 2020, 1, 482–493. [Google Scholar] [CrossRef]
- Grimmelpont, M.; Milinkovitch, T.; Dubillot, E.; Lefrançois, C. Individual Aerobic Performance and Anaerobic Compensation in a Temperate Fish during a Simulated Marine Heatwave. Sci. Total Environ. 2023, 863, 160844. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Puebla, O.; Kunzmann, A. Nutritional Mitigation of Heatwave Stress in European Seabass, Dicentrarchus labrax: Metabolic, Cellular, and Molecular Responses. Aquac. Rep. 2024, 36, 102168. [Google Scholar] [CrossRef]
- D’Abramo, L.R.; Slater, M.J. Climate Change: Response and Role of Global Aquaculture. J. World Aquac. Soc. 2019, 50, 710–714. [Google Scholar] [CrossRef]
- Hassaan, M.S.; El Nagar, A.G.; Salim, H.S.; Fitzsimmons, K.; El-Haroun, E.R. Nutritional Mitigation of Winter Thermal Stress in Nile Tilapia by Propolis-Extract: Associated Indicators of Nutritional Status, Physiological Responses and Transcriptional Response of Delta-9-Desaturase Gene. Aquaculture 2019, 511, 734256. [Google Scholar] [CrossRef]
- Herrera, M.; Mancera, J.M.; Costas, B. The Use of Dietary Additives in Fish Stress Mitigation: Comparative Endocrine and Physiological Responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef]
- Ale, A.; Bacchetta, C.; Rossi, A.S.; Scarabotti, P.A.; Cazenave, J. Low Temperature Stress in a Cultured Fish (Piaractus Mesopotamicus) Fed with Pyropia columbina Red Seaweed-Supplemented Diet. Fish Physiol. Biochem. 2021, 47, 829–839. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Paul, N.A. Meta-analysis of the Use of Seaweeds and Their Extracts as Immunostimulants for Fish: A Systematic Review. Rev. Aquac. 2021, 13, 907–933. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary Inclusion of the Red Seaweed Asparagopsis taxiformis Boosts Production, Stimulates Immune Response and Modulates Gut Microbiota in Atlantic Salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Nahas, R.; Abatis, D.; Anagnostopoulou, M.; Kefalas, P.; Vagias, C.; Roussis, V. Radical-Scavenging Activity of Aegean sea Marine Algae. Food Chem. 2007, 102, 577–581. [Google Scholar] [CrossRef]
- Wang, B.-G.; Zhang, W.-W.; Duan, X.-J.; Li, X.-M. In Vitro Antioxidative Activities of Extract and Semi-Purified Fractions of the Marine Red Alga, Rhodomela confervoides (Rhodomelaceae). Food Chem. 2009, 113, 1101–1105. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current Knowledge and Future Perspectives of the Use of Seaweeds for Livestock Production and Meat Quality: A Systematic Review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef] [PubMed]
- Wassef, E.; El-sayed, A.; Kandeel, K.; Sakr, E. Evaluation of Pterocla dia (Rhodophyta) and Ulva (Chlorophyta) Meals as Additives to Gilthead Sea Bream Sparus aurata Diets. Egypt. J. Aquat. Res. 2005, 31, 321–332. [Google Scholar]
- Peixoto, M.J.; Salas-Leitón, E.; Pereira, L.F.; Queiroz, A.; Magalhães, F.; Pereira, R.; Abreu, H.; Reis, P.A.; Gonçalves, J.F.M.; Ozório, R.O.D.A. Role of Dietary Seaweed Supplementation on Growth Performance, Digestive Capacity and Immune and Stress Responsiveness in European Seabass (Dicentrarchus labrax). Aquac. Rep. 2016, 3, 189–197. [Google Scholar] [CrossRef]
- Lozano Muñoz, I.; Wacyk, J.; Perez, C.; Carrasco, J.; Martin, M.C.-S. Diets Enriched in Red Seaweed (Pyropia columbina and Gracilaria chilensis) Cryo Concentrates Modulate the Immune-Relevant Gene Encoding the Mx Antiviral Protein in Salmon (Salmo salar) White Blood Cells. J. Appl. Phycol. 2019, 31, 1415–1424. [Google Scholar] [CrossRef]
- Pezeshk, F.; Babaei, S.; Abedian Kenari, A.; Hedayati, M.; Naseri, M. The Effect of Supplementing Diets with Extracts Derived from Three Different Species of Macroalgae on Growth, Thermal Stress Resistance, Antioxidant Enzyme Activities and Skin Colour of Electric Yellow Cichlid (Labidochromis caeruleus). Aquac. Nutr. 2019, 25, 436–443. [Google Scholar] [CrossRef]
- Marino, F.; Di Caro, G.; Gugliandolo, C.; Spanò, A.; Faggio, C.; Genovese, G.; Morabito, M.; Russo, A.; Barreca, D.; Fazio, F.; et al. Preliminary Study on the In Vitro and In Vivo Effects of Asparagopsis taxiformis Bioactive Phycoderivates on Teleosts. Front. Physiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Gofas, S.; Verlaque, M.; Cinar, M.E.; Garcia Raso, J.E.; Bianchi, C.N.; Morri, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien Species in the Mediterranean Sea by 2010. A Contribution to the Application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial Distribution. Mediterr. Mar. Sci. 2010, 11, 381. [Google Scholar] [CrossRef]
- Zanolla, M.; Altamirano, M.; Carmona, R.; De La Rosa, J.; Sherwood, A.; Andreakis, N. Photosynthetic Plasticity of the Genus Asparagopsis (Bonnemaisoniales, Rhodophyta) in Response to Temperature: Implications for Invasiveness. Biol. Invasions 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- FAO Fisheries and Aquaculture Department. Species Fact Sheets: Diplodus sargus (Valenciennes, 1830). 2018. Available online: http://www.fao.org/fishery/species/2370/en (accessed on 1 February 2024).
- Santos, C.; Soares, F.; Candeias-Mendes, A.; Pousão-Ferreira, P.; Dinis, M.T.; Cortes Valente Oliveira, C. Characterization of Spawning Rhythms of a Sparidae Aquaculture Species, the White Seabream (Diplodus sargus), in the South of Portugal. Aquac. Res. 2022, 53, 1424–1434. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C. Thermal Tolerance and Potential Impacts of Climate Change on Coastal and Estuarine Organisms. J. Sea Res. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Madeira, D.; Costa, P.M.; Vinagre, C.; Diniz, M.S. When Warming Hits Harder: Survival, Cellular Stress and Thermal Limits of Sparus aurata Larvae under Global Change. Mar. Biol. 2016, 163, 91. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Urea, E.; Sanz, A. Comparative Study of Digestive Enzymes in Fish with Different Nutritional Habits. Proteolytic and Amylase Activities. Aquaculture 1999, 170, 267–283. [Google Scholar] [CrossRef]
- Kolkovski, S. Digestive Enzymes in Fish Larvae and Juveniles—Implications and Applications to Formulated Diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Furné, M.; García-Gallego, M.; Hidalgo, M.C.; Morales, A.E.; Domezain, A.; Domezain, J.; Sanz, A. Effect of Starvation and Refeeding on Digestive Enzyme Activities in Sturgeon (Acipenser naccarii) and Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, M.; Omstedt, A. Recent sea surface temperature trends and future scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Hobday, A.J.; Oliver, E.C.J.; Gupta, A.S.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J.; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorizing and Naming Marine Heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Custódio, A.; Anacleto, P.; Repolho, T.; Pousão Ferreira, P.; Rosa, R.; Marques, A.; Diniz, M. Ecophysiological Responses of Juvenile Seabass (Dicentrarchus labrax) Exposed to Increased Temperature and Dietary Methylmercury. Sci. Total Environ. 2017, 586, 551–558. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Marmelo, I.; Dias, M.; Grade, A.; Pousão-Ferreira, P.; Diniz, M.S.; Marques, A.; Maulvault, A.L. Immunomodulatory and Antioxidant Effects of Functional Aquafeeds Biofortified with Whole Laminaria digitata in Juvenile Gilthead Seabream (Sparus aurata). Front. Mar. Sci. 2024, 11, 1325244. [Google Scholar] [CrossRef]
- Rosa, R.; Ricardo Paula, J.; Sampaio, E.; Pimentel, M.; Lopes, A.R.; Baptista, M.; Guerreiro, M.; Santos, C.; Campos, D.; Almeida-Val, V.M.F.; et al. Neuro-Oxidative Damage and Aerobic Potential Loss of Sharks under Elevated CO2 and Warming. Mar. Biol. 2016, 163, 119. [Google Scholar] [CrossRef]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory Effects of Edible Seaweeds, Polyphenolics and Alginates on the Activities of Porcine Pancreatic α-Amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Anson, M.L. The Estimation of Pepsin, Ttypsin, Papain, and Cathepsin with Hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Worthington, T.M. Enzyme and Related Biochemicals; Biochemical Products Division; Worthington Diagnostics System Inc.: Freehold, NJ, USA, 1982. [Google Scholar]
- Comabella, Y.; Mendoza, R.; Aguilera, C.; Carrillo, O.; Hurtado, A.; García-Galano, T. Digestive Enzyme Activity during Early Larval Development of the Cuban Gar Atractosteus tristoechus. Fish Physiol. Biochem. 2006, 32, 147–157. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The Preparation and Properties of Two New Chromogenic Substrates of Trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B. Proteolytic Degradation of Sardine (Sardinella gibbosa) Proteins by Trypsin from Skipjack Tuna (Katsuwonus pelamis) Spleen. Food Chem. 2006, 98, 14–22. [Google Scholar] [CrossRef]
- Caspers, H. An Atlas of Fish Histology. Normal and Pathological Features. In International Review of Hydrobiology; Hibiya, T., Ed.; John Wiley and Sons: Stuttgart, NY, USA, 1984; Volume 69, p. 406. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Thiele, R.; Slater, M.J. Effects of Extreme Ambient Temperature in European Seabass, Dicentrarchus labrax Acclimated at Different Salinities: Growth Performance, Metabolic and Molecular Stress Responses. Sci. Total Environ. 2020, 735, 139371. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, E.; Jafari, M. Effects of Dietary Supplementation with Red Seaweed, Gracilaria pygmaea, on Growth, Carcass Composition and Hematology of Juvenile Rainbow Trout, Oncorhynchus mykiss. Aquac. Int. 2017, 25, 1857–1867. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vidhya Hindu, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Differential Solvent Extraction of Two Seaweeds and Their Efficacy in Controlling Aeromonas salmonicida Infection in Oreochromis mossambicus: A Novel Therapeutic Approach. Aquaculture 2015, 443, 56–64. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and Nutritional Value of the Chilean Seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Castro, C.; Peréz-Jiménez, A.; Coutinho, F.; Díaz-Rosales, P.; Serra, C.A.D.R.; Panserat, S.; Corraze, G.; Peres, H.; Oliva-Teles, A. Dietary Carbohydrate and Lipid Sources Affect Differently the Oxidative Status of European Sea Bass (Dicentrarchus labrax) Juveniles. Br. J. Nutr. 2015, 114, 1584–1593. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Rufino-Palomares, E.; Ordóñez-Grande, B.; Gallardo, M.A.; Oliva-Teles, A.; Peres, H. Dietary Glutamine Supplementation Effects on Amino Acid Metabolism, Intestinal Nutrient Absorption Capacity and Antioxidant Response of Gilthead Sea Bream (Sparus aurata) Juveniles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Engrola, S.; Conceição, L.E.C.; Dias, L.; Pereira, R.; Ribeiro, L.; Dinis, M.T. Improving Weaning Strategies for Senegalese sole: Effects of Body Weight and Digestive Capacity. Aquac. Res. 2007, 38, 696–707. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; López, G.; Sáez, M.I.; Jiménez, J.A.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Effects of the Microalga Scenedesmus almeriensis as Fishmeal Alternative in Diets for Gilthead Sea Bream, Sparus aurata, Juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Mazumder, S.K.; Das, S.K.; Rahim, S.M.; Ghaffar, M.A. Temperature and Diet Effect on the Pepsin Enzyme Activities, Digestive Somatic Index and Relative Gut Length of Malabar Blood Snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Aquac. Rep. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Sandblom, E.; Clark, T.D.; Gräns, A.; Ekström, A.; Brijs, J.; Sundström, L.F.; Odelström, A.; Adill, A.; Aho, T.; Jutfelt, F. Physiological Constraints to Climate Warming in Fish Follow Principles of Plastic Floors and Concrete Ceilings. Nat. Commun. 2016, 7, 11447. [Google Scholar] [CrossRef]
- Anacleto, P.; Figueiredo, C.; Baptista, M.; Maulvault, A.L.; Camacho, C.; Pousão-Ferreira, P.; Valente, L.M.P.; Marques, A.; Rosa, R. Fish Energy Budget under Ocean Warming and Flame Retardant Exposure. Environ. Res. 2018, 164, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Barbosa, V.; Alves, R.; Anacleto, P.; Camacho, C.; Cunha, S.; Fernandes, J.O.; Ferreira, P.P.; Rosa, R.; Marques, A.; et al. Integrated Multi-Biomarker Responses of Juvenile Seabass to Diclofenac, Warming and Acidification Co-Exposure. Aquat. Toxicol. 2018, 202, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Filipe, D.; Gonçalves, M.; Fernandes, H.; Oliva-Teles, A.; Peres, H.; Belo, I.; Salgado, J.M. Shelf-Life Performance of Fish Feed Supplemented with Bioactive Extracts from Fermented Olive Mill and Winery By-Products. Foods 2023, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.E.; Alagawany, M.; Patra, A.K.; El-Kholy, A.I.; Amer, M.S.; Abd El-Hack, M.E. Beneficial Impacts and Health Benefits of Macroalgae Phenolic Molecules on Fish Production. Aquaculture 2021, 534, 736186. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative Stress in Marine Enviroments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, H.; Zhang, D.; Wang, C.; Hao, R.; Ru, X.; Hu, Q.; Huang, Y.; Zhu, C. Effect of Marine Heatwaves on Juvenile Greater Amberjack (Seriola dumerili). Mar. Environ. Res. 2024, 193, 106302. [Google Scholar] [CrossRef] [PubMed]
- Banh, S.; Wiens, L.; Sotiri, E.; Treberg, J.R. Mitochondrial Reactive Oxygen Species Production by Fish Muscle Mitochondria: Potential Role in Acute Heat-Induced Oxidative Stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 191, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Bacchetta, C.; Cazenave, J. Effect of Thermal Stress on Metabolic and Oxidative Stress Biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol. Indic. 2017, 79, 361–370. [Google Scholar] [CrossRef]
- Pörtner, H. Integrating Climate-Related Stressor Effects on Marine Organisms: Unifying Principles Linking Molecule to Ecosystem-Level Changes. Mar. Ecol. Prog. Ser. 2012, 470, 273–290. [Google Scholar] [CrossRef]
- Strobel, A.; Leo, E.; Pörtner, H.O.; Mark, F.C. Elevated Temperature and PCO2 Shift Metabolic Pathways in Differentially Oxidative Tissues of Notothenia rossii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 166, 48–57. [Google Scholar] [CrossRef]
- Kumar, V.; Sahu, N.P.; Pal, A.K.; Kumar, S.; Sinha, A.K.; Ranjan, J.; Baruah, K. Modulation of Key Enzymes of Glycolysis, Gluconeogenesis, Amino Acid Catabolism, and TCA Cycle of the Tropical Freshwater Fish Labeo rohita Fed Gelatinized and Non-Gelatinized Starch Diet. Fish Physiol. Biochem. 2010, 36, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Makri, V.; Giantsis, I.A.; Nathanailides, C.; Feidantsis, K.; Antonopoulou, E.; Theodorou, J.A.; Michaelidis, B. Seasonal Energy Investment and Metabolic Patterns in a Farmed Fish. J. Therm. Biol. 2024, 123, 103894. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of Temperature on Feeding and Digestive Processes in Fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.F.; Peixoto, M.J.; Carvalho, P.; Sansuwan, K.; Santos, G.A.; Gonçalves, J.F.M.; Ozório, R.O.A. Cross-Effects of Dietary Probiotic Supplementation and Rearing Temperature on Growth Performance, Digestive Enzyme Activities, Cumulative Mortality and Innate Immune Response in Seabass (Dicentrarchus labrax). Aquac. Nutr. 2018, 24, 453–460. [Google Scholar] [CrossRef]
- Alexander, C.; Sahu, N.P.; Pal, A.K.; Akhtar, M.S.; Saravanan, S.; Xavier, B.; Munilkumar, S. Higher Water Temperature Enhances Dietary Carbohydrate Utilization and Growth Performance in Labeo rohita (Hamilton) Fingerlings: Carbohydrate Utilization in Labeo rohita. J. Anim. Physiol. Anim. Nutr. 2011, 95, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A. Improving the Mechanical Characteristics of Faecal Waste in Rainbow Trout: The Influence of Fish Size and Treatment with a Non-Starch Polysaccharide (Guar Gum). Aquac. Nutr. 2009, 15, 229–240. [Google Scholar] [CrossRef]
- Xuan, X.; Wen, X.; Li, S.; Zhu, D.; Li, Y. Potential Use of Macro-Algae Gracilaria lemaneiformis in Diets for the Black Sea Bream, Acanthopagrus schlegelii, Juvenile. Aquaculture 2013, 412, 167–172. [Google Scholar] [CrossRef]
- Ikegami, S.; Tsuchihashi, F.; Harada, H.; Tsuchihashi, N.; Nishide, E.; Innami, S. Effect of Viscous Indigestible Polysaccharides on Pancreatic-Biliary Secretion and Digestive Organs in Rats. J. Nutr. 1990, 120, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A Review of Interactions between Dietary Fibre and the Intestinal Mucosa, and Their Consequences on Digestive Health in Young Non-Ruminant Animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Kokou, F.; Rigos, G.; Kentouri, M.; Alexis, M. Effects of DL-Methionine-Supplemented Dietary Soy Protein Concentrate on Growth Performance and Intestinal Enzyme Activity of Gilthead Sea Bream (Sparus aurata L.). Aquac. Int. 2016, 24, 257–271. [Google Scholar] [CrossRef]

| Diets | ||||
|---|---|---|---|---|
| Ingredients (%) | CTR | 1.5-AT | 3-AT | 6-AT |
| Fishmeal Super Prime a | 25.0 | 25.0 | 25.0 | 25.0 |
| Fish protein concentrate b | 2.0 | 2.0 | 2.0 | 2.0 |
| Soy protein concentrate c | 10.0 | 10.0 | 10.0 | 10.0 |
| Pea protein concentrate d | 3.0 | 3.0 | 3.0 | 3.0 |
| Wheat gluten e | 6.5 | 6.5 | 6.5 | 6.5 |
| Corn gluten meal f | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean meal 44 g | 6.0 | 6.0 | 6.0 | 6.0 |
| Rapeseed meal h | 6.0 | 6.0 | 6.0 | 6.0 |
| Wheat meal i | 10.8 | 9.3 | 7.8 | 4.8 |
| Faba beans (low tannins) j | 6.0 | 6.0 | 6.0 | 6.0 |
| Vitamin and mineral premix k | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride 50% l | 0.2 | 0.2 | 0.2 | 0.2 |
| Monoammonium phosphate m | 1.2 | 1.2 | 1.2 | 1.2 |
| Fish oil n | 5.0 | 5.0 | 5.0 | 5.0 |
| Soybean oil o | 7.3 | 7.3 | 7.3 | 7.3 |
| Asparagopsis taxiformis p | - | 1.5 | 3.0 | 6.0 |
| Proximate composition (%) | ||||
| Dry matter, DM (%) | 94.2 | 94.0 | 93.9 | 94.1 |
| Crude protein | 46.0 | 46.0 | 45.9 | 45.7 |
| Crude fat | 16.0 | 16.0 | 16.1 | 16.1 |
| Fiber | 1.8 | 1.9 | 2.0 | 2.1 |
| Starch | 13.7 | 12.8 | 11.8 | 9.9 |
| Ash | 6.8 | 7.1 | 7.4 | 8.0 |
| Gross energy (MJ/kg feed) | 21.0 | 21.0 | 20.9 | 20.8 |
| W (g) | TL (cm) | K | HSI (%) | WG (%) | SGR (% day−1) | FCR | ||
|---|---|---|---|---|---|---|---|---|
| T30 | CTR | 48.98 ± 7.41 | 13.95 ± 0.75 | 1.80 ± 0.23 | 0.67 ± 0.10 | 83.36 ± 16.17 | 1.71 ± 0.25 | 0.74 ± 0.16 |
| 1.5-AT | 52.23 ± 9.18 | 14.28 ± 0.78 | 1.79 ± 0.23 | 0.59 ± 0.05 | 92.06 ± 26.74 | 2.37 ± 0.36 | 0.69 ± 0.18 | |
| 3-AT | 56.47 ± 11.32 | 14.38 ± 0.86 | 1.88 ± 0.09 | 0.60 ± 0.10 | 83.66 ± 19.90 | 1.78 ± 0.35 | 0.75 ± 0.18 | |
| 6-AT | 50.31 ± 8.11 | 14.28 ± 0.90 | 1.73 ± 0.24 | 0.58 ± 0.14 | 66.86 ± 17.63 | 1.70 ± 0.26 | 0.95 ± 0.23 | |
| T53 | CTR | 64.78 ± 10.29 | 15.18 ± 0.99 | 1.85 ± 0.20 | 0.70 ± 0.18 | 138.63 ± 32.83 | 1.56 ± 0.31 | 0.78 ± 0.18 |
| CTR-HW | 56.55 ± 10.49 | 14.55 ± 0.82 | 1.82 ± 0.06 | 0.50 ± 0.08 | 107.42 ± 32.83 | 1.29 ± 0.31 | 1.05 ± 0.35 | |
| 1.5-AT | 53.49 ± 9.12 | 14.47 ± 0.95 | 1.76 ± 0.18 | 0.54 ± 0.09 | 96.71 ± 25.87 | 1.19 ± 0.33 | 1.14 ± 0.30 | |
| 3-AT | 57.41 ± 7.49 | 15.03 ± 0.74 | 1.69 ± 0.10 | 0.52 ± 0.09 | 94.36 ± 22.12 | 1.33 ± 0.25 | 1.15 ± 0.24 | |
| 6-AT | 55.95 ± 9.71 | 14.92 ± 0.71 | 1.67 ± 0.12 | 0.49 ± 0.07 | 84.19 ± 18.58 | 1.27 ± 0.32 | 1.29 ± 0.29 | |
| T61 | CTR | 65.28 ± 13.17 | 15.22 ± 1.13 | 1.84 ± 0.16 a | 0.54 ± 0.25 | 155.31 ± 20.12 a | 1.37 ± 0.38 | 0.77 ± 0.10 |
| CTR-HW | 56.01 ± 9.82 | 14.83 ± 0.79 | 1.70 ± 0.15 ab | 0.77 ± 0.19 | 109.33 ± 15.96 b | 1.12 ± 0.34 | 1.10 ± 0.16 | |
| 1.5-AT | 58.86 ± 6.96 | 15.15 ± 0.55 | 1.69 ± 0.15 ab | 0.48 ± 0.14 | 111.81 ± 23.15 bc | 1.22 ± 0.20 | 1.22 ± 0.25 | |
| 3-AT | 56.03 ± 5.79 | 14.82 ± 0.64 | 1.72 ± 0.05 ab | 0.64 ± 0.13 | 102.96 ± 14.63 bc | 1.14 ± 0.18 | 1.22 ± 0.12 | |
| 6-AT | 54.69 ± 11.60 | 14.90 ± 0.99 | 1.64 ± 0.09 b | 0.72 ± 0.29 | 76.22 ± 15.16 c | 1.08 ± 0.33 | 1.33 ± 0.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Marmelo, I.; Dias, M.; Silva, A.C.; Grade, A.C.; Barata, M.; Pousão-Ferreira, P.; Dias, J.; Anacleto, P.; Marques, A.; et al. Asparagopsis taxiformis as a Novel Antioxidant Ingredient for Climate-Smart Aquaculture: Antioxidant, Metabolic and Digestive Modulation in Juvenile White Seabream (Diplodus sargus) Exposed to a Marine Heatwave. Antioxidants 2024, 13, 949. https://doi.org/10.3390/antiox13080949
Pereira A, Marmelo I, Dias M, Silva AC, Grade AC, Barata M, Pousão-Ferreira P, Dias J, Anacleto P, Marques A, et al. Asparagopsis taxiformis as a Novel Antioxidant Ingredient for Climate-Smart Aquaculture: Antioxidant, Metabolic and Digestive Modulation in Juvenile White Seabream (Diplodus sargus) Exposed to a Marine Heatwave. Antioxidants. 2024; 13(8):949. https://doi.org/10.3390/antiox13080949
Chicago/Turabian StylePereira, Alícia, Isa Marmelo, Marta Dias, Ana Catarina Silva, Ana Catarina Grade, Marisa Barata, Pedro Pousão-Ferreira, Jorge Dias, Patrícia Anacleto, António Marques, and et al. 2024. "Asparagopsis taxiformis as a Novel Antioxidant Ingredient for Climate-Smart Aquaculture: Antioxidant, Metabolic and Digestive Modulation in Juvenile White Seabream (Diplodus sargus) Exposed to a Marine Heatwave" Antioxidants 13, no. 8: 949. https://doi.org/10.3390/antiox13080949
APA StylePereira, A., Marmelo, I., Dias, M., Silva, A. C., Grade, A. C., Barata, M., Pousão-Ferreira, P., Dias, J., Anacleto, P., Marques, A., Diniz, M. S., & Maulvault, A. L. (2024). Asparagopsis taxiformis as a Novel Antioxidant Ingredient for Climate-Smart Aquaculture: Antioxidant, Metabolic and Digestive Modulation in Juvenile White Seabream (Diplodus sargus) Exposed to a Marine Heatwave. Antioxidants, 13(8), 949. https://doi.org/10.3390/antiox13080949









