From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review
Abstract
1. Introduction
2. An Analysis of the Biological Potential of Bioactive Compounds
2.1. General Information
2.2. Bioactive Compounds Present in Biowaste from the Wine Industry
2.3. Current Perspectives on Methods of Extraction and Release of Bioactive Compounds from Grapes
2.3.1. Conventional Extraction Methods: Solid–Liquid Extraction
- Water: It is one of the most widely used solvents due to its wide availability, low toxicity and ability to dissolve a variety of polar compounds. Water extraction is particularly effective for organic and inorganic compounds that are soluble in water.
- Chloroform: Despite its toxicity, chloroform is used in the extraction of organic compounds due to its high capacity to dissolve a variety of substances. However, its use has been reduced due to environmental and health concerns.
- Hexane: This organic solvent is ideal for the extraction of non-polar compounds such as fats, oils and aromatics. It is highly volatile and is commonly used in the food and pharmaceutical industries.
- Acetic acid: It is used in the extraction of acidic or basic organic compounds, since it can form water-soluble salts with these compounds, facilitating their extraction.
- Methanol: It is known for its high polarity, making it suitable for the extraction of some semi-polar polar compounds. However, it is highly toxic if ingested, inhaled or absorbed through the skin. It is generally used for the extraction of alkaloids, phenols, and other polar compounds.
- Ethanol: It is highly used for the extraction of polar and semi-polar compounds. It has moderate toxicity, so it is relatively safe in small quantities, but can be toxic in large doses. It is easy to handle and accepted for use in the food and pharmaceutical industry.
- Acetone: Suitable for a wide range of organic compounds, including polar and non-polar compounds. Its most common use is the extraction of essential oils, resins, and a wide variety of organic compounds. Excellent dissolving capacity for many organic compounds; fast drying due to its low boiling point; but exhibits high flammability and risk of inhalation toxicity.
- Ethyl acetate: Can be used for a wide range of organic compounds, especially semi-polar and non-polar compounds. With moderate toxicity, it is relatively less toxic than other organic solvents, but can cause irritation and negative effects with prolonged exposure. Its most common uses are the extraction of alkaloids, terpenes, flavonoids, and other semi-polar compounds.
- Solubility of the Solid in the Solvent: The solvent-to-solid ratio must be high enough to ensure that the solid is completely dissolved in the solvent. This ensures efficient extraction of the soluble components of the solid.
- Objectives of Extraction: The specific objectives of the extraction process, such as the purity of the final product and the yield of the process, will influence the selection of the solvent-to-solid ratio. In some cases, a higher ratio may be chosen to maximize extraction, while in other cases, a lower ratio may be preferable to minimize costs.
- Practical Considerations: Practical factors, such as the capacity of the extraction equipment, available time and operating costs, also influence the selection of the solvent-to-solid ratio. It is important to find a balance between process efficiency and economic viability.
2.3.2. Emerging Technologies for the Extraction of Bioactive Compounds—News
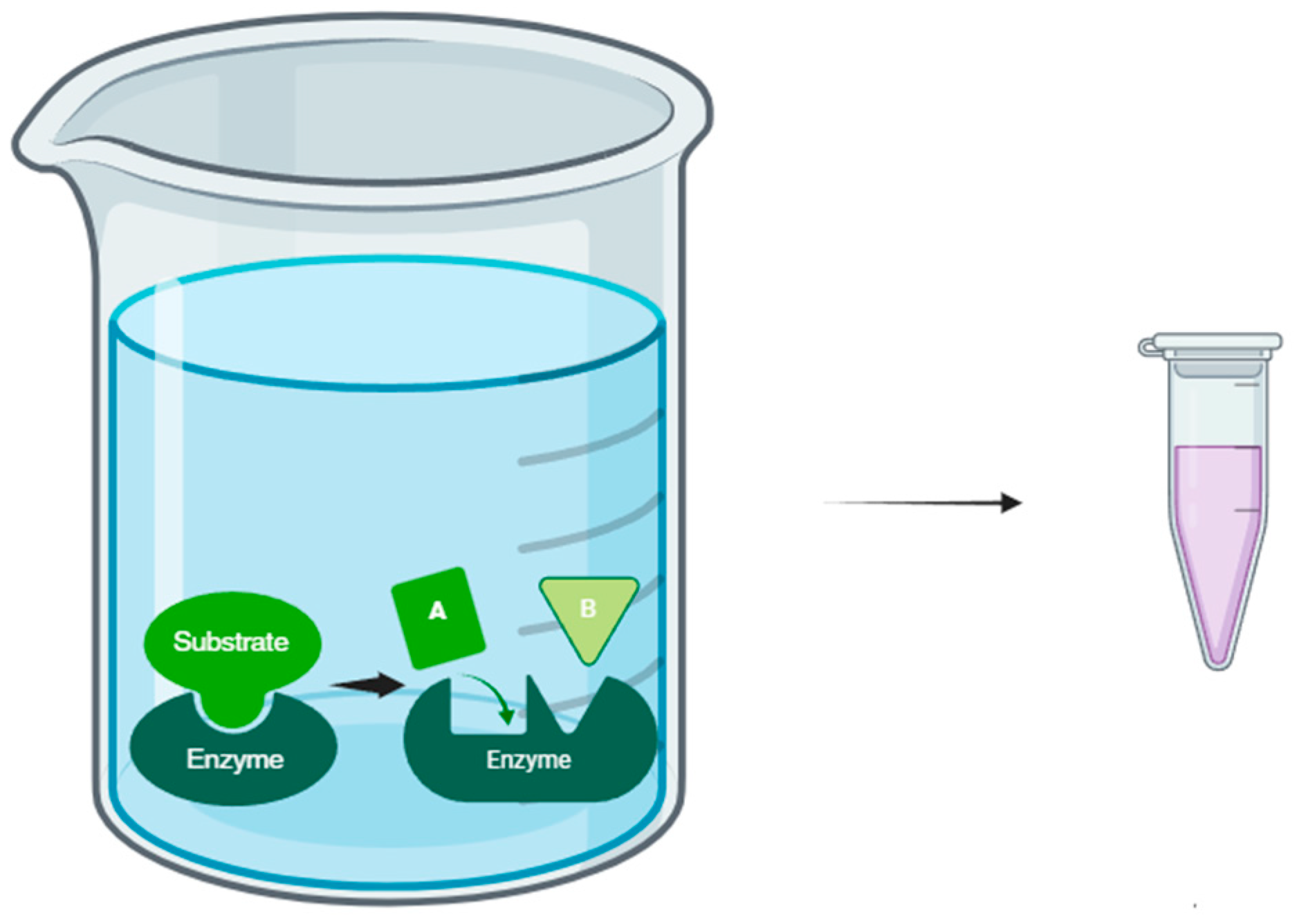
Enzyme-Assisted Extraction
- Selectivity: Enzymes can act selectively on specific substrates, allowing the extraction of compounds of interest with high purity and selectivity.
- Efficiency: Enzymatic action can improve extraction efficiency by facilitating the release of compounds from the feedstock matrix in a short time.
- Sustainability: Enzyme-assisted extraction is a green and sustainable process using biodegradable enzymes and mild operating conditions, which reduces energy consumption and waste generated.
- Added value: This approach allows the recovery of bioactive compounds and metabolites with high added value, which can have applications in the formulation of functional products and nutraceuticals.
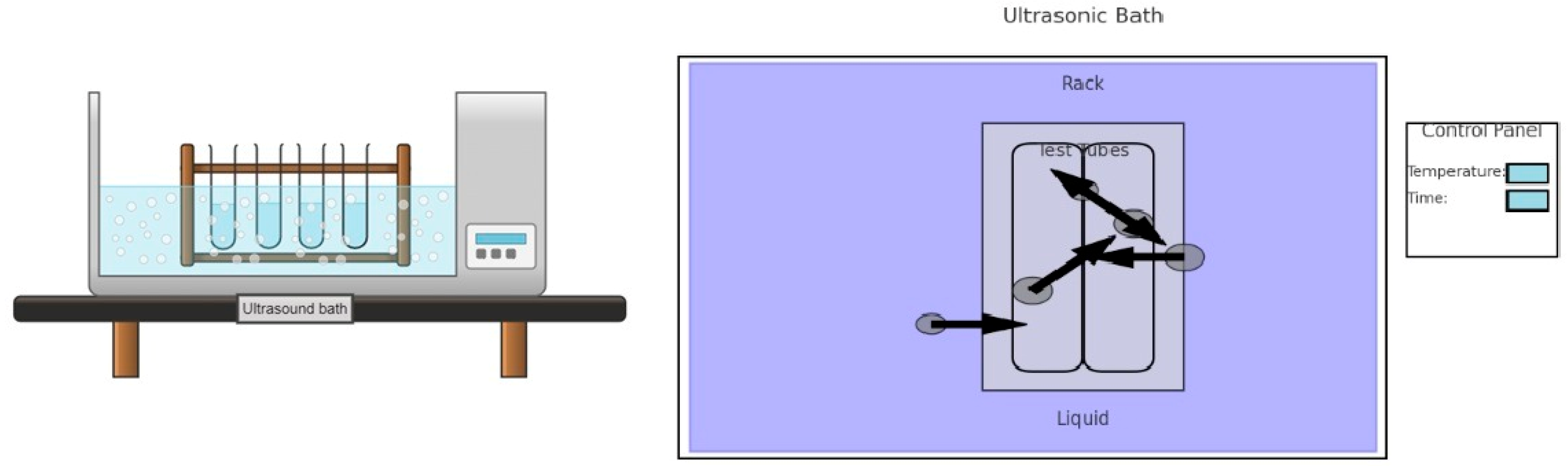
Ultrasound-Assisted Extraction
Supercritical Fluid Extraction
Microwave-Assisted Extraction
Pressurized Liquid Extraction
Hydrostatic High-Pressure Extraction
NaDES-Assisted Extraction
Discussion
2.3.3. Preservation of Polyphenolic Extracts—Focus on Microencapsulation
3. Conclusions
Funding
Conflicts of Interest
References
- Castaldo, L.; Izzo, L.; Pascale, S.D.; Narváez, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Çatak, J.; Uğur, H.; Gürbüz, M.; Belli, İ.; Tanyildiz, S.N.; Yildirim, H.; Cengiz, S.; Yavuz, B.B.; Kişmiroğlu, C.; et al. The Bioaccessibility of Water-Soluble Vitamins: A Review. Trends Food Sci. Technol. 2021, 109, 552–563. [Google Scholar] [CrossRef]
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.; Vilas-Boas, M. Assessment of Bioactive Compounds under Simulated Gastrointestinal Digestion of Bee Pollen and Bee Bread: Bioaccessibility and Antioxidant Activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J.; García-Viguera, C. Anthocyanin-Based Natural Colorants: A New Source of Antiradical Activity for Foodstuff. J. Agric. Food Chem. 2000, 48, 1588–1592. [Google Scholar] [CrossRef]
- Farias, D.d.P.; Neri-Numa, I.A.; Araújo, F.F.d.; Pastore, G.M. A Critical Review of Some Fruit Trees from the Myrtaceae Family as Promising Sources for Food Applications with Functional Claims. Food Chem. 2020, 306, 125630. [Google Scholar] [CrossRef] [PubMed]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A Green Ultrasound-Assisted Extraction Process for the Recovery of Antioxidant Polyphenols and Pigments from Onion Solid Wastes Using Box–Behnken Experimental Design and Kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxidative Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.; Gasson, M.; Narbad, A. Antimicrobial Activity of Flavonoids Extracted from Bergamot (Citrus Bergamia Risso) Peel, a Byproduct of the Essential Oil Industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent Updates on Bioaccessibility of Phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Kunat-Budzyńska, M.; Rysiak, A.; Wiater, A.; Grąz, M.; Andrejko, M.; Budzyński, M.; Bryś, M.S.; Sudziński, M.; Tomczyk, M.; Gancarz, M.; et al. Chemical Composition and Antimicrobial Activity of New Honey Varietals. Int. J. Environ. Res. Public Health 2023, 20, 2458. [Google Scholar] [CrossRef]
- Ziółkiewicz, A.; Kasprzak-Drozd, K.; Rusinek, R.; Markut-Miotła, E.; Oniszczuk, A. The Influence of Polyphenols on Atherosclerosis Development. Int. J. Mol. Sci. 2023, 24, 7146. [Google Scholar] [CrossRef]
- Huey, S.L.; Mehta, N.H.; Konieczynski, E.M.; Bhargava, A.; Friesen, V.M.; Krisher, J.T.; Mbuya, M.N.N.; Monterrosa, E.; Nyangaresi, A.M.; Boy, E.; et al. Bioaccessibility and Bioavailability of Biofortified Food and Food Products: Current Evidence. Crit. Rev. Food Sci. Nutr. 2022, 64, 4500–4522. [Google Scholar] [CrossRef]
- Rein, M.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.d.S. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Biluca, F.C.; Gonzaga, L.V.; Magnani, M.; Borges, G.d.S.C.; Vitali, L.; Micke, G.A.; Gois, J.S.d.; Almeida, T.S.; Borges, D.L.G.; et al. Bioaccessibility of Bioactive Compounds and Antioxidant Potential of Juçara Fruits (Euterpe Edulis Martius) Subjected to in Vitro Gastrointestinal Digestion. Food Chem. 2017, 228, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Rusinek, R.; Dobrzański, B., Jr.; Gawrysiak-Witulska, M.; Siger, A.; Żytek, A.; Karami, H.; Umar, A.; Lipa, T.; Gancarz, M. Effect of the Roasting Level on the Content of Bioactive and Aromatic Compoundsin Arabica Coffee Beans. Int. Agrophys. 2023, 38, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]
- Soler Cantero, A.; Motilva Casado, M.J.; Romero Fabregat, M.P. Estudio de la Capacidad Antioxidante y la Biodisponibilidad de los Compuestos Fenólicos del Aceitede Oliva. In Primeras Etapas en el Desarrollo de un Aceite de Oliva Funcional; Universitat de Lleida: Lleida, Spain, 2010. [Google Scholar]
- Manzi, L.V.; Mayz, J.C. Valorando Los Microorganismos. Rev. Soc. Venez. Microbiol. 2003, 23, 1. [Google Scholar]
- Pimentel, G.; Pimentel, G.; José, L.G.S.; José, L. Microorganismos de Las Cuevas Volcánicas de La Palma (Islas Canarias): Diversidad y Potencial Uso Bioteconológico. null 2019. Available online: https://digital.csic.es/handle/10261/185148 (accessed on 28 June 2024).
- Geagea, M.F. Micosis de Interés Clínico y Su Diagnóstico Diferencial; Facultad de Medicina, Universidad Nacional de Colombia: Bogotá, Colombia, 2023. [Google Scholar]
- Agarwal, A.; Rizwana; Tripathi, A.D.; Kumar, T.; Sharma, K.P.; Patel, S.K.S. Nutritional and Functional New Perspectives and Potential Health Benefits of Quinoa and Chia Seeds. Antioxidants 2023, 12, 1413. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya De Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Nowak, W.; Jeziorek, M. The Role of Flaxseed in Improving Human Health. Healthcare 2023, 11, 395. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Kumar, S.; Banerjee, S.; Kaur, A.; Sasi, M.; Kumari, S.; Sachdev, A.; Dahuja, A. Isoflavones Play a Potential Role in Off-Flavour Scavenging, with a Key Role of IFS2 in Isoflavone Accumulation in Soybean Seeds. Food Technol. Biotechnol. 2023, 61, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and Pharmacological Effects and Nutritional Impact of Phytosterols: A Comprehensive Review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Pryandoko, D.; Wahyuni, C.D.; Marthania, M.; Kusuma, H.S.W.; Handayani, T.; Rizal. Antioxidant Properties of Soybean (Glycine max L.) Extract and Isoflavone. In 2021 IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce); IEEE: Medan, Indonesia, 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Brenjian, S.; Moini, A.; Yamini, N.; Kashani, L.; Faridmojtahedi, M.; Bahramrezaie, M.; Khodarahmian, M.; Amidi, F. Resveratrol Treatment in Patients with Polycystic Ovary Syndrome Decreased Pro-Inflammatory and Endoplasmic Reticulum Stress Markers. Am. J. Reprod. Immunol. 2020, 83, e13186. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef]
- Mansour, A.; Samadi, M.; Sanginabadi, M.; Gerami, H.; Karimi, S.; Hosseini, S.; Shirzad, N.; Hekmatdoost, A.; Mahdavi-Gorabi, A.; Mohajeri-Tehrani, M.R.; et al. Effect of Resveratrol on Menstrual Cyclicity, Hyperandrogenism and Metabolic Profile in Women with PCOS. Clin. Nutr. 2021, 40, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Ostwal, V.; Ramaswamy, A.; Bhargava, P.; Srinivas, S.; Mandavkar, S.; Chaugule, D.; Peelay, Z.; Baheti, A.; Tandel, H.; Jadhav, V.K.; et al. A Pro-Oxidant Combination of Resveratrol and Copper Reduces Chemotherapy-Related Non-Haematological Toxicities in Advanced Gastric Cancer: Results of a Prospective Open Label Phase II Single-Arm Study (RESCU III Study). Med. Oncol. 2022, 40, 17. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Zaw, J.J.T.; Xian, C.J.; Howe, P.R.C. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable Green Processing of Grape Pomace for the Production of Value-Added Products: An Overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Pham, T.-N.; Cazier, E.A.; Gormally, E.; Lawrence, P. Valorization of Biomass Polyphenols as Potential Tyrosinase Inhibitors. Drug Discov. Today 2024, 29, 103843. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Baek, K.-H. Valorization of Agro-Industrial Biowaste to Green Nanomaterials for Wastewater Treatment: Approaching Green Chemistry and Circular Economy Principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Sirohi, R.; Varjani, S.; Kim, S.H.; Wong, J.W.C. Sustainable Processing of Food Waste for Production of Bio-Based Products for Circular Bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef] [PubMed]
- Matiacevich, S.; Soto-Madrid, D.; Gutiérrez-Cutiño, M. Economía circular: Obtención y encapsulación de compuestos polifenólicos provenientes de residuos agroindustriales. RIVAR 2023, 10, 77–100. [Google Scholar] [CrossRef]
- López-Astorga, M.; Molina-Domínguez, C.C.; Ovando-Martínez, M.; Leon-Bejarano, M. Orujo de Uva: Más Que Un Residuo, Una Fuente de Compuestos Bioactivos. Epistemus 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Ide, W.; Sabando, C.; Castaño, J.; Pettinelli, N.; Bustos, R.; Linares, A.; Mora, L.; Müller, N.; Pascual, G.; Rodríguez-Llamazares, S. Grape (Vitis vinifera L. cv. País) Juices Obtained by Steam Extraction. Processes 2021, 9, 1670. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of Solid–Liquid Extraction Kinetics of Total Polyphenols from Grape Seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar] [CrossRef]
- Lizárraga-Chaidez, M.; Lucía, A.-G.; De Jesús, M.-S.M.; Eric Leonardo, H.-M.; Mendoza-Sánchez, M. Optimization of the Green Extraction Process of Antioxidants Derived from Grape Pomace. Sustain. Chem. Pharm. 2024, 37, 101396. [Google Scholar] [CrossRef]
- De Quiros, A.R.-B.; Lopez-Hernandez, J.; Lage-Yusty, M.A. Liquid Chromatographic Determination of Malvidin-3-O-Glucoside and Malvidin 3,5-O-Diglucoside in Wine Samples by Direct Injection. Open Food Sci. J. 2008, 2, 68–71. [Google Scholar] [CrossRef]
- Lutz, M.; Cajas, Y.; Henríquez, C. Phenolics Content and Antioxidant Capacity of Chilean grapes cv. País and Cabernet Sauvignon. CyTA-J. Food 2012, 10, 251–257. [Google Scholar] [CrossRef][Green Version]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Mikšovsky, P.; Kornpointner, C.; Parandeh, Z.; Goessinger, M.; Bica-Schröder, K.; Halbwirth, H. Enzyme-Assisted Supercritical Fluid Extraction of Flavonoids from Apple Pomace (Malus × domestica). ChemSusChem 2024, 17, e202301094. [Google Scholar] [CrossRef]
- Available online: https://biomodel.uah.es/en/DIY/JSME/draw.es.htm (accessed on 28 June 2024).
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Rabanal-Atalaya, M.; Medina-Hoyos, A. Análisis de Antocianinas En El Maíz Morado (Zea mays L.) Del Perú y Sus Propiedades Antioxidantes. Terra Latinoam. 2021, 39. [Google Scholar] [CrossRef]
- Dudek, A.; Spiegel, M.; Strugała, P.; Strugała-Danak, P.; Gabrielska, J. Analytical and Theoretical Studies of Antioxidant Properties of Chosen Anthocyanins; A Structure-Dependent Relationships. Int. J. Mol. Sci. 2022, 23, 5432. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Z.; Chang, M.; Zhang, X.; Liu, X.; Wang, J. Three Anthocyanin-Rich Berry Extracts Regulate the in Vitro Digestibility of Corn Starch: Physicochemical Properties, Structure and α-Amylase. Int. J. Biol. Macromol. 2023, 253, 127484. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animal 2019, 9, 856. [Google Scholar] [CrossRef]
- Kim, H.I.; Hur, Y.Y.; Im, D.J.; Lee, D.H.; Jung, S.M.; Park, S.J.; Kim, S.J. Solvent Extraction Conditions for the Analysis of Condensed Tannins from Grape Skin and Seeds. Korean J. Food Preserv. 2019, 26, 808–813. [Google Scholar] [CrossRef]
- Bai, L.; Tian, X.; Wang, Y.; Zhang, K.; Guo, J.; Ma, C.; Shen, R.; Wang, X.; Wang, W. Antioxidant Activity during in Vitro Gastrointestinal Digestion and the Mode of Action with Tannins of Cowhide-Derived Collagen Hydrolysates: The Effects of Molecular Weight. Food Biosci. 2023, 53, 102773. [Google Scholar] [CrossRef]
- Wimalasiri, P.M.; Harrison, R.; Hider, R.; Donaldson, I.; Kemp, B.; Tian, B. Development of Tannins and Methoxypyrazines in Grape Skins, Seeds, and Stems of Two Pinot Noir Clones during Ripening. J. Agric. Food Chem. 2023, 71, 15754–15765. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Fuentes, I.A.; Quimis-Ponce, K.L.; Sorroza-Rojas, N.A.; García-Larreta, F.S.; Mariscal-Santi, W.E.; Mariscal-Garcia, R.E. Determinación de Taninos y Cumarinas Presente En La Planta Tres Filos. Polo del Conoc. 2017, 2, 500. [Google Scholar] [CrossRef]
- Céspedes Loayza, A.L.; Muñoz Guillen, G.D.R.M. Influencia De La Temperatura, Solvente Y Tipo De Vaina En La Extraccion De Taninos De Caesalpinia Spinosa (Tara) Por Percolacion Y Relacion Con Su Actividad Antioxidante; Universidad Católica de Santa María: Arequipa, Perú, 2013. [Google Scholar]
- Isaza, M.J.H. Taninos o Polifenoles Vegetales. Sci. Tech. 2007, 1, 13–17. [Google Scholar]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Sieniawska, E. Activities of Tannins–From In Vitro Studies to Clinical Trials. Nat. Prod. Commun. 2015, 10, 1934578X1501001118. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313. [Google Scholar] [CrossRef] [PubMed]
- Sekharan, T.R.; Chandira, R.M.; Tamilvanan, S.; Rajesh, S.C.; Venkateswarlu, B.S. Deep Eutectic Solvents as an Alternate to Other Harmful Solvents. Biointerface Res. Appl. Chem. 2021, 12, 847–860. [Google Scholar] [CrossRef]
- Cicci, A.; Sed, G.; Bravi, M. Potential of Choline Chloride-Based Natural Deep Eutectic Solvents (Nades) in the Extraction of Microalgal Metabolites. Chem. Eng. Trans. 2017, 57, 61–66. [Google Scholar] [CrossRef]
- Amendola, D.; Faveri, D.M.D.; Spigno, G. Grape Marc Phenolics: Extraction Kinetics, Quality and Stability of Extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Cerón-Montes, G.I.; Garulo-Fuentes, A.L.; Valencia-Pérez, N.S.; Garrido-Hernández, A.; Yáñez-Fernández, J. Modelación de La Extracción de Polifenoles de Semillas de Uva. PÄDI Boletín Científico De Cienc. Básicas E Ing. Del ICBI 2021, 9, 179–186. [Google Scholar] [CrossRef]
- Jia, M.-Z.; Fu, X.-Q.; Deng, L.; Li, Z.-L.; Dang, Y.-Y. Phenolic Extraction from Grape (Vitis vinifera) Seed via Enzyme and Microwave Co-Assisted Salting-out Extraction. Food Biosci. 2021, 40, 100919. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Kontić, J.K. Recovery of Flavonoids from Grape Skins by Enzyme-Assisted Extraction. Sep. Sci. Technol. 2016, 51, 255–268. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Sousa, A.M.; Gando-Ferreira, L.; Quina, M. Grape Pomace as a Natural Source of Phenolic Compounds: Solvent Screening and Extraction Optimization. Molecules 2023, 28, 2715. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of Grape Pomace Phenolic Compounds through Optimized Extraction and Adsorption Processes. Chem. Eng. Process. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; Vietro, N.D.; Massari, F.; Zambonin, C.G. Determination of Polyphenols and Vitamins in Wine-Making by-Products by Supercritical Fluid Extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Porto, C.D.; Natolino, A.; Scalet, M. Improved Sustainability in Wine Industry Byproducts: A Scale-up and Economical Feasibility Study for High-Value Compounds Extraction Using Modified SC-CO2. ACS Omega 2022, 7, 33845–33857. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.O.; Poejo, J.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J.; Mato, R.B. Microwave Pretreatment to Improve Extraction Efficiency and Polyphenol Extract Richness from Grape Pomace. Effect on Antioxidant Bioactivity. Food Bioprod. Process. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Álvarez-Casas, M.; Lores, M. Antioxidant White Grape Seed Phenolics: Pressurized Liquid Extracts from Different Varieties. Antioxidants 2015, 4, 737. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of Anthocyanins from Grape Skins Assisted by High Hydrostatic Pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Chañi-Paucar, L.O.; Silva, J.W.L.; Maciel, M.I.S.; Lima, V.L.A.G.D. Simplified Process of Extraction of Polyphenols from Agroindustrial Grape Waste. Food Sci. Technol 2021, 41 (Suppl. S2), 723–731. [Google Scholar] [CrossRef]
- Sokač Cvetnić, T.; Krog, K.; Benković, M.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J.; Radojčić Redovniković, I.; Jurinjak Tušek, A. Solid–Liquid Extraction of Bioactive Molecules from White Grape Skin: Optimization and Near-Infrared Spectroscopy. Separations 2023, 10, 452. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; Faveri, D.M.D. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M.J. Assisted Extraction of Rosemary Antioxidants with Green Solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef]
- Orozco-Flores, L.; Salas, E.; González-Sánchez, G.; Chávez-Flores, D.; Ramírez-García, R.; Rocha-Gutiérrez, B.; Peralta-Pérez, M.; Ballinas-Casarrubias, M. Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace. Processes 2022, 10, 1112. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaciu, M.-I.; Sălăgean, C.-D.; Ranga, F.; Fărcaș, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C. A. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus Edulis and Cantharellus Cibarius Mushrooms from Romania. Molecules 2021, 26, 7508. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A Powerful and Greener Alternative to the Latest Solid-Liquid Extraction Techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Elboughdiri, N. Effect of Time, Solvent-Solid Ratio, Ethanol Concentration and Temperature on Extraction Yield of Phenolic Compounds from Olive Leaves. Eng. Technol. Appl. Sci. Res. 2018, 8, 2805–2808. [Google Scholar] [CrossRef]
- Norshazila, S.; Koy, C.N.; Rashidi, O.; Ho, L.H.; Azrina, I.; Zaizuliana, N.R.; Zarinah, Z. The Effect of Time, Temperature and Solid to Solvent Ratio on Pumpkin Carotenoids Extracted Using Food Grade Solvents. Sains Malays. 2017, 46, 231–237. [Google Scholar] [CrossRef]
- Alara, O.R.; Nour, A.H.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; He, J.; Wang, H.; Li, C.; Wu, G.; Zhu, K.; Chen, X.; Zhang, Y.; Tan, L. Comparison of Microwave, Ultrasound and Ultrasound-Microwave Assisted Solvent Extraction Methods on Phenolic Profile and Antioxidant Activity of Extracts from Jackfruit (Artocarpus heterophyllus Lam.) Pulp. LWT 2023, 173, 114395. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Puzeryte, V.; Martusevice, P.; Sousa, S.; Balciunaitiene, A.; Viskelis, J.; Gomes, A.M.; Viskelis, P.; Cesoniene, L.; Urbonaviciene, D. Optimization of Enzyme-Assisted Extraction of Bioactive Compounds from Sea Buckthorn (Hippophae rhamnoides L.) Leaves: Evaluation of Mixed-Culture Fermentation. Microorganisms 2023, 11, 2180. [Google Scholar] [CrossRef] [PubMed]
- Cascaes Teles, A.S.; Hidalgo Chávez, D.W.; Zarur Coelho, M.A.; Rosenthal, A.; Fortes Gottschalk, L.M.; Tonon, R.V. Combination of Enzyme-Assisted Extraction and High Hydrostatic Pressure for Phenolic Compounds Recovery from Grape Pomace. J. Food Eng. 2021, 288, 110128. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Considerations on the Use of Enzyme-Assisted Extraction in Combination with Pressurized Liquids to Recover Bioactive Compounds from Algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Aguilar-Zárate, P.; Veana, F.; Muñiz-Márquez, D.B. Impacto de Las Tecnologías de Extracción Verdes Para La Obtención de Compuestos Bioactivos de Los Residuos de Frutos Cítricos. TIP Rev. Espec. En Cienc. Químico-Biológicas 2020, 23, 1–11. [Google Scholar] [CrossRef]
- Mustafa, A.; Mustafa, A.; Turner, C.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. Liq.-Phase Extr. 2020, 375–398. [Google Scholar] [CrossRef]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Vidal-San Martín, C.; Bastías-Montes, J.M.; Villagra-Jorquera, C.; Salinas-Huenchulao, G.; Flores-Ríos, A.; Gonzáles-Díaz, N.; Tamarit-Pino, Y.; Muñoz-Fariña, O.; Quevedo-León, R. Effect of Cryoconcentration Assisted by Centrifugation-Filtration on Bioactive Compounds and Microbiological Quality of Aqueous Maqui (Aristotelia chilensis (Mol.) Stuntz) and Calafate (Berberis microphylla G. Forst) Extracts Pretreated with High-Pressure Homogenization. Processes 2021, 9, 692. [Google Scholar] [CrossRef]
- Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High Hydrostatic Pressure to Increase the Biosynthesis and Extraction of Phenolic Compounds in Food: A Review. Molecules 2022, 27, 1502. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, R.; Kim, D.-S.; Salleh, L.M.; Lim, S.-B. Optimization of High Hydrostatic Pressure Extraction of Bioactive Compounds from Noni Fruits. J. Food Meas. Charact. 2020, 14, 2810–2818. [Google Scholar] [CrossRef]
- Silva, N.M.d.; Bona, E.; Cardozo-Filho, L.; Santos, O.d.O.; Heck, S.C.; Feihrmann, A.C. Optimization of the Extraction of Phenolic Compounds from the Leaves of Yerba Mate (Ilex paraguariensis) through High Hydrostatic Pressure System Using Mixture Design with Process Variables. J. Food Sci. 2023, 88, 4122–4130. [Google Scholar] [CrossRef] [PubMed]
- Torres Palacio, P.C. Síntesis, Propiedades, Caracterización y Aplicaciones Químicas de Novedosos Disolventes Eutécticos Profundos: DES (Deep Eutectic Solvents). Ph.D. Thesis, Universitat de Lleida, Departament de Química, Barcelona, Spain, 2021. [Google Scholar]
- López-Luna, S.; Pérez-Uribe, V.; Camilo Villa, C.; Blach-vargas, D. Solventes Nades Como Medios de Reacción Sustentables Para la Síntesis de Nanopartículas Funcionales. J. Res. Univ. Quindio 2022, 122, 1–291. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Shah, E.; Patel, A. Technical Assessment of Natural Deep Eutectic Solvent (NADES) Mediated Biorefinery Process: A Case Study. J. Mol. Liq. 2018, 260, 313–322. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Cecone, C.; Hoti, G.; Bracco, P.; Trotta, F. Natural Deep Eutectic Solvents (NADES)-Progress in Polymer Synthesis and Pharmaceutical Application. Pharm. Sci. 2022, 28, 492–495. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents Providing Enhanced Stability of Natural Colorants from Safflower (Carthamus tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased Natural Deep Eutectic System as Versatile Solvents: Structure, Interaction and Advanced Applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef]
- Guerrero, M.S.; Torres, J.S.; Núñez, M.J. Extraction of Polyphenols from White Distilled Grape Pomace: Optimization and Modelling. Bioresour. Technol. 2008, 99, 1311–1318. [Google Scholar] [CrossRef]
- Porto, C.D.; Porretto, E.; Decorti, D. Comparison of Ultrasound-Assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis vinifera L.) Seeds. Ultrason. Sonochemistry 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between Total Antioxidant Capacity, Polyphenol and Fatty Acid Content of Native Grape Seed and Pomace of Four Different Grape Varieties in Hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Impact of Eutectic Solvents Utilization in the Microwave Assisted Extraction of Proanthocyanidins from Grape Pomace. Molecules 2021, 27, 246. [Google Scholar] [CrossRef] [PubMed]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural Deep Eutectic Solvents as a Green Extraction of Polyphenols from Spent Coffee Ground with Enhanced Bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers Based on Natural Deep Eutectic Mixtures to Enhance Anthocyanins Isolation from Grape Pomace by Pressurized Hot Water Extraction. Lwt-Food Sci. Technol. 2021, 149, 111889. [Google Scholar] [CrossRef]
- Islam, M.; Malakar, S.; Rao, M.V.; Kumar, N.; Sahu, J.K. Recent Advancement in Ultrasound-Assisted Novel Technologies for the Extraction of Bioactive Compounds from Herbal Plants: A Review. Food Sci. Biotechnol. 2023, 32, 1763–1782. [Google Scholar] [CrossRef] [PubMed]
- Tsevdou, M.; Ntzimani, A.; Katsouli, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Comparative Study of Microwave, Pulsed Electric Fields, and High Pressure Processing on the Extraction of Antioxidants from Olive Pomace. Molecules 2024, 29, 2303. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical Fluid Extraction of Bioactive Compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Guerrero, I.; Maldonado, L.; Marcia, J.; Aleman, R.; Moncada, M.; Fernández, V.M.; Reyes Jose, T.; Montero-Fernandez, I. Freeze Drying Optimization of Canola Oil with Phytosterols Using Alginate and Maltodextrin. Chem. Eng. Trans. 2022, 93, 139–144. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of Oregano Essential Oil (Origanum vulgare) by Complex Coacervation between Gelatin and Chia Mucilage and Its Properties after Spray Drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almonaitytė, A.; Jensen, I.-J.; Kurek, M.; Lerfall, J. Alginate Microbeads Incorporated with Anthocyanins from Purple Corn (Zea mays L.) Using Electrostatic Extrusion: Microencapsulation Optimization, Characterization, and Stability Studies. Int. J. Biol. Macromol. 2023, 246, 125684. [Google Scholar] [CrossRef] [PubMed]
- Radünz, M.; Hackbart, H.C.d.S.; Camargo, T.M.; Nunes, C.F.P.; Barros, F.A.P.d.; Magro, J.D.; Filho, P.J.S.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; et al. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Microencapsulation of Mentha Spicata Essential Oil by Spray Drying: Optimization, Characterization, Release Kinetics of Essential Oil from Microcapsules in Food Models. Ind. Crops Prod. 2020, 154, 112694. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepúlveda, E.; Chávez, J.; Sáenz, C. Encapsulation of Polyphenols and Anthocyanins from Pomegranate (Punica granatum) by Spray Drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Jeykumari, A.; Elavarasan, K.; Binsi, P.K. Encapsulation of bioactive ingredients: Application and challenges. In Recent Advances in Harvest and Post-Harvest Technologies in Fisheries; Remya, S., Parvathy, U., Jeyanthi, P.J., Mohanty, A.K., Bindu, J., Eds.; Central Institute of Fisheries Technology: Cochin, India, 2022; pp. 31–35. [Google Scholar]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G. Microencapsulation Techniques, Factors Influencing Encapsulation Efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Phupaboon, S.; Matra, M.; Prommachart, R.; Totakul, P.; Supapong, C.; Wanapat, M. Extraction, Characterization, and Chitosan Microencapsulation of Bioactive Compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants 2022, 11, 2103. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, C.; Chung, M.M.S.; Dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an Anthocyanin-Rich Blackberry (Rubus spp.) by-Product Extract by Freeze-Drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Le, T.V.A.; Dang, N.N.; Nguyen, D.C.; Nguyen, P.T.N.; Tran, T.T.; Nguyen, Q.V.; Bach, L.G.; Thuy Nguyen Pham, T.D. Microencapsulation of Essential Oils by Spray-Drying and Influencing Factors. J. Food Qual. 2021, 2021, 5525879. [Google Scholar] [CrossRef]
- Jafari, S.; Jafari, S.M.; Ebrahimi, M.; Kijpatanasilp, I.; Assatarakul, K. A Decade Overview and Prospect of Spray Drying Encapsulation of Bioactives from Fruit Products: Characterization, Food Application and in Vitro Gastrointestinal Digestion. Food Hydrocoll. 2023, 134, 108068. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Arozarena, I.; Marín-Arroyo, M.R. Optimization of a Wall Material Formulation to Microencapsulate a Grape Seed Extract Using a Mixture Design of Experiments. Food Bioprocess Technol 2013, 6, 941–951. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Choque-Chávez, M.C.; Alarcón-Enos, J.; Quevedo-León, R.; Muñoz-Fariña, O.; Vidal-San-Martín, C. Effect of Spray Drying at 150, 160, and 170 °C on the Physical and Chemical Properties of Maqui Extract (Aristotelia chilensis (Molina) Stuntz). Chil. J. Agric. Res. 2019, 79, 144–152. [Google Scholar] [CrossRef]

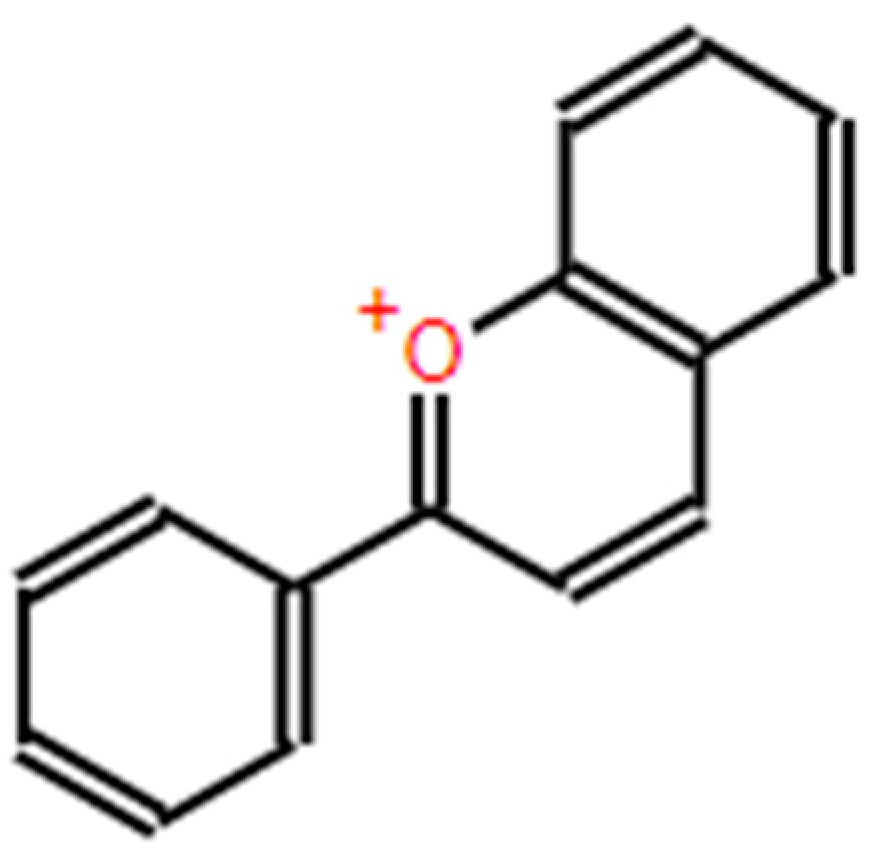
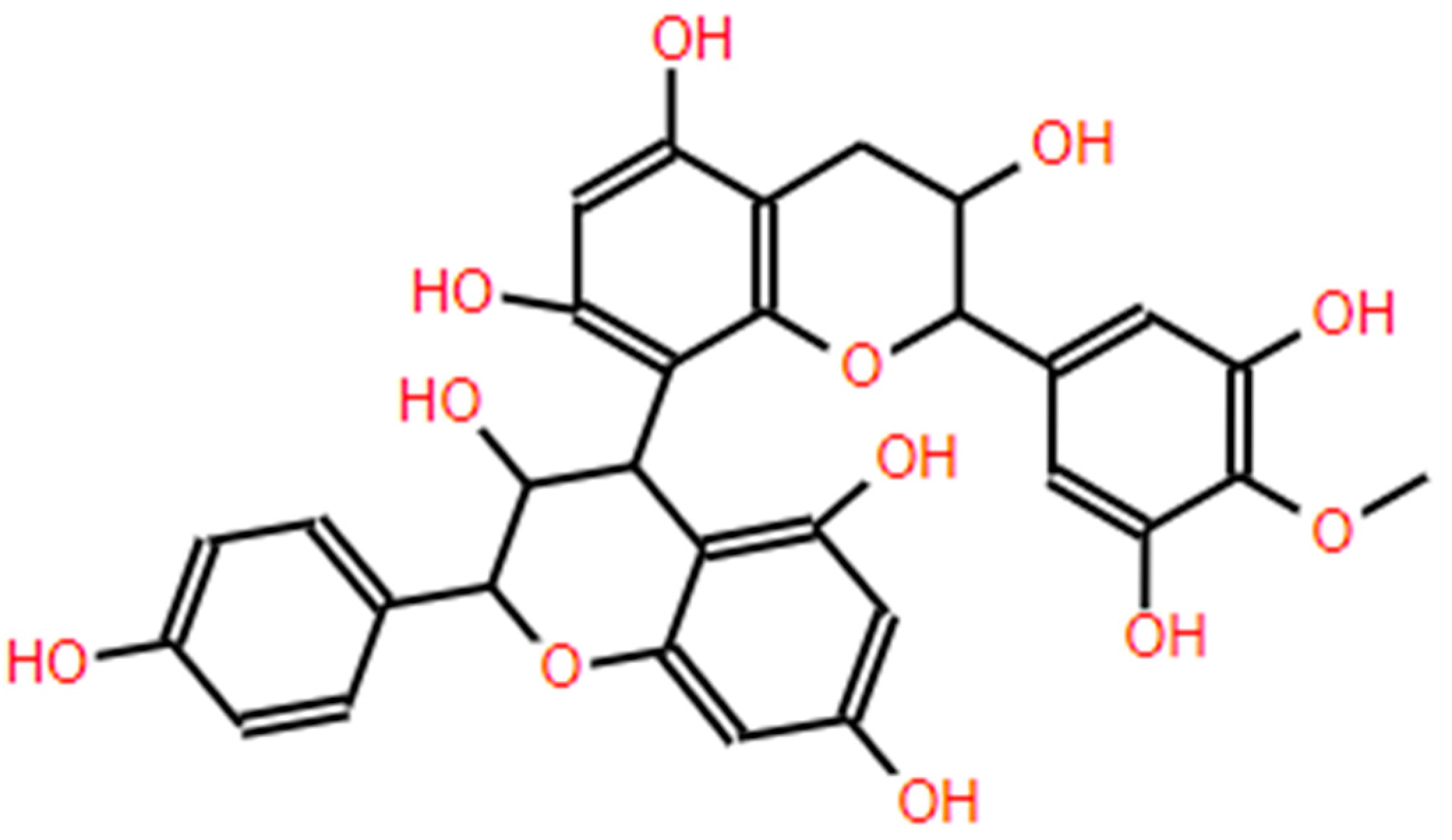
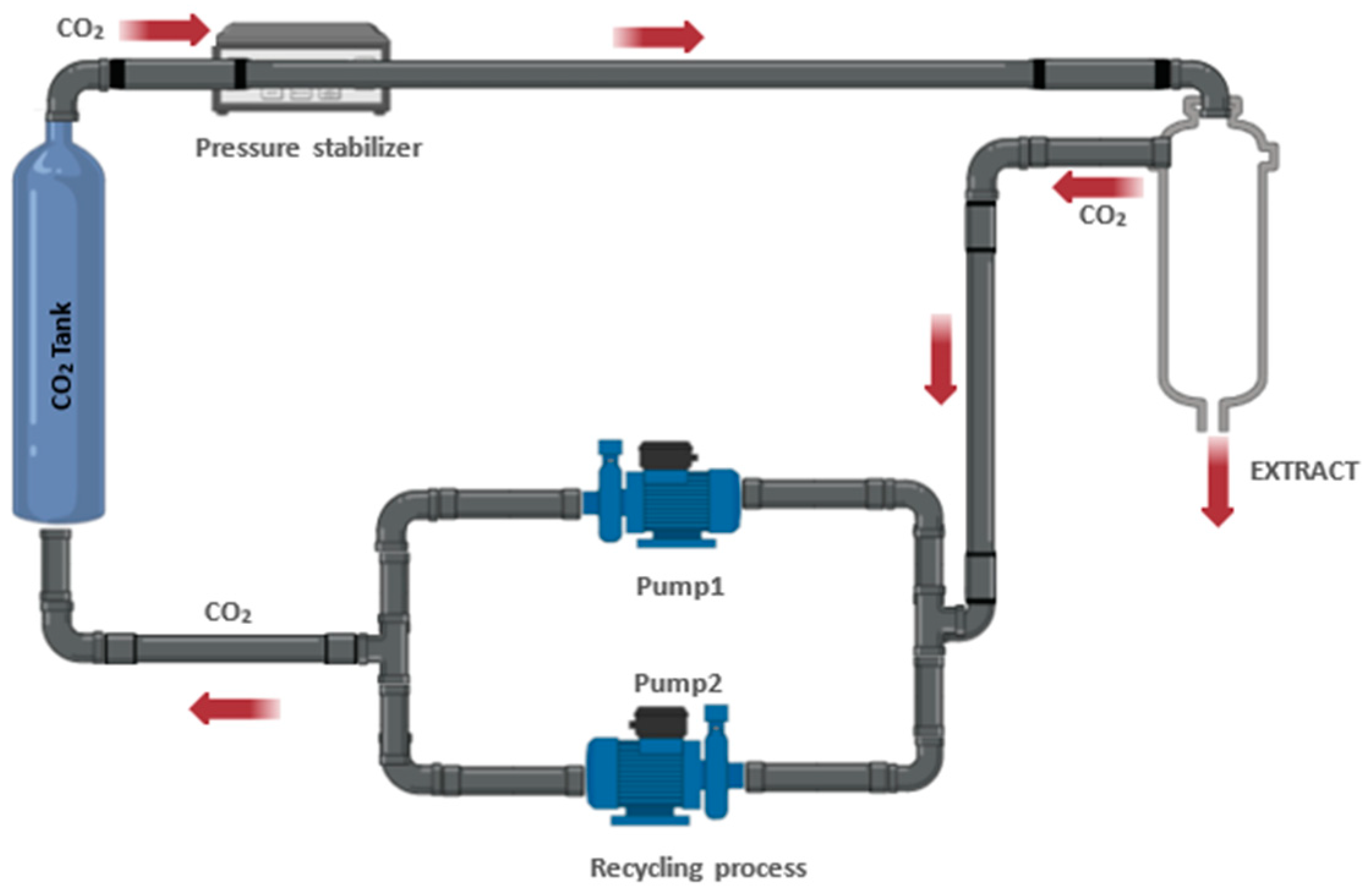

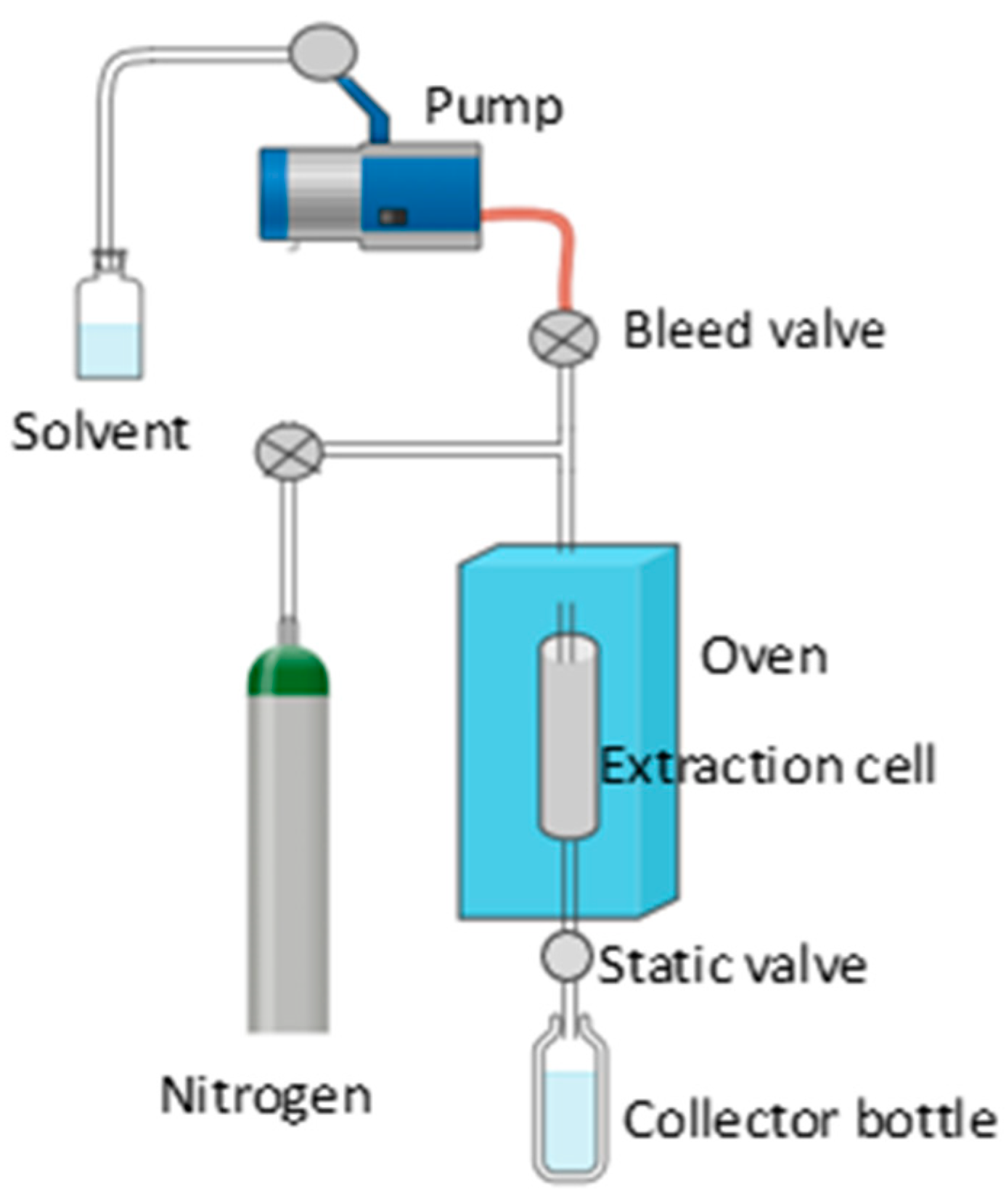

| Extraction Method | Substrate | Advantages | Disadvantages | References |
|---|---|---|---|---|
| SLE | Grape pomace Grape seeds | Simple and widely used method. It requires simple equipment and is suitable for a wide range of samples. | May be less efficient compared to more advanced methods. The duration of the process can be long and some compounds may not be completely extracted. | [67,68] |
| EAE | Grape skins Grape seeds | Selective and gentle. Can improve the extraction of specific compounds without the need for high temperatures. Lower risk of degradation. | Requires specific conditions for each enzyme and can be slower. Enzyme can be expensive and requires precise control of conditions. | [69,70] |
| L/S | Grape pomace | Allows the solvent concentration to be adjusted to suit the sample. Controlling the ratio can influence extraction efficiency. | It may require testing and adjustment to determine the best ratio. In some conditions, an incorrect ratio may adversely affect extraction. | [71] |
| UAE | Grape pomace | Fast and efficient. The use of ultrasound improves mass transfer and can reduce extraction time. Can be used with a variety of solvents. | It can generate heat, which may affect the stability of some compounds. Efficiency may depend on the ultrasonic frequency and power used. | [72] |
| SFE | Grape pomace | It uses supercritical CO2, which is safe and evaporates easily. It allows adjustable selectivity and efficient extraction of lipophilic compounds. | It requires specialized equipment and is more expensive. Optimization of conditions is crucial. Not suitable for all compounds. | [73,74] |
| MAE | Grape pomace | Fast and efficient. Microwave energy can accelerate the release of compounds. It can reduce extraction time and improve yields. | Non-uniform energy distribution can result in uneven extractions. Care is needed to avoid thermal degradation of some compounds. | [75] |
| PLE | Grape seeds | Uses pressurized solvents to improve extraction efficiency. Can reduce extraction time and improve yields. | It requires specialized equipment and can be more expensive. Selection of solvent and operating conditions is critical. | [76] |
| HHP | Grape skin | Uses high pressures to improve extraction. Can better preserve the characteristics of heat-sensitive compounds. | It requires specialized equipment and is more expensive. Optimization of pressure conditions is critical. Not suitable for all sample types. | [77] |
| NaDES AE | Grape pomace | NaDES are made from natural components, which gives them a more sustainable profile. They exhibit low toxicity compared to other solvents. They can be designed to be highly selective in the extraction of certain compounds. | NaDES can be expensive to obtain or synthesize compared to other extraction methods. They may require specific temperature, pressure or pH conditions to achieve effective extraction. Research is still ongoing to determine their feasibility on a large scale. | [78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Portillo, G.-C.; Acuña-Nelson, S.-M.; Bastías-Montes, J.-M. From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review. Antioxidants 2024, 13, 992. https://doi.org/10.3390/antiox13080992
Peña-Portillo G-C, Acuña-Nelson S-M, Bastías-Montes J-M. From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review. Antioxidants. 2024; 13(8):992. https://doi.org/10.3390/antiox13080992
Chicago/Turabian StylePeña-Portillo, Glenda-Caridad, Sergio-Miguel Acuña-Nelson, and José-Miguel Bastías-Montes. 2024. "From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review" Antioxidants 13, no. 8: 992. https://doi.org/10.3390/antiox13080992
APA StylePeña-Portillo, G.-C., Acuña-Nelson, S.-M., & Bastías-Montes, J.-M. (2024). From Waste to Wealth: Exploring the Bioactive Potential of Wine By-Products—A Review. Antioxidants, 13(8), 992. https://doi.org/10.3390/antiox13080992






