Spermidine Improves Freezing Tolerance by Regulating H2O2 in Brassica napus L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Freezing Treatment
2.3. Determination of H2O2 Concentration
2.4. RNA-Seq
2.5. Quantitative Real-Time PCR Analysis
2.6. Statistics and Analysis
3. Results
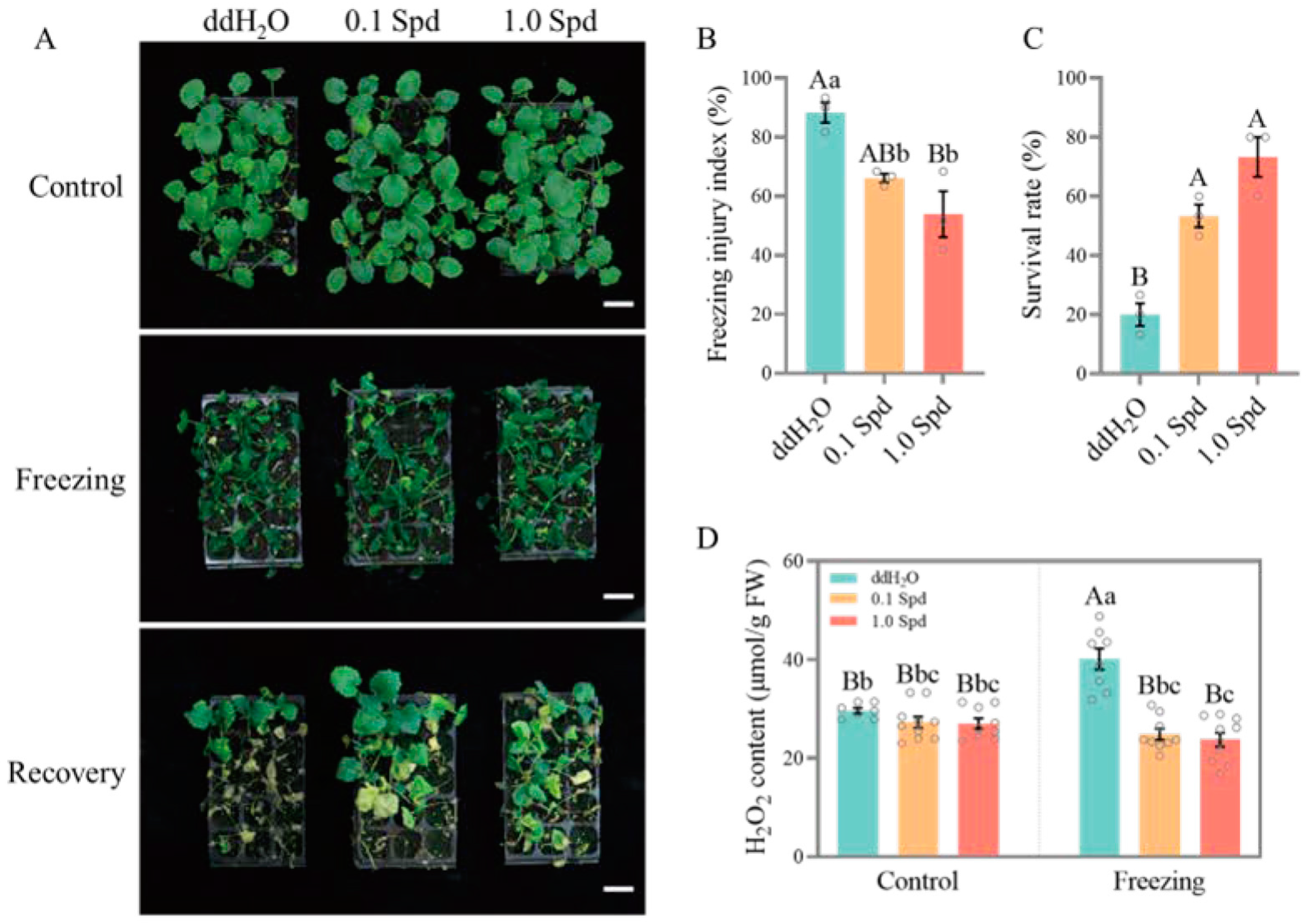
3.1. Two Concentrations of Exogenous Spd Improve the Freezing Tolerance of Seedlings
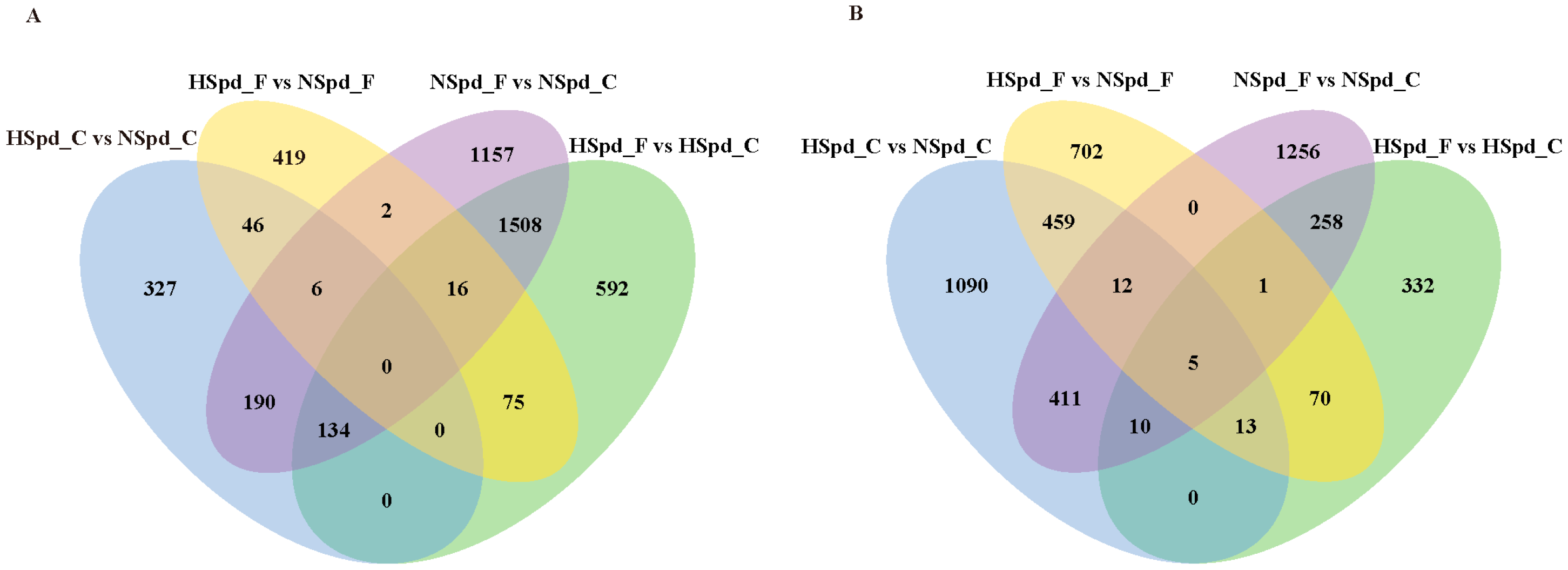
3.2. Transcriptome Sequencing Analysis of the Effect of Spd on Rapeseed Seedlings under Freezing Treatment
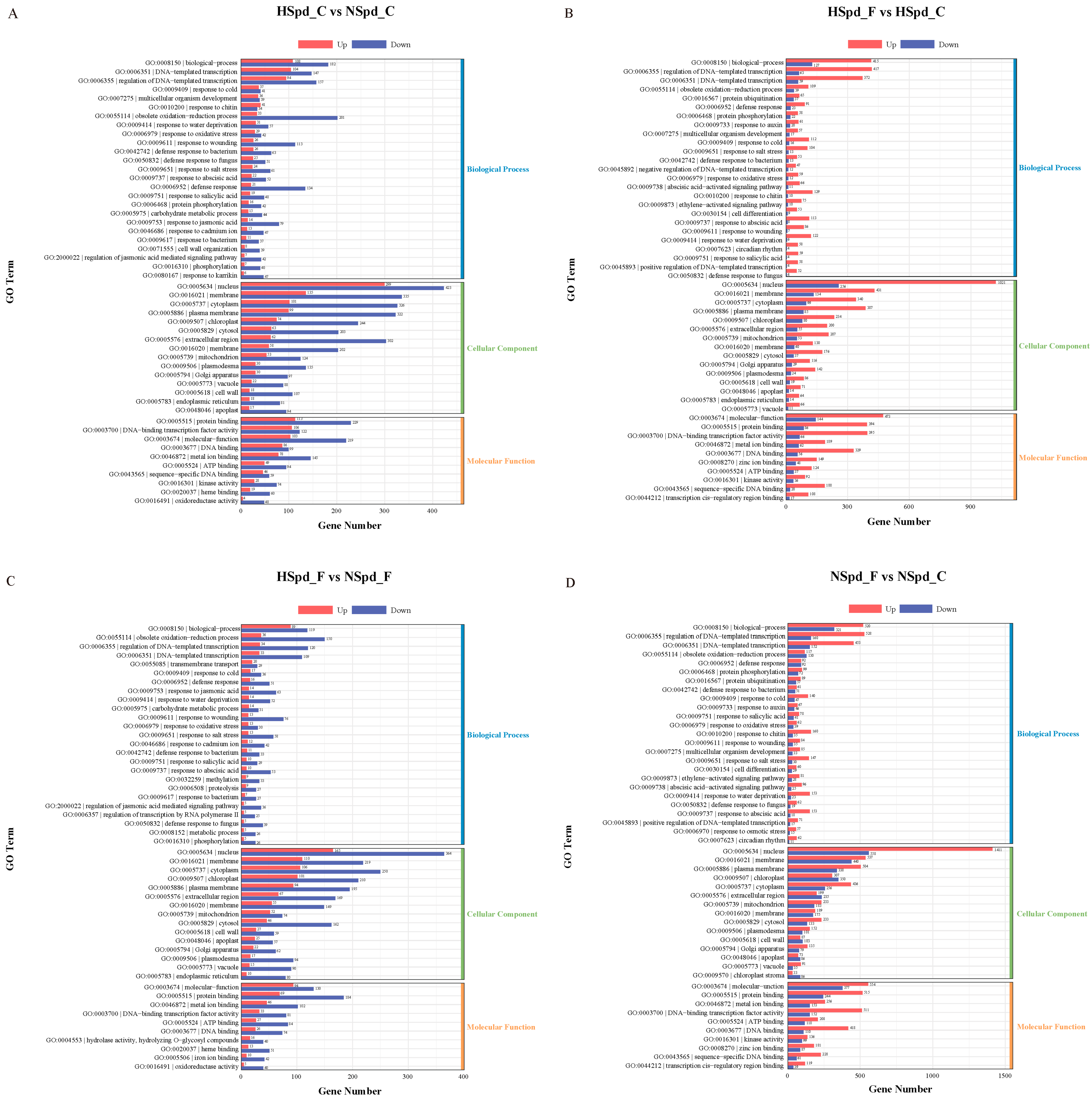
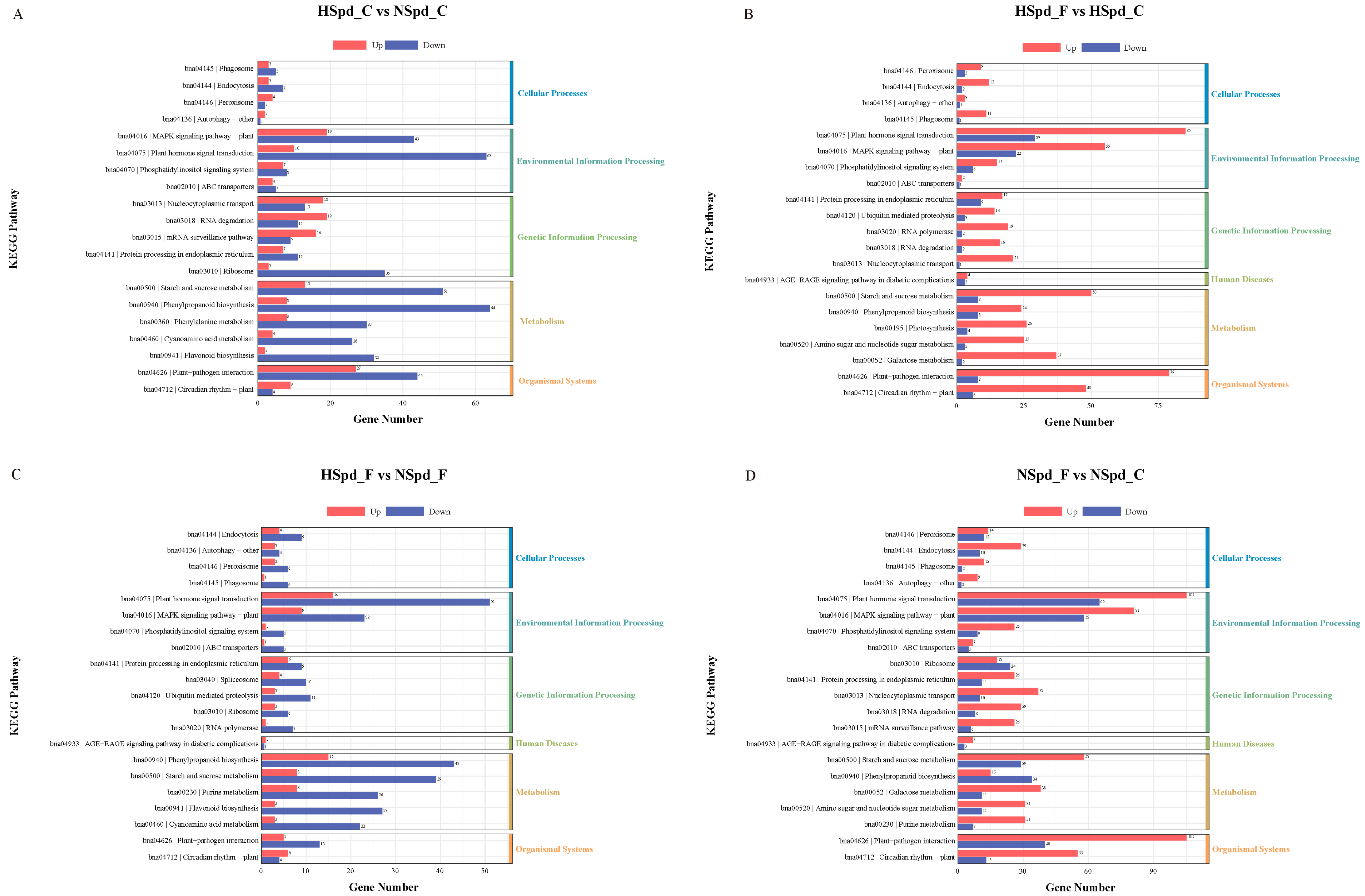
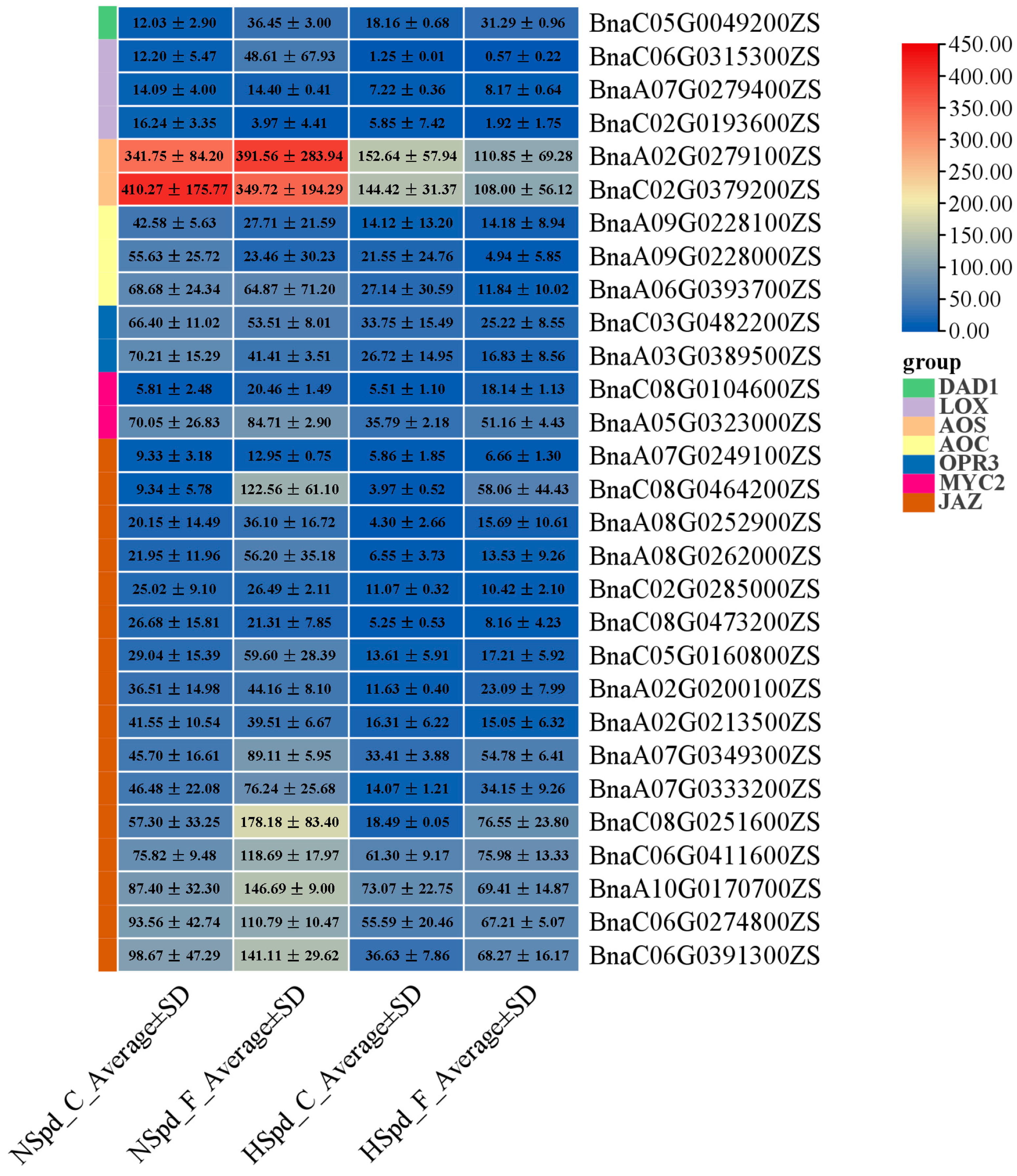
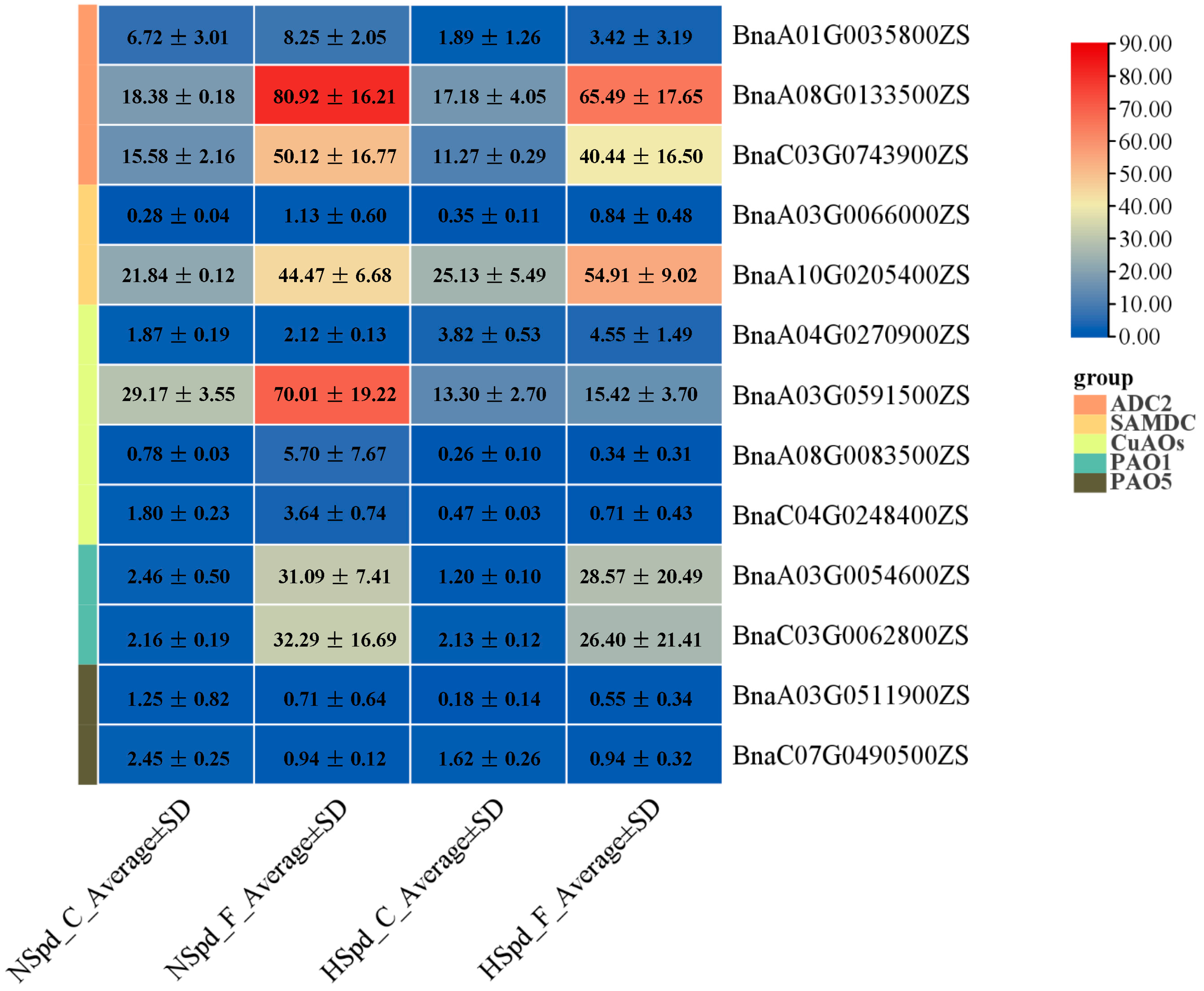
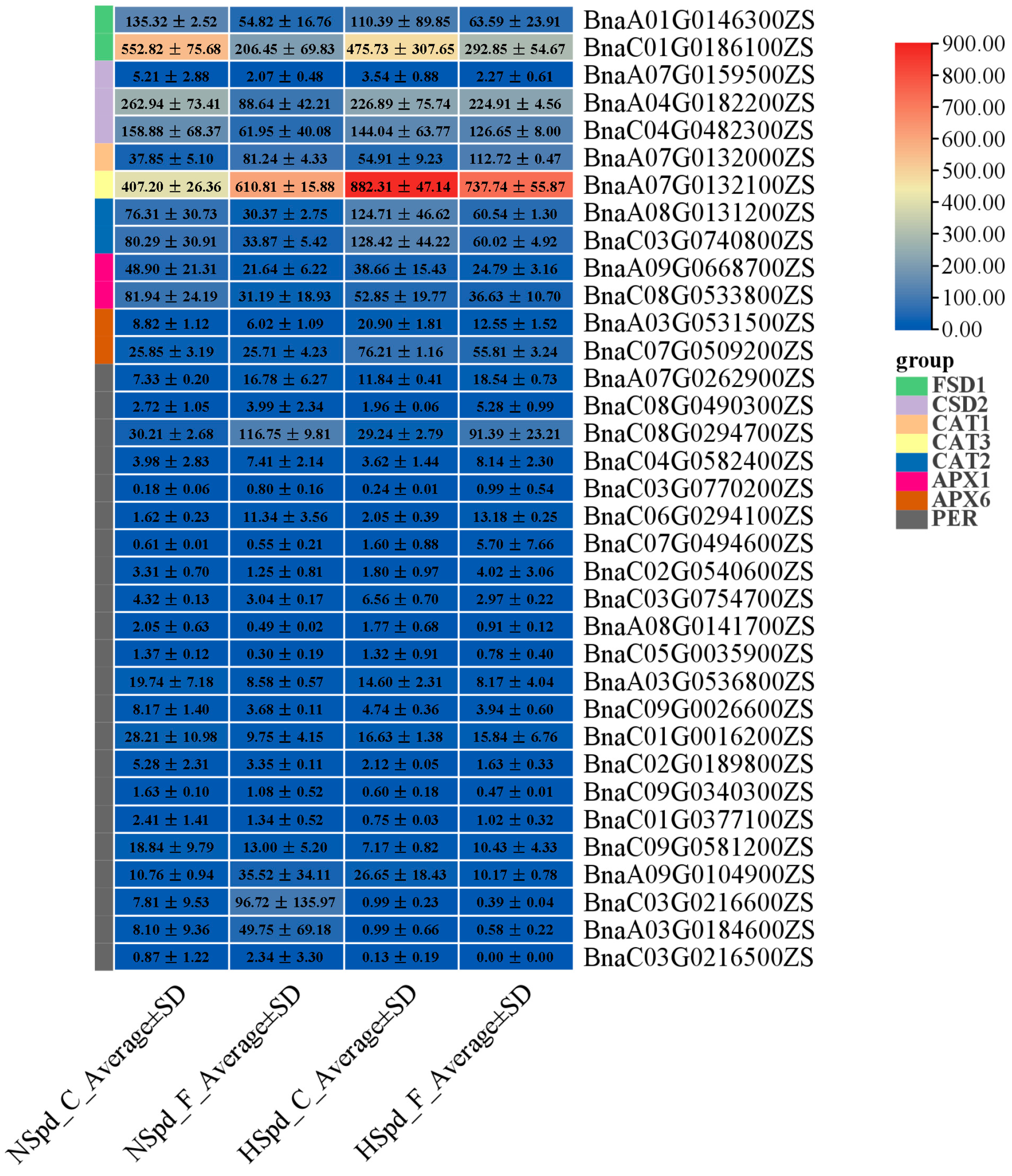
3.3. Gene Ontology Functional and Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analysis
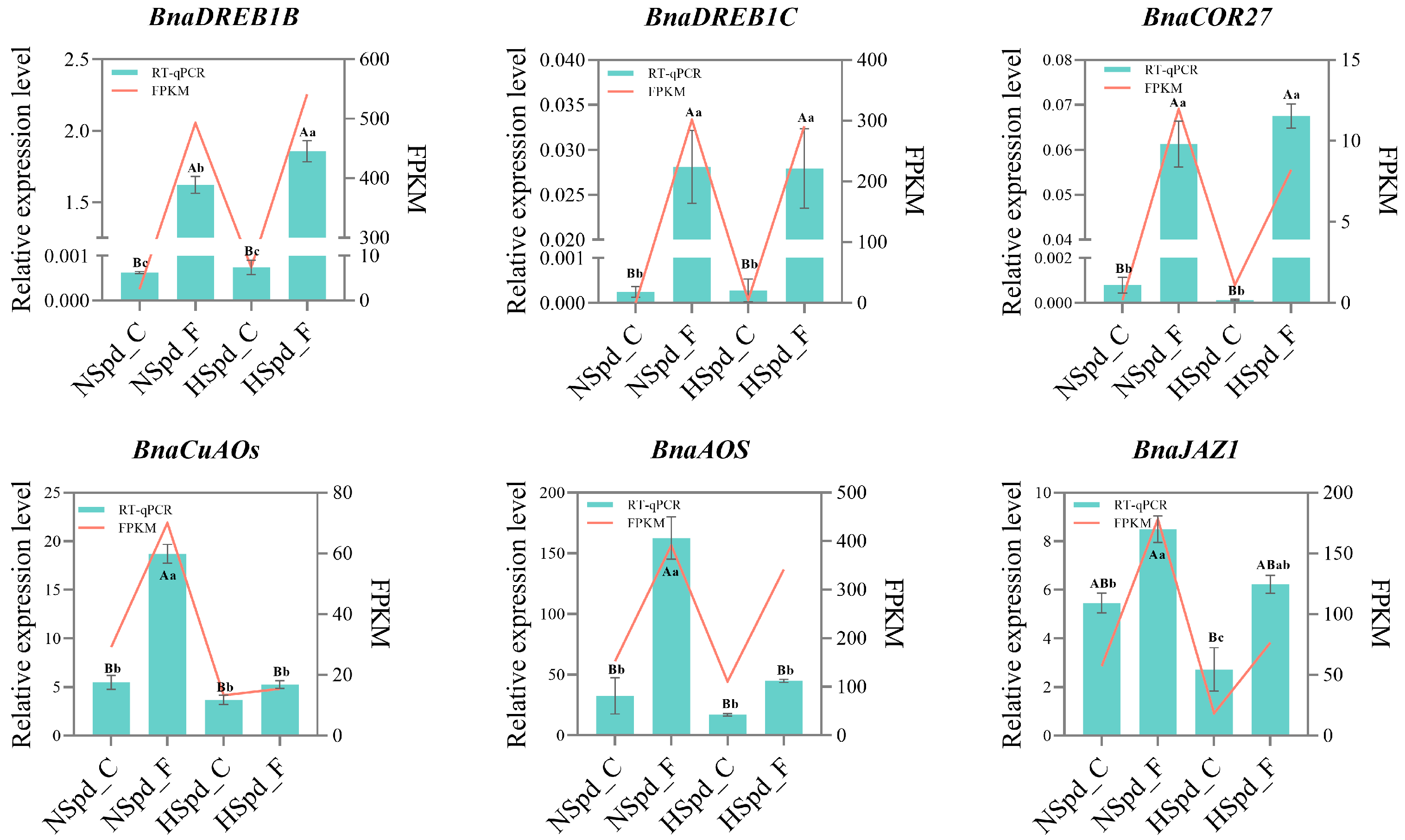
3.4. qRT-PCR Validation of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Primer Name | ZS11 Gene ID | Primer Sequence (5′→3′) |
|---|---|---|
| Darmor Gene ID | ||
| Actin-F | - | GGTTGGGATGGACCAGAAGG |
| Actin-R | BnaC05g34300D | TCAGGAGCAATACGGAGC |
| DREB1C_qF | BnaA03G0486800ZS | CGGCAAATCAGCTTGTCTCAA |
| DREB1C_qR | - | GCAGCCGCCTTCTGAAATCT |
| AZF2_qF | BnaA05G0364000ZS | CAAGACGCAACCGCAAGAAT |
| AZF2_qR | BnaA05g20490D | GTTGTTGAATCGTCGTCGGC |
| COR27_qF | BnaA06G0444700ZS | GCCAAAACGGAAGCTCCAAG |
| COR27_qR | BnaA06g37010D | AGTGGAACAACCTATACGCCA |
| SPE2_qF | BnaA08G0133500ZS | CCGTATCTAGCGACTGAGCC |
| SPE2_qR | BnaA08g10990D | CCACACGACTACCCTTGTCA |
| CML4_qF | BnaA09G0541500ZS | ACCGACGACAAGATCACACC |
| CML4_qR | BnaA09g37780D | GCCGTCTCCGTTCTTGTCA |
| ERF019_qF | BnaC05G0198700ZS | TAGTTTCCAAGACCTCGCCG |
| ERF019_qR | BnaC05g18050D | CGTGATCGTGACCGTCCAAA |
| NAC055_qF | BnaC05G0447000ZS | TACGTTACGTTAACCGGGTCG |
| NAC055_qR | BnaC05g38150D | TACTTCCTGTCCCTTGGGCT |
| DREB1B_qF | BnaC08G0165900ZS | GAGTCTCCGGTAGGTGGTGA |
| DREB1B_qR | - | GTGACGTGTCTCCCGAAACT |
| JAZ1_qF | BnaC08G0251600ZS | CATCGCTCCTACCTCGAACC |
| JAZ1_qR | BnaC08g18640D | TGAGATAGATTACCGCATGCCA |
| AOS-qF | BnaA02G0279100ZS | TTCTCTTCACGCTCGGCTTCAC |
| AOS-qR | BnaA02g23180D | ACGGCTAGATCAGGAGGAGTCTC |
| AOC2-qF | BnaA06G0393700ZS | TTACAGCTTCTACTTCGGCGACTAC |
| AOC2-qR | BnaA06g33410D | GGTGATGGCGAGGAACGAGTC |
| CuAOs_qF | BnaA03G0591500ZS | CTTGATTCGGGTCGGGTCGTTTC |
| CuAOs_qR | - | GCGATACCTGAGAAGCCACGATG |
Appendix B


References
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Fakhari, A.R.; Ghanati, F. Selective regulation of nicotine and polyamines biosynthesis in tobacco cells by enantiomers of ornithine. Chirality 2013, 25, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Taylor, M.; Altabella, T.; Tiburcio, A.F. Recent advances in polyamine research. Trends Plant Sci. 1997, 2, 124–130. [Google Scholar] [CrossRef]
- Mustafavi, S.H.; Naghdi Badi, H.; Sękara, A.; Mehrafarin, A.; Janda, T.; Ghorbanpour, M.; Rafiee, H. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol. Plant. 2018, 40, 1–19. [Google Scholar] [CrossRef]
- de Oliveira, L.F.; Elbl, P.; Navarro, B.V.; Macedo, A.F.; Dos Santos, A.L.; Floh, E.I.; Cooke, J. Elucidation of the polyamine biosynthesis pathway during Brazilian pine (Araucaria angustifolia) seed development. Tree Physiol. 2017, 37, 116–130. [Google Scholar] [CrossRef]
- Blazquez, M.A. Polyamines: Their Role in Plant Development and Stress. Annu. Rev. Plant Biol. 2024, 75, 95–117. [Google Scholar] [CrossRef]
- de Oliveira, L.F.; Navarro, B.V.; Cerruti, G.V.; Elbl, P.; Minocha, R.; Minocha, S.C.; Dos Santos, A.L.W.; Floh, E.I.S. Polyamine- and Amino Acid-Related Metabolism: The Roles of Arginine and Ornithine are Associated with the Embryogenic Potential. Plant Cell Physiol. 2018, 59, 1084–1098. [Google Scholar] [CrossRef]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front. Plant Sci. 2022, 13, 1003155. [Google Scholar] [CrossRef]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Ono, Y.; Kim, D.W.; Watanabe, K.; Sasaki, A.; Niitsu, M.; Berberich, T.; Kusano, T.; Takahashi, Y. Constitutively and highly expressed Oryza sativa polyamine oxidases localize in peroxisomes and catalyze polyamine back conversion. Amino Acids 2012, 42, 867–876. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-Containing Amine Oxidases and FAD-Dependent Polyamine Oxidases Are Key Players in Plant Tissue Differentiation and Organ Development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Yang YunYing, Y.Y.; Yang Xian, Y.X. Effect of temperature on endogenous polyamines content of leaves in Chinese Kale (Brassica alboglabra Bailey) seedlings. J. S. China Agric. Univ. (Nat. Sci. Ed.) 2002, 23, 9–12. [Google Scholar]
- He, L.; Nada, K.; Tachibana, S. Effects of spermidine pretreatment through the roots on growth and photosynthesis of chilled cucumber plants (Cucumis sativus L.). J. Jpn. Soc. Hortic. Sci. 2002, 71, 490–498. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of Polyamines, Abscisic Acid, Nitric Oxide, and Hydrogen Peroxide under Chilling Stress in Tomato (Lycopersicon esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous Spermidine Alleviates Low Temperature Injury in Mung Bean (Vigna radiata L.) Seedlings by Modulating Ascorbate-Glutathione and Glyoxalase Pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Moghym, S. Effect of polyamines on thermotolerance and membrane stability of soybean seedling. Afr. J. Biotechnol. 2011, 10, 9673–9676. [Google Scholar]
- Khorshidi, M.; Hamedi, F. Effect of putrescine on lemon balm under salt stress. Int. J. Agric. Crop Sci. 2014, 7, 601–609. [Google Scholar]
- Zhang, N.; Shi, X.; Guan, Z.; Zhao, S.; Zhang, F.; Chen, S.; Fang, W.; Chen, F. Treatment with spermidine protects chrysanthemum seedlings against salinity stress damage. Plant Physiol. Biochem. 2016, 105, 260–270. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]
- Hussain, S.; Farooq, M.; Wahid, M.A.; Wahid, A. Seed priming with putrescine improves the drought resistance of maize hybrids. Int. J. Agric. Biol. 2013, 15, 1349–1353. [Google Scholar]
- Liu, Y.; Xu, H.; Wen, X.-X.; Liao, Y.-C. Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J. Integr. Agric. 2016, 15, 2759–2774. [Google Scholar] [CrossRef]
- Sadeghipour, O. Polyamines protect mung bean [Vigna radiata (L.) Wilczek] plants against drought stress. Biol. Futur. 2019, 70, 71–78. [Google Scholar] [CrossRef]
- Moschou, P.N.; Roubelakis-Angelakis, K.A. Polyamines and programmed cell death. J. Exp. Bot. 2014, 65, 1285–1296. [Google Scholar] [CrossRef]
- Mehta, R.A.; Cassol, T.; Li, N.; Ali, N.; Handa, A.K.; Mattoo, A.K. Engineered polyamine accumulation in tomatoenhances phytonutrient content, juice quality, and vine life. Nat. Biotechnol. 2002, 20, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Banik, N.; Bhattacharjee, S. Redox-regulation of germination during imbibitional oxidative and chilling stress in an indica rice cultivar (Oryza sativa L., Cultivar Ratna). Physiol. Mol. Biol. Plants 2019, 25, 649–665. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Sun, X.; Han, L. Effects of spermidine and spermine on phytohormones content and polyamine metabolism of zoysiagrass under low temperature condition. Acta Agrestia Sin. 2015, 23, 804. [Google Scholar]
- Diao, Q.; Song, Y.; Qi, H. Exogenous spermidine enhances chilling tolerance of tomato (Solanum lycopersicum L.) seedlings via involvement in polyamines metabolism and physiological parameter levels. Acta Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, S.; Liu, S.; Chen, J.; Ma, H.; Cui, Z.; Zhang, X.; Ge, C.; Liu, R.; Li, Y.; et al. Comparative Transcriptome Analysis Provides Insights into the Seed Germination in Cotton in Response to Chilling Stress. Int. J. Mol. Sci. 2020, 21, 2067. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, G.; Xu, H.; Liu, J.; Luo, J.; Shen, Y. Exogenous Spermidine Improves Chilling Tolerance in Sweet Corn Seedlings by Regulation on Abscisic Acid, ROS and Ca2+ Pathways. J. Plant Biol. 2021, 64, 487–499. [Google Scholar] [CrossRef]
- Marco, F.; Alcazar, R.; Tiburcio, A.F.; Carrasco, P. Interactions between polyamines and abiotic stress pathway responses unraveled by transcriptome analysis of polyamine overproducers. OMICS 2011, 15, 775–781. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.; Peng, Y.; Merewitz, E.; Ma, X.; Huang, L.; Yan, Y. The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ. Exp. Bot. 2016, 124, 22–38. [Google Scholar] [CrossRef]
- Cuevas, J.C.; Lopez-Cobollo, R.; Alcazar, R.; Zarza, X.; Koncz, C.; Altabella, T.; Salinas, J.; Tiburcio, A.F.; Ferrando, A. Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol. 2008, 148, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Bing, Y.U.; Chen, M.D.; Wang, Y.G. Advances of Plant Trihelix Transcription Factor Family Interacting with Environmental Factors. J. Plant Genet. Resour. 2019, 20, 1134–1140. [Google Scholar]
- Wang, Y.; Gong, X.; Liu, W.; Kong, L.; Si, X.; Guo, S.; Sun, J. Gibberellin mediates spermidine-induced salt tolerance and the expression of GT-3b in cucumber. Plant Physiol. Biochem. 2020, 152, 147–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahammed, G.J.; Cao, K.; Shah Jahan, M.; Shu, S.; Wang, J.; et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890. [Google Scholar] [CrossRef]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Asija, S.; Seth, T.; Umar, S.; Gupta, R. Polyamines and Their Crosstalk with Phytohormones in the Regulation of Plant Defense Responses. J. Plant Growth Regul. 2022, 42, 5224–5246. [Google Scholar] [CrossRef]
- Wafaa, M.; Haggag, Y.S.M.; Eman, M.F. Signaling Necessities and Function of Polyamines/Jasmonate Dependent Induced Resistance in Sugar Beet Against Beet Mosaic Virus (BtMV) Infection. N. Y. Sci. J. 2010, 3, 95–103. [Google Scholar]
- Haggag, W.M.; Abd-El-Kareem, F. Methyl jasmonate stimulates polyamines biosynthesisand resistance against leaf rust in wheat plants. Arch. Phytopathol. Plant Protect. 2009, 42, 16–31. [Google Scholar] [CrossRef]
- Zhang, C.; Atanasov, K.E.; Murillo, E.; Vives-Peris, V.; Zhao, J.; Deng, C.; Gomez-Cadenas, A.; Alcazar, R. Spermine deficiency shifts the balance between jasmonic acid and salicylic acid-mediated defence responses in Arabidopsis. Plant Cell Environ. 2023, 46, 3949–3970. [Google Scholar] [CrossRef]
- Stefania, B.; Sonia, S.; Francesca, C.; Maddalena Altamura, M.; Patrizia, T. Methyl jasmonate upregulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J. Exp. Bot. 2001, 52, 231–242. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Y.; Kang, Y.; Liu, W.; Wang, W.; Wang, Z.; Xia, X.; Chen, X.; Wang, C.; He, X. Spermidine Improves Freezing Tolerance by Regulating H2O2 in Brassica napus L. Antioxidants 2024, 13, 1032. https://doi.org/10.3390/antiox13091032
Li S, Liu Y, Kang Y, Liu W, Wang W, Wang Z, Xia X, Chen X, Wang C, He X. Spermidine Improves Freezing Tolerance by Regulating H2O2 in Brassica napus L. Antioxidants. 2024; 13(9):1032. https://doi.org/10.3390/antiox13091032
Chicago/Turabian StyleLi, Shun, Yan Liu, Yu Kang, Wei Liu, Weiping Wang, Zhonghua Wang, Xiaoyan Xia, Xiaoyu Chen, Chen Wang, and Xin He. 2024. "Spermidine Improves Freezing Tolerance by Regulating H2O2 in Brassica napus L." Antioxidants 13, no. 9: 1032. https://doi.org/10.3390/antiox13091032





