Role of Anonychium africanum (Plantae, Fabaceae) in Metal Oxido-Inflammatory Response: Protection Evidence in Gonad of Male Albino Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Anonychium africanum (Prosopis africana, PA) and Preparation of Crude Extract
2.2. PA Preparation for Analysis by Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Acute Toxicity Testing (LD50)
2.4. Animal Ethics and Maintenance
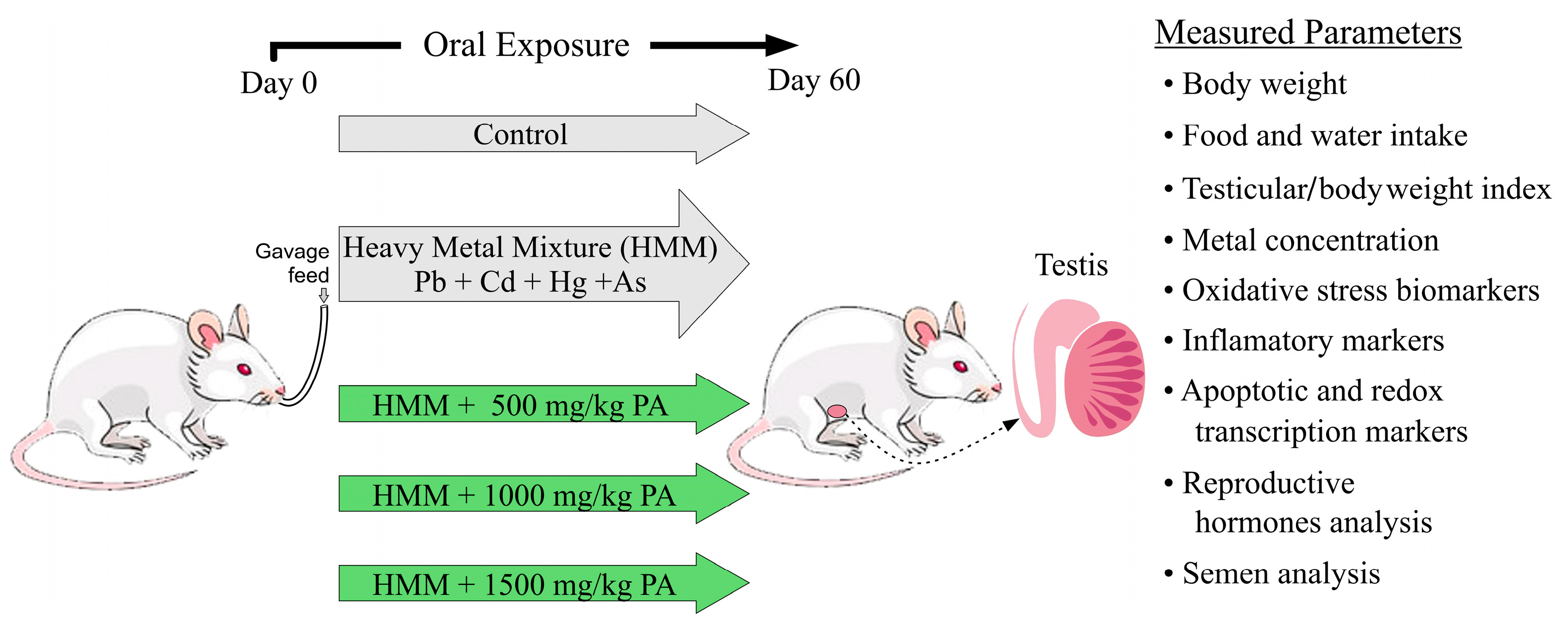
2.5. Experimental Design and Dose Administration
- -
- Group 1. Negative Control: This control group of rats was given deionized water orally once daily for 60 days.
- -
- Group 2. Positive Control, HMM: This group received only the heavy metal mixture at the dose standards described above daily for 60 days.
- -
- Group 3. HMM + PA (500 mg/kg): This groups received the same heavy metal mixture as the positive control but was treated with Prosopis africana aqueous extract at daily doses of 500 mg/kg body weight for 60 days.
- -
- Group 4. HMM + PA (1000 mg/kg): This group received the same heavy metal mixture as the positive control but was treated with Prosopis africana aqueous extract at a daily dose of 1000 mg/kg body weight for 60 days.
- -
- Group 5. HMM + PA (1500 mg/kg): This group received the same heavy metal mixture as the positive control but was treated with Prosopis africana aqueous extract at a daily dose of 1500 mg/kg body weight for 60 days.
2.6. Body Weight Measurement
2.7. Measurement of Feed and Water Intake
2.8. Necropsy, Tissues and Organ Collection and Processing
2.9. Body Organ Index
2.10. Metal Concentrations in Tissue Samples
2.11. Oxidative Stress Markers
2.12. Measurement of Inflammatory Markers
2.13. Measurement of Apoptotic and Redox Transcription Markers
2.14. Reproductive Hormones Analysis
2.15. Semen Analysis
2.16. Statistical Analysis
3. Results
3.1. Phytoconstituents in Aqueous Extract of Anonychium africanum (Prosopis africana, PA)
3.2. Acute Toxicity Test of Prosopis africana Aqueous Extract
3.3. Effect of Prosopis africana on the Body Weight and Absolute and Relative Weight of Testis of Male Albino Rats Exposed to HMM
3.4. Prosopis africana Effect on Male Albino Rat Semen Exposed to Heavy Metal Mixture (HMM)
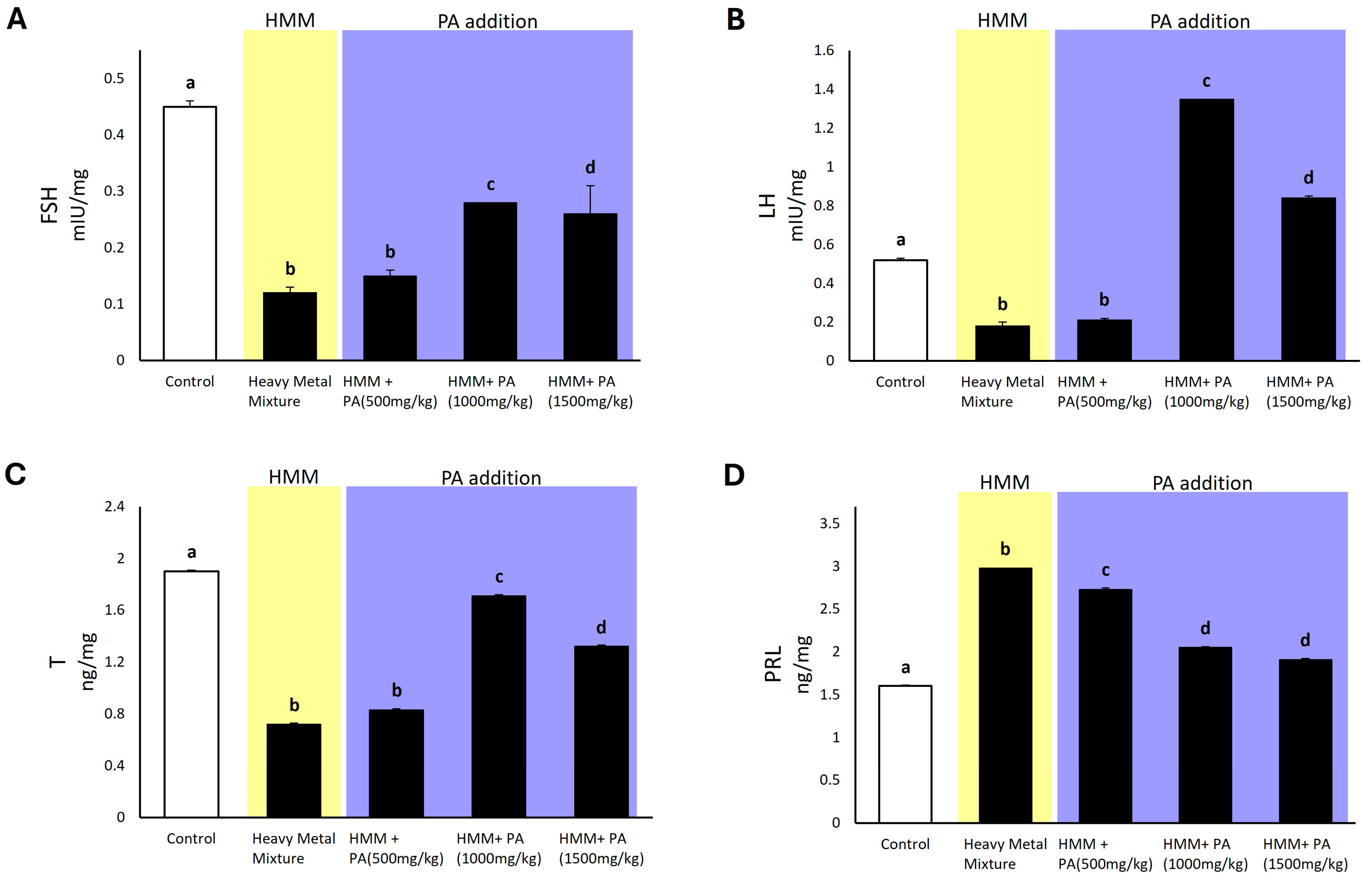
3.5. Prosopis africana Effect on Hormonal Profile of Male Albino Rats Exposed to HMM
3.6. Effect of Prosopis africana in Bioaccumulation of Heavy Metals in Rat Testis
3.7. Effect of Prosopis africana on Oxidative Stress Markers of Male Albino Rat Testis Exposed to HMM
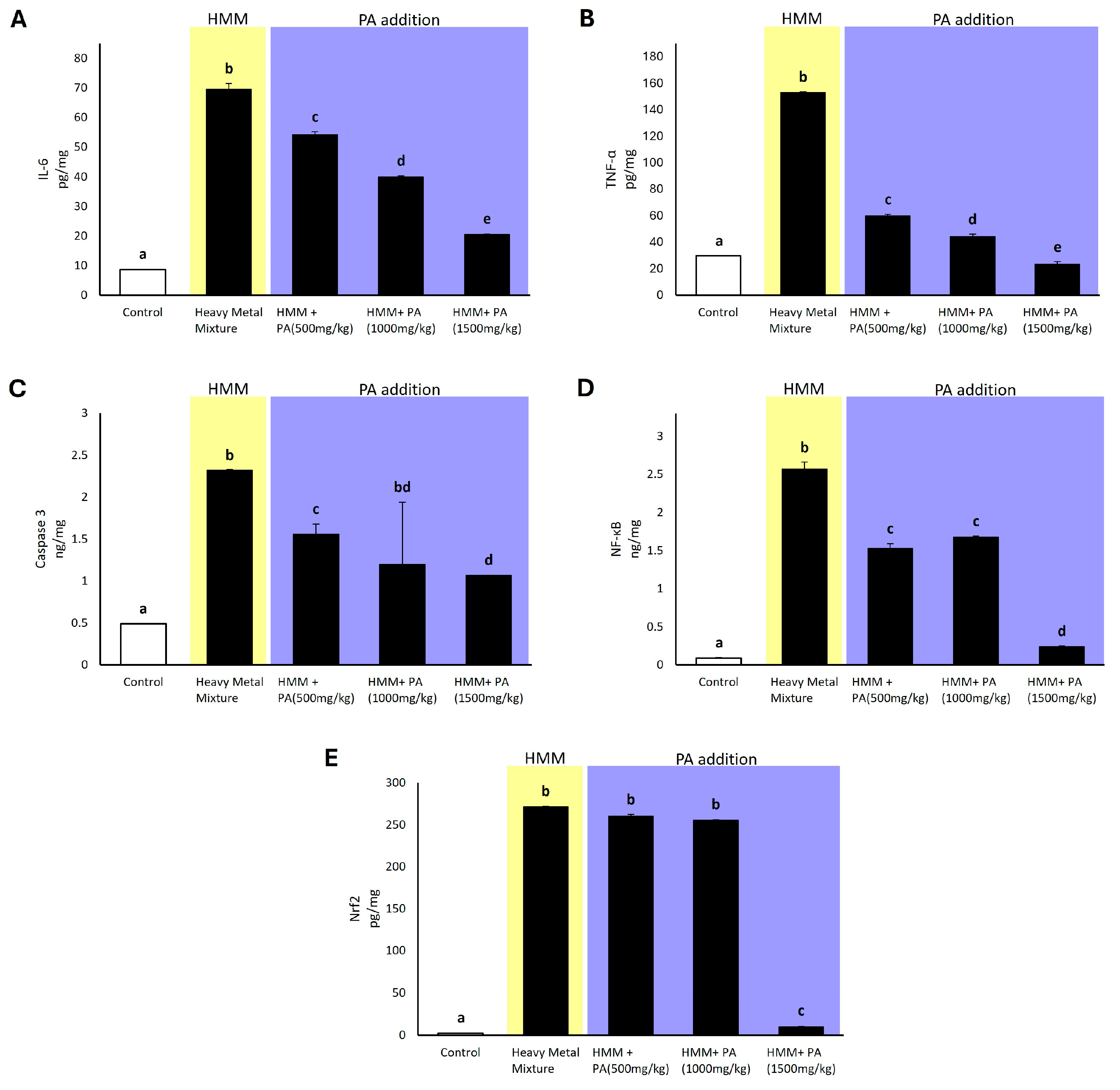
3.8. Effect of Prosopis africana on Expression of Pro-Inflammatory Factors and Apoptotic and Transcriptional Factors in Male Albino Rat Testis Exposed to HMM
4. Discussion
4.1. Chemical Characteristics and Relevant Activity of Prosopis africana
4.2. Effect of Prosopis africana on the Body Weight and Absolute and Relative Weight of Testis of Male Albino Rats Exposed to Heavy Metal Mixture (HMM)
4.3. Effect of Prosopis africana in Bioaccumulation of HMM in Rat Testis
4.4. Prosopis africana Affects Oxidative Stress Markers of Male Albino Rat Testis Exposed to HMM
4.5. Prosopis africana Effect on Expression of Pro-Inflammatory Factors and Apoptotic and Transcriptional Factors in Male Albino Rat Testis Exposed to HMM
4.6. Effect of Prosopis africana on Hormonal Profile of the Male Albino Rat Exposed to HMM
4.7. Correlation Analysis of Biochemical Parameters in HMM-Exposed Male Albino Rat Testis
4.8. Effect of Prosopis africana on Semen Analysis of Male Albino Rat Exposed to HMM
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiati Kenston, S.S.; Su, H.; Li, Z.; Kong, L.; Wang, Y.; Song, X.; Gu, Y.; Barber, T.; Aldinger, J.; Hua, Q.; et al. The Systemic Toxicity of Heavy Metal Mixtures in Rats. Toxicol. Res. 2018, 7, 396–407. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Bassem, S.M.; Khalil, W.K.; Temraz, T.A.; Ciarcia, G.; Abdel-Gawad, F.K. Temperature changes and marine fish species (Epinephelus coioides and Sparus aurata): Role of oxidative stress biomarkers in toxicological food studies. Emir. J. Food Agric. 2018, 30, 205–211. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of Heavy Metals on Fish Physiology—A Review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, P.; Guezgouz, N.; Di Iorio, E.; Colombo, C.; Guerriero, G.; De Marco, A. Anthropic Impact on Soil Heavy Metal Contamination in Riparian Ecosystems of Northern Algeria. Chemosphere 2023, 313, 137522. [Google Scholar] [CrossRef]
- Calogero, A.E.; Fiore, M.; Giacone, F.; Altomare, M.; Asero, P.; Ledda, C.; Romeo, G.; Mongioì, L.M.; Copat, C.; Giuffrida, M.; et al. Exposure to multiple metals/metalloids and human semen quality: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 215, 112165. [Google Scholar] [CrossRef]
- Skakkebaek, N.; De Meyts, E.R.; Main, K.M. Testicular Dysgenesis Syndrome: An Increasingly Common Developmental Disorder with Environmental Aspects. APMIS 2001, 109, S22–S30. [Google Scholar] [CrossRef]
- Koyama, H.; Kamogashira, T.; Yamasoba, T. Heavy metal exposure: Molecular pathways, clinical implications, and protective strategies. Antioxidants 2024, 13, 76. [Google Scholar] [CrossRef]
- Badr, F.M.; El-Habit, O. Heavy Metal Toxicity Affecting Fertility and Reproduction of Males. In Bioenvironmental Issues Affecting Men’s Reproductive and Sexual Health; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–304. ISBN 978-0-12-801299-4. [Google Scholar]
- Anyanwu, B.O.; Ezejiofor, A.N.; Nwaogazie, I.L.; Akaranta, O.; Orisakwe, O.E. Low-dose Heavy Metal Mixture (Lead, Cadmium and Mercury)-induced Testicular Injury and Protective Effect of Zinc and Costus afer in Wistar Albino Rats. Andrologia 2020, 52, e13697. [Google Scholar] [CrossRef]
- Ozoani, H.; Ezejiofor, A.N.; Okolo, K.O.; Orish, C.N.; Cirovic, A.; Cirovic, A.; Orisakwe, O.E. Zinc and Selenium Attenuate Quaternary Heavy Metal Mixture-Induced Testicular Damage via Amplification of the Antioxidant System, Reduction in Metal Accumulation, Inflammatory and Apoptotic Biomarkers. Toxicol. Res. 2023, 39, 497–515. [Google Scholar] [CrossRef]
- Acharya, U.R.; Mishra, M.; Tripathy, R.R.; Mishra, I. Testicular Dysfunction and Antioxidative Defense System of Swiss Mice after Chromic Acid Exposure. Reprod. Toxicol. 2006, 22, 87–91. [Google Scholar] [CrossRef]
- Momparler, R.L. Cancer Epigenetics. Oncogene 2003, 22, 6479–6483. [Google Scholar] [CrossRef]
- Ceramella, J.; De Maio, A.C.; Basile, G.; Facente, A.; Scali, E.; Andreu, I.; Sinicropi, M.S.; Iacopetta, D.; Catalano, A. Phytochemicals involved in mitigating silent toxicity induced by heavy metals. Foods 2024, 13, 978. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The Duckweed, Lemna minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Badawi, K.; El Sharazly, B.M.; Negm, O.; Khan, R.; Carter, W.G. Is Cadmium Genotoxicity due to the Induction of Redox Stress and Inflammation? A Systematic Review. Antioxidants 2024, 13, 932. [Google Scholar] [CrossRef]
- Ramos-Treviño, J.; Bassol-Mayagoitia, S.; Hernández-Ibarra, J.A.; Ruiz-Flores, P.; Nava-Hernández, M.P. Toxic Effect of Cadmium, Lead, and Arsenic on the Sertoli Cell: Mechanisms of Damage Involved. DNA Cell Biol. 2018, 37, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.W.; Lee, S.J.; Kim, S.G. Molecular Mechanism of Nrf2 Activation by Oxidative Stress. Antioxid. Redox Signal. 2005, 7, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Vitale, L.; Bianchi, A.R.; Mistretta, C.; Vitale, E.; Parisi, C.; Guerriero, G.; Magliulo, V.; De Maio, A. The ageing process affects the antioxidant defences and the poly (ADPribosyl) ation activity in Cistus incanus L. leaves. Antioxidants 2019, 8, 528. [Google Scholar] [CrossRef]
- Matsumaru, D.; Motohashi, H. The KEAP1-NRF2 System in Healthy Aging and Longevity. Antioxidants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Parisi, C.; Guerriero, G. Antioxidative Defense and Fertility Rate in the Assessment of Reprotoxicity Risk Posed by Global Warming. Antioxidants 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Falade, K.O.; Akeem, S.A. Physicochemical Properties, Protein Digestibility and Thermal Stability of Processed African Mesquite Bean (Prosopis africana) Flours and Protein Isolates. Food Meas. 2020, 14, 1481–1496. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Evelyn, U.E.; Ikpeama, C.N.; Orish, A.N.; Ezejiofor, K.O.; Okolo, A.; Cirovic, A.; Cirovic, I.; Nwaogazie, L.; Onoyima, C.S. Prosopis africana exerts neuroprotective activity against quaternary metal mixture-induced memory impairment mediated by oxido-inflammatory response via Nrf2 pathway, AIMS. Neuroscience 2024, 11, 118–143. [Google Scholar] [CrossRef]
- Nielsen, I.; Keay, R.W.J. 1989. Trees of Nigeria. Clarendon Press Oxford. Nord. J. Bot. 1991, 11, 322. [Google Scholar] [CrossRef]
- Ezike, A.; Akah, P.; Okoli, C.; Udegbunam, S.; Okwume, N.; Okeke, C.; Iloani, O. Medicinal Plants Used in Wound Care: A Study of Prosopis africana (Fabaceae) Stem Bark. Indian J. Pharm. Sci. 2010, 72, 334. [Google Scholar] [CrossRef]
- Hossain, M.A.; Shah, M.D.; Sakari, M. Gas Chromatography–Mass Spectrometry Analysis of Various Organic Extracts of Merremia borneensis from Sabah. Asian Pac. J. Trop. Med. 2011, 4, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Bagewadi, Z.K.; Siddanagouda, R.S.; Baligar, P.G. Phytoconstituents Investigation by LC-MS and Evaluation of Anti-Microbial and Anti-Pyretic Properties of Cynodon Dactylon. Int. J. Pharm. Sci. Res. 2014, 5, 2874. [Google Scholar]
- Lorke, D. A New Approach to Practical Acute Toxicity Testing. Arch. Toxicol. 1983, 54, 275–287. [Google Scholar] [CrossRef]
- Messarah, M.; Klibet, F.; Boumendjel, A.; Abdennour, C.; Bouzerna, N.; Boulakoud, M.S.; El Feki, A. Hepatoprotective Role and Antioxidant Capacity of Selenium on Arsenic-Induced Liver Injury in Rats. Exp. Toxicol. Pathol. 2012, 64, 167–174. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Zhao, T.; Zhang, Z.; Feng, W.; Wang, W.; Li, Q.; Wu, X.; et al. Toxicity Assessment due to Sub-Chronic Exposure to Individual and Mixtures of Four Toxic Heavy Metals. J. Hazard. Mater. 2015, 294, 109–120. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants 2019, 9, 10. [Google Scholar] [CrossRef]
- Ozoani, H.A.; Ezejiofor, A.N.; Amadi, C.N.; Chijioke-Nwauche, I.; Orisakwe, O.E. Safety of Honey Consumed in Enugu State, Nigeria: A Public Health Risk Assessment of Lead and Polycyclic Aromatic Hydrocarbons. Rocz. Panstw. Zakl. Hig. 2020, 71, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Oktem, G.; Uysal, A.; Oral, O.; Sezer, E.D.; Olukman, M.; Erol, A.; Akgur, S.A.; Bilir, A. Resveratrol Attenuates Doxorubicin-Induced Cellular Damage by Modulating Nitric Oxide and Apoptosis. Exp. Toxicol. Pathol. 2012, 64, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Di Finizio, A.; Ciarcia, G. Stress-Induced Changes of Plasma Antioxidants in Aquacultured Sea Bass, Dicentrarchus labrax. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 132, 205–211. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Qiu, Q.; Kuo, A.; Todd, H.; Dias, J.A.; Gould, J.E.; Overstreet, J.W.; Lasley, B.L. Enzyme Immunoassay Method for Total Urinary Follicle-Stimulating Hormone (FSH) Beta Subunit and Its Application for Measurement of Total Urinary FSH. Fertil. Steril. 1998, 69, 278–285. [Google Scholar] [CrossRef]
- Frank, L.H.; Rushlow, C. A Group of Genes Required for Maintenance of the Amnioserosa Tissue in Drosophila. Development 1996, 122, 1343–1352. [Google Scholar] [CrossRef]
- Vanderpump; French; Appleton; Tunbridge; Kendall-Taylor. The Prevalence of Hyperprolactinaemia and Association with Markers of Autoimmune Thyroid Disease in Survivors of the Whickham Survey Cohort. Clin. Endocrinol. 1998, 48, 39–44. [Google Scholar] [CrossRef]
- Guerriero, G.; Roselli, C.E.; Paolucci, M.; Botte, V.; Ciarcia, G. Estrogen Receptors and Aromatase Activity in the Hypothalamus of the Female Frog, Rana esculenta. Fluctuations throughout the Reproductive Cycle. Brain Res. 2000, 880, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Chapin, R.E.; Filler, R.S.; Gulati, D.; Heindel, J.J.; Katz, D.F.; Mebus, C.A.; Obasaju, F.; Perreault, S.D.; Russell, S.R.; Schrader, S.; et al. Methods for assessing rat sperm motility. Reprod. Toxicol. 1992, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Moskovtsev, S.I.; Librach, C.L. Methods of Sperm Vitality Assessment. In Spermatogenesis; Carrell, D.T., Aston, K.I., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 13–19. [Google Scholar]
- Gupta, A.; Kumar, A.; Naqvi, S.; Flora, S.J.S. Chronic exposure to multi-metals on testicular toxicity in rats. Toxicol. Mech. Methods 2021, 31, 53–66. [Google Scholar] [CrossRef]
- Maremanda, K.P.; Khan, S.; Jena, G. Zinc protects cyclophosphamide-induced testicular damage in rat: Involvement of metallothionein, tesmin and Nrf2. Biochem. Biophys. Res. Commun. 2014, 445, 591–596. [Google Scholar] [CrossRef]
- Lin, M.C.; Tsai, T.C.; Yang, Y.S. Measurement of viscosity of human semen with a rotational viscometer. J. Formos. Med. Assoc. 1992, 91, 419–423. [Google Scholar] [PubMed]
- Mohammadi, F.; Nikzad, H.; Taherian, A.; Amini Mahabadi, J.; Salehi, M. Effects of Herbal Medicine on Male Infertility. Anat. Sci. J. 2013, 10, 3–16. [Google Scholar]
- Hammer, Ø.; Harper, D.A. Past: Paleontological Statistics Software Package for Educaton and Data Anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Study on Chemical Composition, Antioxidant and Anti-Inflammatory Activities of Hot Water Extract from Picea Mariana Bark and Its Proanthocyanidin-Rich Fractions. Food Chem. 2009, 113, 897–902. [Google Scholar] [CrossRef]
- Ajiboye, A.A.; Agboola, D.A.; Fadimu, O.Y.; Afolabi, A.O. Antibacterial, Phytochemical and Proximate Analysis of Prosopis africana (Linn) Seed and Pod Extract. FUTA J. Sci. Res. 2013, 1, 101–109. [Google Scholar]
- Olajide, O.B.; Fadimu, O.Y.; Osaguona, P.O.; Saliman, M.I. Ethnobotanical and Phytochemical Studies of Some Selected Species of Leguminoseae of Northern Nigeria: A Study of Borgu Local Government Area, Niger State. Nigeria. Int. J. Nat. Sci. 2013, 4, 546–551. [Google Scholar]
- Manchope, M.F.; Casagrande, R.; Verri, W.A. Naringenin: An Analgesic and Anti-Inflammatory Citrus Flavanone. Oncotarget 2017, 8, 3766–3767. [Google Scholar] [CrossRef] [PubMed]
- El-desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-Inflammatory and Antioxidant Activities of Naringin Isolated from Carissa Carandas L.: In Vitro and in Vivo Evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.D.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential Anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid. Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef] [PubMed]
- Erden-İnal, M.; Sunal, E.; Kanbak, G. Age-related Changes in the Glutathione Redox System. Cell Biochem. Funct. 2002, 20, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Hazato, T.; Ashino, H.; Yamamoto, Y.; Iwasaki, E.; Tobe, H.; Yamamoto, K.; Yamamoto, S. Inhibition of Angiogenesis by Humulone, a Bitter Acid from Beer Hop. Biochem. Biophys. Res. Commun. 2001, 289, 220–224. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, Anti-inflammatory, and Antitumor Activities of Phenolic Compounds from White, Red, and Black Chenopodium Quinoa Seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Gawlik-Dziki, U.; Nowak, R. Antioxidant, Anti-Inflammatory, and Anti-Diabetic Activity of Phenolic Acids Fractions Obtained from Aerva lanata (L.) Juss. Molecules 2021, 26, 3486. [Google Scholar] [CrossRef]
- Brown, K.S.; Jamieson, P.; Wu, W.; Vaswani, A.; Alcazar Magana, A.; Choi, J.; Mattio, L.M.; Cheong, P.H.-Y.; Nelson, D.; Reardon, P.N.; et al. Computation-Assisted Identification of Bioactive Compounds in Botanical Extracts: A Case Study of Anti-Inflammatory Natural Products from Hops. Antioxidants 2022, 11, 1400. [Google Scholar] [CrossRef]
- Wang, J.; Tian, B.; Cheng, J.; Yang, J.; Liu, Y. Isolation, Characterization, Crystal Structure, Antioxidant and Antibacterial Properties of Co-Lupulone. J. Mol. Struct. 2022, 1250, 131718. [Google Scholar] [CrossRef]
- Valaei, K.; Mehrabani, J.; Wong, A. Effects of L-Citrulline Supplementation on Nitric Oxide and Antioxidant Markers after High-Intensity Interval Exercise in Young Men: A Randomised Controlled Trial. Br. J. Nutr. 2022, 127, 1303–1312. [Google Scholar] [CrossRef]
- Mirenayat, M.S.; Faramarzi, M.; Ghazvini, M.R.; Karimian, J.; Hadi, A.; Heidari, Z.; Rouhani, M.H.; Naeini, A.A. The Effects of Short Term Citrulline Malate Supplementation on Oxidative Stress and Muscle Damage in Trained Soccer Players. Hum. Nutr. Metab. 2024, 36, 200242. [Google Scholar] [CrossRef]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant Role of Catechin in Health and Disease. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–271. ISBN 978-0-12-398456-2. [Google Scholar]
- Morrison, M.; Van Der Heijden, R.; Heeringa, P.; Kaijzel, E.; Verschuren, L.; Blomhoff, R.; Kooistra, T.; Kleemann, R. Epicatechin Attenuates Atherosclerosis and Exerts Anti-Inflammatory Effects on Diet-Induced Human-CRP and NFκB in Vivo. Atherosclerosis 2014, 233, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Matta, F.V.; Grace, M.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Phenolic Content, Anti-Inflammatory Properties, and Dermal Wound Repair Properties of Industrially Processed and Non-Processed Acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.A.; Hozayen, W.G.; Abo Sree, H.T.; Al-Muzafar, H.M.; Amin, K.A.; Ahmed, O.M. Naringin and Hesperidin Counteract Diclofenac-Induced Hepatotoxicity in Male Wistar Rats via Their Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Oxid. Med. Cell. Longev. 2021, 2021, 9990091. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Cabrita, A.R.; Fonseca, A.J.; ValentÃ, P.; Andrade, P.B. Alkaloids in the Valorization of European Lupinus spp. Seeds Crop. Ind. Crops Prod. 2017, 95, 286–295. [Google Scholar]
- Bermúdez-Torres, K.; Ferval, M.; Hernández-Sánchez, A.M.; Tei, A.; Gers, C.; Wink, M.; Legal, L. Molecular and Chemical Markers to Illustrate the Complex Diversity of the Genus Lupinus (Fabaceae). Diversity 2021, 13, 263. [Google Scholar] [CrossRef]
- Ramírez-Betancourt, A.; Hernández-Sánchez, A.M.; Salcedo-Morales, G.; Ventura-Zapata, E.; Robledo, N.; Wink, M.; Bermúdez-Torres, K. Unraveling the Biosynthesis of Quinolizidine Alkaloids Using the Genetic and Chemical Diversity of Mexican Lupins. Diversity 2021, 13, 375. [Google Scholar] [CrossRef]
- Erdemoglu, N.; Ozkan, S.; Tosun, F. Alkaloid Profile and Antimicrobial Activity of Lupinus angustifolius L. Alkaloid Extract. Phytochem. Rev. 2007, 6, 197–201. [Google Scholar] [CrossRef]
- Chukwuemeka, E.S.; Ogoamaka, O.P.; Obodoike, E.C.; Ifeoma, I.A.S.; Ishola, A.A.; Chukwunonye, U.C.E.; Uchenna, O.E.; Nnaemeka, A.T.; Chiedu, O.F.B. Molecular Docking and Anti-inflammatory Studies on Extracts of Prosopis africana (Guill. & Perr.) Taubert and Parkia biglobossa (Jacq.) Benth (Fabaceae). Asian J. Adv. Res. 2024, 7, 14–22. [Google Scholar]
- Abdullah, R.R.H.; Abd El-Wahab, A.H.; Abd El-Salam, S.A. Insecticidal Activity and Possible Modes of Action of Secondary Metabolites of Some Fungal Strains and Wild Plants as Natural Pesticides against Spodoptera Frugiperda. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 9. [Google Scholar] [CrossRef]
- De Santis, M.; Pan, B.; Lian, J.; Huang, X.-F.; Deng, C. Different Effects of Bifeprunox, Aripiprazole, and Haloperidol on Body Weight Gain, Food and Water Intake, and Locomotor Activity in Rats. Pharmacol. Biochem. Behav. 2014, 124, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Kundu, S.N.; Khuda Bukhsh, A.R. Efficacy of a Potentized Homoeopathic Drug (Arsenicum Album-30) in Reducing Toxic Effects Produced by Arsenic Trioxide in Mice: II. On Alterations in Body Weight, Tissue Weight and Total Protein. Complement Ther. Clin. Pract. 1999, 7, 24–34. [Google Scholar] [CrossRef]
- Su, Y.; Quan, C.; Li, X.; Shi, Y.; Duan, P.; Yang, K. Mutual Promotion of Apoptosis and Autophagy in Prepubertal Rat Testes Induced by Joint Exposure of Bisphenol A and Nonylphenol. Environ. Pollut. 2018, 243, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, Q.; Chen, Y.; Wang, S.; Huang, D. Resveratrol Attenuates Inflammation and Oxidative Stress in Epididymal White Adipose Tissue: Implications for Its Involvement in Improving Steroidogenesis in Diet-induced Obese Mice. Mol. Reprod. Dev. 2015, 82, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Dong, W.; Li, Y.; Zheng, X.; Piao, F.; Li, S. Subchronic Exposure to Lead Acetate Inhibits Spermatogenesis and Downregulates the Expression of Ddx3y in Testis of Mice. Reprod. Toxicol. 2013, 42, 242–250. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, X.; Ge, R.-S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-Induced Testicular Injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Skuza, L.; Szućko-Kociuba, I.; Filip, E.; Bożek, I. Natural Molecular Mechanisms of Plant Hyperaccumulation and Hypertolerance towards Heavy Metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef]
- Eleiwa, N.Z.H.; Galal, A.A.A.; Abd El-Aziz, R.M.; Hussin, E.M. Antioxidant Activity of Spirulina Platensis Alleviates Doxorubicin-Induced Oxidative Stress and Reprotoxicity in Male Rats. Orient. Pharm. Exp. Med. 2018, 18, 87–95. [Google Scholar] [CrossRef]
- Guerriero, G.; Di Finizio, A.; Ciarcia, G. Oxidative defenses in the sea bass, Dicentrarchus labrax. In Oxygen Transport to Tissue XXIV; Springer: Berlin/Heidelberg, Germany, 2003; pp. 681–688. [Google Scholar]
- Guerriero, G.; D’Errico, G.; Di Giaimo, R.; Rabbito, D.; Olanrewaju, O.S.; Ciarcia, G. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environ. Sci. Pollut. Res. 2018, 25, 18286–18296. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Kasperczyk, A.; Dobrakowski, M.; Czuba, Z.P.; Horak, S.; Kasperczyk, S. Environmental Exposure to Lead Induces Oxidative Stress and Modulates the Function of the Antioxidant Defense System and the Immune System in the Semen of Males with Normal Semen Profile. Toxicol. Appl. Pharmacol. 2015, 284, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Mognetti, B.; Franco, F.; Castrignano, C.; Bovolin, P.; Berta, G.N. Mechanisms of Phytoremediation by Resveratrol against Cadmium Toxicity. Antioxidants 2024, 13, 782. [Google Scholar] [CrossRef]
- Buha, A.; Baralić, K.; Djukic-Cosic, D.; Bulat, Z.; Tinkov, A.; Panieri, E.; Saso, L. The Role of Toxic Metals and Metalloids in Nrf2 Signaling. Antioxidants 2021, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The Therapeutic Effect of Resveratrol: Focusing on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Ateşşahin, A.; Ürk, G.T.; Karahan, İ.; Yılmaz, S.; Çeribaşı, A.O.; Bulmuş, Ö. Lycopene Prevents Adriamycin-Induced Testicular Toxicity in Rats. Fertil. Steril. 2006, 85, 1216–1222. [Google Scholar] [CrossRef]
- McVey, M.J.; Cooke, G.M.; Curran, I.H.A.; Chan, H.M.; Kubow, S.; Lok, E.; Mehta, R. Effects of Dietary Fats and Proteins on Rat Testicular Steroidogenic Enzymes and Serum Testosterone Levels. Food Chem. Toxicol. 2008, 46, 259–269. [Google Scholar] [CrossRef]
- Badkoobeh, P.; Parivar, K.; Kalantar, S.M.; Hosseini, S.D.; Salabat, A. Effect of Nano-Zinc Oxide on Doxorubicin-Induced Oxidative Stress and Sperm Disorders in Adult Male Wistar Rats. Iran. J. Reprod. Med. 2013, 11, 355. [Google Scholar] [PubMed]
- Guerriero, G.; Prins, G.S.; Birch, L.; Ciarcia, G. Neurodistribution of androgen receptor immunoreactivity in the male frog, Rana esculenta. Ann. N. Y. Acad. Sci. 2005, 1040, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Mobasher, M.A.; Alsirhani, A.M.; Alwaili, M.A.; Baakdah, F.; Eid, T.M.; Alshanbari, F.A.; Alzahri, R.Y.; Alkhodair, S.A.; El-Said, K.S. Annona squamosa Fruit Extract Ameliorates Lead Acetate-Induced Testicular Injury by Modulating JAK-1/STAT-3/SOCS-1 Signaling in Male Rats. Int. J. Mol. Sci. 2024, 25, 5562. [Google Scholar] [CrossRef] [PubMed]
- Al-Nahari, H.; Al Eisa, R. Effect of turmeric (Curcuma longa) on some pituitary, thyroid and testosterone hormone against aluminum chloride (AlCl3) induced toxicity in rat. Adv. Environ. Biol. 2016, 10, 250–261. [Google Scholar]
- Bashandy, S.A.E.; El Awdan, S.A.; Ebaid, H.; Alhazza, I.M. Antioxidant Potential of Spirulina Platensis Mitigates Oxidative Stress and Reprotoxicity Induced by Sodium Arsenite in Male Rats. Oxid. Med. Cell. Longev. 2016, 2016, 7174351. [Google Scholar] [CrossRef]
- Farag, M.R.; Abd EL-Aziz, R.M.; Ali, H.A.; Ahmed, S.A. Evaluating the Ameliorative Efficacy of Spirulina Platensis on Spermatogenesis and Steroidogenesis in Cadmium-Intoxicated Rats. Environ. Sci. Pollut. Res. 2016, 23, 2454–2466. [Google Scholar] [CrossRef]
- Ezejiofor, A.N.; Orisakwe, O.E. The protective effect of Costus afer Ker Gawl aqueous leaf extract on lead-induced reproductive changes in male albino Wistar rats. JBRA Assist. Reprod. 2019, 23, 215. [Google Scholar] [CrossRef]
- Adelakun, S.A.; Ukwenya, V.O.; Akingbade, G.T.; Omotoso, O.D.; Aniah, J.A. Interventions of aqueous extract of Solanum melongena fruits (garden eggs) on mercury chloride induced testicular toxicity in adult male Wistar rats. Biomed. J. 2020, 43, 174–182. [Google Scholar] [CrossRef]
- Barros, M.E.; Schor, N.; Boim, M.A. Effects of an Aqueous Extract from Phyllantus niruri on Calcium Oxalate Crystallization in Vitro. Urol. Res. 2003, 30, 374–379. [Google Scholar] [CrossRef]
- Guerriero, G.; Ferro, R.; Ciarcia, G. Correlations between Plasma Levels of Sex Steroids and Spermatogenesis during the Sexual Cycle of the Chub, Leuciscus cephalus L. (Pisces: Cyprinidae). Zool. Stud. 2005, 44, 228. [Google Scholar]


| Treatment | Feed Intake (g) | Fluid Intake (mL) | Absolute Testicular Weight (g) | Relative Testicular Weight (%) | Body Weight (g) | ||
|---|---|---|---|---|---|---|---|
| Initial Weight | Final Weight | % Body wt Difference | |||||
| Control | 164.75 ± 18.80 d | 225.08 ± 58.95 d | 3.18 ± 0.02 c | 1.1 ± 0.06 b | 175.0 ± 4.35 | 270.0 ± 13.73 | 54.29 a |
| HMM | 78.78 ± 27.54 a | 102.30 ± 20.03 a | 3.06 ± 0.32 a | 1.28 ± 0.39 a | 158.0 ± 2.00 | 240.0 ± 16.63 | 51.90 a |
| HMM + PA (500 mg/kg) | 88.53 ± 20.90 b | 148.10 ± 27.13 b | 2.84 ± 0.38 bc | 1.23 ± 0.08 b | 151.0 ± 1.00 | 230.3 ± 14.57 | 52.54 a |
| HMM + PA (1000 mg/kg) | 130.03 ± 18.48 c | 190.20 ± 56.50 c | 2.26 ± 0.11 bc | 1.06 ± 0.18 b | 146.0 ± 1.00 | 213.0 ± 27.15 | 45.89 a |
| HMM + PA (1500 mg/kg) | 158.55 ± 18.40 d | 218.31 ± 58.98 d | 2.01 ± 0.44 b | 0.96 ± 0.32 b | 141.0 ± 1.73 | 208.3 ± 20.81 | 47.75 a |
| Treatment | pH | Viable Cell Count (×106 cells/mL) | Viscosity | Sperm Morphology (%) | Sperm Motility (%) | Sperm Count (×106 cells/mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | Active | Sluggish | Immotile | |||||
| Control | 8.5 ± 0.06 a | 0.85 ± 0.05 a | Slightly viscous | 85 ± 5 a | 15 ± 5 b | 82 ± 4 a | 8 ± 2 b | 10 ± 1 d | 716.7 ± 104.1 a |
| HMM | 8.1 ± 0.03 a | 0.61 ± 0.07 c | Non viscous | 68 ± 7 c | 31 ± 7 a | 58 ± 2 c | 11 ± 2 a | 30 ± 0 a | 266.7 ± 115.5 d |
| HMM + PA (500 mg/kg) | 8.1 ± 0.09 a | 0.70 ± 0.08 a | Non viscous | 73 ± 5 b | 26 ± 5 a | 70 ± 8 b | 11 ± 2 a | 18 ± 7 a | 383.3 ± 76.4 c |
| HMM + PA (1000 mg/kg) | 8.3 ± 0.02 a | 0.78 ± 0.02 b | Non viscous | 75 ± 10 b | 25 ± 10 a | 63 ± 5 c | 10 ± 1 a | 26 ± 5 a | 600.0 ± 173.2 b |
| HMM + PA (1500 mg/kg) | 8.5 ± 0.04 a | 0.85 ± 0.05 a | Non viscous | 83 ± 5 a | 15 ± 5 b | 85 ± 5 a | 8 ± 5 b | 8 ± 2 c | 733.3 ± 57.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozoani, H.A.; Orisakwe, O.E.; Parisi, C.; Assisi, L.; Ezejiofor, A.N.; Okolo, K.O.; Orish, C.N.; Vangone, R.; Sivieri, E.M.; Guerriero, G. Role of Anonychium africanum (Plantae, Fabaceae) in Metal Oxido-Inflammatory Response: Protection Evidence in Gonad of Male Albino Rat. Antioxidants 2024, 13, 1028. https://doi.org/10.3390/antiox13091028
Ozoani HA, Orisakwe OE, Parisi C, Assisi L, Ezejiofor AN, Okolo KO, Orish CN, Vangone R, Sivieri EM, Guerriero G. Role of Anonychium africanum (Plantae, Fabaceae) in Metal Oxido-Inflammatory Response: Protection Evidence in Gonad of Male Albino Rat. Antioxidants. 2024; 13(9):1028. https://doi.org/10.3390/antiox13091028
Chicago/Turabian StyleOzoani, Harrison A., Orish Ebere Orisakwe, Costantino Parisi, Loredana Assisi, Anthonet N. Ezejiofor, Kenneth O. Okolo, Chinna N. Orish, Rubina Vangone, Emidio M. Sivieri, and Giulia Guerriero. 2024. "Role of Anonychium africanum (Plantae, Fabaceae) in Metal Oxido-Inflammatory Response: Protection Evidence in Gonad of Male Albino Rat" Antioxidants 13, no. 9: 1028. https://doi.org/10.3390/antiox13091028
APA StyleOzoani, H. A., Orisakwe, O. E., Parisi, C., Assisi, L., Ezejiofor, A. N., Okolo, K. O., Orish, C. N., Vangone, R., Sivieri, E. M., & Guerriero, G. (2024). Role of Anonychium africanum (Plantae, Fabaceae) in Metal Oxido-Inflammatory Response: Protection Evidence in Gonad of Male Albino Rat. Antioxidants, 13(9), 1028. https://doi.org/10.3390/antiox13091028










