Sea Cucumber Viscera Processed by Protease Hydrolysis Combined with Cordyceps militaris Fermentation Protect Caco-2 Cells against Oxidative Damage via Enhancing Antioxidant Capacity, Activating Nrf2/HO-1 Pathway and Improving Cell Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Experimental Samples and Component Detection
2.2. Cell Culture and Treatments
2.3. Effect of Samples on Cell Growth Cycle and Apoptosis
2.4. Measurement of Cells’ Membrane Potential
2.5. Measurement of ROS and Related Enzyme Levels in Cells
2.6. Real-Time Quantitative PCR (RT-qPCR) Analysis
2.7. Western Blot Analysis
2.8. Untargeted Metabolomics Analysis
2.9. Statistical Analysis
3. Results
3.1. The Contents of SVH and FSVH
3.2. Cell Viability and Oxidative Damage Model
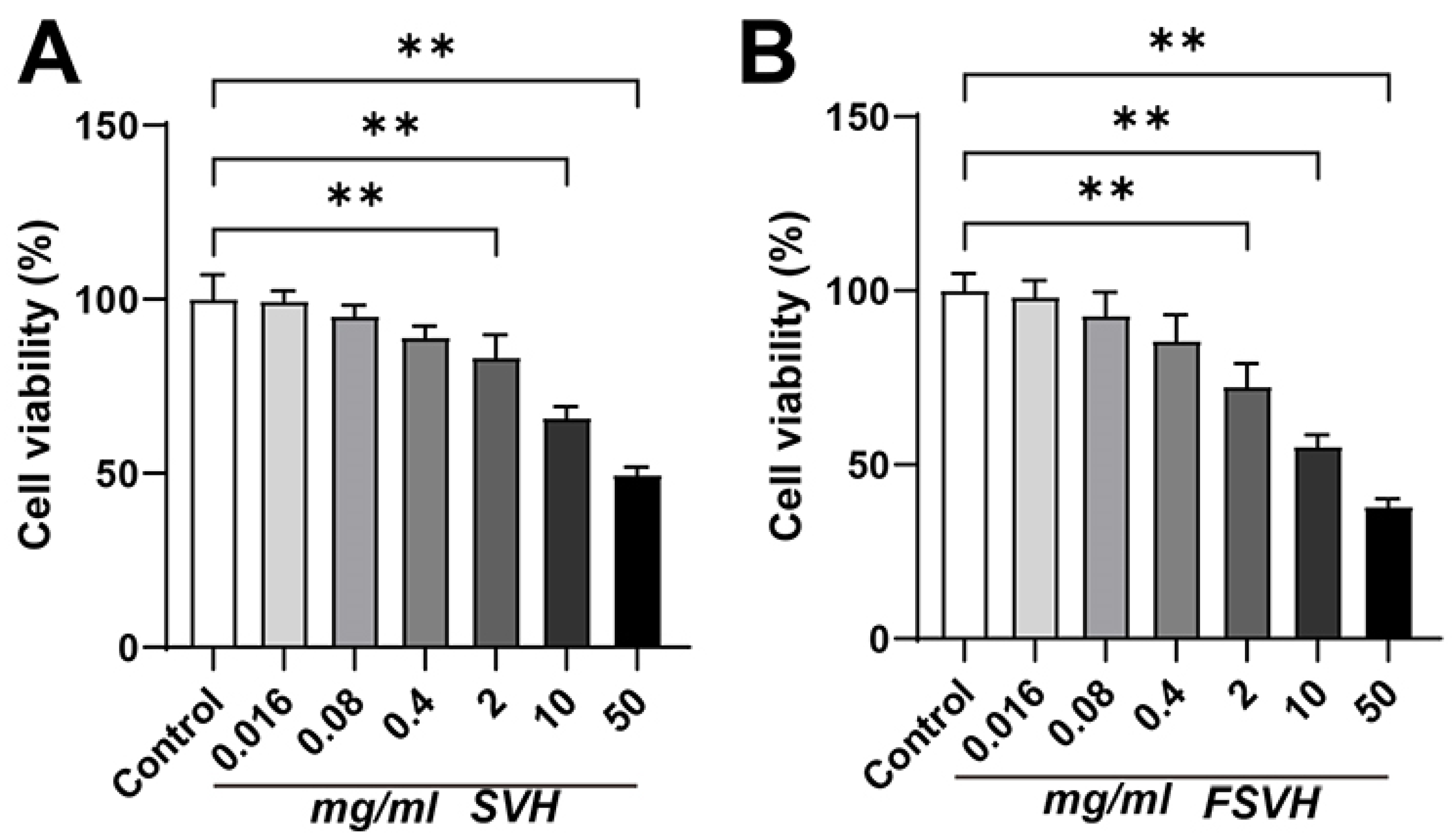
3.2.1. Cell Viability
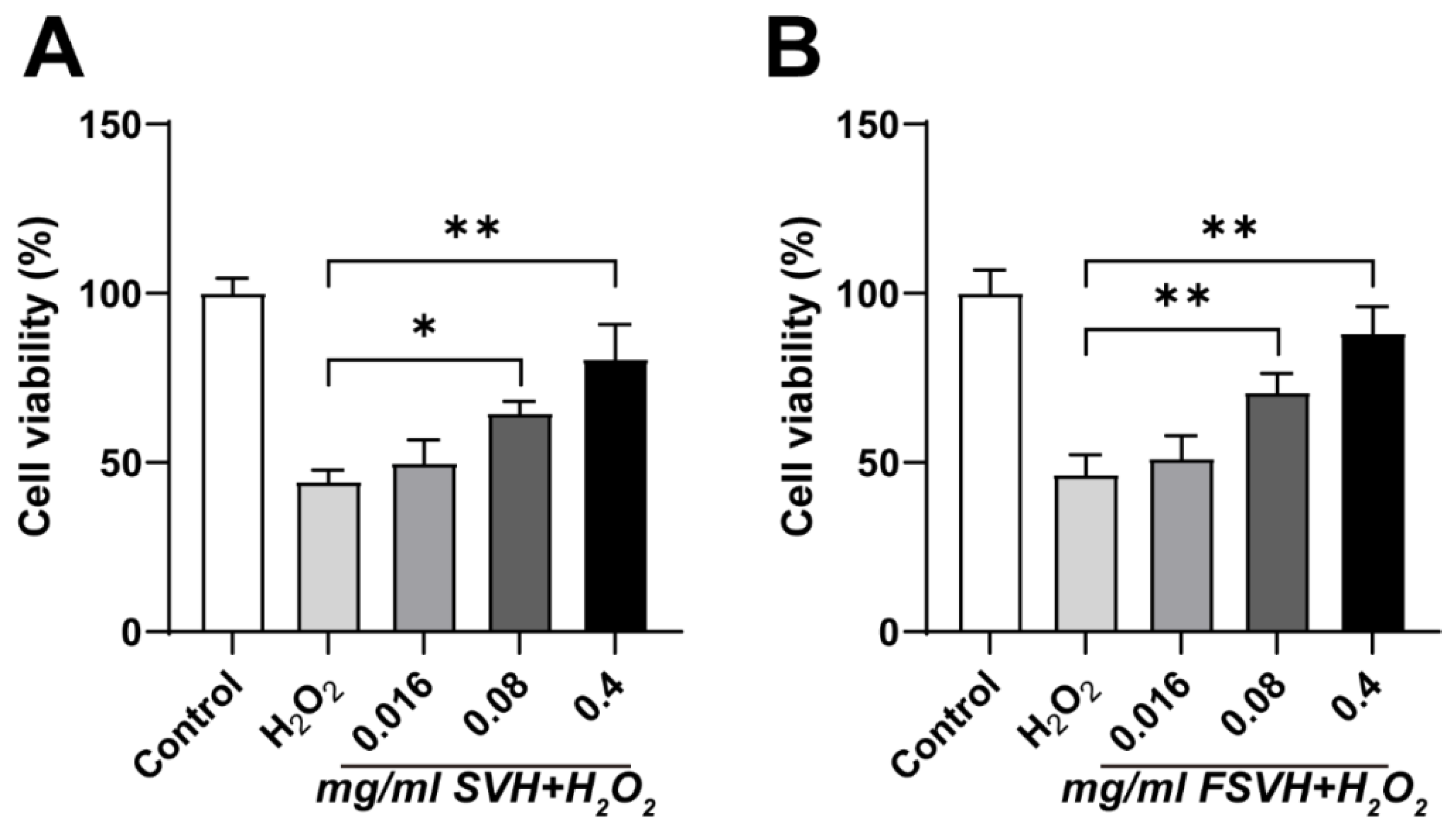
3.2.2. Establishment of Oxidative Damage Model
3.3. Effects of SVH and FSVH on Oxidative Damage
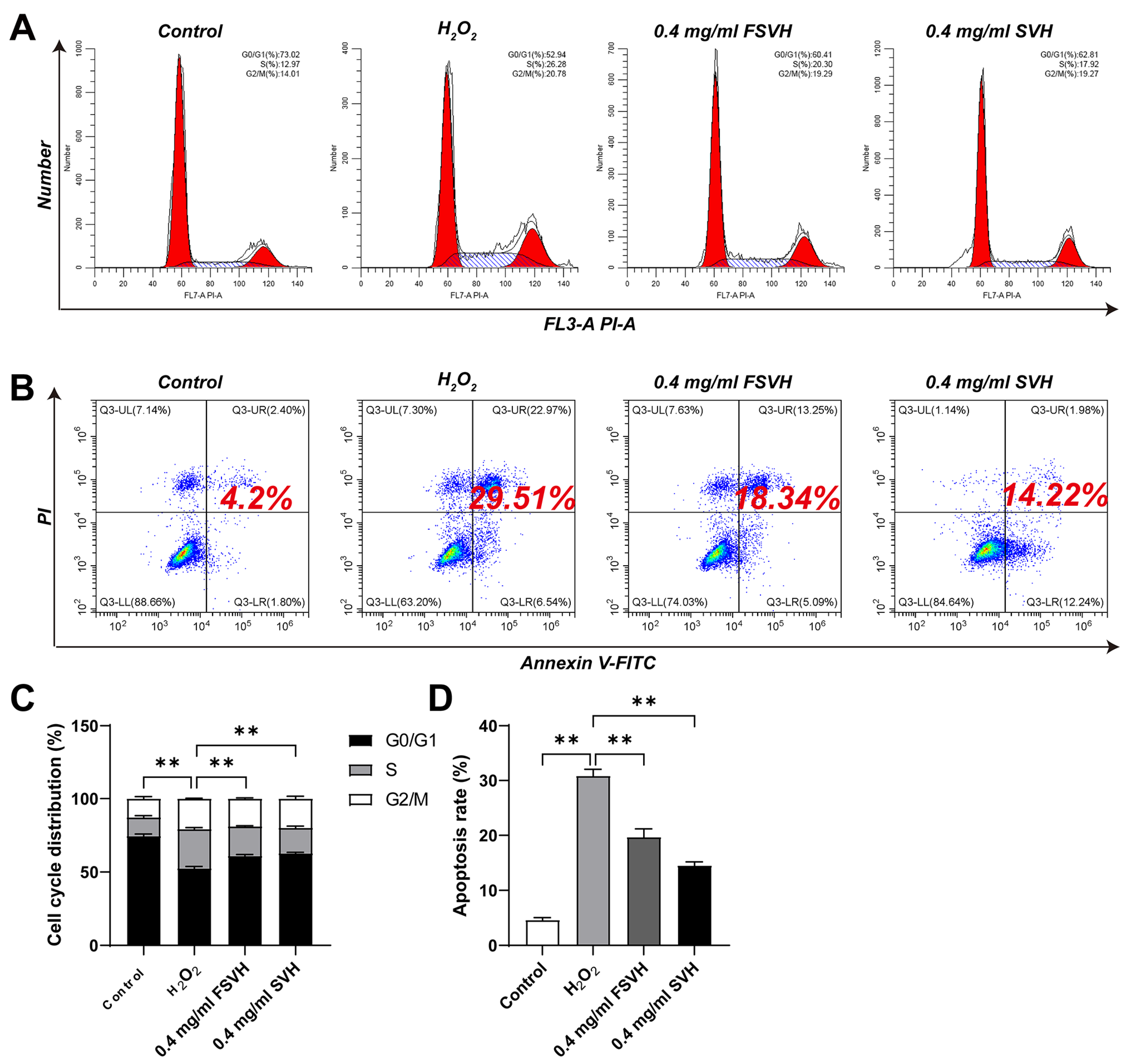
3.3.1. Cell Cycle
3.3.2. Cell Apoptosis
3.3.3. Cells’ Membrane Potential
3.3.4. The ROS Level
3.3.5. Cytoprotective Effect
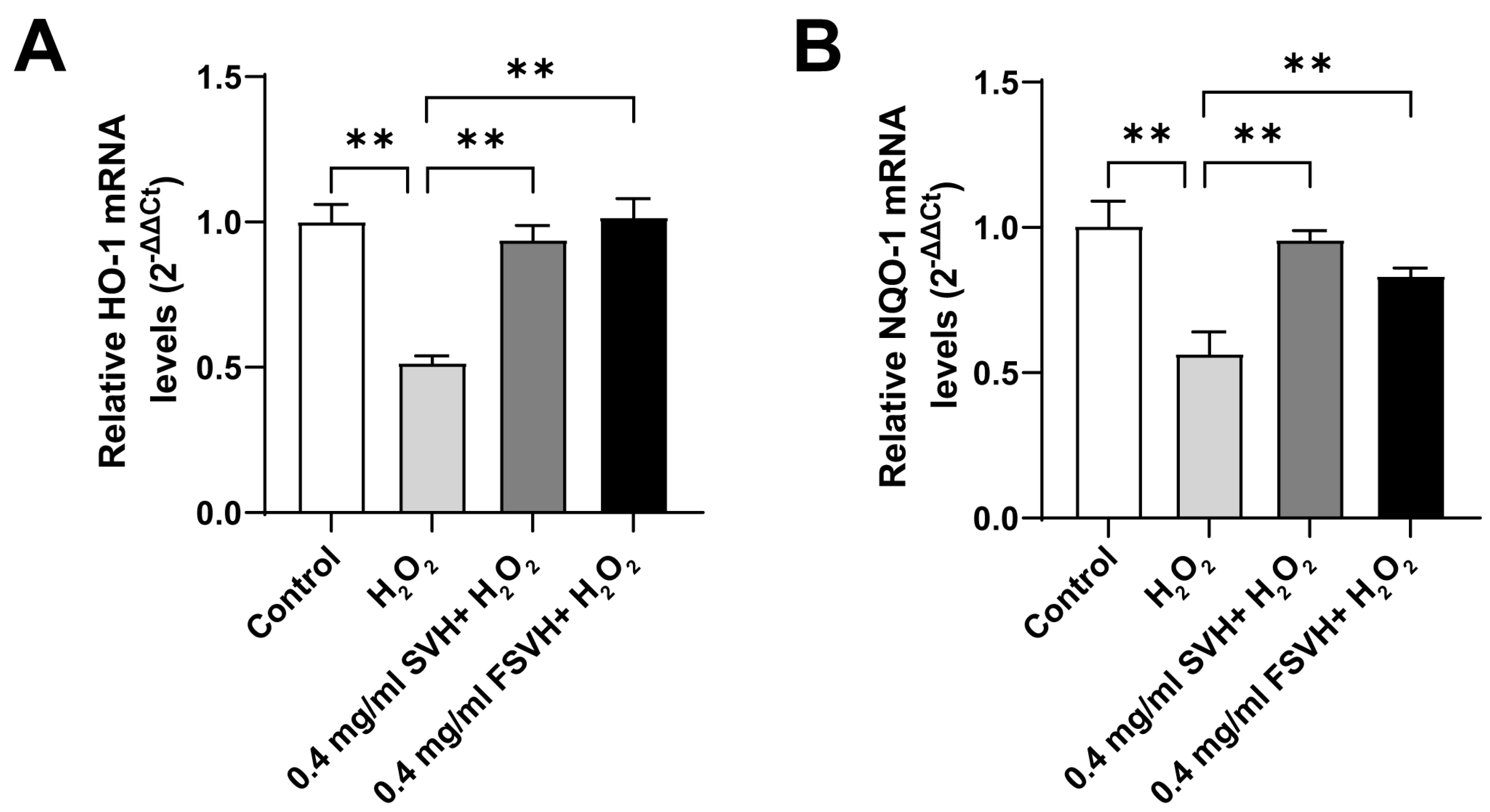
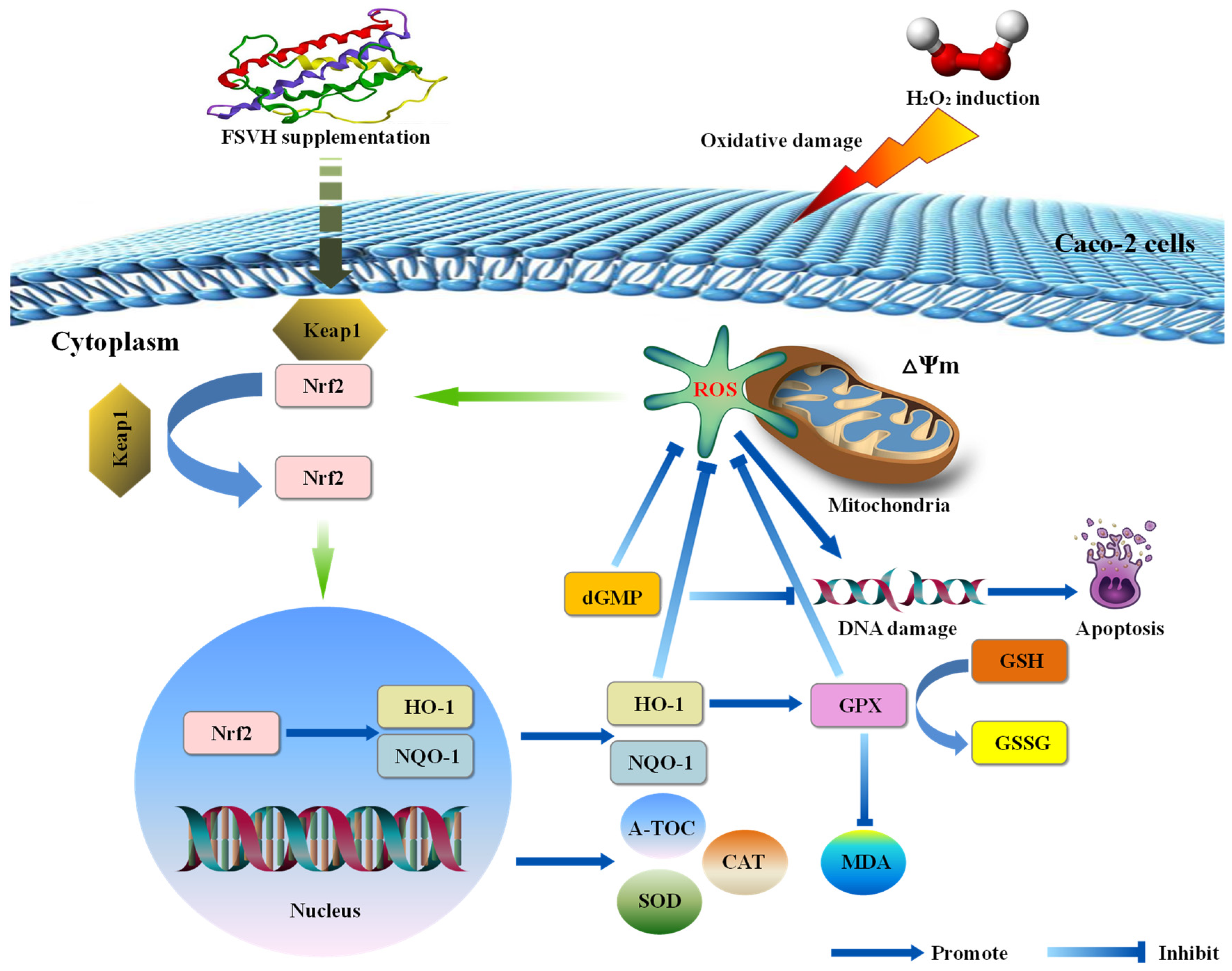
3.4. FSVH Promoted Antioxidant Defense via Activation of the Nrf2 Signaling Pathway
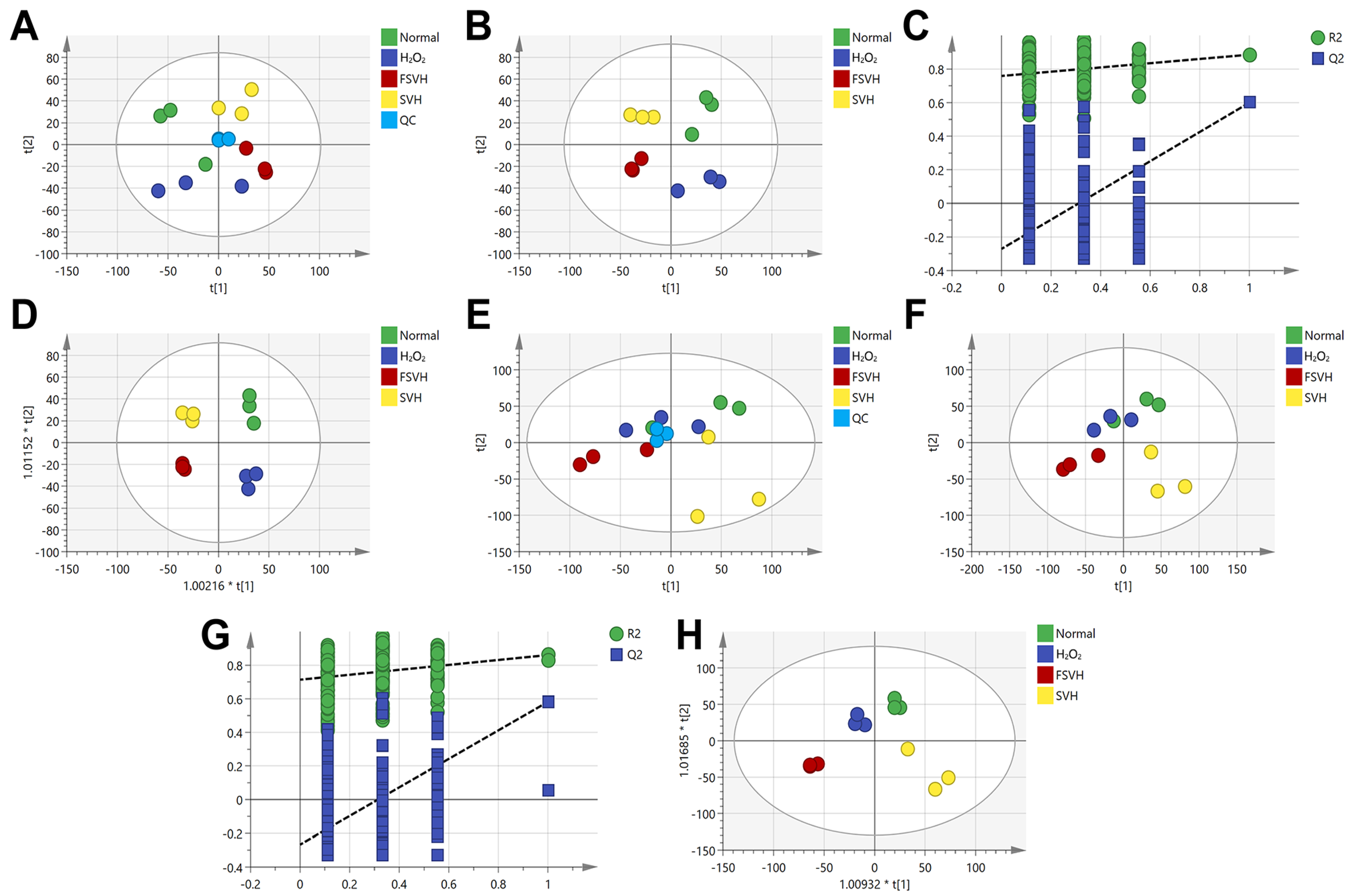
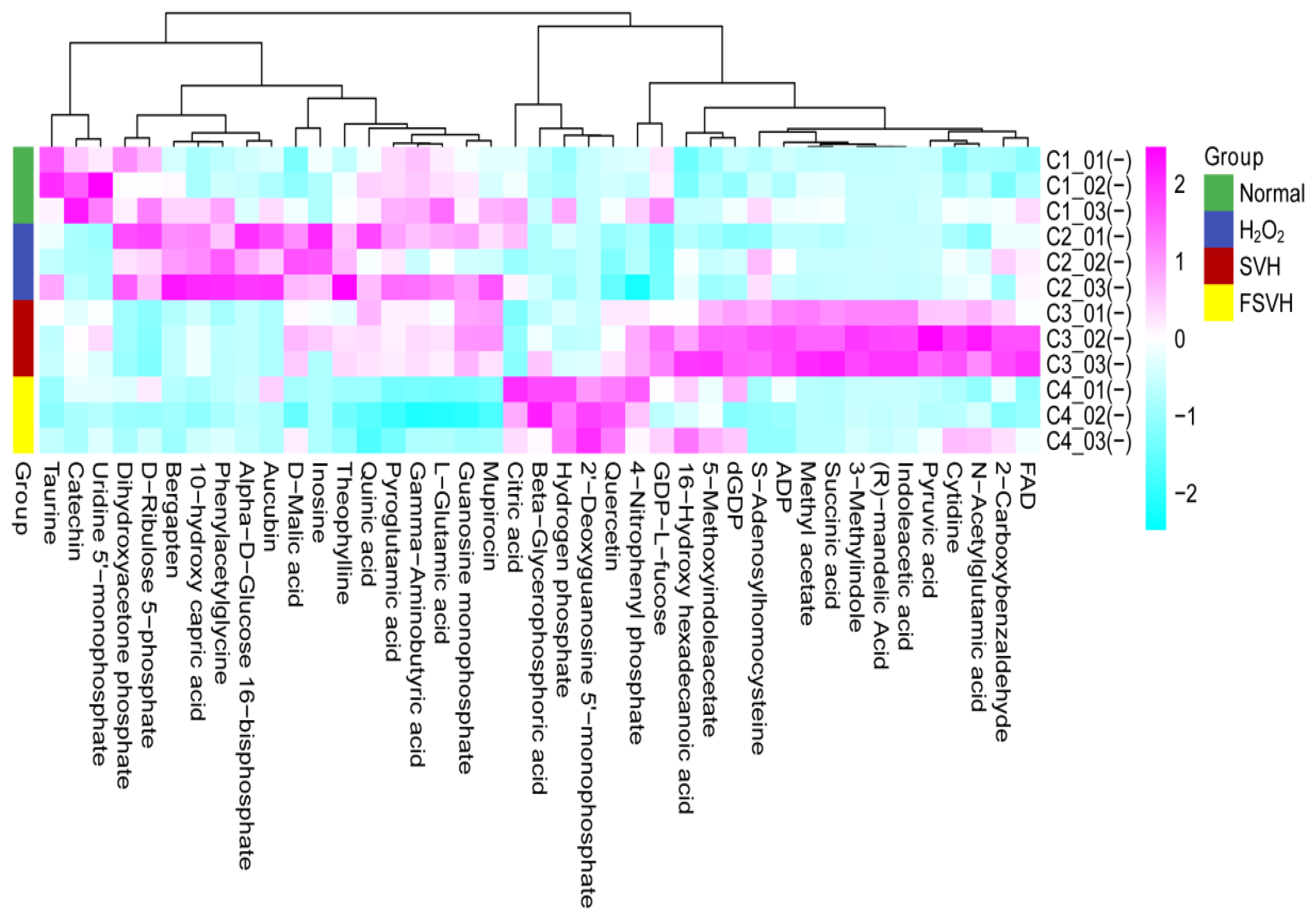
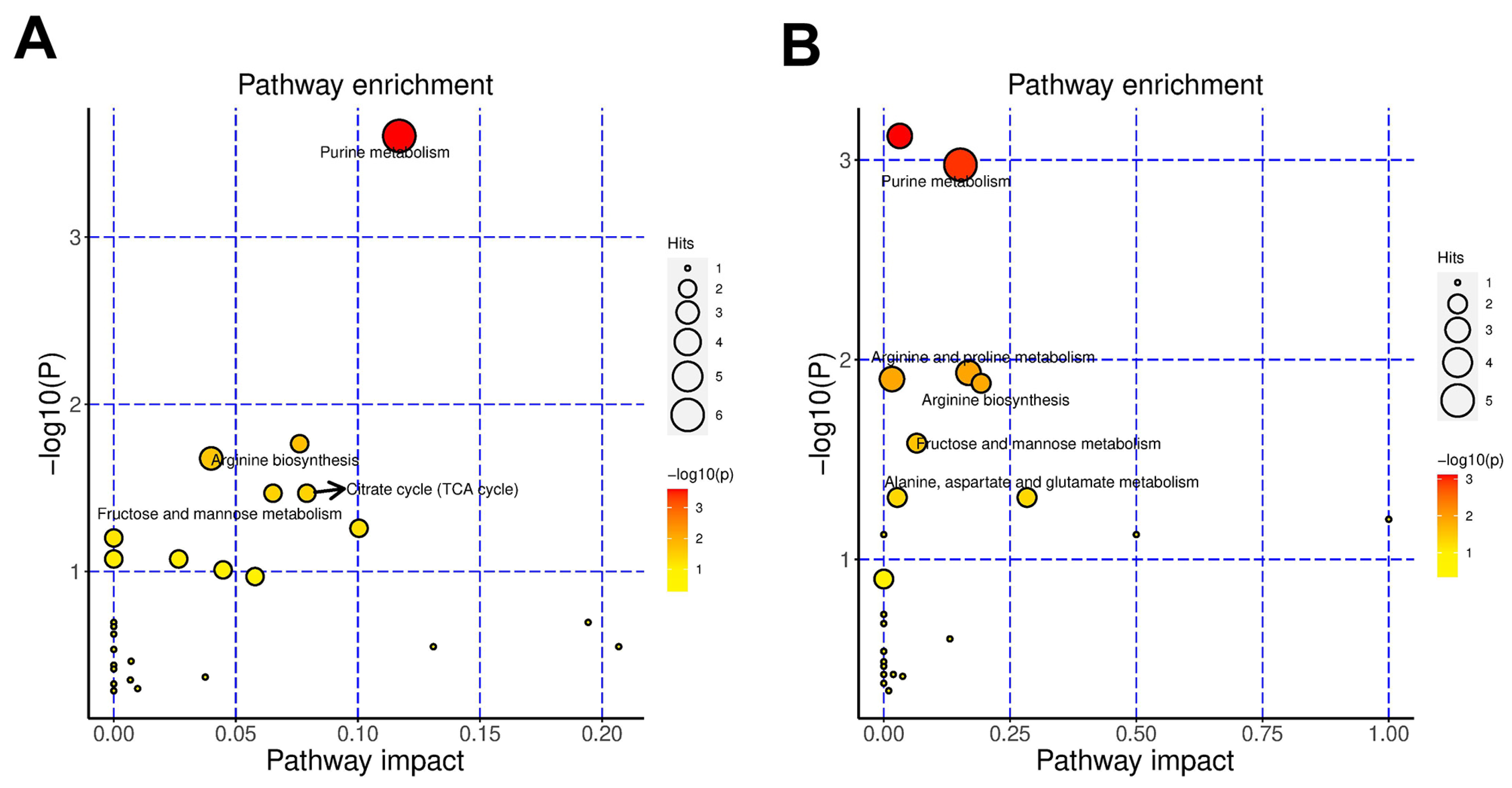
3.5. Variations in the Cells’ Metabolome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, Q.; Lan, J.; Pan, Y.; Wang, Y.; Song, T.; Yang, Y.; Tian, X.; Chen, L.; Gu, Z.; Ding, Y. Effects of Dityrosine on Lactic Acid Metabolism in Mice Gastrocnemius Muscle During Endurance Exercise via the Oxidative Stress-Induced Mitochondria Damage. J. Agric. Food Chem. 2024, 72, 5269–5282. [Google Scholar] [CrossRef]
- Roginskaya, M.; Razskazovskiy, Y. Oxidative DNA Damage and Repair: Mechanisms, Mutations, and Relation to Diseases. Antioxidants 2023, 12, 2623. [Google Scholar] [CrossRef]
- Checa, J.; Fiol, P.; Guevara, M.; Aran, J. TNFRSF1B Signaling Blockade Protects Airway Epithelial Cells from Oxidative Stress. Antioxidants 2024, 13, 368. [Google Scholar] [CrossRef]
- Kruidenier, L.; Verspaget, H.W. Oxidative stress as a pathogenic factor in inflammatory bowel disease-radicals or ridiculous. Aliment. Pharm. Ther. 2002, 16, 1997–2015. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Cao, Y.; Tian, X.; Zeng, R.; Liao, D.; Cao, D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid. Med. Cell. Longev. 2016, 2016, 9875298. [Google Scholar] [CrossRef]
- Moura, F.A.; De Andrade, K.Q.; Dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef]
- Ning, K.; Duan, Y.M.; Tong, W.W.; Chen, Y.; Zhang, Q.H.; Xie, Q.H.; Xiang, H.Y. Protective Effects of Different Molecular Weights of Purslane (Portulaca oleracea L.) Aqueous Extract on DSS-Induced Ulcerative Colitis in Mice. Antioxidants 2023, 12, 1400. [Google Scholar] [CrossRef]
- Mitani, T.; Yoshioka, Y.; Furuyashiki, T.; Yamashita, Y.; Shirai, Y.; Ashida, H. Enzymatically synthesized glycogen inhibits colitis through decreasing oxidative stress. Free Radical Bio. Med. 2017, 106, 355–367. [Google Scholar] [CrossRef]
- Irrazabal, T.; Thakur, B.K.; Kang, M.S.; Malaise, Y.; Martin, A. Limiting oxidative DNA damage reduces microbe-induced colitis-associated colorectal cancer. Nat. Commun. 2020, 11, 1802. [Google Scholar] [CrossRef]
- Li, M.; Qi, Y.; Mu, L.; Li, Z.; Zhao, Q.; Sun, J.; Jiang, Q. Effects of processing method on chemical compositions and nutritional quality of ready-to-eat sea cucumber (Apostichopus japonicus). Food Sci. Nutr. 2019, 7, 755–763. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Ding, Y.; Li, S.; Sang, X.; Li, T.; Zhao, Q.; Yu, S. Structure, in vitro digestive characteristics and effect on gut microbiota of sea cucumber polysaccharide fermented by Bacillus subtilis Natto. Food Res. Int. 2023, 169, 112872. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, J.; Zhong, H.; Abdullah; Zhuang, J.; Zhang, J.; Wang, J.; Zhang, X.; Feng, F. High-degree hydrolysis sea cucumber peptides improve exercise performance and exert antifatigue effect via activating the NRF2 and AMPK signaling pathways in mice. J. Funct. Foods. 2021, 86, 104677. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs. 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Qi, X.; Xu, Q.; Yuan, C.; Han, H. A new sulfated triterpene glycoside from the sea cucumber Colochirus quadrangularis, and evaluation of its antifungal, antitumor and immunomodulatory activities. Bioorg. Med. Chem. 2021, 41, 116188. [Google Scholar] [CrossRef]
- Nakchum, L.; Kim, S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem.Biotech. 2016, 46, 123–130. [Google Scholar] [CrossRef]
- Zang, Y.; Tian, X.; Dong, S.; Dong, Y. Growth, metabolism and immune responses to evisceration and the regeneration of viscera in sea cucumber, Apostichopus japonicas. Aquaculture 2012, 2358–359, 50–60. [Google Scholar] [CrossRef]
- Mi, R.; Zhou, Z.; Meng, N. Skin care efficacy evaluation on the enzymatic hydrolysates of viscera and the body wall extracts of sea cucumber Apostichopus japonicas. Mod. Food Sci. Technol. 2023, 39, 222–229. [Google Scholar]
- Graham, A.E.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]
- Joung, H.J.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Jeong, J.H.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Anti-inflammatory effects of extract from Haliotis discus hannai fermented with Cordyceps militaris mycelia in RAW264.7 macrophages through TRIF-dependent signaling pathway. Fish Shellfish Immun. 2014, 38, 184–189. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Y.; Wang, L.; Wu, H.; Azi, F.; Tekliye, M.; Zhou, J.; Liu, X.; Dong, M.; Xia, X. Neuroprotective potency of a soy whey fermented by Cordyceps militaris SN-18 against hydrogen peroxide-induced oxidative injury in PC12 cells. Eur. J. Nutr. 2022, 61, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, J.; Kong, W.; Liu, Y.; Liu, S.; Su, L. Cordyceps militaris polysaccharide alleviates ovalbumin-induced allergic asthma through the Nrf2/HO-1 and NF-κB signaling pathways and regulates the gut microbiota. Int. J. Biol. Macromol. 2023, 238, 124333. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xu, R.; Zhou, J.; Chen, J.; Wang, L.; Liu, X.; Liang, X.; Liu, B.; Lu, R.; Wu, J.; et al. Cordyceps militaris polysaccharides exerted protective effects on diabetic nephropathy in mice via regulation of autophagy. Food Funct. 2019, 10, 5102–5114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Q.; Wang, J.; Li, Y.; Chen, B.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Xie, Y.; et al. Novel selenium peptides obtained from selenium-enriched Cordyceps militaris alleviate neuroinflammation and gut microbiota dysbacteriosis in LPS-injured mice. J. Agric. Food Chem. 2022, 70, 3194–3206. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Du, X.; Meng, N.; Li, Y.; Mi, R.; Li, X.; Li, S. Tussah silkmoth pupae improve anti-tumor properties of Cordyceps militaris (L.) Link by increasing the levels of major metabolite cordycepin. RSC Adv. 2019, 9, 5480–5491. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Qian, Y.; Fan, X.; Cao, H.; Tian, Y. Nutritional properties of Europen eel (Anguilla anguilla) bone peptide-calcium and its apoptosis effect on Caco-2 cells. Food Sci. Hum. Well. 2022, 11, 1482–1490. [Google Scholar] [CrossRef]
- Zhou, Y.; She, X.; Chen, Z.; Wei, Y.; Xiao, Y.; Zhou, X. Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) protein-derived antioxidant peptides: Mechanisms of action and structure-activity relationship in Caco-2 cell models. Food Sci. Hum. Well. 2022, 11, 1580–1590. [Google Scholar] [CrossRef]
- Xu, Y.; Zang, J.; Regenstein, J.M.; Xia, W. Technological roles of microorganisms in fish fermentation: A review. Crit. Rev. Food Sci. 2021, 61, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, D.; Li, M.; Liu, L.; Deng, B.; Jia, L.; Yang, F. Coprinus comatus polysaccharides ameliorated carbon tetrachloride-induced liver fibrosis through modulating inflammation and apoptosis. Food Funct. 2022, 13, 11125–11141. [Google Scholar] [CrossRef]
- Du, K.; Zheng, X.; Lv, J.; Zhong, X.; Wei, M.; Liu, M. Cordycepin exacerbates cadmium-induced neurotoxicity via promoting endoplasmic reticulum stress-associated apoptosis. J. Funct. Foods. 2022, 89, 104935. [Google Scholar] [CrossRef]
- Sah, D.K.; Arjunan, A.; Park, S.Y.; Lee, B.; Jung, Y.D. Sulforaphane Inhibits IL-1β-Induced IL-6 by Suppressing ROS Production, AP-1, and STAT3 in Colorectal Cancer HT-29 Cells. Antioxidants 2024, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Ri, M. Endoplasmic-reticulum stress pathway-associated mechanisms of action of proteasome inhibitors in multiple myeloma. Int. J. Hematol. 2016, 104, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mitophagy: In sickness and in health. Mol. Cell. Oncol. 2016, 3, e1056332. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well. 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Xie, S.; Wu, X.; Fu, J. Mechanism of action of cordycepin in the treatment of hepatocellular carcinoma via regulation of the Hippo signaling pathway. Food Sci. Hum. Well. 2023, 13, 1040–1054. [Google Scholar] [CrossRef]
- Tang, X.; Fu, X.; Liu, Y.; Yu, D.; Cai, S.; Yang, C. Blockade of glutathione metabolism in IDH1-mutated glioma. Mol. Cancer Ther. 2020, 19, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Ge, T.; Liu, J.; Xiao, J.; Wu, X.; Wang, H.; Zhu, Y.; Xia, D.; Hu, B. Trehalose inhibits ferroptosis via NRF2/HO-1 pathway and promotes functional recovery in mice with spinal cord injury. Aging 2022, 14, 3216–3232. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; He, Y.; Wu, Q.; Zhang, Y.; Li, F.; Jiao, D.; Liu, X.; Wang, C. Novel Antioxidant Peptides Derived from Feather Keratin Alleviate H2O2-Induced Oxidative Damage in HepG2 Cells via Keap1/Nrf2 Pathway. J. Agric. Food Chem. 2023, 71, 20062–20072. [Google Scholar] [CrossRef] [PubMed]
- Sihvola, V.; Levonen, A.L. Keap1 as the redox sensor of the antioxidant response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef]
- Baird, L.; Swift, S.; Llères, D.; Dinkova-Kostova, A.T. Monitoring Keap1-Nrf2 interactions in single live cells. Biotechnol. Adv. 2014, 32, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, M.; Zhang, P.; Li, H.; Qu, K.; Xu, R.; Gao, N.; Zhu, H. Cordycepin alleviated metabolic inflammation in Western diet-fed mice by targeting intestinal barrier integrity and intestinal flora. Pharmacol. Res. 2022, 178, 106191. [Google Scholar] [CrossRef] [PubMed]
- Bulst, S.; Abicht, A.; Holinski-Feder, E.; Müller-Ziermann, S.; Koehler, U.; Thirion, C.; Walter, M.C.; Stewart, J.D.; Chinnery, P.F.; Lochmüller, H.; et al. In vitro supplementation with dAMP/dGMP leads to partial restoration of mtDNA levels in mitochondrial depletion syndromes. Hum. Mol. Genet. 2009, 18, 1590–1599. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holen, E.; Jonsson, R. Dietary nucleotides and intestinal cell lines: I. Modulation of growth. Nutr. Res. 2004, 24, 197–207. [Google Scholar] [CrossRef]
- Holen, E.; Bjørge, O.A.; Jonsson, R. Dietary nucleotides and human immune cells. II. Modulation of PBMC growth and cytokine secretion. Nutrition 2006, 22, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Gentry, B.G.; Gentry, S.N.; Jackson, T.L.; Zemlicka, J.; Drach, J.C. Phosphorylation of antiviral and endogenous nucleotides to di- and triphosphates by guanosine monophosphate kinase. Biochem. Pharmacol. 2011, 81, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Olou, A.A.; King, R.J.; Yu, F.; Singh, P.K. MUC1 oncoprotein mitigates ER stress via CDA-mediated reprogramming of pyrimidine metabolism. Oncogene 2020, 39, 3381–3395. [Google Scholar] [CrossRef] [PubMed]
- Mullen, N.J.; Singh, P.K. Nucleotide metabolism: A pan-cancer metabolic dependency. Nat. Rev. Cancer. 2023, 23, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and metabolic regulator of stem cell state and function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Tang, N.; Ding, Z.; Zhang, S.; Luo, D.; Liu, X.; Bao, X.; Liu, C.; Liu, Z. Nanoassemblies derived from natural flavonoid compounds as new antioxidant oral preparations for targeted inflammatory bowel disease therapy. Adv. Funct. Mater. 2023, 33, 2305133. [Google Scholar] [CrossRef]
- Balakina, A.A.; Raevskaya, T.A.; Pavlov, V.S.; Mumyatova, V.A.; Goncharova, S.A.; Terent’ev, A.A. Nrf2-dependent expression of glutathione antioxidant system genes and redox status in cells of in vivo drug-resistant murine P388 leukemia strains. Bull. Exp. Biol. Med. 2020, 169, 249–253. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequence (5′→3′) | |
|---|---|---|
| HO-1 | Forward | 5′-AACTTTCAGAAGGGCCAGGT-3′ |
| Reverse | 5′-AGACTGGGCTCTCCTTGTTG-3′ | |
| NQO-1 | Forward | 5′-GGGATCCACGGGGACATGAATG-3′ |
| Reverse | 5′-ATTTGAATTCGGGCGTCTGCTG-3′ | |
| β-actin | Forward | 5′-GGAAATCGTGCGTGACATTA-3′ |
| Reverse | 5′-GGAGCAATGATCTTGATCTTC-3′ | |
| Functional Components | SVH | FSVH |
|---|---|---|
| Protein and peptide (g/100 g) | 37.05 ± 2.89 | 32.35 ± 3.89 |
| Polysaccharide (g/100 g) | 13.85 ± 2.25 | 19.53 ± 2.56 * |
| Saponin (mg/kg) | 5187.42 ± 339.15 | 5537.93 ± 323.14 |
| Cordycepin (mg/kg) | 0 | 852.19 ± 83.54 * |
| Group Name | Up | Down |
|---|---|---|
| H2O2 vs. Normal | 18 | 8 |
| SVH vs. H2O2 | 23 | 13 |
| FSVH vs. H2O2 | 13 | 28 |
| No. | Name | VIP | p-Value | FC (H2O2 vs. Normal) | FC (SVH vs. H2O2) | FC (FSVH vs. H2O2) | Pathway |
|---|---|---|---|---|---|---|---|
| 1 | 2′-Deoxyguanosine 5′-monophosphate | 1.24 | 0.000026 | 0.00 | 2441.43 | 56076.55 | Purine metabolism |
| 2 | GDP-L-fucose | 1.02 | 0.005034 | 0.01 | 121.51 | 71.05 | Fructose and mannose metabolism |
| 3 | Catechin | 1.15 | 0.001565 | 0.03 | 11.97 | 3.44 | Flavonoid biosynthesis |
| 4 | Uridine 5′-monophosphate | 1.10 | 0.011365 | 0.11 | 4.27 | 2.77 | Pyrimidine metabolism |
| 5 | Methyl acetate | 1.11 | 0.000007 | 0.18 | 36.39 | 0.06 | Butanoate metabolism |
| 6 | Succinic acid | 1.07 | 0.000053 | 0.24 | 22.71 | 0.13 | Citrate cycle (TCA cycle) |
| 7 | Alpha-D-Glucose 1,6-bisphosphate | 1.52 | 0.000067 | 12.72 | 0.02 | 0.02 | Starch and sucrose metabolism |
| 8 | Inosine | 1.57 | 0.003841 | 9.36 | 0.30 | 0.00 | Purine metabolism |
| 9 | Phenylacetylglycine | 1.39 | 0.007821 | 2.14 | 0.24 | 0.28 | Phenylalanine metabolism |
| 10 | Bergapten | 1.55 | 0.000607 | 2.05 | 0.25 | 0.17 | Biosynthesis of various plant secondary metabolites |
| 11 | Theophylline | 1.55 | 0.007128 | 1.95 | 0.64 | 0.22 | Caffeine metabolism |
| 12 | Dihydroxyacetone phosphate | 1.43 | 0.003620 | 1.56 | 0.03 | 0.13 | Glycolysis/Gluconeogenesis |
| 13 | Indoleacetic acid | 1.30 | 0.000001 | 1.51 | 79.36 | 2.96 | Tryptophan metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, R.; Fu, Z.; Jiang, J.; Gao, S.; Guan, X.; Wang, X.; Zhou, Z. Sea Cucumber Viscera Processed by Protease Hydrolysis Combined with Cordyceps militaris Fermentation Protect Caco-2 Cells against Oxidative Damage via Enhancing Antioxidant Capacity, Activating Nrf2/HO-1 Pathway and Improving Cell Metabolism. Antioxidants 2024, 13, 988. https://doi.org/10.3390/antiox13080988
Mi R, Fu Z, Jiang J, Gao S, Guan X, Wang X, Zhou Z. Sea Cucumber Viscera Processed by Protease Hydrolysis Combined with Cordyceps militaris Fermentation Protect Caco-2 Cells against Oxidative Damage via Enhancing Antioxidant Capacity, Activating Nrf2/HO-1 Pathway and Improving Cell Metabolism. Antioxidants. 2024; 13(8):988. https://doi.org/10.3390/antiox13080988
Chicago/Turabian StyleMi, Rui, Zhiyu Fu, Jingwei Jiang, Shan Gao, Xiaoyan Guan, Xuda Wang, and Zunchun Zhou. 2024. "Sea Cucumber Viscera Processed by Protease Hydrolysis Combined with Cordyceps militaris Fermentation Protect Caco-2 Cells against Oxidative Damage via Enhancing Antioxidant Capacity, Activating Nrf2/HO-1 Pathway and Improving Cell Metabolism" Antioxidants 13, no. 8: 988. https://doi.org/10.3390/antiox13080988
APA StyleMi, R., Fu, Z., Jiang, J., Gao, S., Guan, X., Wang, X., & Zhou, Z. (2024). Sea Cucumber Viscera Processed by Protease Hydrolysis Combined with Cordyceps militaris Fermentation Protect Caco-2 Cells against Oxidative Damage via Enhancing Antioxidant Capacity, Activating Nrf2/HO-1 Pathway and Improving Cell Metabolism. Antioxidants, 13(8), 988. https://doi.org/10.3390/antiox13080988





