Exploring Plasma Coenzyme Q10 Status in Paediatric Dyslipidaemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Samples and Laboratory Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crane, F.L. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion 2007, 7, S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef] [PubMed]
- Tran, U.P.C.; Clarke, C.F. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 2007, 7, S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, Z.; Zhao, D.; Liang, Y.; Dai, S.; Liu, M.; Hou, S.; Dong, X.; Zhaxinima; Yang, Y. Effects of Coenzyme Q10 Supplementation on Lipid Profiles in Adults: A Meta-analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 108, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, J.; Wang, C.; Zhao, J.; Wang, H.; Zhang, Y.; Sun, H.; Liu, M. Coenzyme Q10 Protects Against Hyperlipidemia-Induced Osteoporosis by Improving Mitochondrial Function via Modulating miR-130b-3p/PGC-1alpha Pathway. Calcif. Tissue Int. 2024, 114, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Yubero, D.; Montero, R.; Artuch, R. What can we expect from blood (plasma) Coenzyme Q10 analysis. In Coenzyme Q10: From Fact to Fiction; Hargreaves, I.P., Ed.; Nova Biomedical: New York, NY, USA, 2015; pp. 253–269. [Google Scholar]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Hear. Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Montero, R.; Yubero, D.; Salgado, M.C.; González, M.J.; Campistol, J.; del Mar O’Callaghan, M.; Pineda, M.; Delgadillo, V.; Maynou, J.; Fernandez, G.; et al. Plasma coenzyme Q10 status is impaired in selected genetic conditions. Sci. Rep. 2019, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.P. Coenzyme Q10 in phenylketonuria and mevalonic aciduria. Mitochondrion 2007, 7, S175–S180. [Google Scholar] [CrossRef]
- Haas, D.; Niklowitz, P.; Hoffmann, G.F.; Andler, W.; Menke, T. Plasma and thrombocyte levels of coenzyme Q10 in children with Smith-Lemli-Opitz syndrome (SLOS) and the influence of HMG-CoA reductase inhibitors. BioFactors 2008, 32, 191–197. [Google Scholar] [CrossRef]
- Menke, T.; Niklowitz, P.; Reinehr, T.; De Sousa, G.J.; Andler, W. Plasma levels of coenzyme Q10 in children with hyperthyroidism. Horm. Res. 2004, 61, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Oudshoorn, J.H.; Lecluse, A.L.Y.; Van Den Berg, R.; Vaes, W.H.J.; Van Der Laag, J.; Houwen, R.H.J. Decreased coenzyme Q10 concentration in plasma of children with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.; Köller, Y.; Surkova, E. Effect of Coenzyme Q10 on statin-associated myalgia and adherence to statin therapy: A systematic review and meta-analysis. Atherosclerosis 2020, 299, 1–8. [Google Scholar] [CrossRef]
- Qu, H.; Meng, Y.Y.; Chai, H.; Liang, F.; Zhang, J.Y.; Gao, Z.Y.; Shi, D.Z. The effect of statin treatment on circulating coenzyme Q10 concentrations: An updated meta-analysis of randomized controlled trials. Eur. J. Med. Res. 2018, 23, 57. [Google Scholar] [CrossRef] [PubMed]
- Wittenstein, B.; Klein, M.; Finckh, B.; Ullrich, K.; Kohlschütter, A. Plasma antioxidants in pediatric patients with glycogen storage disease, diabetes mellitus, and hypercholesterolemia. Free Radic. Biol. Med. 2002, 33, 103–110. [Google Scholar] [CrossRef]
- Paredes-Fuentes, A.J.; Oliva, C.; Montero, R.; Alcaide, P.; Ruijter, G.J.G.; García-Villoria, J.; Ruiz-Sala, P.; Artuch, R. Technical Aspects of Coenzyme Q10 Analysis: Validation of a New HPLC-ED Method. Antioxidants 2022, 11, 528. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2023. [Google Scholar]
- Gutierrez-Mariscal, F.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Alcalá-Diaz, J.F.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 and Cardiovascular Diseases. Antioxidants 2021, 10, 906. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; de la Mata, M.; Villanueva-Paz, M.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Munuera, M.; et al. Atherosclerosis and Coenzyme Q10. Int. J. Mol. Sci. 2019, 20, 5195. [Google Scholar] [CrossRef]
- Bredefeld, C.; Hussain, M.M.; Averna, M.; Black, D.D.; Brin, M.F.; Burnett, J.R.; Charrière, S.; Cuerq, C.; Davidson, N.O.; Deckelbaum, R.J.; et al. Guidance for the diagnosis and treatment of hypolipidemia disorders. J. Clin. Lipidol. 2022, 16, 797–812. [Google Scholar] [CrossRef]
- Tarugi, P.; Averna, M.; Di Leo, E.; Cefalù, A.B.; Noto, D.; Magnolo, L.; Cattin, L.; Bertolini, S.; Calandra, S. Molecular diagnosis of hypobetalipoproteinemia: An ENID review. Atherosclerosis 2007, 195, e19–e27. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Tekle, M.; Dallner, G.; Brismar, K.; Gustafsson, J.Å.; Steffensen, K.R.; Catrina, S.B. Influence of liver-X-receptor on tissue cholesterol, coenzyme Q and dolichol content. Mol. Membr. Biol. 2012, 29, 299–308. [Google Scholar] [CrossRef]
- Peretti, N.; Sassolas, A.; Roy, C.C.; Deslandres, C.; Charcosset, M.; Castagnetti, J.; Pugnet-Chardon, L.; Moulin, P.; Labarge, S.; Bouthillier, L.; et al. Guidelines for the diagnosis and management of chylomicron retention disease based on a review of the literature and the experience of two centers. Orphanet J. Rare Dis. 2010, 5, 24. [Google Scholar] [CrossRef]
- Asselta, R.; Paraboschi, E.M.; Duga, S. Hereditary hypofibrinogenemia with hepatic storage. Int. J. Mol. Sci. 2020, 21, 7830. [Google Scholar] [CrossRef] [PubMed]
- Callea, F.; Giovannoni, I.; Sari, S.; Gulda, E.; Dalgic, B.; Akyol, G.; Sogo, T.; Al-Hussaini, A.; Maggiore, G.; Bartuli, A.; et al. Fibrinogen gamma chain mutations provoke fibrinogen and apolipoprotein B plasma deficiency and liver storage. Int. J. Mol. Sci. 2017, 18, 2717. [Google Scholar] [CrossRef] [PubMed]
- Mašek, J.; Andersson, E.R. Jagged-mediated development and disease: Mechanistic insights and therapeutic implications for Alagille syndrome. Curr. Opin. Cell Biol. 2024, 86, 102302. [Google Scholar] [CrossRef]
- Hannoush, Z.C.; Puerta, H.; Bauer, M.S.; Goldberg, R.B. New JAG1 mutation causing alagille syndrome presenting with severe hypercholesterolemia: Case report with emphasis on genetics and lipid abnormalities. J. Clin. Endocrinol. Metab. 2017, 102, 350–353. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Fernandez-Twinn, D.S.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Martin-Gronert, M.S.; McConnell, J.M.; Ozanne, S.E. Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am. J. Clin. Nutr. 2016, 103, 579–588. [Google Scholar] [CrossRef]
- Tao, L.; Xue, Y.-F.; Sun, F.-F.; He, X.; Wang, H.-Q.; Tong, C.-C.; Zhang, C.; Xu, D.-X.; Chen, X. MitoQ protects against carbon tetrachloride-induced hepatocyte ferroptosis and acute liver injury by suppressing mtROS-mediated ACSL4 upregulation. Toxicol. Appl. Pharmacol. 2024, 486, 116914. [Google Scholar] [CrossRef]
- Martinefski, M.R.; Yamasato, M.F.; Di Carlo, M.B.; Daruich, J.R.; Tripodi, V.P. Coenzyme Q10 deficiency in patients with hereditary hemochromatosis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101624. [Google Scholar] [CrossRef]
- Liaghat, M.; Yaghoubzad-Maleki, M.; Nabi-Afjadi, M.; Fathi, Z.; Zalpoor, H.; Heidari, N.; Bahreini, E. A Review of the Potential Role of CoQ10 in the Treatment of Hepatocellular Carcinoma. Biochem. Genet. 2024, 62, 575–593. [Google Scholar] [CrossRef] [PubMed]
- Vrentzos, E.; Ikonomidis, I.; Pavlidis, G.; Katogiannis, K.; Korakas, E.; Kountouri, A.; Pliouta, L.; Michalopoulou, E.; Pelekanou, E.; Boumpas, D.; et al. Six-month supplementation with high dose coenzyme Q10 improves liver steatosis, endothelial, vascular and myocardial function in patients with metabolic-dysfunction associated steatotic liver disease: A randomized double-blind, placebo-controlled trial. Cardiovasc. Diabetol. 2024, 23, 245. [Google Scholar] [CrossRef] [PubMed]

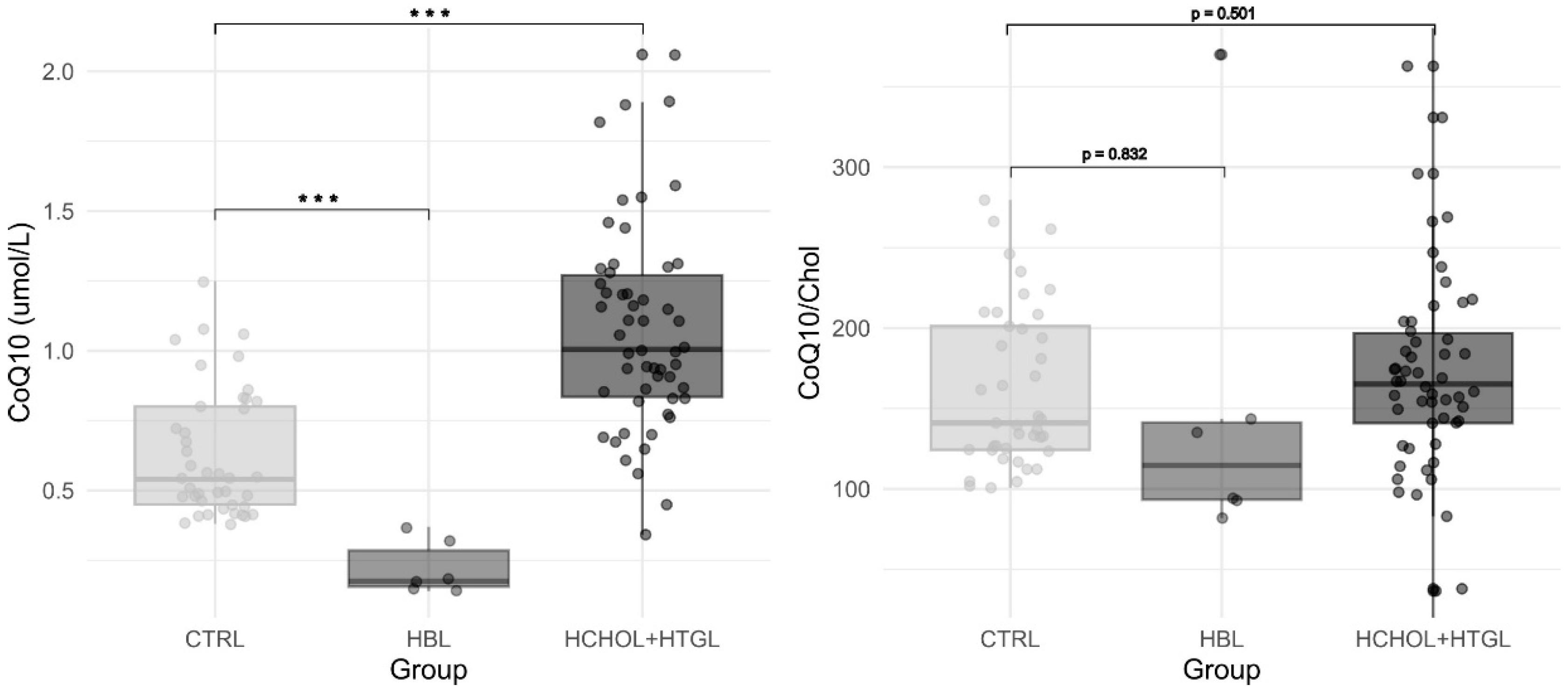
| Hypercholesterolemia/ Hypertriglyceridemia (n = 54) | Hypobetalipoproteinemia (n = 6) | Reference Values | |||||
|---|---|---|---|---|---|---|---|
| µ | Range | SD | µ | Range | SD | RI | |
| CoQ (μmol/L) | 1.07 | 0.34–2.06 | 0.36 | 0.2 | 0.14–0.37 | 0.097 | 0.38–1.25 |
| CoQ/Chol (µmol/mol Chol) | 171 | 36.6–362 | 96 | 153 | 81.9–370 | 109.2 | 101–280 |
| Chol (mmol/L) | 6.63 | 4.78–22.6 | 2.48 | 1.6 | 1.0–2.2 | 0.48 | 2.47–5.2 |
| Triglycerides (mmol/L) | 1.57 | 0.13–28.7 | 4.05 | 0.7 | 0.13–2.22 | 0.76 | 0.5–1.85 |
| HDL cholesterol (mmol/L) | 1.52 | 0.76–4.07 | 0.53 | 0.7 | 0.25–1.17 | 0.36 | >1.04 |
| LDL cholesterol (mmol/L) | 4.78 | 2.77–21.2 | 2.54 | 0.7 | 0.52–1.17 | 0–52 | <3.36 |
| VLDL-cholesterol (mmol/L) | 0.39 | 0.12–1.07 | 0.21 | 0.3 | 0.06–1.0 | 0.34 | 0.01–0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minguez, B.; de Los Santos, M.; Garcia-Volpe, C.; Molera, C.; Paredes-Fuentes, A.J.; Oliva, C.; Arias, A.; Rodriguez-Gonzalez, H.; Yubero, D.; Tondo, M.; et al. Exploring Plasma Coenzyme Q10 Status in Paediatric Dyslipidaemia. Antioxidants 2024, 13, 966. https://doi.org/10.3390/antiox13080966
Minguez B, de Los Santos M, Garcia-Volpe C, Molera C, Paredes-Fuentes AJ, Oliva C, Arias A, Rodriguez-Gonzalez H, Yubero D, Tondo M, et al. Exploring Plasma Coenzyme Q10 Status in Paediatric Dyslipidaemia. Antioxidants. 2024; 13(8):966. https://doi.org/10.3390/antiox13080966
Chicago/Turabian StyleMinguez, Beatriz, Mariela de Los Santos, Camila Garcia-Volpe, Cristina Molera, Abraham J. Paredes-Fuentes, Clara Oliva, Angela Arias, Helena Rodriguez-Gonzalez, Delia Yubero, Mireia Tondo, and et al. 2024. "Exploring Plasma Coenzyme Q10 Status in Paediatric Dyslipidaemia" Antioxidants 13, no. 8: 966. https://doi.org/10.3390/antiox13080966







