Identification and Validation of the miR/RAS/RUNX2 Autophagy Regulatory Network in AngII-Induced Hypertensive Nephropathy in MPC5 Cells Treated with Hydrogen Sulfide Donors
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Analysis of Differentially Expressed miRNAs
2.3. Prediction and Identification of Hub Target Genes
2.4. Selection of Autophagy-Related Genes
2.5. Functional and Pathway Enrichment Analysis of ADEGs
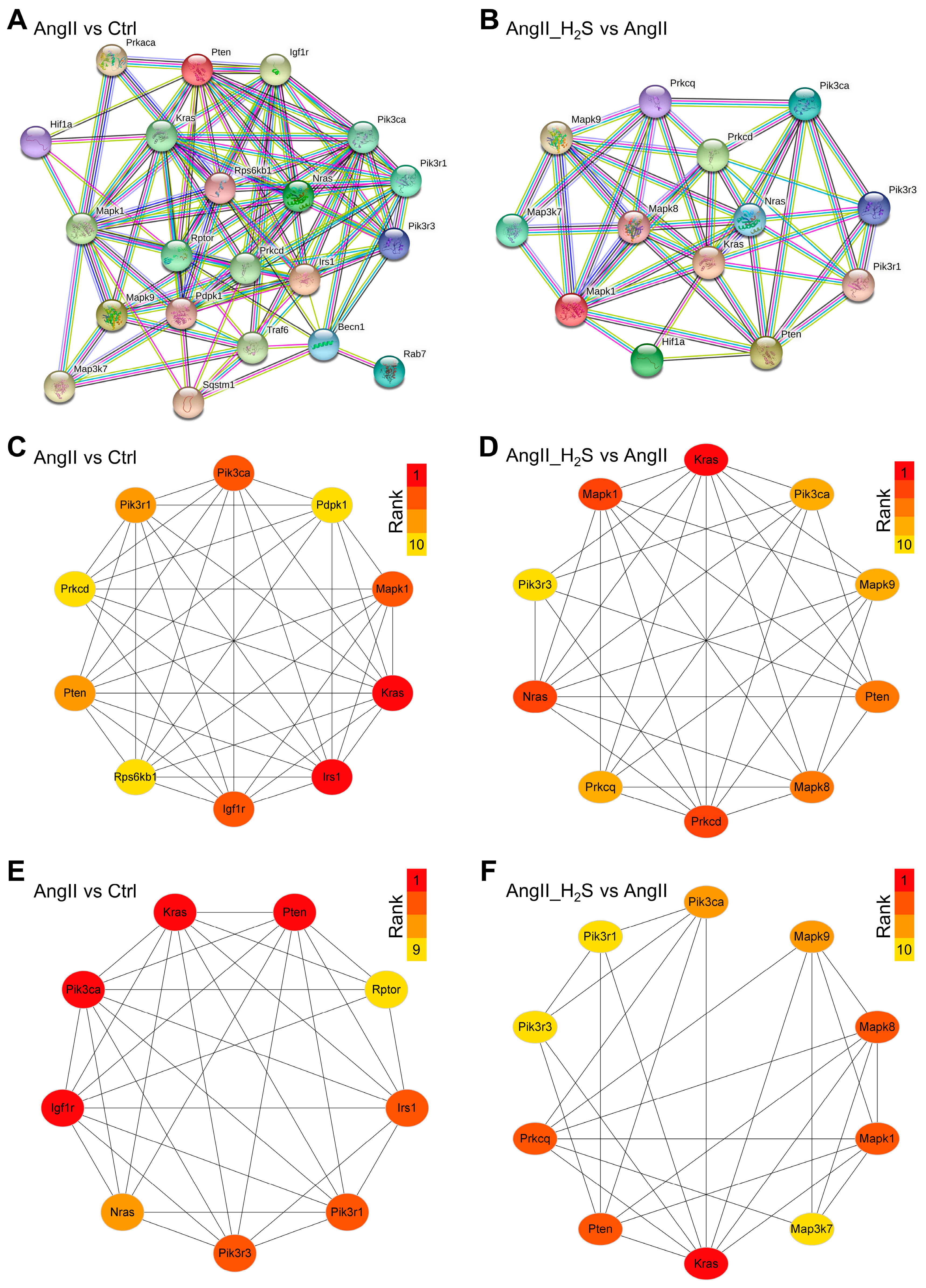
2.6. PPI Network and Recognition of Hub ADEGs and Core Modules
2.7. Construction of miRNA-mRNA-TF Regulatory Network
2.8. Drugs and Reagents
2.9. Cell Culture
2.10. Quantitative PCR
2.11. Western Blot
2.12. H2S Content Assay
2.13. Statistics
3. Results
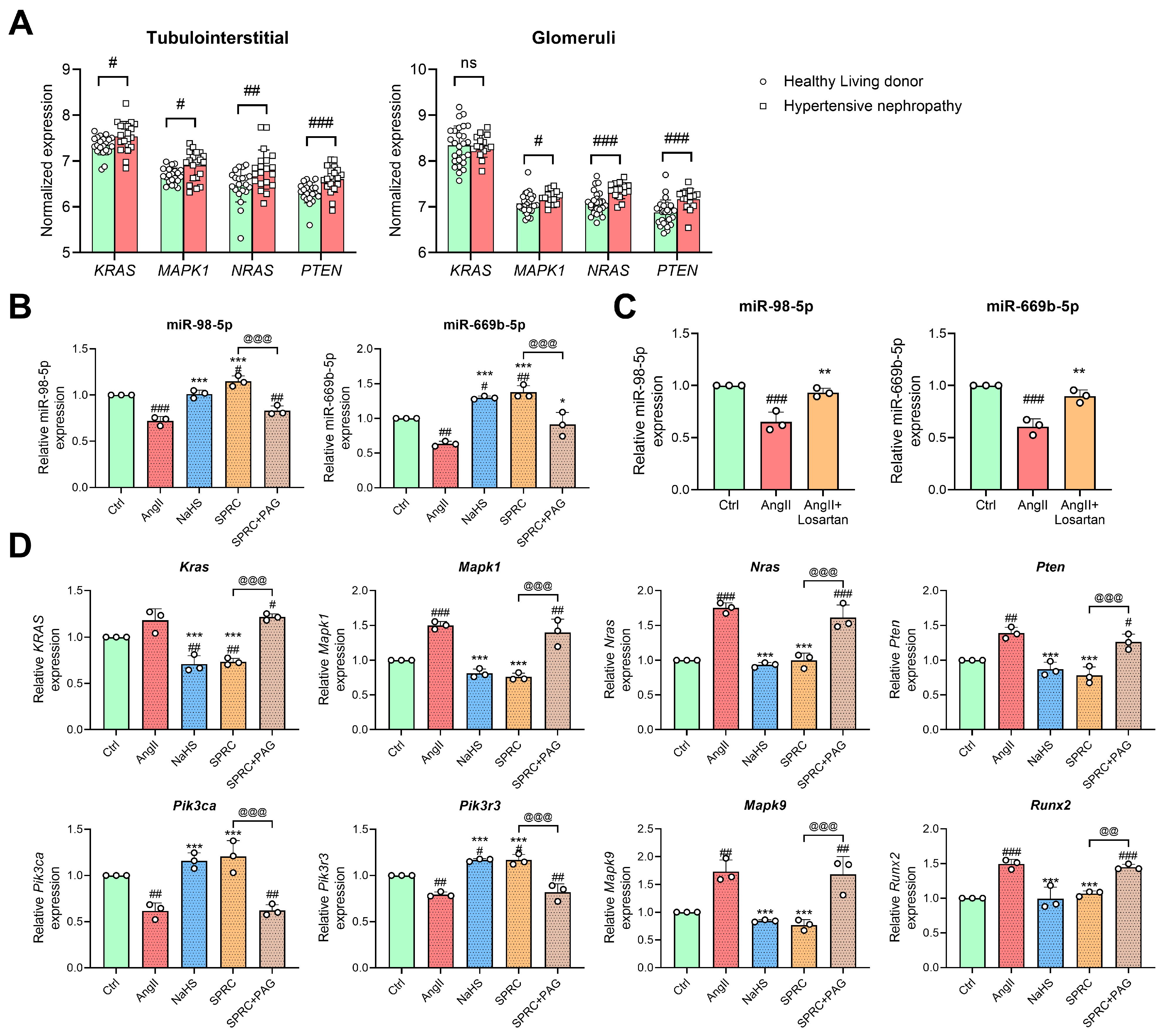
3.1. MiRNAs Were Dysregulated in Mouse Kidney after AngII Induction and H2S Donor Treatment
3.2. The Target Genes of These Dysregulated miRNAs Were Mainly Concentrated in the Autophagy Pathway
3.3. The Hub ADEGs of These Dysregulated miRNAs Were Analyzed
3.4. Construction of the miRNA-mRNA-TF Autophagy Regulatory Network for H2S Donor and/or AngII Treatment
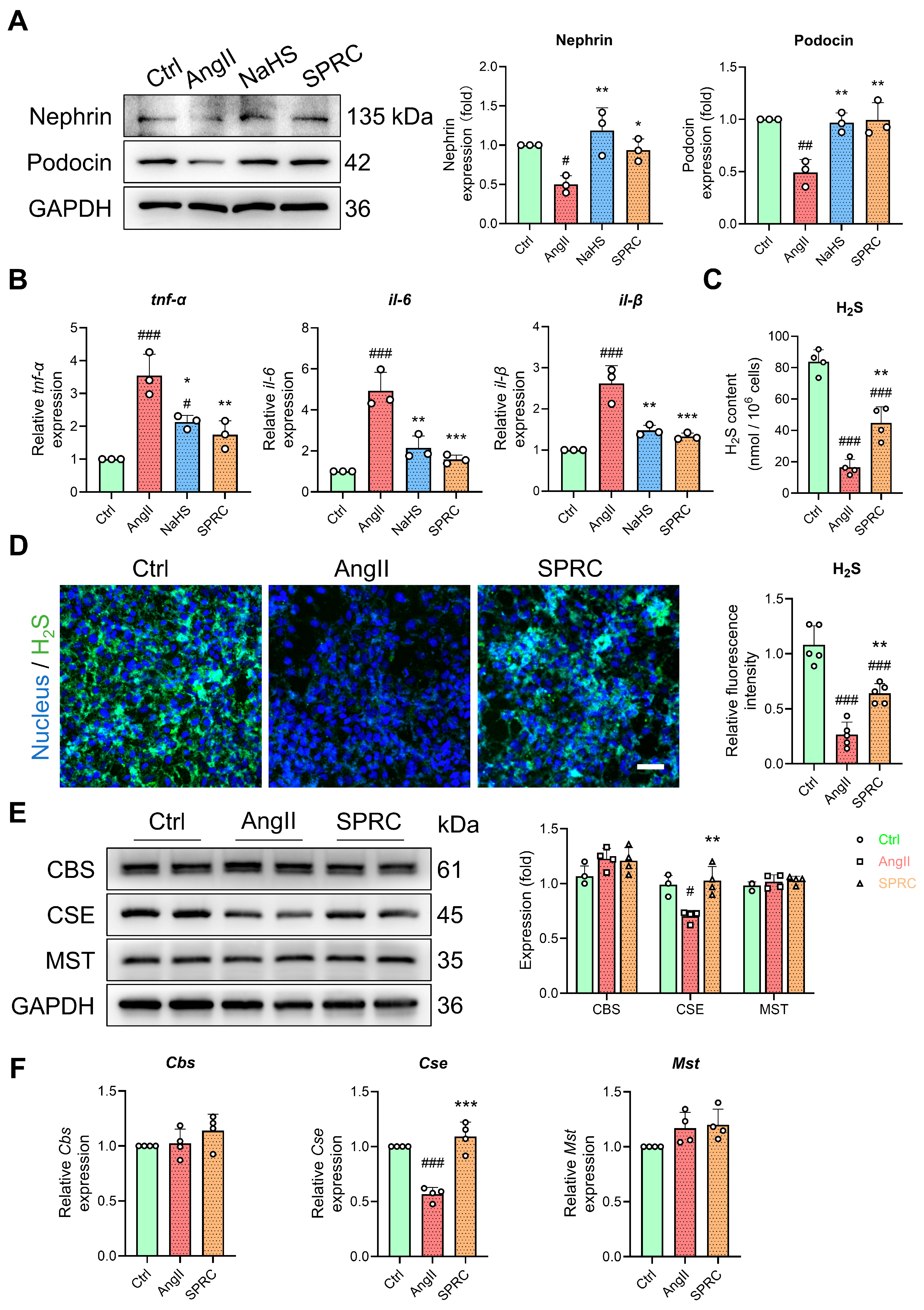
3.5. In AngII-Treated MPC5 Cells, the Hydrogen Sulfide Pathway Is Dysregulated
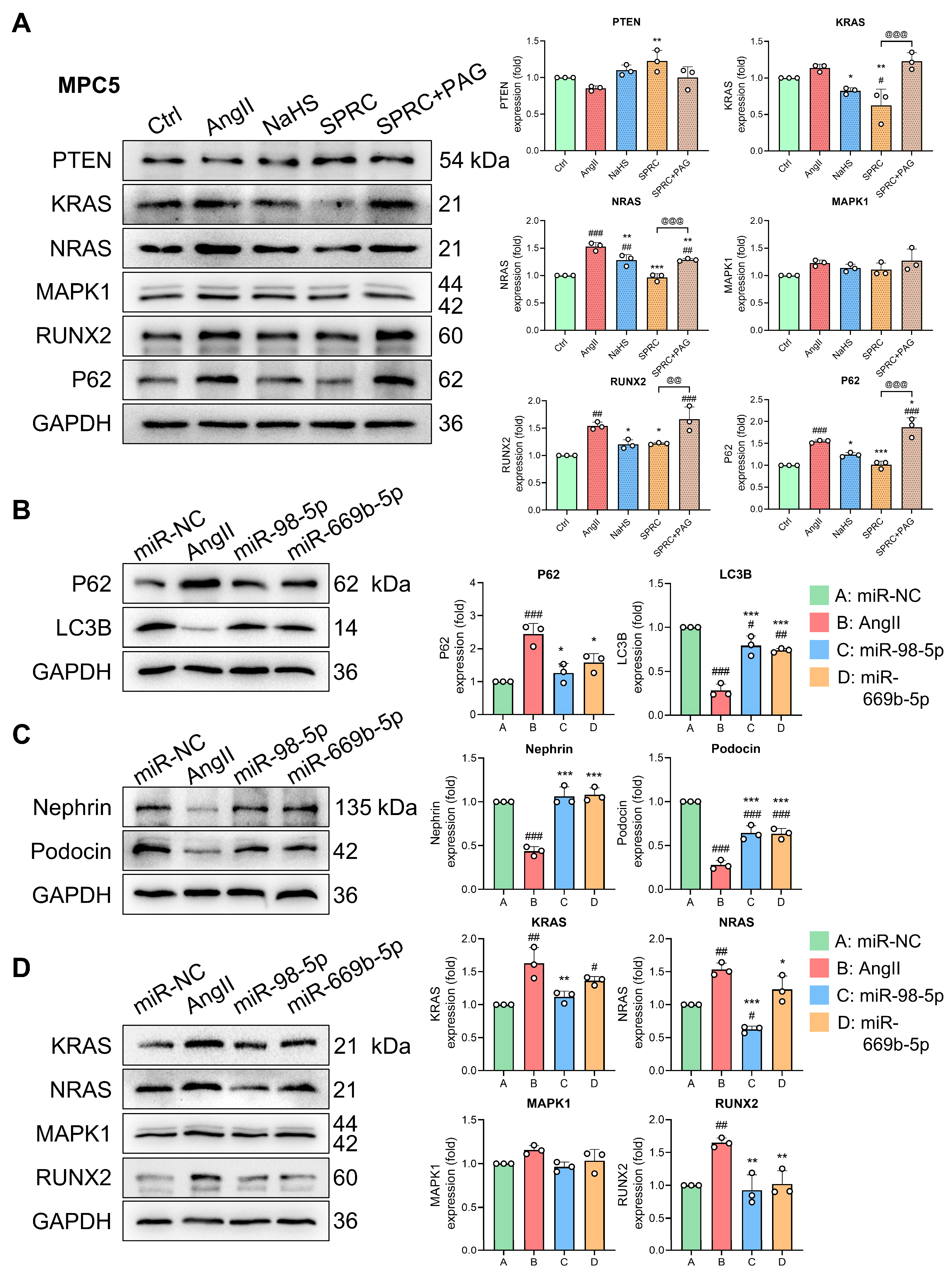
3.6. Changes in miR-98/669b, Related ADEGs, and Transcription Factors Were Observed in the Autophagy Regulatory Network
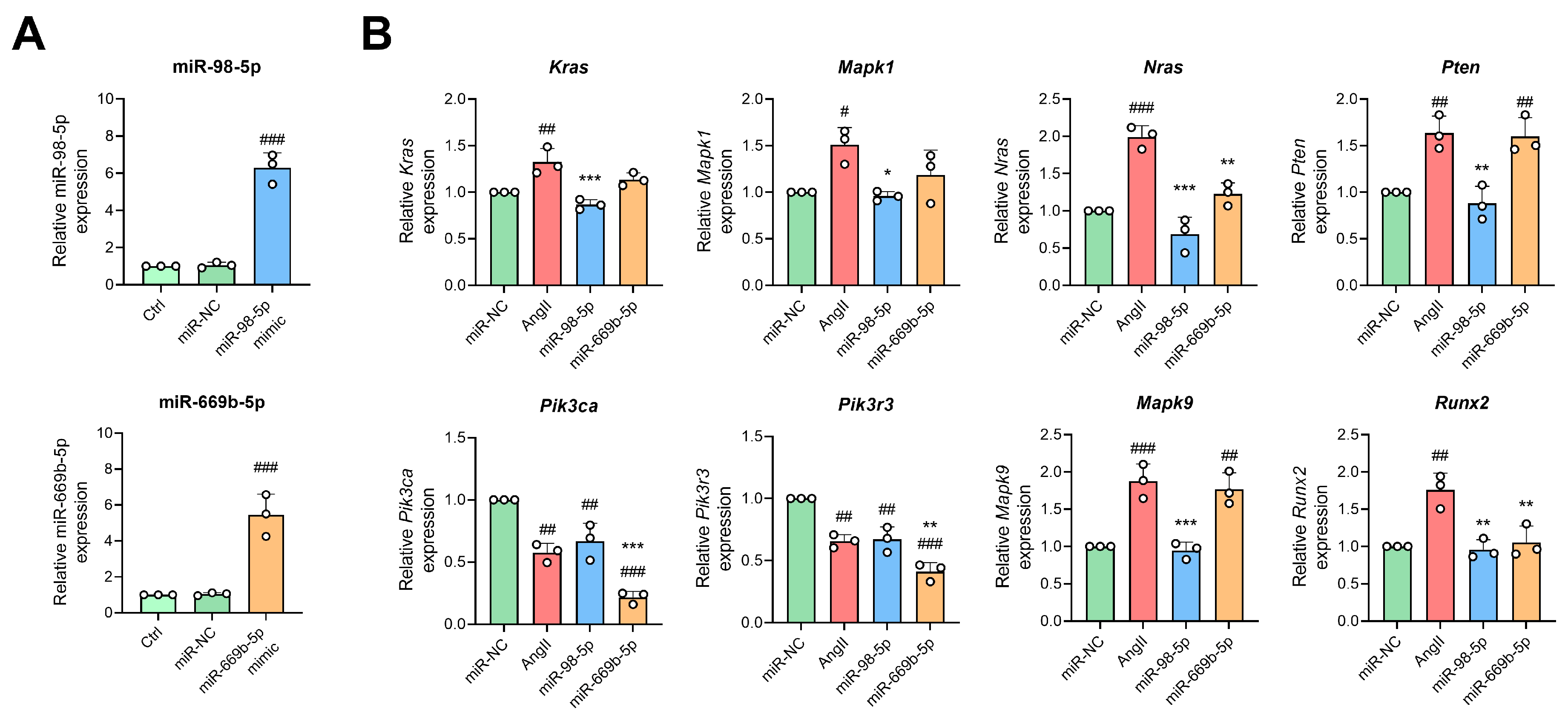
3.7. H2S Donors and miR-98/669b Overexpression Reversed Target Gene Changes, Restored Autophagy Flux, and Reduced Cell Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liang, M. Renal metabolism and hypertension. Nat. Commun. 2021, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Bidani, A.K.; Griffin, K.A. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 2004, 44, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.L.; Ferrao, F.M.; Zheng, Y.; Li, X.C. New frontiers in the intrarenal Renin-Angiotensin system: A critical review of classical and new paradigms. Front. Endocrinol. 2013, 4, 166. [Google Scholar] [CrossRef] [PubMed]
- Laffer, C.L.; Elijovich, F.; Sahinoz, M.; Pitzer, A.; Kirabo, A. New Insights into the Renin-Angiotensin System in Chronic Kidney Disease. Circ. Res. 2020, 127, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, X.R.; Lan, H.Y. Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am. J. Physiol. Ren. Physiol. 2012, 302, F986–F997. [Google Scholar] [CrossRef] [PubMed]
- Bensaada, I.; Robin, B.; Perez, J.; Salemkour, Y.; Chipont, A.; Camus, M.; Lemoine, M.; Guyonnet, L.; Lazareth, H.; Letavernier, E.; et al. Calpastatin prevents Angiotensin II-mediated podocyte injury through maintenance of autophagy. Kidney Int. 2021, 100, 90–106. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Ding, Y.; Choi, M.E. Autophagy in diabetic nephropathy. J. Endocrinol. 2015, 224, R15–R30. [Google Scholar] [CrossRef]
- Wang, B.; Huang, C.; Chen, L.; Xu, D.; Zheng, G.; Zhou, Y.; Wang, X.; Zhang, X. The Emerging Roles of the Gaseous Signaling Molecules NO, H2S, and CO in the Regulation of Stem Cells. ACS Biomater. Sci. Eng. 2020, 6, 798–812. [Google Scholar] [CrossRef]
- Wang, D.; Yang, H.; Zhang, Y.; Hu, R.; Hu, D.; Wang, Q.; Liu, Y.; Liu, M.; Meng, Z.; Zhou, W.; et al. Inhibition of cystathionine β-synthase promotes apoptosis and reduces cell proliferation in chronic myeloid leukemia. Signal Transduct. Target. Ther. 2021, 6, 52. [Google Scholar] [CrossRef]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Vaziri, N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 498–504. [Google Scholar] [CrossRef]
- Han, S.J.; Noh, M.R.; Jung, J.M.; Ishii, I.; Yoo, J.; Kim, J.I.; Park, K.M. Hydrogen sulfide-producing cystathionine γ-lyase is critical in the progression of kidney fibrosis. Free Radic. Biol. Med. 2017, 112, 423–432. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Xie, F.; Tan, W.; Wang, S.; Huang, L.; Tao, L.; Xing, Q.; Yuan, Q. Loss of the Protein Cystathionine β-Synthase during Kidney Injury Promotes Renal Tubulointerstitial Fibrosis. Kidney Blood Press. Res. 2017, 42, 428–443. [Google Scholar] [CrossRef]
- Feng, J.; Lu, X.; Li, H.; Wang, S. The roles of hydrogen sulfide in renal physiology and disease states. Ren. Fail. 2022, 44, 1289–1308. [Google Scholar] [CrossRef]
- Ngowi, E.E.; Sarfraz, M.; Afzal, A.; Khan, N.H.; Khattak, S.; Zhang, X.; Li, T.; Duan, S.F.; Ji, X.Y.; Wu, D.D. Roles of Hydrogen Sulfide Donors in Common Kidney Diseases. Front. Pharmacol. 2020, 11, 564281. [Google Scholar] [CrossRef] [PubMed]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as therapeutic targets in cardiovascular disease. J. Clin. Investig. 2022, 132, e159179. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Mahtal, N.; Lenoir, O.; Tinel, C.; Anglicheau, D.; Tharaux, P.L. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kwan, B.C.; Lai, F.M.; Choi, P.C.; Chow, K.M.; Li, P.K.; Szeto, C.C. Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. Am. J. Hypertens. 2010, 23, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Macconi, D.; Tomasoni, S.; Romagnani, P.; Trionfini, P.; Sangalli, F.; Mazzinghi, B.; Rizzo, P.; Lazzeri, E.; Abbate, M.; Remuzzi, G.; et al. MicroRNA-324-3p promotes renal fibrosis and is a target of ACE inhibition. J. Am. Soc. Nephrol. 2012, 23, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Hackfort, B.T.; Mishra, P.K. Emerging role of hydrogen sulfide-microRNA crosstalk in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H802–H812. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.J.; Pushpakumar, S.B.; Sen, U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H874–H885. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Data analysis. In ggplot2; Springer: Berlin, Germany, 2016; pp. 189–201. [Google Scholar]
- Ru, Y.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, H.; Yao, C.; Shuai, J.; Sun, X. Effect of Dynamic Interaction between microRNA and Transcription Factor on Gene Expression. BioMed. Res. Int. 2016, 2016, 2676282. [Google Scholar] [CrossRef] [PubMed]
- Martinez, N.J.; Walhout, A.J. The interplay between transcription factors and microRNAs in genome-scale regulatory networks. Bioessays 2009, 31, 435–445. [Google Scholar] [CrossRef]
- Janky, R.; Verfaillie, A.; Imrichová, H.; Van de Sande, B.; Standaert, L.; Christiaens, V.; Hulselmans, G.; Herten, K.; Naval Sanchez, M.; Potier, D.; et al. iRegulon: From a gene list to a gene regulatory network using large motif and track collections. PLoS Comput. Biol. 2014, 10, e1003731. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Jia, W.W.; Liu, X.H.; Pan, L.L.; Zhang, Q.Y.; Yang, D.; Shen, X.Y.; Liu, L.; Zhu, Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.R.; Mu, Q.; Rose, P.; Zhu, Y.Z. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: The contribution of the hydrogen sulfide-mediated pathway. J. Cardiovasc. Pharmacol. 2009, 54, 139–146. [Google Scholar] [CrossRef]
- Mundel, P.; Reiser, J.; Zúñiga Mejía Borja, A.; Pavenstädt, H.; Davidson, G.R.; Kriz, W.; Zeller, R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 1997, 236, 248–258. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, J.; Zhang, C.; Yi, B.; Kazama, K.; Liu, W.; Sun, X.; Liu, Y.; Sun, J. Endothelial PRMT5 plays a crucial role in angiogenesis after acute ischemic injury. JCI Insight 2022, 7, e152481. [Google Scholar] [CrossRef]
- Johansen, D.; Ytrehus, K.; Baxter, G.F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury—Evidence for a role of K ATP channels. Basic Res. Cardiol. 2006, 101, 53–60. [Google Scholar] [CrossRef]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef]
- Tsikas, D.; Cooper, A.J. Letter by Tsikas and Cooper regarding article, “dysregulation of hydrogen sulfide (H2S) producing enzyme cystathionine γ-lyase (CSE) contributes to maternal hypertension and placental abnormalities in preeclampsia”. Circulation 2014, 129, e516. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2022, 24, 167–185. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, M.; Hu, C.; Zhang, X.; Li, C.; Nie, S.; Huang, Z.; Zhao, Z.; Hou, F.F.; et al. Prevalence of Chronic Kidney Disease in China: Results from the Sixth China Chronic Disease and Risk Factor Surveillance. JAMA Intern. Med. 2023, 183, 298–310. [Google Scholar] [CrossRef]
- Livingston, M.J.; Shu, S.; Fan, Y.; Li, Z.; Jiao, Q.; Yin, X.M.; Venkatachalam, M.A.; Dong, Z. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy 2023, 19, 256–277. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Choi, M.E. Autophagy in Kidney Disease. Annu. Rev. Physiol. 2020, 82, 297–322. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791. [Google Scholar] [CrossRef]
- Yang, D.; Livingston, M.J.; Liu, Z.; Dong, G.; Zhang, M.; Chen, J.K.; Dong, Z. Autophagy in diabetic kidney disease: Regulation, pathological role and therapeutic potential. Cell. Mol. Life Sci. 2018, 75, 669–688. [Google Scholar] [CrossRef]
- Nowak, K.L.; Edelstein, C.L. Apoptosis and autophagy in polycystic kidney disease (PKD). Cell. Signal. 2020, 68, 109518. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C. Hydrogen Sulfide: A Key Role in Autophagy Regulation from Plants to Mammalians. Antioxidants 2022, 11, 327. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, S.; Zhang, K.; Zhang, Z.; Li, C.; Gao, B.; Zhang, W.; Wang, Y. Exogenous H2S Inhibits Autophagy in Unilateral Ureteral Obstruction Mouse Renal Tubule Cells by Regulating the ROS-AMPK Signaling Pathway. Cell. Physiol. Biochem. 2018, 49, 2200–2213. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Floege, J.; Biessen, E.A.L.; Jankowski, J.; van der Vorst, E.P.C. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int. J. Mol. Sci. 2020, 21, 6547. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Hydrogen Sulfide in Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2018, 19, 1438. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Liang, W.; Li, J.; Feng, L.; Zheng, P.; Ji, T.; Bai, S. miR-98-5p Alleviated Epithelial-to-Mesenchymal Transition and Renal Fibrosis via Targeting Hmga2 in Diabetic Nephropathy. Int. J. Endocrinol. 2019, 2019, 4946181. [Google Scholar] [CrossRef]
- Xiao, M.; Bai, S.; Chen, J.; Li, Y.; Zhang, S.; Hu, Z. CDKN2B-AS1 participates in high glucose-induced apoptosis and fibrosis via NOTCH2 through functioning as a miR-98-5p decoy in human podocytes and renal tubular cells. Diabetol. Metab. Syndr. 2021, 13, 107. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H.; Guo, J.; Yu, Y.; Yang, D.; He, F.; Du, Z. MicroRNA-98 negatively regulates myocardial infarction-induced apoptosis by down-regulating Fas and caspase-3. Sci. Rep. 2017, 7, 7460. [Google Scholar] [CrossRef]
- Neiburga, K.D.; Vilne, B.; Bauer, S.; Bongiovanni, D.; Ziegler, T.; Lachmann, M.; Wengert, S.; Hawe, J.S.; Güldener, U.; Westerlund, A.M.; et al. Vascular Tissue Specific miRNA Profiles Reveal Novel Correlations with Risk Factors in Coronary Artery Disease. Biomolecules 2021, 11, 1683. [Google Scholar] [CrossRef]
- Kolosowska, N.; Gotkiewicz, M.; Dhungana, H.; Giudice, L.; Giugno, R.; Box, D.; Huuskonen, M.T.; Korhonen, P.; Scoyni, F.; Kanninen, K.M.; et al. Intracerebral overexpression of miR-669c is protective in mouse ischemic stroke model by targeting MyD88 and inducing alternative microglial/macrophage activation. J. Neuroinflamm. 2020, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Crippa, S.; Montecchiani, C.; Camps, J.; Cornaglia, A.I.; Boldrin, L.; Morgan, J.; Calligaro, A.; Casasco, A.; Orlacchio, A.; et al. Long-term miR-669a therapy alleviates chronic dilated cardiomyopathy in dystrophic mice. J. Am. Heart Assoc. 2013, 2, e000284. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, R.; de Bruin, R.G.; van Solingen, C.; van Gils, J.M.; Duijs, J.M.; van der Veer, E.P.; Rabelink, T.J.; Humphreys, B.D.; van Zonneveld, A.J. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int. 2016, 89, 1268–1280. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Tod, P.; Róka, B.; Kaucsár, T.; Szatmári, K.; Vizovišek, M.; Vidmar, R.; Fonovič, M.; Szénási, G.; Hamar, P. Time-Dependent miRNA Profile during Septic Acute Kidney Injury in Mice. Int. J. Mol. Sci. 2020, 21, 5316. [Google Scholar] [CrossRef]
- Liu, Y.; Usa, K.; Wang, F.; Liu, P.; Geurts, A.M.; Li, J.; Williams, A.M.; Regner, K.R.; Kong, Y.; Liu, H.; et al. MicroRNA-214-3p in the Kidney Contributes to the Development of Hypertension. J. Am. Soc. Nephrol. 2018, 29, 2518–2528. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, D.; Wang, F.; Liu, J.; Huang, B.; Baker, M.A.; Yin, J.; Wu, R.; Liu, X.; Regner, K.R.; et al. Endogenous miR-204 Protects the Kidney against Chronic Injury in Hypertension and Diabetes. J. Am. Soc. Nephrol. 2020, 31, 1539–1554. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wang, F.; Liang, M. miR-204: Molecular Regulation and Role in Cardiovascular and Renal Diseases. Hypertension 2021, 78, 270–281. [Google Scholar] [CrossRef]
- Chen, J.; Lai, W.; Deng, Y.; Liu, M.; Dong, M.; Liu, Z.; Wang, T.; Li, X.; Zhao, Z.; Yin, X.; et al. MicroRNA-363-3p promotes apoptosis in response to cadmium-induced renal injury by down-regulating phosphoinositide 3-kinase expression. Toxicol. Lett. 2021, 345, 12–23. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Chuan, L.; Wei, S.; Wang, J.; Yang, X.; Ru, J. miR-129-5p alleviates LPS-induced acute kidney injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int. Immunopharmacol. 2020, 89, 107016. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, J.; Lin, C.; Zhang, L.; Liu, B.; Ouyang, L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm. Sin. B 2020, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Boyer-Guittaut, M.; Dodson, M.; Chatham, J.; Darley-Usmar, V.; Zhang, J. Regulation of autophagy by protein post-translational modification. Lab. Investig. 2015, 95, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hu, W.; Li, F.; Marshall, R.S.; Zarza, X.; Munnik, T.; Vierstra, R.D. AUTOPHAGY-RELATED14 and Its Associated Phosphatidylinositol 3-Kinase Complex Promote Autophagy in Arabidopsis. Plant Cell 2020, 32, 3939–3960. [Google Scholar] [CrossRef]
- Du, S.; Zheng, H. Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. 2021, 11, 188. [Google Scholar] [CrossRef]
- Guo, X.; Li, Z.; Zhu, X.; Zhan, M.; Wu, C.; Ding, X.; Peng, K.; Li, W.; Ma, X.; Lv, Z.; et al. A coherent FOXO3-SNAI2 feed-forward loop in autophagy. Proc. Natl. Acad. Sci. USA 2022, 119, e2118285119. [Google Scholar] [CrossRef]
- Yao, J.; Wang, J.; Xu, Y.; Guo, Q.; Sun, Y.; Liu, J.; Li, S.; Guo, Y.; Wei, L. CDK9 inhibition blocks the initiation of PINK1-PRKN-mediated mitophagy by regulating the SIRT1-FOXO3-BNIP3 axis and enhances the therapeutic effects involving mitochondrial dysfunction in hepatocellular carcinoma. Autophagy 2022, 18, 1879–1897. [Google Scholar] [CrossRef]
- Dossou, A.S.; Basu, A. The Emerging Roles of mTORC1 in Macromanaging Autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef]
- Gonzalez, C.D.; Carro Negueruela, M.P.; Nicora Santamarina, C.; Resnik, R.; Vaccaro, M.I. Autophagy Dysregulation in Diabetic Kidney Disease: From Pathophysiology to Pharmacological Interventions. Cells 2021, 10, 2497. [Google Scholar] [CrossRef]
- Majumder, S.; Pushpakumar, S.; Juin, S.K.; Jala, V.R.; Sen, U. Toll-like receptor 4 mutation protects the kidney from Ang-II-induced hypertensive injury. Pharmacol. Res. 2022, 175, 106030. [Google Scholar] [CrossRef]
- Bryant, K.L.; Stalnecker, C.A.; Zeitouni, D.; Klomp, J.E.; Peng, S.; Tikunov, A.P.; Gunda, V.; Pierobon, M.; Waters, A.M.; George, S.D.; et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 2019, 25, 628–640. [Google Scholar] [CrossRef]
- Kinsey, C.G.; Camolotto, S.A.; Boespflug, A.M.; Guillen, K.P.; Foth, M.; Truong, A.; Schuman, S.S.; Shea, J.E.; Seipp, M.T.; Yap, J.T.; et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 2019, 25, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Xiang, Y.; Tang, Y.; Ge, Z.; Li, Q.; Zhang, Y. Circ-ADAM9 targeting PTEN and ATG7 promotes autophagy and apoptosis of diabetic endothelial progenitor cells by sponging mir-20a-5p. Cell Death Dis. 2020, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, H.; Yu, J.; Zhao, Z.; Xiao, F.; Xia, T.; Wang, C.; Li, K.; Deng, J.; Guo, Y.; et al. MAPK1/3 regulate hepatic lipid metabolism via ATG7-dependent autophagy. Autophagy 2016, 12, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, H.; Yang, B. MicroRNA-98-5p modulates cervical cancer progression via controlling PI3K/AKT pathway. Bioengineered 2021, 12, 10596–10607. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Z.; Cai, Z.; Sun, Y.; Li, L.; Yao, F.; Yang, L.; Zhou, Y.; Zhu, H.; Fu, Y.; et al. Runx2 (Runt-Related Transcription Factor 2)-Mediated Microcalcification Is a Novel Pathological Characteristic and Potential Mediator of Abdominal Aortic Aneurysm. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1352–1369. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Wang, J.C.; Hsu, Y.J.; Chiu, Y.L.; Lin, C.Y.; Lu, C.Y.; Tsai, S.H. miR-424/322 protects against abdominal aortic aneurysm formation by modulating the Smad2/3/runt-related transcription factor 2 axis. Mol. Ther. Nucleic Acids 2022, 27, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, Y.; Sun, Z. Deficiency in the anti-aging gene Klotho promotes aortic valve fibrosis through AMPKα-mediated activation of RUNX2. Aging Cell 2016, 15, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Raaz, U.; Schellinger, I.N.; Chernogubova, E.; Warnecke, C.; Kayama, Y.; Penov, K.; Hennigs, J.K.; Salomons, F.; Eken, S.; Emrich, F.C.; et al. Transcription Factor Runx2 Promotes Aortic Fibrosis and Stiffness in Type 2 Diabetes Mellitus. Circ. Res. 2015, 117, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, W.; Su, X.; Luo, D.; Li, Z.; Zhou, Y.; Zhu, Y.; Zhang, M.; Chen, J.; Liu, B.; et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease. J. Clin. Investig. 2022, 132, e150051. [Google Scholar] [CrossRef] [PubMed]
- Ruffenach, G.; Chabot, S.; Tanguay, V.F.; Courboulin, A.; Boucherat, O.; Potus, F.; Meloche, J.; Pflieger, A.; Breuils-Bonnet, S.; Nadeau, V.; et al. Role for Runt-related Transcription Factor 2 in Proliferative and Calcified Vascular Lesions in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2016, 194, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, L.; Xie, F.; Guo, S.; Liu, F.; Dong, N.; Wang, Y. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc. Res. 2018, 114, 168–179. [Google Scholar] [CrossRef]
- Tandon, M.; Othman, A.H.; Ashok, V.; Stein, G.S.; Pratap, J. The role of Runx2 in facilitating autophagy in metastatic breast cancer cells. J. Cell. Physiol. 2018, 233, 559–571. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Ren, M.; Fan, D.; Mao, Y.; Zhu, Y.-Z. Identification and Validation of the miR/RAS/RUNX2 Autophagy Regulatory Network in AngII-Induced Hypertensive Nephropathy in MPC5 Cells Treated with Hydrogen Sulfide Donors. Antioxidants 2024, 13, 958. https://doi.org/10.3390/antiox13080958
Ye Q, Ren M, Fan D, Mao Y, Zhu Y-Z. Identification and Validation of the miR/RAS/RUNX2 Autophagy Regulatory Network in AngII-Induced Hypertensive Nephropathy in MPC5 Cells Treated with Hydrogen Sulfide Donors. Antioxidants. 2024; 13(8):958. https://doi.org/10.3390/antiox13080958
Chicago/Turabian StyleYe, Qing, Mi Ren, Di Fan, Yicheng Mao, and Yi-Zhun Zhu. 2024. "Identification and Validation of the miR/RAS/RUNX2 Autophagy Regulatory Network in AngII-Induced Hypertensive Nephropathy in MPC5 Cells Treated with Hydrogen Sulfide Donors" Antioxidants 13, no. 8: 958. https://doi.org/10.3390/antiox13080958
APA StyleYe, Q., Ren, M., Fan, D., Mao, Y., & Zhu, Y.-Z. (2024). Identification and Validation of the miR/RAS/RUNX2 Autophagy Regulatory Network in AngII-Induced Hypertensive Nephropathy in MPC5 Cells Treated with Hydrogen Sulfide Donors. Antioxidants, 13(8), 958. https://doi.org/10.3390/antiox13080958







