The Dietary Inflammatory Index and Its Associations with Biomarkers of Nutrients with Antioxidant Potential, a Biomarker of Inflammation and Multiple Long-Term Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. EPIC-Norfolk Study Design
2.2. Study Population
2.3. Assessment of Dietary Intake and Supplement Use
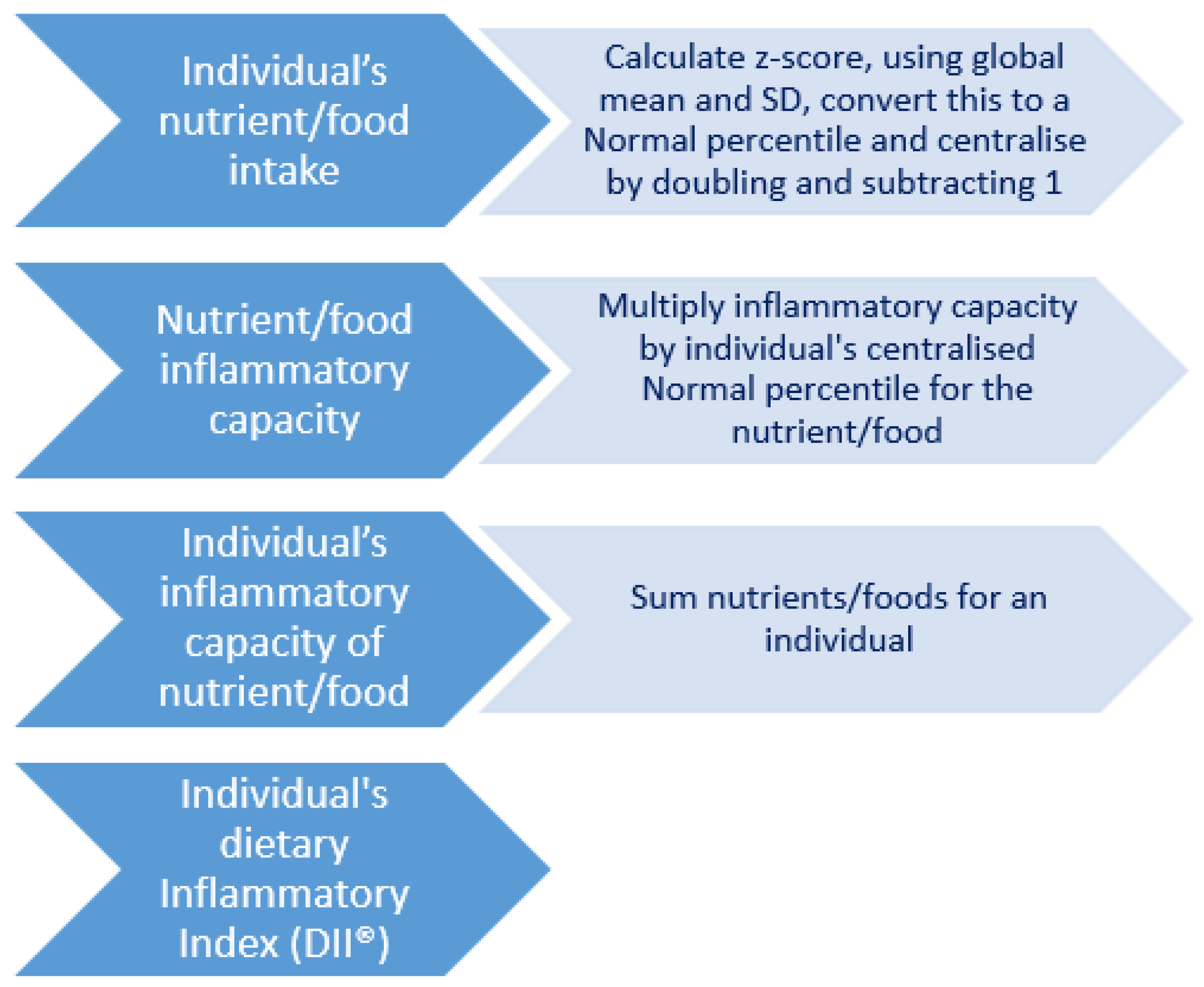
2.4. The Dietary Inflammatory Index (DII®)
2.5. Creation of the DII®
2.6. Blood Sample and Biomarker Analyses
2.7. Calculation of the MLTC Score
2.8. Measurement of Other Associated Variables
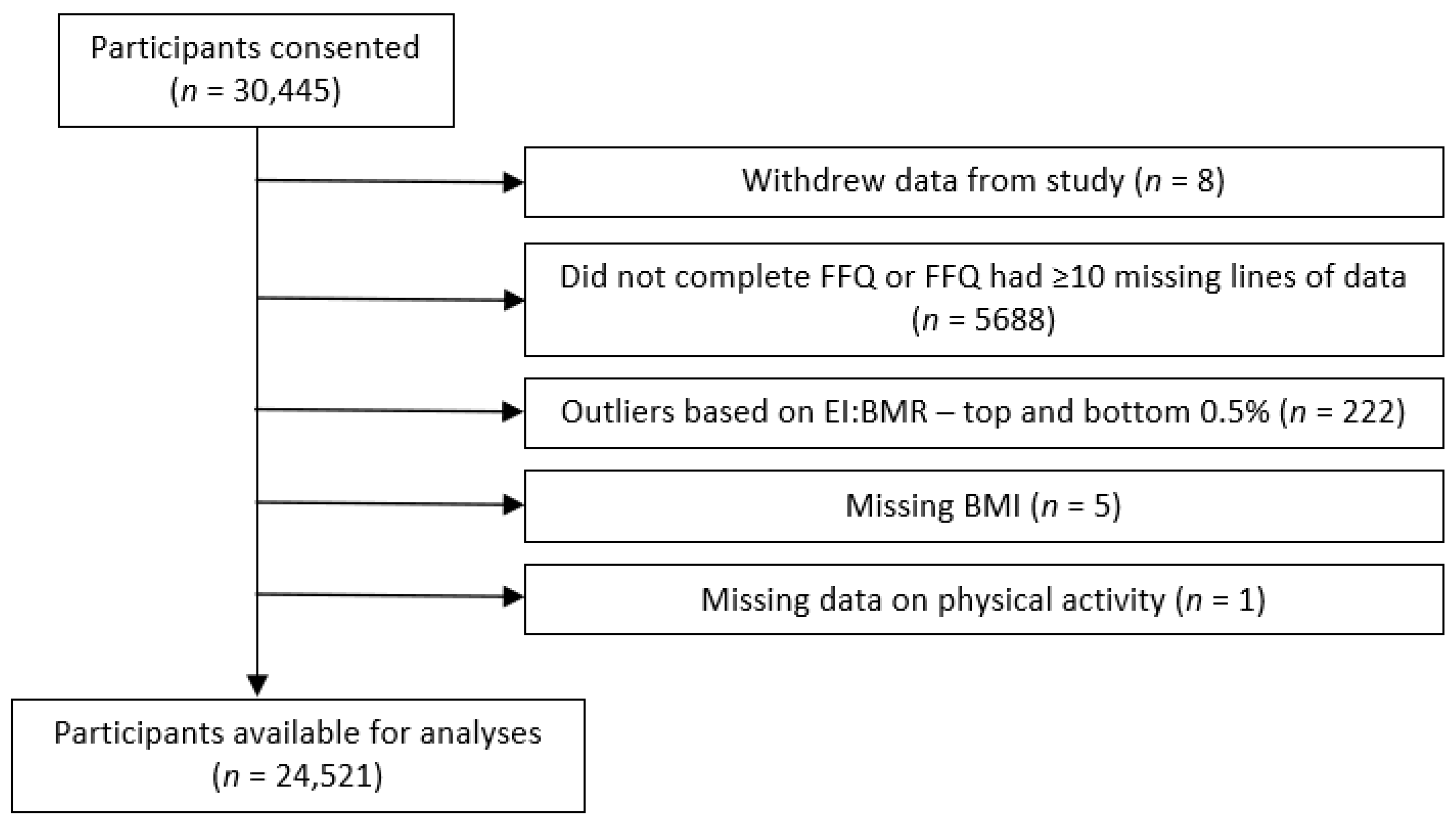
2.9. Inclusion and Exclusion Criteria for Analysis
2.10. Statistical Analyses
2.10.1. Descriptive Analyses
2.10.2. Associative Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Food Group Consumption
3.3. Validation of the DII® Score
3.4. Associations between the DII® Score and MLTCs
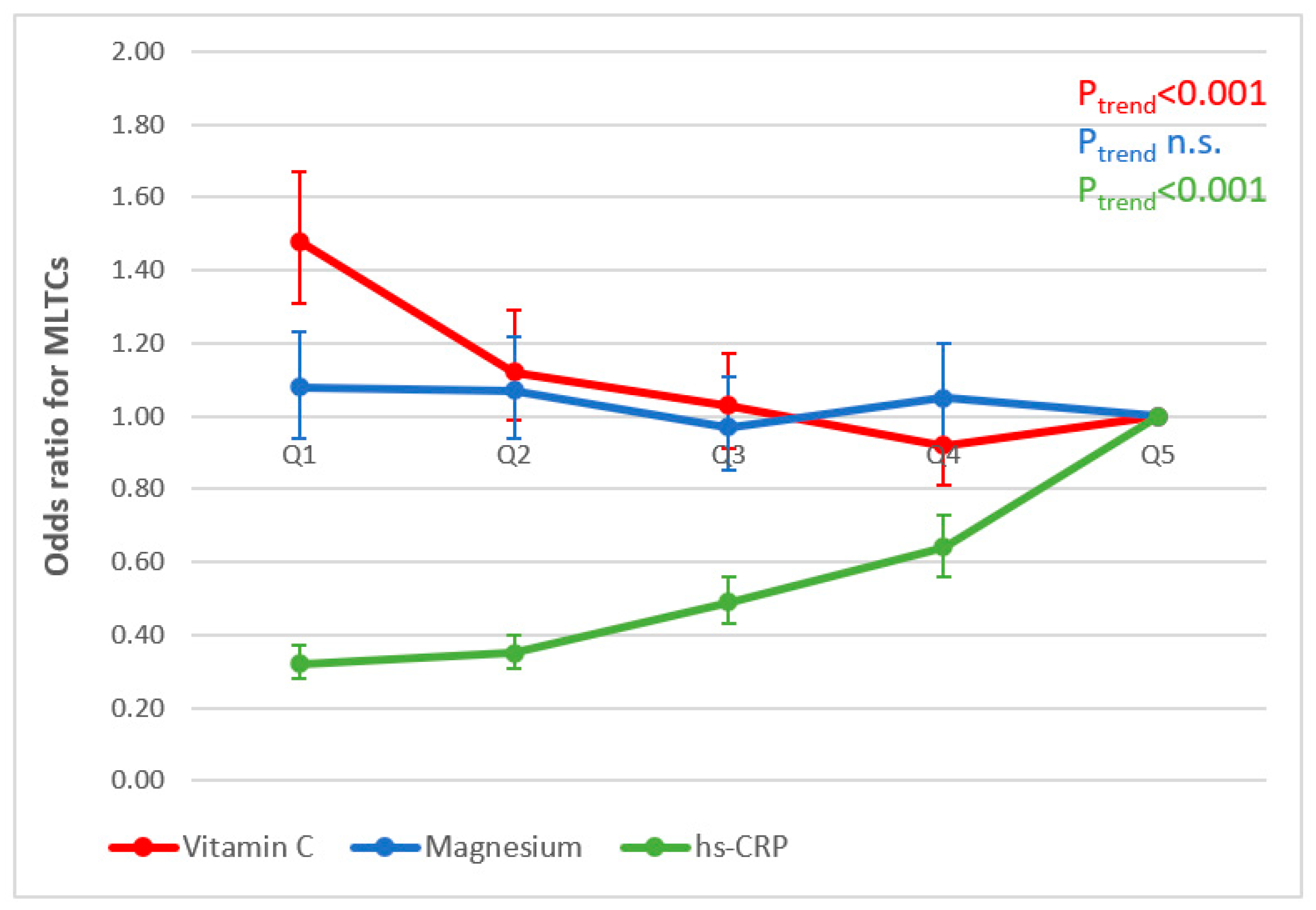
3.5. Associations between Nutritional Biomarker Concentrations, Inflammation and MLTCs
3.6. Associations between hs-CRP and Nutritional Biomarkers (Research Question 6)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age- Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-derived antioxidants and their role in inflammation, obesity and gut microbiota modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S.; Daglia, M.; Rengasamy, K.R. Dietary carotenoids in cancer chemoprevention and chemotherapy: A review of emerging evidence. Pharmacol. Res. 2020, 157, 104830. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, Y. Associations of Dietary Vitamin C and E Intake with Depression. A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 857823. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose–Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Luben, R.N.; A Welch, A.; A Bingham, S.; Wareham, N.J.; Khaw, K.-T. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer-Norfolk prospective population study. Am. J. Clin. Nutr. 2008, 87, 64–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, C.; Zhang, Z.; He, P.; Li, Q.; Liu, C.; Qin, X. Inverse association between dietary vitamin A intake and new-onset hypertension. Clin. Nutr. 2021, 40, 2868–2875. [Google Scholar] [CrossRef]
- Myint, P.K.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T. Association between plasma vitamin C concentrations and blood pressure in the European prospective investigation into cancer-norfolk population-based study. Hypertension 2011, 58, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Sharp, S.J.; Imamura, F.; Chowdhury, R.; E Gundersen, T.; Steur, M.; Sluijs, I.; van der Schouw, Y.T.; Agudo, A.; Aune, D.; et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 2020, 370, 3–5. [Google Scholar] [CrossRef]
- Marcelino, G.; Machate, D.J.; Freitas, K.d.C.; Hiane, P.A.; Maldonade, I.R.; Pott, A.; Asato, M.A.; Candido, C.J.; Guimarães, R.d.C.A. β-Carotene: Preventive Role for Type 2 Diabetes Mellitus and Obesity: A Review. Molecules 2020, 25, 5803. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Multimorbidity. Available online: https://cks.nice.org.uk/topics/multimorbidity/ (accessed on 3 June 2023).
- Johnston, M.C.; Crilly, M.; Black, C.; Prescott, G.J.; Mercer, S.W. Defining and measuring multimorbidity: A systematic review of systematic reviews. Eur. J. Public Health 2019, 29, 182–189. [Google Scholar] [CrossRef]
- Stirland, L.; Gonzalez-Saavedra, L.; Mullin, D.; Ritchie, C.; Muniz-Terrera, G.; Russ, T. Measuring multimorbidity beyond counting diseases: Systematic review of community and population studies and guide to index choice. BMJ 2020, 368, m127. [Google Scholar] [CrossRef]
- Ho, I.S.-S.; Azcoaga-Lorenzo, A.; Akbari, A.; Black, C.; Davies, J.; Hodgins, P.; Khunti, K.; Kadam, U.; A Lyons, R.; McCowan, C.; et al. Examining variation in the measurement of multimorbidity in research: A systematic review of 566 studies. Lancet Public Health 2021, 6, e587–e597. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Guo, Y.-Z.; Pan, L.; Du, C.-J.; Ren, D.-Q.; Xie, X.-M. Association between C-reactive protein and risk of cancer: A meta-analysis of prospective cohort studies. Asian Pac. J. Cancer Prev. 2013, 14, 243–248. [Google Scholar] [CrossRef]
- Sarwar, N.; Thompson, A.J.; Di Angelantonio, E. Markers of inflammation and risk of coronary heart disease. Dis. Markers 2009, 26, 217–225. [Google Scholar] [CrossRef]
- Black, P.H. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun. 2003, 17, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Derry, H.M.; Fagundes, C.P. Inflammation: Depression fans the flames and feasts on the heat. Am. J. Psychiatry 2015, 172, 1075–1091. [Google Scholar] [CrossRef]
- Mora, S.; Musunuru, K.; Blumenthal, R.S. The clinical utility of high-sensitivity C-reactive protein in cardiovascular disease and the potential implication of JUPITER on current practice guidelines. Clin. Chem. 2009, 55, 219–228. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Pruimboom, L.; Dijck-Brouwer, D.J.; Muskiet, F.A. Lifestyle and nutritional imbalances associated with Western diseases: Causes and consequences of chronic systemic low-grade inflammation in an evolutionary context. J. Nutr. Biochem. 2013, 24, 1183–1201. [Google Scholar] [CrossRef]
- Smidowicz, A.; Regula, J. Effect of nutritional status and dietary patterns on human serum c-reactive protein and interleukin-6 concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.; Yildiz, V.; E Steck, S.; Hébert, J.R.; Ma, Y.; Cauley, J.A.; Li, W.; Mossavar-Rahmani, Y.; Johnson, K.C.; Sattari, M.; et al. Dietary Inflammatory Index, Bone Mineral Density, and Risk of Fracture in Postmenopausal Women: Results from the Women’s Health Initiative. J. Bone Miner. Res. 2017, 32, 1136–1146. [Google Scholar] [CrossRef]
- Kingston, A.; Robinson, L.; Booth, H.; Knapp, M.; Jagger, C. Projections of multi-morbidity in the older population in England to 2035: Estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing 2018, 47, 374–380. [Google Scholar] [CrossRef]
- Office for National Statistics. Healthcare Expenditure, UK Health Accounts—Provisional Estimates: 2019; Office for National Statistics: Newport, Wales, 2021.
- Menotti, A.; Mulder, I.; Nissinen, A.; Giampaoli, S.; Feskens, E.J.; Kromhout, D. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortalitym: The FINE study (Finland, Italy, Netherlands, elderly). J. Clin. Epidemiol. 2001, 54, 680–686. [Google Scholar] [CrossRef]
- Luben, R.; Hayat, S.; Wareham, N.; Pharoah, P.P.; Khaw, K.-T. Sociodemographic and lifestyle predictors of incident hospital admissions with multimorbidity in a general population, 1999–2019: The EPIC-Norfolk cohort. BMJ Open 2020, 10, e042115. [Google Scholar] [CrossRef]
- Makovski, T.T.; Schmitz, S.; Zeegers, M.P.; Stranges, S.; Akker, M.v.D. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res. Rev. 2019, 53, 100903. [Google Scholar] [CrossRef]
- Mohammadi, S.; Hosseinikia, M.; Ghaffarian-Bahraman, A.; Clark, C.C.T.; Davies, I.G.; Rad, E.Y.; Saboori, S. Dietary inflammatory index and elevated serum C-reactive protein: A systematic review and meta-analysis. Food Sci. Nutr. 2023, 11, 5786–5798. [Google Scholar] [CrossRef]
- Schöttker, B.; Saum, K.-U.; Jansen, E.H.J.M.; Holleczek, B.; Brenner, H. Associations of metabolic, inflammatory and oxidative stress markers with total morbidity and multi-morbidity in a large cohort of older German adults. Age Ageing 2016, 45, 127–135. [Google Scholar] [CrossRef]
- Vázquez-Fernández, A.; Lana, A.; A Struijk, E.; Vega-Cabello, V.; Cárdenas-Valladolid, J.; Salinero-Fort, M.; Rodríguez-Artalejo, F.; Lopez-Garcia, E.; Caballero, F.F. Cross-sectional Association between Plasma Biomarkers and Multimorbidity Patterns in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad249. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E. Nutrition and cancer: Background and rationale of the European prospective investigation into cancer and nutrition (EPIC). Ann. Oncol. 1992, 3, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Day, N.; Oakes, S.; Luben, R.; Khaw, K.T.; Bingham, S.A.; Welch, A.; Wareham, N. EPIC-Norfolk: Study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br. J. Cancer 1999, 80, 95–103. [Google Scholar] [PubMed]
- Bingham, S.A.; Cassidy, A.; Cole, T.J.; Welch, A.; Runswick, S.A.; Black, A.E.; Thurnham, D.; Bates, C.; Khaw, K.T.; Key, T.J.A.; et al. Validation of weighed records and other methods of dietary assessment using the 24 h urine nitrogen technique and other biological markers. Br. J. Nutr. 1995, 73, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Gill, C.; Welch, A.; Cassidy, A.; Runswick, S.A.; Oakes, S.; Lubin, R.; Thurnham, D.I.; Key, T.J.; Roe, L.; et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int. J. Epidemiol. 1997, 26 (Suppl. S1), 137–151. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; E Day, N.; A Welch, A.; A Runswick, S.; Luben, R.N.; A Mulligan, A.; McTaggart, A.; A Bingham, S. Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am. J. Clin. Nutr. 2001, 74, 188–196. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef]
- Welch, A.A.; Luben, R.; Khaw, K.T.; Bingham, S.A. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J. Hum. Nutr. Diet. 2005, 18, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Henry, C. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary Fat and Coronary Heart Disease: A Comparison of Approaches for Adjusting for Total Energy Intake and Modeling Repeated Dietary Measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Lentjes, M.A.H.; Mulligan, A.A.; Welch, A.A.; Bhaniani, A.; Luben, R.N.; Khaw, K. Contribution of cod liver oil-related nutrients (vitamins A, D, E and eicosapentaenoic acid and docosahexaenoic acid) to daily nutrient intake and their associations with plasma concentrations in the EPIC-Norfolk cohort. J. Hum. Nutr. Diet. 2015, 28, 568–582. [Google Scholar] [CrossRef]
- Thurnham, D.I.; Davies, J.A.; Crump, B.J.; Situnayake, R.D.; Davis, M. The use of different lipids to express serum tocopherol: Lipids ratios for the measurement of vitamin E status. Ann. Clin. Biochem. 1986, 23, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.A.; Hayhoe, R.P.G.; Luben, R.N.; Welch, A.A. Positive Associations of Dietary Intake and Plasma Concentrations of Vitamin E with Skeletal Muscle Mass, Heel Bone Ultrasound Attenuation and Fracture Risk in the EPIC-Norfolk Cohort. Antioxidants 2021, 10, 159. [Google Scholar] [CrossRef]
- Steghens, J.-P.; van Kappel, A.L.; Riboli, E.; Collombel, C. Simultaneous measurement of seven carotenoids, retinol and α-tocopherol in serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1997, 694, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, J.P.; Keck, E. Fluorometric assay of vitamin C in biological materials using a centrifugal analyser with fluorescence attachment. J. Micronutr. Anal. 1989, 5, 25–34. [Google Scholar]
- NHS. Quality and Outcomes Framework (QOF) 2021–2022 Prevalence. Available online: https://app.powerbi.com/view?r=eyJrIjoiYWI4Y2VkZTEtMThhMi00ZGZkLTgxYWEtNTU3NGM1ZGE3OTI0IiwidCI6IjUwZjYwNzFmLWJiZmUtNDAxYS04ODAzLTY3Mzc0OGU2MjllMiIsImMiOjh9 (accessed on 4 June 2023).
- National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management (NG136). Available online: https://www.nice.org.uk/guidance/ng136 (accessed on 21 October 2023).
- Shohaimi, S.; Luben, R.; Wareham, N.; Day, N.; Bingham, S.; Welch, A.; Oakes, S.; Khaw, K.-T. Residential area deprivation predicts smoking habit independently of individual educational level and occupational social class. A cross sectional study in the Norfolk cohort of the European Investigation into Cancer (EPIC-Norfolk). J. Epidemiol. Community Health 2003, 57, 270–276. [Google Scholar] [CrossRef]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of the EPIC-Norfolk physical activity questionnaire. Int. J. Epidemiol. 2002, 31, 168–174. [Google Scholar] [CrossRef]
- Wareham, N.J.; Jakes, R.W.; Rennie, K.L.; Schuit, J.; Mitchell, J.; Hennings, S.; Day, N.E. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Jakes, R.; Bingham, S.; Welch, A.; Luben, R.; Day, N.; Wareham, N. Work and leisure time physical activity assessed using a simple, pragmatic, validated questionnaire and incident cardiovascular disease and all-cause mortality in men and women: The European Prospective Investigation into Cancer in Norfolk prospective pop. Int. J. Epidemiol. 2006, 35, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Sampson, L.; Willett, W.C. The association between a nutritional quality index and risk of chronic disease. Am. J. Prev. Med. 2011, 40, 505–513. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary patterns and associations with biomarkers of inflammation in adults: A systematic review of observational studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Woodside, J.V.; Draper, J.; Lloyd, A.; McKinley, M.C. Use of biomarkers to assess fruit and vegetable intake. Proc. Nutr. Soc. 2017, 76, 308–315. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Bingham, S.; Welch, A.; Luben, R.; Wareham, N.; Oakes, S.; Day, N. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: A prospective population study. Lancet 2001, 357, 657–663. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Carrillo-Larco, R.M.; Lim, C.C.; Mishra, S.R.; Yuan, C.; Xu, X. Association of dietary patterns and food groups intake with multimorbidity: A prospective cohort study. Clin. Nutr. ESPEN 2022, 51, 359–366. [Google Scholar] [CrossRef]
- Wu, J.; Carter, A. Abnormal Laboratory Results: Magnesium: The forgotten electrolyte. Aust. Prescr. 2007, 30, 102–105. [Google Scholar] [CrossRef]
- Brandner, M.; Zhang, L.; MacGregor, A.; Traka, M.; Welch, A. Associations between dietary intake and multiple long-term conditions in adults: A scoping review. Proc. Nutr. Soc. 2024, 83, E219. [Google Scholar] [CrossRef]
- Ruel, G.; Shi, Z.; Zhen, S.; Zuo, H.; Kröger, E.; Sirois, C.; Lévesque, J.-F.; Taylor, A.W. Association between nutrition and the evolution of multimorbidity: The importance of fruits and vegetables and whole grain products. Clin. Nutr. 2014, 33, 513–520. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, J.; Lee, H.; Kim, D.-Y.; Lim, H. Association of cardiometabolic multimorbidity pattern with dietary factors among adults in South Korea. Nutrients 2020, 12, 2730. [Google Scholar] [CrossRef]
- Shi, J.; Guo, Y.; Li, Z.; Liang, Z.; Pan, L.; Yu, Y.; Zhu, W.; Shao, A.; Chen, W.; Gao, C.; et al. Sociodemographic and behavioral influences on multimorbidity among adult residents of northeastern China. BMC Public Health 2022, 22, 342. [Google Scholar] [CrossRef]
- Shang, X.; Peng, W.; Wu, J.; He, M.; Zhang, L. Leading determinants for multimorbidity in middle-aged Australian men and women: A nine-year follow-up cohort study. Prev. Med. 2020, 141, 106260. [Google Scholar] [CrossRef]
- Park, Y.; Dodd, K.W.; Kipnis, V.; E Thompson, F.; Potischman, N.; A Schoeller, D.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D. Reverse Causality in Cardiovascular Epidemiological Research. Circulation 2017, 135, 2369–2372. [Google Scholar] [CrossRef]
- Lindsted, K.D.; Fraser, G.E.; Steinkohl, M.; Beeson, W. Healthy volunteer effect in a cohort study: Temporal resolution in the Adventist Health Study. J. Clin. Epidemiol. 1996, 49, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.; Dodd, T.; Flatley, J.; Freeth, S.; Bolling, K. Health Survey for England 1993; HMSO: London, UK, 1995.
- Diederichs, C.; Berger, K.; Bartels, D.B. The measurement of multiple chronic diseases—A systematic review on existing multimorbidity indices. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2011, 66, 301–311. [Google Scholar] [CrossRef]
- Dodds, R.M.; Bunn, J.G.; Hillman, S.J.; Granic, A.; Murray, J.; Witham, M.D.; Robinson, S.M.; Cooper, R.; Sayer, A.A. Simple approaches to characterising multiple long-term conditions (multimorbidity) and rates of emergency hospital admission: Findings from 495,465 UK Biobank participants. J. Intern. Med. 2023, 293, 100–109. [Google Scholar] [CrossRef]
- MacRae, C.; McMinn, M.; Mercer, S.W.; Henderson, D.; McAllister, D.A.; Ho, I.; Jefferson, E.; Morales, D.R.; Lyons, J.; Lyons, R.A.; et al. The impact of varying the number and selection of conditions on estimated multimorbidity prevalence: A cross-sectional study using a large, primary care population dataset. PLoS Med. 2023, 20, e1004208. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.S.S.; Azcoaga-Lorenzo, A.; Akbari, A.; Davies, J.; Khunti, K.; Kadam, U.T.; A Lyons, R.; McCowan, C.; Mercer, S.W.; Nirantharakumar, K.; et al. Measuring multimorbidity in research: Delphi consensus study. BMJ Med. 2022, 1, e000247. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Almirall, J.; Nicholson, K. Development of a Research Tool to Document Self-Reported Chronic Conditions in Primary Care. J. Comorb. 2017, 7, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Luben, R.N.; Lentjes, M.A.H.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.E.; Schroeter, H.; Kuhnle, G.G.C. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. Br. J. Nutr. 2014, 111, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.A.; Kuhnle, G.G.; Lentjes, M.A.; van Scheltinga, V.; A Powell, N.; McTaggart, A.; Bhaniani, A.; Khaw, K.-T. Intakes and sources of isoflavones, lignans, enterolignans, coumestrol and soya-containing foods in the Norfolk arm of the European prospective investigation into cancer and nutrition (EPIC-Norfolk), from 7 d food diaries, using a newly updated database. Public Health Nutr. 2013, 16, 1454–1462. [Google Scholar] [CrossRef]
- Drexler, H.; Hornig, B. Endothelial Dysfunction in Human Disease. J. Mol. Cell Cardiol. 1999, 31, 51–60. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Verbeuren, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Mechanisms of disease: Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 46–56. [Google Scholar] [CrossRef]
- Kuper, H.; Adami, H.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2001, 249, 171–183. [Google Scholar] [CrossRef]
- Singhal, G.; Jaehne, E.J.; Corrigan, F.; Toben, C.; Baune, B.T. Inflammasomes in neuroinflammation and changes in brain function: A focused review. Front. Neurosci. 2014, 8, 315. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and oxidative stress in human diseases: From molecular mechanisms to novel treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]



| Quintile 1: Most Anti-Inflammatory | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5: Most Pro-Inflammatory | p Trend | |
|---|---|---|---|---|---|---|
| MEN | n = 2223 | n = 2223 | n = 2222 | n = 2223 | n = 2222 | |
| DII® range | −6.76 to −1.31 | −1.31 to −0.02 | −0.02 to 1.03 | 1.03 to 2.15 | 2.15 to 7.60 | |
| DII®(median) | −2.24 | −0.62 | 0.50 | 1.55 | 2.95 | |
| MLTCs (n, %) | 839 (38) | 768 (35) | 710 (32) | 713 (32) | 655 (29) | |
| Age (years) | 60.5 (9.0) | 60.1 (9.3) | 59.4 (9.2) | 59.5 (9.4) | 58.9 (9.5) | <0.001 |
| Weight (kg) | 80.9 (11.2) | 80.7 (11.4) | 80.7 (11.2) | 80.0 (11.4) | 79.0 (11.5) | <0.001 |
| BMI (kg/m2) | 26.6 (3.3) | 26.6 (3.3) | 26.6 (3.2) | 26.5 (3.3) | 26.2 (3.2) | <0.001 |
| Supplement user, % | 50 | 42 | 38 | 32 | 29 | <0.001 |
| Social class, % | <0.001 | |||||
| Non-manual | 65 | 61 | 58 | 55 | 48 | |
| Manual | 33 | 37 | 40 | 44 | 49 | |
| Missing | 2 | 2 | 2 | 1 | 2 | |
| Education, % | <0.001 | |||||
| No qualifications | 25 | 27 | 29 | 32 | 38 | |
| O level and above | 75 | 73 | 71 | 68 | 61 | |
| Missing | 0 | 0 | 0 | 0 | 0 | |
| Smoking status, % | <0.001 | |||||
| Current | 5 | 7 | 11 | 15 | 22 | |
| Former | 58 | 58 | 55 | 53 | 48 | |
| Never | 36 | 35 | 34 | 32 | 30 | |
| Missing | 1 | 0 | 1 | 1 | 1 | |
| Physical activity, % | <0.001 | |||||
| Inactive | 27 | 28 | 31 | 34 | 33 | |
| Moderately inactive | 26 | 26 | 26 | 25 | 20 | |
| Moderately active | 24 | 23 | 23 | 22 | 24 | |
| Active | 24 | 22 | 20 | 20 | 23 | |
| BMI, % | 0.012 | |||||
| Underweight | 0 | 0 | 0 | 0 | 0 | |
| Normal weight | 31 | 32 | 31 | 32 | 36 | |
| Overweight | 56 | 54 | 55 | 54 | 52 | |
| Obese | 14 | 14 | 13 | 13 | 12 | |
| Quintile 1: Most Anti-Inflammatory | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5: Most Pro-Inflammatory | pTrend | |
| WOMEN | n = 2682 | n = 2682 | n = 2681 | n = 2682 | n = 2681 | |
| DII® range | −6.62 to −2.17 | −2.17 to −1.02 | −1.02 to 0.01 | 0.01 to 1.18 | 1.18 to 6.71 | |
| DII® (median) | −2.95 | −1.55 | −0.50 | 0.55 | 2.08 | |
| MLTCs (n, %) | 1081 (40) | 1073 (40) | 1018 (38) | 1045 (39) | 1043 (39) | |
| Age (years) | 59.1 (9.1) | 58.8 (9.2) | 58.7 (9.2) | 58.7 (9.3) | 58.9 (9.6) | 0.569 |
| Weight (kg) | 68.6 (12.0) | 68.1 (11.6) | 67.8 (11.1) | 68.1 (12.1) | 67.1 (11.8) | <0.001 |
| BMI (kg/m2) | 26.3 (4.3) | 26.2 (4.3) | 26.1 (4.0) | 26.3 (4.5) | 26.0 (4.4) | 0.001 |
| Supplement user, % | 64 | 58 | 53 | 49 | 42 | <0.001 |
| Social class, % | <0.001 | |||||
| Non-manual | 65 | 64 | 60 | 58 | 55 | |
| Manual | 32 | 34 | 38 | 39 | 42 | |
| Missing | 2 | 2 | 2 | 3 | 3 | |
| Education, % | <0.001 | |||||
| No qualifications | 35 | 39 | 40 | 45 | 50 | |
| O level and above | 65 | 61 | 60 | 55 | 50 | |
| Missing | 0 | 0 | 0 | 0 | 0 | |
| Smoking status, % | <0.001 | |||||
| Current | 6 | 7 | 9 | 13 | 20 | |
| Former | 36 | 34 | 32 | 30 | 29 | |
| Never | 58 | 58 | 58 | 56 | 50 | |
| Missing | 1 | 1 | 1 | 1 | 1 | |
| Physical activity, % | <0.001 | |||||
| Inactive | 25 | 27 | 30 | 32 | 36 | |
| Moderately inactive | 31 | 33 | 34 | 34 | 30 | |
| Moderately active | 24 | 24 | 22 | 21 | 21 | |
| Active | 21 | 16 | 14 | 13 | 13 | |
| BMI, % | 0.005 | |||||
| Underweight | 1 | 1 | 0 | 1 | 1 | |
| Normal weight | 42 | 43 | 43 | 43 | 46 | |
| Overweight | 41 | 40 | 41 | 38 | 37 | |
| Obese | 17 | 17 | 16 | 18 | 16 |
| Quintile 1: Most Anti-Inflammatory | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5: Most Pro-Inflammatory | Q5–Q1 Diff | % Diff | p Trend | |
|---|---|---|---|---|---|---|---|---|
| MEN | n = 2223 | n = 2223 | n = 2222 | n = 2223 | n = 2222 | |||
| hs-CRP (nmol/L) | 27.4 (51.2) (n = 1603) | 27.7 (50.4) (n = 1609) | 26.5 (45.2) (n = 1590) | 27.5 (54.0) (n = 1622) | 33.1 (72.7) (n = 1610) | 5.7 | 20.9 | 0.006 |
| β-carotene (µmol/L) | 0.42 (0.25) (n = 761) | 0.39 (0.25) (n = 737) | 0.35 (0.22) (n = 727) | 0.33 (0.18) (n = 741) | 0.30 (0.18) (n = 707) | −0.12 | −28.6 | <0.001 |
| Vitamin A (µmol/L) | 1.87 (0.44) (n = 761) | 1.86 (0.45) (n = 737) | 1.84 (0.43) (n = 727) | 1.82 (0.46) (n = 741) | 1.77 (0.43) (n = 707) | −0.1 | −5.4 | <0.001 |
| Vitamin E, adjusted for cholesterol (μmol/mmol) | 4.56 (1.18) (n = 755) | 4.42 (1.13) (n = 723) | 4.39 (0.98) (n = 714) | 4.31 (1.00) (n = 734) | 4.02 (0.92) (n = 697) | −0.55 | −12.0 | <0.001 |
| Vitamin C (µmol/L) | 54.3 (17.5) (n = 1993) | 50.9 (17.7) (n = 1973) | 47.5 (17.6) (n = 1967) | 44.2 (18.6) (n = 1981) | 38.7 (18.8) (n = 1952) | −15.6 | −28.8 | <0.001 |
| Magnesium (mmol/L) | 0.82 (0.12) (n = 1602) | 0.81 (0.12) (n = 1608) | 0.82 (0.12) (n = 1591) | 0.81 (0.12) (n = 1617) | 0.81 (0.12) (n = 1611) | −0.001 | −0.15 | <0.001 |
| WOMEN | n = 2682 | n = 2682 | n = 2681 | n = 2682 | n = 2681 | |||
| hs-CRP (nmol/L) | 27.5 (48.2) (n = 1960) | 29.4 (65.2) (n = 1997) | 27.7 (56.5) (n = 2003) | 30.7 (66.2) (n = 1976) | 31.7 (62.5) (n = 1925) | 4.2 | 15.2 | 0.125 |
| β-carotene (µmol/L) | 0.59 (0.35) (n = 693) | 0.49 (0.27) (n = 696) | 0.48 (0.29) (n = 723) | 0.44 (0.28) (n = 704) | 0.40 (0.23) (n = 701) | −0.19 | −32.4 | <0.001 |
| Vitamin A (µmol/L) | 1.78 (0.44) (n = 693) | 1.80 (0.48) (n = 696) | 1.75 (0.42) (n = 723) | 1.72 (0.45) (n = 704) | 1.70 (0.42) (n = 701) | −0.08 | −4.3 | <0.001 |
| Vitamin E, adjusted for cholesterol (μmol/mmol) | 4.64 (1.11) (n = 690) | 4.50 (1.09) (n = 687) | 4.45 (1.05) (n = 711) | 4.37 (1.02) (n = 699) | 4.14 (0.97) (n = 689) | −0.51 | −10.9 | <0.001 |
| Vitamin C (µmol/L) | 65.4 (18.6) (n = 2360) | 62.1 (18.3) (n = 2351) | 59.8 (18.4) (n = 2363) | 57.0 (19.4) (n = 2323) | 49.6 (21.2) (n = 2305) | −15.9 | −24.3 | <0.001 |
| Magnesium (mmol/L) | 0.79 (0.13) (n = 1959) | 0.80 (0.12) (n = 1990) | 0.80 (0.12) (n = 1998) | 0.80 (0.12) (n = 1966) | 0.80 (0.12) (n = 1920) | 0.002 | 0.2 | 0.610 |
| Q1 (Most Anti-Inflammatory) | Q2 | Q3 | Q4 | Q5 (Most Pro-Inflammatory) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | p Trend | |
| MEN (n = 11,113) | ||||||||||
| Model 1 | 1.45 | 1.28–1.64 | 1.26 | 1.11–1.43 | 1.12 | 0.99–1.28 | 1.13 | 0.99–1.28 | 1.00 | <0.001 |
| Model 2 | 1.35 | 1.19–1.54 | 1.20 | 1.05–1.36 | 1.10 | 0.96–1.25 | 1.10 | 0.96–1.25 | 1.00 | <0.001 |
| Model 3 | 1.40 | 1.23–1.60 | 1.22 | 1.07–1.39 | 1.11 | 0.97–1.26 | 1.09 | 0.95–1.24 | 1.00 | <0.001 |
| WOMEN (n = 13,408) | ||||||||||
| Model 1 | 1.06 | 0.95–1.18 | 1.05 | 0.94–1.17 | 0.96 | 0.86–1.07 | 1.00 | 0.90–1.12 | 1.00 | 0.209 |
| Model 2 | 1.06 | 0.94–1.19 | 1.06 | 0.95–1.19 | 0.97 | 0.87–1.09 | 1.02 | 0.91–1.14 | 1.00 | 0.243 |
| Model 3 | 1.12 | 1.00–1.26 | 1.10 | 0.98–1.24 | 1.00 | 0.89–1.12 | 1.03 | 0.92–1.16 | 1.00 | 0.024 |
| MEN | WOMEN | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Exp (Coeff) | 95% CI | N | Mean (SD) | Exp (Coeff) | 95% CI | ||
| β-carotene | 2767 | 19.4 (12.1) | 0.81 | 0.78–0.84 | 2642 | 25.5 (15.8) | 0.75 | 0.72–0.78 | |
| Vitamin A | 2767 | 52.6 (12.8) | 0.90 | 0.87–0.94 | 2642 | 49.9 (12.4) | 1.04 | 0.99–1.08 | |
| Vitamin C | 7728 | 46.9 (18.6) | 0.80 | 0.78–0.82 | 9499 | 58.7 (19.8) | 0.81 | 0.79–0.83 | |
| Vitamin E | 2725 | 4.35 (1.02) | 1.07 | 1.02–1.12 | 2612 | 4.34 (1.06) | 1.05 | 1.01–1.09 | |
| Magnesium | 7678 | 0.81 (0.12) | 1.17 | 1.14–1.20 | 9430 | 0.80 (0.12) | 1.16 | 1.13–1.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulligan, A.A.; Lentjes, M.A.H.; Skinner, J.; Welch, A.A. The Dietary Inflammatory Index and Its Associations with Biomarkers of Nutrients with Antioxidant Potential, a Biomarker of Inflammation and Multiple Long-Term Conditions. Antioxidants 2024, 13, 962. https://doi.org/10.3390/antiox13080962
Mulligan AA, Lentjes MAH, Skinner J, Welch AA. The Dietary Inflammatory Index and Its Associations with Biomarkers of Nutrients with Antioxidant Potential, a Biomarker of Inflammation and Multiple Long-Term Conditions. Antioxidants. 2024; 13(8):962. https://doi.org/10.3390/antiox13080962
Chicago/Turabian StyleMulligan, Angela A., Marleen A. H. Lentjes, Jane Skinner, and Ailsa A. Welch. 2024. "The Dietary Inflammatory Index and Its Associations with Biomarkers of Nutrients with Antioxidant Potential, a Biomarker of Inflammation and Multiple Long-Term Conditions" Antioxidants 13, no. 8: 962. https://doi.org/10.3390/antiox13080962
APA StyleMulligan, A. A., Lentjes, M. A. H., Skinner, J., & Welch, A. A. (2024). The Dietary Inflammatory Index and Its Associations with Biomarkers of Nutrients with Antioxidant Potential, a Biomarker of Inflammation and Multiple Long-Term Conditions. Antioxidants, 13(8), 962. https://doi.org/10.3390/antiox13080962







