Sex Differences in Hepatic Inflammation, Lipid Metabolism, and Mitochondrial Function Following Early Lipopolysaccharide Exposure in Epileptic WAG/Rij Rats
Abstract
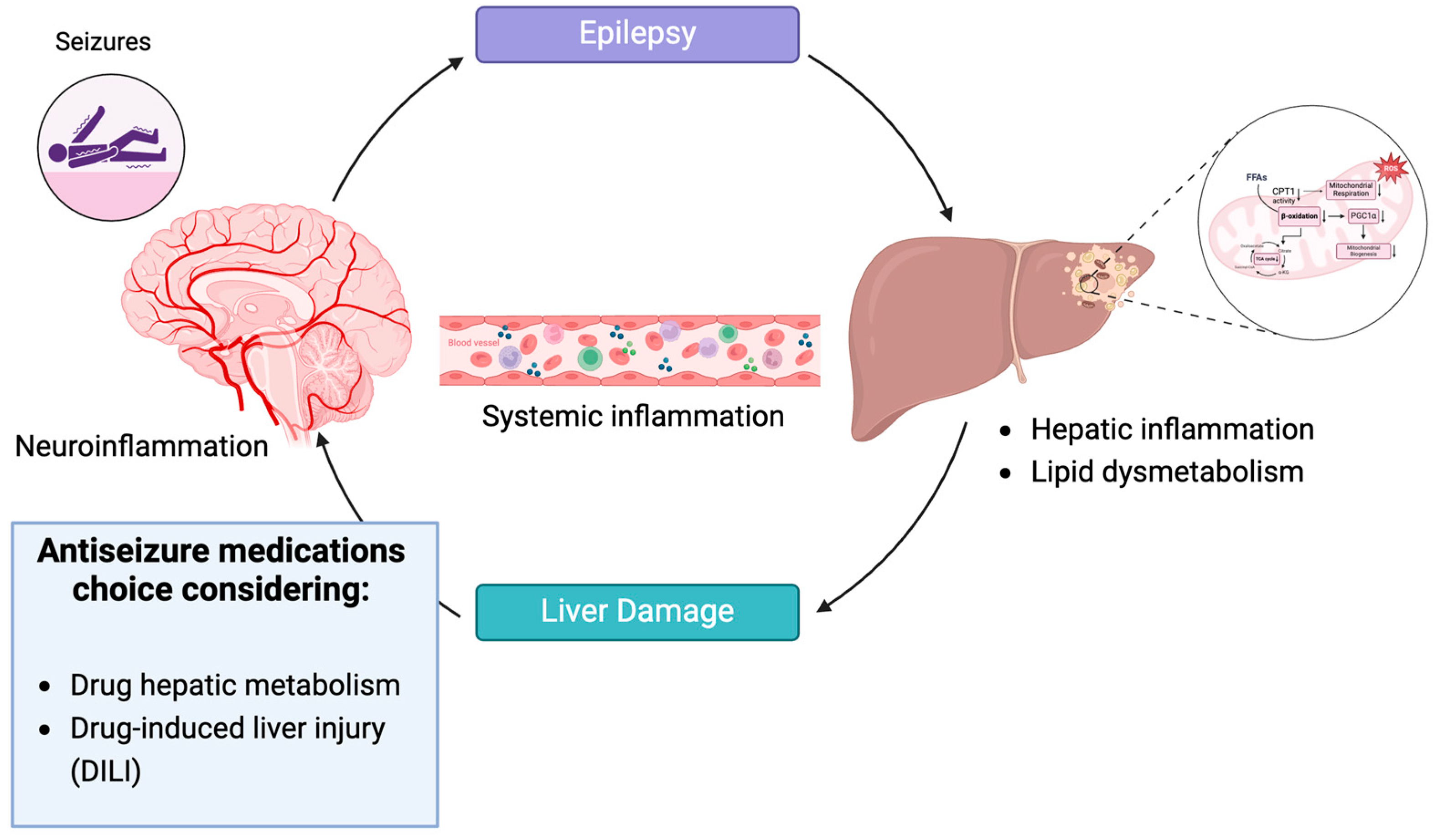
:1. Introduction
2. Materials and Methods
2.1. In Vivo Experimental Procedures and Ethics Statement
2.2. Biochemical Evaluations of Serum Hepatic Parameters, Lipid Profile, and Inflammatory/Immune Mediators
2.3. Mitochondrial Bioenergetics and Redox Status Evaluation
2.4. ROS Assay
2.5. RNA Extraction and Semi-Quantitative Real-Time (RT)-PCR
2.6. Statistical Analysis
3. Results
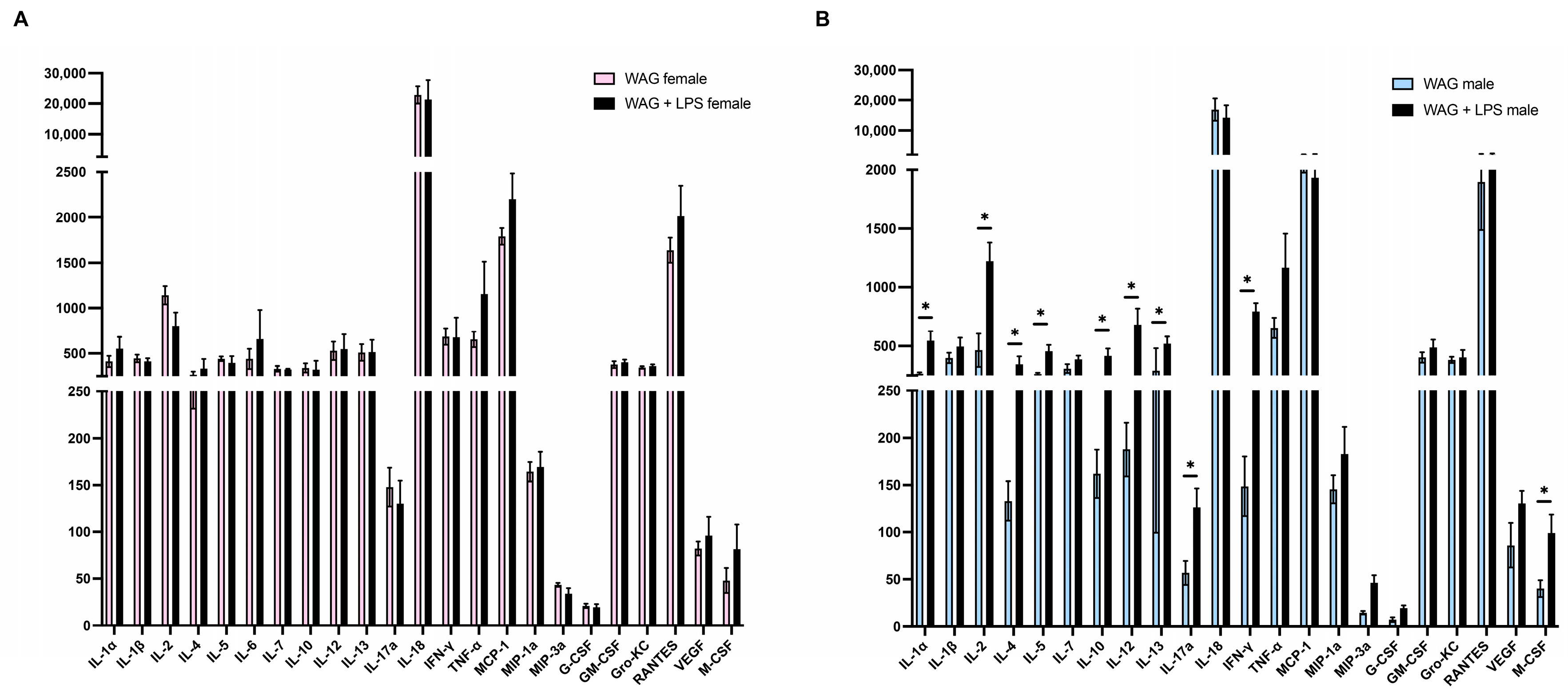
3.1. Gender Differences and LPS Effect on Biochemical Parameters and Inflammatory Mediators in Serum of WAG/Rij Rats
3.2. Hepatic Inflammation and Immune Response of Male and Female Epileptic Rats: Effect of an Early LPS Challenge
3.3. LPS-Driven Metabolic Alterations in the Liver of Epileptic Rats: Sex-Related Differences
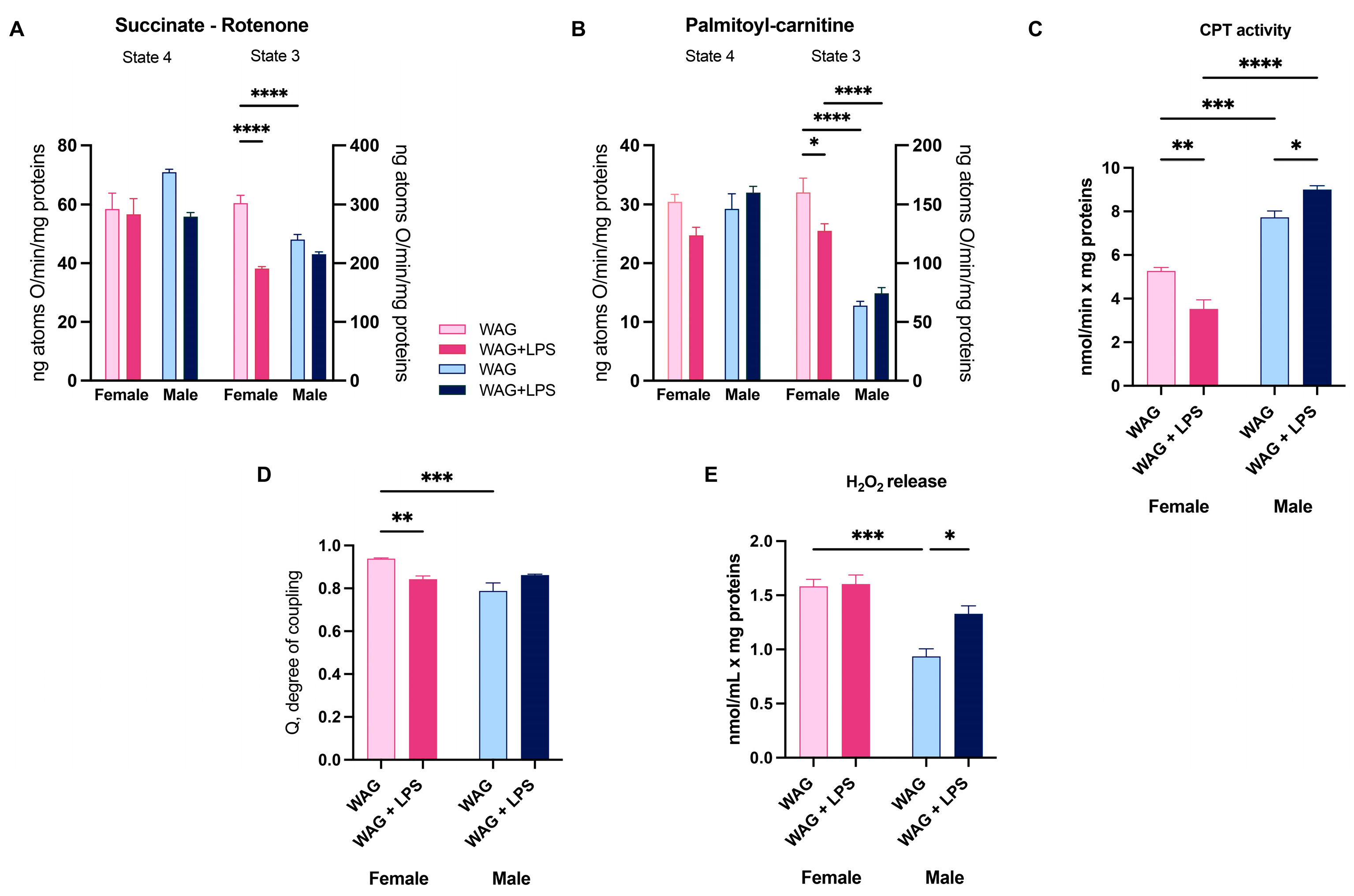
3.4. Effect of LPS on Hepatic Mitochondrial Bioenergetics in Male and Female Epileptic Rats
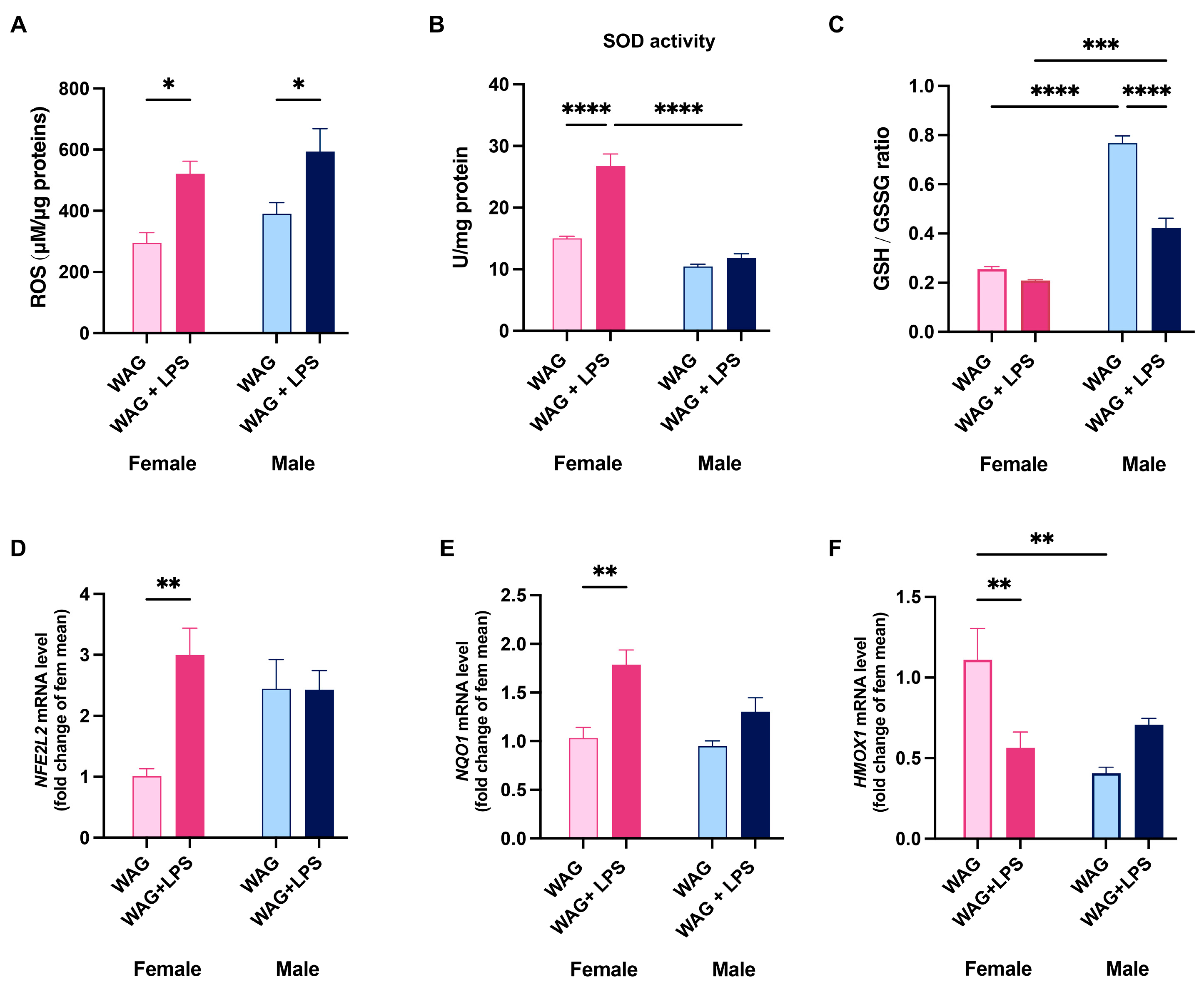
3.5. Sex-Related Susceptibility to Oxidative Stress of WAG/Rij Rats: Effect of LPS Injection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakagaki, B.N.; Mafra, K.; de Carvalho, E.; Lopes, M.E.; Carvalho-Gontijo, R.; de Castro-Oliveira, H.M.; Campolina-Silva, G.H.; de Miranda, C.D.M.; Antunes, M.M.; Silva, A.C.C.; et al. Immune and metabolic shifts during neonatal development reprogram liver identity and function. J. Hepatol. 2018, 69, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef]
- Szabo, G.; Romics, L., Jr.; Frendl, G. Liver in sepsis and systemic inflammatory response syndrome. Clin. Liver Dis. 2002, 6, 1045–1066. [Google Scholar] [CrossRef]
- Gao, B.; Jeong, W.I.; Tian, Z. Liver: An organ with predominant innate immunity. Hepatology 2008, 47, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M. Structural Modifications of Bacterial Lipopolysaccharide that Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Zhai, R.; Liang, H.; Song, G.; Yuan, Y.; Xu, Y.; Yan, Y.; Qiu, L.; Sun, T. Metformin attenuated sepsis-associated liver injury and inflammatory response in aged mice. Bioengineered 2022, 13, 4598–4609. [Google Scholar] [CrossRef]
- Hastings, K.L.; Green, M.D.; Gao, B.; Ganey, P.E.; Roth, R.A.; Burleson, G.R. Beyond Metabolism: Role of the Immune System in Hepatic Toxicity. Int. J. Toxicol. 2020, 39, 151–164. [Google Scholar] [CrossRef]
- Gu, J.; Luo, L.; Wang, Q.; Yan, S.; Lin, J.; Li, D.; Cao, B.; Mei, H.; Ying, B.; Bin, L.; et al. Maresin 1 attenuates mitochondrial dysfunction through the ALX/cAMP/ROS pathway in the cecal ligation and puncture mouse model and sepsis patients. Lab. Investig. 2018, 98, 715–733. [Google Scholar] [CrossRef]
- Markley, M.A.; Pierro, A.; Eaton, S. Hepatocyte mitochondrial metabolism is inhibited in neonatal rat endotoxaemia: Effects of glutamine. Clin. Sci. 2002, 102, 337–344. [Google Scholar] [CrossRef]
- Brealey, D.; Singer, M. Mitochondrial Dysfunction in Sepsis. Curr. Infect. Dis. Rep. 2003, 5, 365–371. [Google Scholar] [CrossRef]
- Ferro, J.M.; Oliveira, S. Neurologic manifestations of gastrointestinal and liver diseases. Curr. Neurol. Neurosci. Rep. 2014, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, D.; Solmaz, V.; Taskiran, D.; Erbas, O. The association between seizure predisposition and inflammation in a rat model of fatty liver disease. Neurol. Sci. 2014, 35, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, J.; Cui, P.; Zhou, Y.; Liu, C.; Wu, X.; Ji, Y.; Wang, S.; Cheng, B.; Ye, H.; et al. TREM2 sustains macrophage-hepatocyte metabolic coordination in nonalcoholic fatty liver disease and sepsis. J. Clin. Investig. 2021, 131, e135197. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and Gender Differences in Bacterial Infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; De Caro, C.; Citraro, R.; Russo, E.; Tallarico, M.; Iannone, M.; Ferrante, M.C.; et al. Butyrate prevents valproate-induced liver injury: In vitro and in vivo evidence. FASEB J. 2020, 34, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Cimmino, F.; Catapano, A.; Carta, G.; Pirozzi, C.; Murru, E.; Lama, A.; Meli, R.; Bergamo, P.; et al. Decreased Metabolic Flexibility in Skeletal Muscle of Rat Fed with a High-Fat Diet Is Recovered by Individual CLA Isomer Supplementation via Converging Protective Mechanisms. Cells 2020, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Cairns, C.B.; Walther, J.; Harken, A.H.; Banerjee, A. Mitochondrial oxidative phosphorylation thermodynamic efficiencies reflect physiological organ roles. Am. J. Physiol. 1998, 274, R1376–R1383. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, P.; Maurano, F.; Rossi, M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic. Biol. Med. 2007, 43, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Harbeson, D.; Francis, F.; Bao, W.; Amenyogbe, N.A.; Kollmann, T.R. Energy Demands of Early Life Drive a Disease Tolerant Phenotype and Dictate Outcome in Neonatal Bacterial Sepsis. Front. Immunol. 2018, 9, 1918. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr. Res. 2020, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Grayck, M.R.; McCarthy, W.C.; Solar, M.; Balasubramaniyan, N.; Zheng, L.; Orlicky, D.J.; Wright, C.J. Implications of neonatal absence of innate immune mediated NFkappaB/AP1 signaling in the murine liver. Pediatr. Res. 2024, 95, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Zarate, M.A.; Nguyen, L.M.; De Dios, R.K.; Zheng, L.; Wright, C.J. Maturation of the Acute Hepatic TLR4/NF-kappaB Mediated Innate Immune Response Is p65 Dependent in Mice. Front. Immunol. 2020, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Dembek, A.; Laggai, S.; Kessler, S.M.; Czepukojc, B.; Simon, Y.; Kiemer, A.K.; Hoppstadter, J. Hepatic interleukin-6 production is maintained during endotoxin tolerance and facilitates lipid accumulation. Immunobiology 2017, 222, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Bonkovsky, H.L.; Stine, J.G.; Gu, J.; Barnhart, H.; Jacobsen, E.; Bjornsson, E.; Fontana, R.J.; Kleiner, D.E.; Hoofnagle, J.H.; et al. Clinical characteristics of antiepileptic-induced liver injury in patients from the DILIN prospective study. J. Hepatol. 2022, 76, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Kamitaki, B.K.; Minacapelli, C.D.; Zhang, P.; Wachuku, C.; Gupta, K.; Catalano, C.; Rustgi, V. Drug-induced liver injury associated with antiseizure medications from the FDA Adverse Event Reporting System (FAERS). Epilepsy Behav. 2021, 117, 107832. [Google Scholar] [CrossRef]
- Fishel, M.A.; Watson, G.S.; Montine, T.J.; Wang, Q.; Green, P.S.; Kulstad, J.J.; Cook, D.G.; Peskind, E.R.; Baker, L.D.; Goldgaber, D.; et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch. Neurol. 2005, 62, 1539–1544. [Google Scholar] [CrossRef]
- Aomatsu, M.; Kato, T.; Kasahara, E.; Kitagawa, S. Gender difference in tumor necrosis factor-alpha production in human neutrophils stimulated by lipopolysaccharide and interferon-gamma. Biochem. Biophys. Res. Commun. 2013, 441, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, A.; Keel, M.; Zellweger, R.; Steckholzer, U.; Trentz, O.; Ertel, W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J. Trauma Acute Care Surg. 2000, 48, 932–937. [Google Scholar] [CrossRef]
- Frink, M.; Pape, H.C.; van Griensven, M.; Krettek, C.; Chaudry, I.H.; Hildebrand, F. Influence of sex and age on mods and cytokines after multiple injuries. Shock 2007, 27, 151–156. [Google Scholar] [CrossRef]
- Knoferl, M.W.; Angele, M.K.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit. Care Med. 2002, 30, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Knoferl, M.W.; Angele, M.K.; Diodato, M.D.; Schwacha, M.G.; Ayala, A.; Cioffi, W.G.; Bland, K.I.; Chaudry, I.H. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann. Surg. 2002, 235, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Kreem, S.; Shweash, M.; Paul, A.; Alexander, J.; Roberts, C.W. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J. Immunol. 2010, 185, 4525–4534. [Google Scholar] [CrossRef]
- Kyurkchiev, D.; Ivanova-Todorova, E.; Hayrabedyan, S.; Altankova, I.; Kyurkchiev, S. Female sex steroid hormones modify some regulatory properties of monocyte-derived dendritic cells. Am. J. Reprod. Immunol. 2007, 58, 425–433. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef]
- Angele, M.K.; Pratschke, S.; Hubbard, W.J.; Chaudry, I.H. Gender differences in sepsis: Cardiovascular and immunological aspects. Virulence 2014, 5, 12–19. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Baker, P.R., II; Friedman, J.E. Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease. J. Clin. Investig. 2018, 128, 3692–3703. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wen, Y.; Li, J.; Zhang, H.; Liu, Y. Prenatal Lipopolysaccharide Exposure Promotes Dyslipidemia in the Male Offspring Rats. Front. Physiol. 2018, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Santos, A.; Armendariz-Borunda, J. PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 8298. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005, 336, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bedoucha, M.; Atzpodien, E.; Boelsterli, U.A. Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J. Hepatol. 2001, 35, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, R.; Masih-Khan, E.; Lo, M.; van Breemen, C.; McManus, B.M.; Dube, G.P. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol. Cell Biochem. 2001, 224, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Brewer, B., Jr.; Reitman, M.L.; Gonzalez, F.J. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Investig. 2003, 111, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Terauchi, Y.; Kubota, N.; Takamoto, I.; Eto, K.; Yamashita, T.; Komeda, K.; Yamauchi, T.; Kamon, J.; Kita, S.; et al. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes 2004, 53, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; bin Ali, A.; Zhang, Q.; Loh, L.M.; Tan, C.E.; Retnam, L.; El Oakley, R.M.; Lim, S.K. Hepatic expression of PPARalpha, a molecular target of fibrates, is regulated during inflammation in a gender-specific manner. FEBS Lett. 2003, 546, 237–240. [Google Scholar] [CrossRef]
- Ohhira, M.; Motomura, W.; Fukuda, M.; Yoshizaki, T.; Takahashi, N.; Tanno, S.; Wakamiya, N.; Kohgo, Y.; Kumei, S.; Okumura, T. Lipopolysaccharide induces adipose differentiation-related protein expression and lipid accumulation in the liver through inhibition of fatty acid oxidation in mice. J. Gastroenterol. 2007, 42, 969–978. [Google Scholar] [CrossRef]
- Rao, M.S.; Reddy, J.K. PPARalpha in the pathogenesis of fatty liver disease. Hepatology 2004, 40, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Hashimoto, T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Sewal, R.K.; Modi, M.; Saikia, U.N.; Chakrabarti, A.; Medhi, B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res. 2017, 135, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Ramalingam, K.; Kumar, R.V. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure 2012, 21, 780–784. [Google Scholar] [CrossRef]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Expert Rev. Neurother. 2018, 18, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- Fabisiak, T.; Patel, M. Crosstalk between neuroinflammation and oxidative stress in epilepsy. Front. Cell Dev. Biol. 2022, 10, 976953. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S. Pathophysiology of mitochondrial disease causing epilepsy and status epilepticus. Epilepsy Behav. 2015, 49, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.P.; Waldbaum, S.; Rowley, S.; Huang, T.T.; Day, B.J.; Patel, M. Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: Attenuation by a lipophilic metalloporphyrin. Neurobiol. Dis. 2012, 45, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Teskey, G.; Abrahem, R.; Cao, R.; Gyurjian, K.; Islamoglu, H.; Lucero, M.; Martinez, A.; Paredes, E.; Salaiz, O.; Robinson, B.; et al. Glutathione as a Marker for Human Disease. Adv. Clin. Chem. 2018, 87, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.C.; Zaretsky, M.D.; Dubs, J.G.; Roederer, M.; Anderson, M.; Green, A.; Mitra, D.; Watanabe, N.; Nakamura, H.; Tjioe, I.; et al. N-acetylcysteine replenishes glutathione in HIV infection. Eur. J. Clin. Investig. 2000, 30, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Nesci, V.; Tallarico, M.; Amodio, N.; Cantafio, E.M.G.; De Sarro, G.; Constanti, A.; Russo, E.; Citraro, R. IL-6 Receptor Blockade by Tocilizumab Has Anti-absence and Anti-epileptogenic Effects in the WAG/Rij Rat Model of Absence Epilepsy. Neurotherapeutics 2020, 17, 2004–2014. [Google Scholar] [CrossRef]
- Tallarico, M.; Leo, A.; Guarnieri, L.; Zito, M.C.; De Caro, C.; Nicoletti, F.; Russo, E.; Constanti, A.; De Sarro, G.; Citraro, R. N-acetylcysteine aggravates seizures while improving depressive-like and cognitive im-pairment comorbidities in the WAG/Rij rat model of absence epilepsy. Mol. Neurobiol. 2022, 59, 2702–2714. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melini, S.; Trinchese, G.; Lama, A.; Cimmino, F.; Del Piano, F.; Comella, F.; Opallo, N.; Leo, A.; Citraro, R.; Trabace, L.; et al. Sex Differences in Hepatic Inflammation, Lipid Metabolism, and Mitochondrial Function Following Early Lipopolysaccharide Exposure in Epileptic WAG/Rij Rats. Antioxidants 2024, 13, 957. https://doi.org/10.3390/antiox13080957
Melini S, Trinchese G, Lama A, Cimmino F, Del Piano F, Comella F, Opallo N, Leo A, Citraro R, Trabace L, et al. Sex Differences in Hepatic Inflammation, Lipid Metabolism, and Mitochondrial Function Following Early Lipopolysaccharide Exposure in Epileptic WAG/Rij Rats. Antioxidants. 2024; 13(8):957. https://doi.org/10.3390/antiox13080957
Chicago/Turabian StyleMelini, Stefania, Giovanna Trinchese, Adriano Lama, Fabiano Cimmino, Filomena Del Piano, Federica Comella, Nicola Opallo, Antonio Leo, Rita Citraro, Luigia Trabace, and et al. 2024. "Sex Differences in Hepatic Inflammation, Lipid Metabolism, and Mitochondrial Function Following Early Lipopolysaccharide Exposure in Epileptic WAG/Rij Rats" Antioxidants 13, no. 8: 957. https://doi.org/10.3390/antiox13080957




