Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases: A Systematic Review
Abstract
:1. Introduction
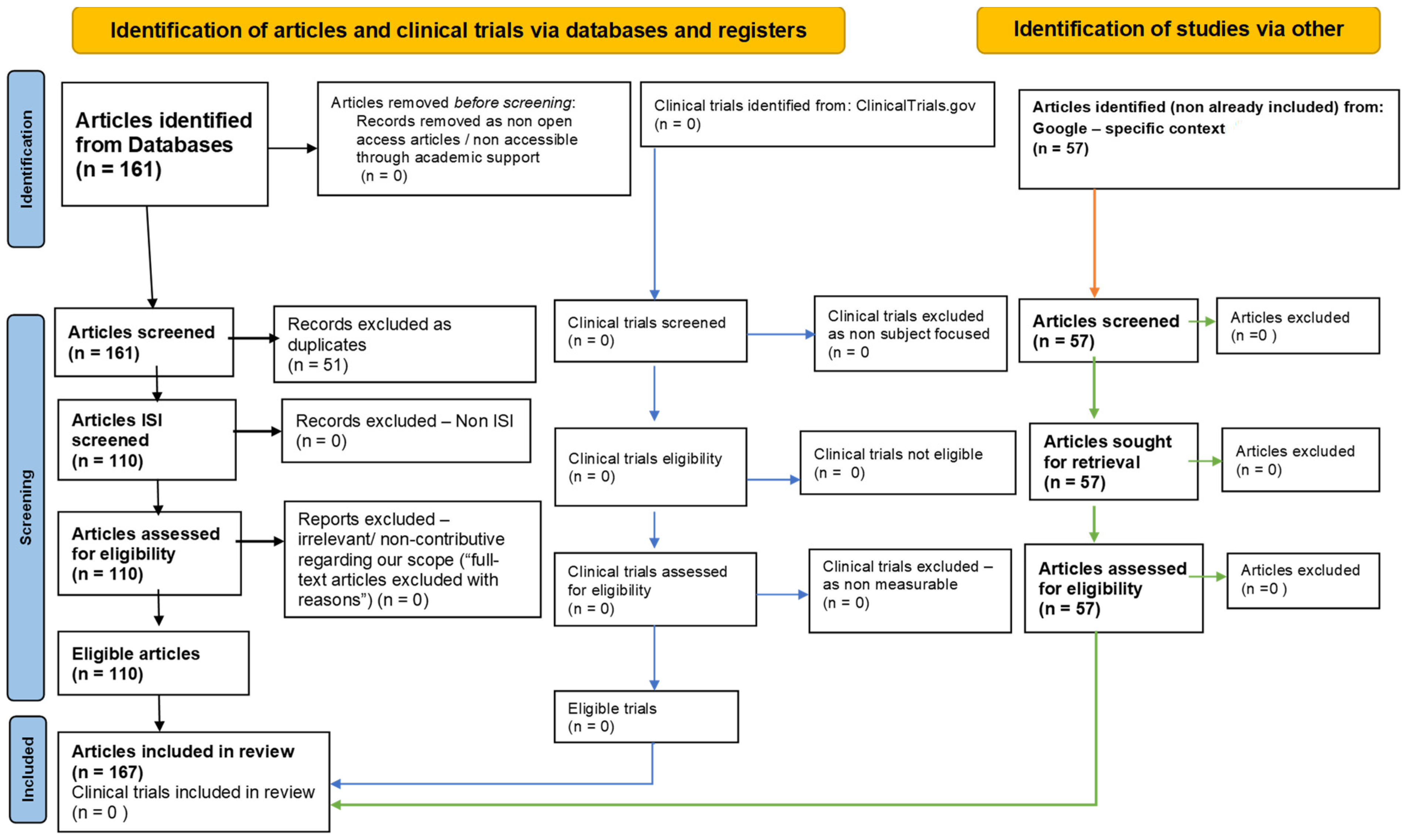
2. Methodology: PRISMA-Type Systematic Review Strategy
- Narrative Synthesis: this part focused on the roles of oxidative stress, gut microbiota, and epigenetic modifications in neurodegenerative diseases.
- Quantitative Synthesis: this part focused on the microorganism’s composition of gut microbiota.
3. Oxidative Stress and Redox Homeostasis in Neurodegenerative Diseases
4. Gut Microbiota and Neurodegenerative Diseases
5. Therapeutic Strategies Targeting Gut Microbiota
| Microorganism Phylum | Relative Abundance (%) in Healthy Individuals | Role in Gut Microbiota | Changes in Neurodegenerative Diseases | Microorganism Species | Number of Mentions in the Selected Articles | Refs. no. |
|---|---|---|---|---|---|---|
| Bacteroidetes | 30 | Metabolism of complex molecules, production of SCFAs (anti-inflammatory properties) | Decrease in abundance, linked to reduced anti-inflammatory properties | Bacteroides | 6 | [104,118,127,129] |
| Prevotella | 4 | [104,129] | ||||
| Firmicutes | 50 | The phylum Firmicutes is one of the major bacterial groups present in the human gut microbiota. Fermentation of dietary fibers, production of SCFAs (gut barrier integrity, immune response modulation) | Variable, but often altered ratio with Bacteroidetes, linked to various diseases | Firmicutes | 6 | [104,118,127] |
| Lactobacillus | 158 | [104,112,117,118,121,125,127] | ||||
| Clostridium | 5 | [104,117,127] | ||||
| Bacillus | 1 | [118] | ||||
| Enterococcus | 2 | [118] | ||||
| Streptococcus | 12 | [118] | ||||
| Ruminococcus | 4 | [118] | ||||
| Eubacterium | 4 | [104,118,129] | ||||
| Proteobacteria | 10 | Includes many pathogenic bacteria, can contribute to inflammation and oxidative stress | Increase in abundance, associated with heightened inflammation and oxidative stress | Escherichia | 6 | [104,118] |
| Helicobacter | 1 | [104] | ||||
| Actinobacteria | 5 | Includes beneficial bacteria such as Bifidobacterium (gut health maintenance, vitamin production) | Decrease in abundance, linked to increased gut permeability and systemic inflammation | Bifidobacterium | 59 | [104,112,118,127] |
| Verrucomicrobia | 2 | Includes Akkermansia muciniphila (mucin degradation, improved metabolic health, reduced inflammation) | Decrease in abundance, related to metabolic health and inflammation | Akkermansia muciniphila | 4 | [104,127] |
6. Hormesis, Nutrients (Polyphenols and Probiotics) in Gut–Brain Axis Disorders
7. Epigenetic Modifications and Oxidative Stress in Neurodegenerative Diseases
8. The Interplay between Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases
9. Limitations of the Study
10. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef]
- Aurelian, S.; Ciobanu, A.; Cărare, R.; Stoica, S.I.; Anghelescu, A.; Ciobanu, V.; Onose, G.; Munteanu, C.; Popescu, C.; Andone, I.; et al. Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer’s Disease-A Systematic Review. Int. J. Mol. Sci. 2023, 24, 16533. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Iordan, D.A.; Hoteteu, M.; Popescu, C.; Postoiu, R.; Onu, I.; Onose, G. Mechanistic Intimate Insights into the Role of Hydrogen Sulfide in Alzheimer’s Disease: A Recent Systematic Review. Int. J. Mol. Sci. 2023, 24, 15481. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Muleiro Alvarez, M.; Cano-Herrera, G.; Osorio Martínez, M.F.; Vega Gonzales-Portillo, J.; Monroy, G.R.; Murguiondo Pérez, R.; Torres-Ríos, J.A.; van Tienhoven, X.A.; Garibaldi Bernot, E.M.; Esparza Salazar, F.; et al. A Comprehensive Approach to Parkinson’s Disease: Addressing Its Molecular, Clinical, and Therapeutic Aspects. Int. J. Mol. Sci. 2024, 25, 7183. [Google Scholar] [CrossRef]
- Gökçal, E.; Gür, V.E.; Selvitop, R.; Babacan Yildiz, G.; Asil, T. Motor and Non-Motor Symptoms in Parkinson’s Disease: Effects on Quality of Life. Noro Psikiyatr. Ars. 2017, 54, 143–148. [Google Scholar] [CrossRef]
- Kobylecki, C. Update on the diagnosis and management of Parkinson’s disease. Clin. Med. 2020, 20, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, A.; Onose, G.; Popescu, C.; Băilă, M.; Stoica, S.I.; Postoiu, R.; Brumă, E.; Petcu, I.R.; Ciobanu, V.; Munteanu, C. Parkinson’s Disease and SARS-CoV-2 Infection: Particularities of Molecular and Cellular Mechanisms Regarding Pathogenesis and Treatment. Biomedicines 2022, 10, 1000. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Munteanu, C.; Turnea, M.A.; Rotariu, M. Hydrogen Sulfide: An Emerging Regulator of Oxidative Stress and Cellular Homeostasis-A Comprehensive One-Year Review. Antioxidants 2023, 12, 1737. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Gorji, A. Neuroinflammation: The Pathogenic Mechanism of Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 5744. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Mark, L.P.; Prost, R.W.; Ulmer, J.L.; Smith, M.M.; Daniels, D.L.; Strottmann, J.M.; Douglas Brown, W.; Hacein-Bey, L. Pictorial review of glutamate excitotoxicity: Fundamental concepts for neuroimaging. Am. J. Neuroradiol. 2001, 22, 1813–1824. [Google Scholar]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, M.Z.; Yang, Z.Y.; Jin, W.L. Microglia in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 270–280. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Ding, Z.B.; Song, L.J.; Wang, Q.; Kumar, G.; Yan, Y.Q.; Ma, C.G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Jackson, W.S.; Bauer, S.; Kaczmarczyk, L.; Magadi, S.S. Selective Vulnerability to Neurodegenerative Disease: Insights from Cell Type-Specific Translatome Studies. Biology 2024, 13, 67. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Soto, C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 2011, 22, 482–487. [Google Scholar] [CrossRef]
- Kocahan, S.; Doğan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1. [Google Scholar] [CrossRef]
- Xu, L.; Pu, J. Alpha-Synuclein in Parkinson’s Disease: From Pathogenetic Dysfunction to Potential Clinical Application. Park. Dis. 2016, 2016, 1720621. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2020, 8, 86–132. [Google Scholar] [CrossRef]
- Rocha, E.; Chamoli, M.; Chinta, S.J.; Andersen, J.K.; Wallis, R.; Bezard, E.; Goldberg, M.; Greenamyre, T.; Hirst, W.; Kuan, W.L.; et al. Aging, Parkinson’s Disease, and Models: What Are the Challenges? Aging Biol. 2023, 1, e20230010. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Andhale, R.; Shrivastava, D. Huntington’s Disease: A Clinical Review. Cureus 2022, 14, e28484. [Google Scholar] [CrossRef]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Stansberry, W.M.; Pierchala, B.A. Neurotrophic factors in the physiology of motor neurons and their role in the pathobiology and therapeutic approach to amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2023, 16, 1238453. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Mortada, I.; Farah, R.; Nabha, S.; Ojcius, D.M.; Fares, Y.; Almawi, W.Y.; Sadier, N.S. Immunotherapies for Neurodegenerative Diseases. Front. Neurol. 2021, 12, 654739. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves third anti-amyloid antibody for Alzheimer disease. Nat. Rev. Drug Discov. 2024, 23, 571. [Google Scholar] [CrossRef]
- Murakami, H.; Shiraishi, T.; Umehara, T.; Omoto, S.; Iguchi, Y. Recent Advances in Drug Therapy for Parkinson’s Disease. Intern. Med. 2023, 62, 33–42. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef]
- Chang, E.; Ghosh, N.; Yanni, D.; Lee, S.; Alexandru, D.; Mozaffar, T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit. Rev. Phys. Rehabil. Med. 2013, 25, 11–22. [Google Scholar] [CrossRef]
- Anderson, K.E.; van Duijn, E.; Craufurd, D.; Drazinic, C.; Edmondson, M.; Goodman, N.; van Kammen, D.; Loy, C.; Priller, J.; Goodman, L.V. Clinical Management of Neuropsychiatric Symptoms of Huntington Disease: Expert-Based Consensus Guidelines on Agitation, Anxiety, Apathy, Psychosis and Sleep Disorders. J. Huntingt. Dis. 2018, 7, 355–366. [Google Scholar] [CrossRef]
- Rosenblatt, A. Neuropsychiatry of Huntington’s disease. Dialogues Clin. Neurosci. 2007, 9, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: Challenges and advances in diagnosis and treatment. Front. Neurosci. 2023, 17, 1263771. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, B.; Maurya, N.; Lee, S.D.; Bharath Kumar, V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Models Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [Google Scholar] [CrossRef]
- Valera, E.; Spencer, B.; Masliah, E. Immunotherapeutic Approaches Targeting Amyloid-β, α-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders. Neurotherapeutics 2016, 13, 179–189. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Philip Mani, A.; Balasubramanian, B.; Mali, L.A.; Joseph, K.S.; Meyyazhagan, A.; Pappuswamy, M.; Joseph, B.V. The Role of the Gut Microbiota in Neurodegenerative Diseases. Microbiol. Res. 2024, 15, 489–507. [Google Scholar] [CrossRef]
- Warren, A.; Nyavor, Y.; Zarabian, N.; Mahoney, A.; Frame, L.A. The microbiota-gut-brain-immune interface in the pathogenesis of neuroinflammatory diseases: A narrative review of the emerging literature. Front. Immunol. 2024, 15, 1365673. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Li, L.; Zhong, C.; Zhang, Y.; Yang, X.; Li, M.; Yang, C. The role of gut microbiota in intestinal disease: From an oxidative stress perspective. Front. Microbiol. 2024, 15, 1328324. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Coppedè, F. The epigenetics of neurodegenerative diseases. In Epigenetics in Human Disease, 3rd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 333–365. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Kim, K.; Yi, S.J. The role of histone modifications: From neurodevelopment to neurodiseases. Signal Transduct. Target. Ther. 2022, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; He, W.; Liou, Y.C. The redox language in neurodegenerative diseases: Oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 2021, 12, 58. [Google Scholar] [CrossRef]
- Glover, S.; Hill, C.; McGuinness, B.; McKnight, A.J.; Hunter, R.F. Exploring the epigenome to identify biological links between the urban environment and neurodegenerative disease: An evidence review. Cities Health, 2024; advance online publication. [Google Scholar] [CrossRef]
- Vitorakis, N.; Piperi, C. Pivotal role of AGE-RAGE axis in brain aging with current interventions. Ageing Res. Rev. 2024, 100, 102429. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef]
- Chui, Z.S.W.; Chan, L.M.L.; Zhang, E.W.H.; Liang, S.; Choi, E.P.H.; Lok, K.Y.W.; Tun, H.M.; Kwok, J.Y.Y. Effects of microbiome-based interventions on neurodegenerative diseases: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 9558. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef]
- Patel, T.A.; Kevadiya, B.D.; Bajwa, N.; Singh, P.A.; Zheng, H.; Kirabo, A.; Li, Y.-L.; Patel, K.P. Role of Nanoparticle-Conjugates and Nanotheranostics in Abrogating Oxidative Stress and Ameliorating Neuroinflammation. Antioxidants 2023, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Zilberter, Y.; Tabuena, D.R.; Zilberter, M. NOX-induced oxidative stress is a primary trigger of major neurodegenerative disorders. Prog. Neurobiol. 2023, 231, 102539. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Mei, S.; Wang, X.; Hu, G.; Lu, M. Focusing on mitochondria in the brain: From biology to therapeutics. Transl. Neurodegener. 2024, 13, 23. [Google Scholar] [CrossRef]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Lv, L.; Long, Z.; Tan, X.; Qin, L.; Yan, W.; Zhang, H.; Ren, F.; Wang, C. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between oxidative stress and Parkinson’s disease. Front. Aging Neurosci. 2024, 16, 1423773. [Google Scholar] [CrossRef]
- Yao, M.F.; Dang, T.; Wang, H.J.; Zhu, X.Z.; Qiao, C. Mitochondrial homeostasis regulation: A promising therapeutic target for Parkinson’s disease. Behav. Brain Res. 2024, 459, 114811. [Google Scholar] [CrossRef]
- Kuntić, M.; Hahad, O.; Münzel, T.; Daiber, A. Crosstalk between Oxidative Stress and Inflammation Caused by Noise and Air Pollution-Implications for Neurodegenerative Diseases. Antioxidants 2024, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Acun, A.D.; Kantar, D. Modulation of oxidative stress and apoptosis by alteration of bioactive lipids in the pancreas, and effect of zinc chelation in a rat model of Alzheimer’s disease. J. Trace Elem. Med. Biol. 2024, 85, 127480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Pham, T.K.; Verber, N.; Turner, M.R.; Malaspina, A.; Collins, M.O.; Mead, R.J.; Shaw, P.J. Glutathione Oxidation in Cerebrospinal Fluid as a Biomarker of Oxidative Stress in Amyotrophic Lateral Sclerosis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Yang, E.J. Protective Effects of a Combined Herbal Medicine against Amyotrophic Lateral Sclerosis-Associated Inflammation and Oxidative Stress. Appl. Sci. 2024, 14, 5386. [Google Scholar] [CrossRef]
- Assoni, A.F.; Guerrero, E.N.; Wardenaar, R.; Oliveira, D.; Bakker, P.L.; Alves, L.M.; Carvalho, V.M.; Okamoto, O.K.; Zatz, M.; Foijer, F. IFNγ protects motor neurons from oxidative stress via enhanced global protein synthesis in FUS-associated amyotrophic lateral sclerosis. Brain Pathol. 2024, 34, e13206. [Google Scholar] [CrossRef] [PubMed]
- Badini, F.; Bayrami, A.; Mirshekar, M.A.; Shahraki, S.; Fanaei, H. Levothyroxine attenuates behavioral impairment and improves oxidative stress and histological alteration 3-nitropropionic acid induced experimental Huntington’s disease in rats. Behav. Brain Res. 2024, 461, 114864. [Google Scholar] [CrossRef] [PubMed]
- Ramos-González, E.; Bitzer-Quintero, O.; Ortiz, G.; Hernández-Cruz, J.; Ramírez-Jirano, L. Relationship between inflammation and oxidative stress and its effect on multiple sclerosis. Neurología 2024, 39, 292–301. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Orfali, R.; Alwatban, A.Z.; Orfali, R.S.; Lau, L.; Chea, N.; Alotaibi, A.M.; Nam, Y.W.; Zhang, M. Oxidative stress and ion channels in neurodegenerative diseases. Front. Physiol. 2024, 15, 1320086. [Google Scholar] [CrossRef]
- Javaid, M.; Arain, F.; Javaid, M.D. Oxidative stress in neurodegenerative diseases. In Fundamental Principles of Oxidative Stress in Metabolism and Reproduction; Academic Press: Cambridge, MA, USA, 2023; pp. 167–183. [Google Scholar] [CrossRef]
- Ekundayo, B.E.; Obafemi, T.O.; Adewale, O.B.; Obafemi, B.A.; Oyinloye, B.E.; Ekundayo, S.K. Oxidative Stress, Endoplasmic Reticulum Stress and Apoptosis in the Pathology of Alzheimer’s Disease. Cell Biochem. Biophys. 2024, 82, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Tsekrekou, M.; Giannakou, M.; Papanikolopoulou, K.; Skretas, G. Protein aggregation and therapeutic strategies in SOD1- and TDP-43- linked ALS. Front. Mol. Biosci. 2024, 11, 1383453. [Google Scholar] [CrossRef] [PubMed]
- Dağcı, Y.; Bilge, N.; Ceylan, M.; Özkan, H.İ. The relationship between melatonin level, oxidative stress, fatigue and sleep disorders in multiple sclerosis patients. Anatol. Curr. Med. J. 2024, 6, 168–174. [Google Scholar] [CrossRef]
- Nadiga, A.P.R.; Suman Krishna, K.L. A novel Zebrafish model of Alzheimer’s disease by Aluminium chloride; involving nitro-oxidative stress, neuroinflammation and cholinergic pathway. Eur. J. Pharmacol. 2024, 965, 176332. [Google Scholar] [CrossRef]
- Akçin, Ş.; Gürsoy, A.E.; Selek, Ş.; Çimen, Y.A.; Köktaşoğlu, F.; Meral, İ.; Üstünova, S. Oxidative Stress, Serum Mineral and Trace Element Levels in Patients with Multiple Sclerosis with or without Restless Legs Syndrome. East. J. Med. 2024, 29, 129–137. [Google Scholar] [CrossRef]
- Bogoje Raspopović, A.; Balta, V.; Vodopić, M.; Drobac, M.; Boroš, A.; Đikić, D.; Demarin, V. The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis. Open Med. 2024, 19, 20240952. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Wang, K.; Chen, X. Protective effect of flavonoids on oxidative stress injury in Alzheimer’s disease. Nat. Prod. Res. 2024, 23, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Chu, C.; Qin, Q.; Shen, H.; Wen, L.; Tang, Y.; Qu, M. Lipid metabolism and oxidative stress in patients with Alzheimer’s disease and amnestic mild cognitive impairment. Brain Pathol. 2024, 34, e13202. [Google Scholar] [CrossRef]
- Guan, Y.; Tang, G.; Li, L.; Shu, J.; Zhao, Y.; Huang, L.; Tang, J. Herbal medicine and gut microbiota: Exploring untapped therapeutic potential in neurodegenerative disease management. Arch. Pharm. Res. 2024, 47, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Watanabe, H.; Dijkstra, J.M.; Nagatsu, T. Parkinson’s Disease: Cells Succumbing to Lifelong Dopamine-Related Oxidative Stress and Other Bioenergetic Challenges. Int. J. Mol. Sci. 2024, 25, 2009. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Hori, R.; Xiao, J.; Khan, M.M. DNA double-strand breaks: A potential therapeutic target for neurodegenerative diseases. Chromosome Res. 2019, 27, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Shadfar, S.; Parakh, S.; Jamali, M.S.; Atkin, J.D. Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 18. [Google Scholar] [CrossRef]
- Ayaz, M.; Mosa, O.F.; Nawaz, A.; Hamdoon, A.A.E.; Elkhalifa, M.E.M.; Sadiq, A.; Ullah, F.; Ahmed, A.; Kabra, A.; Khan, H.; et al. Neuroprotective potentials of Lead phytochemicals against Alzheimer’s disease with focus on oxidative stress-mediated signaling pathways: Pharmacokinetic challenges, target specificity, clinical trials and future perspectives. Phytomedicine 2024, 124, 155272. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Ardianto, C.; Celia, C.; Sidharta, V.M.; Sasmita, P.K.; Satriotomo, I.; Turana, Y. Brain-derived neurotrophic factor interplay with oxidative stress: Neuropathology approach in potential biomarker of Alzheimer’s disease. Dement. Neuropsychol. 2023, 17, e20230012. [Google Scholar] [CrossRef] [PubMed]
- Valvaikar, S.; Vaidya, B.; Sharma, S.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Supplementation of probiotic Bifidobacterium breve Bif11 reverses neurobehavioural deficits, inflammatory changes and oxidative stress in Parkinson’s disease model. Neurochem. Int. 2024, 174, 105691. [Google Scholar] [CrossRef]
- Muresan, S.; Slevin, M. C-reactive Protein: An Inflammatory Biomarker and a Predictor of Neurodegenerative Disease in Patients with Inflammatory Bowel Disease? Cureus 2024, 16, e59009. [Google Scholar] [CrossRef]
- Aburto, M.R.; Cryan, J.F. Gastrointestinal and brain barriers: Unlocking gates of communication across the microbiota-gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 222–247, Erratum in Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 365. [Google Scholar] [CrossRef]
- Sun, L.J.; Li, J.N.; Nie, Y.Z. Gut hormones in microbiota-gut-brain cross-talk. Chin. Med. J. 2020, 133, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, H.Y.; Choi, Y.Y.; Mo, S.J.; Jeon, S.; Ha, J.H.; Park, S.D.; Shim, J.J.; Lee, J.; Chung, B.G. Effect of gut microbiota-derived metabolites and extracellular vesicles on neurodegenerative disease in a gut-brain axis chip. Nano Converg. 2024, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharifa, M.; Ghosh, T.; Daher, O.A.; Bhusal, P.; Alaameri, Y.A.; Naz, J.; Ekhator, C.; Bellegarde, S.B.; Bisharat, P.; Vaghani, V.; et al. Unraveling the Gut-Brain Axis in Multiple Sclerosis: Exploring Dysbiosis, Oxidative Stress, and Therapeutic Insights. Cureus 2023, 15, e47058. [Google Scholar] [CrossRef] [PubMed]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative stress in Alzheimer’s disease: Current knowledge of signaling pathways and therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef]
- Beltrán-Velasco, A.I.; Reiriz, M.; Uceda, S.; Echeverry-Alzate, V. Lactiplantibacillus (Lactobacillus) plantarum as a Complementary Treatment to Improve Symptomatology in Neurodegenerative Disease: A Systematic Review of Open Access Literature. Int. J. Mol. Sci. 2024, 25, 3010. [Google Scholar] [CrossRef]
- Ghosh, S.; Dhungel, S.; Shaikh, M.F.; Sinha, J.K. Editorial: World digestive health day: Investigating the link between neurodegenerative disease and gut microbiota. Front. Aging Neurosci. 2023, 15, 1351855. [Google Scholar] [CrossRef]
- Gahtani, R.M.; Shoaib, S.; Hani, U.; Jayachithra, R.; Alomary, M.N.; Chauhan, W.; Jahan, R.; Tufail, S.; Ansari, M.A. Combating Parkinson’s disease with plant-derived polyphenols: Targeting oxidative stress and neuroinflammation. Neurochem. Int. 2024, 178, 105798. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.O.; Holtzman, D.M. Current understanding of the Alzheimer’s disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Uceda, S.; Echeverry-Alzate, V.; Reiriz-Rojas, M.; Martínez-Miguel, E.; Pérez-Curiel, A.; Gómez-Senent, S.; Beltrán-Velasco, A.I. Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach-A Comprehensive Narrative Review. Int. J. Mol. Sci. 2023, 24, 13294. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 15, 1–16. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Lu, S.; Xu, J.; Liu, X.; Yang, D.; Yang, Y.; Hou, L.; Li, N. A crazy trio in Parkinson’s disease: Metabolism alteration, α-synuclein aggregation, and oxidative stress. Mol. Cell. Biochem. 2024. [Google Scholar] [CrossRef]
- Castelli, V.; d’Angelo, M.; Quintiliani, M.; Benedetti, E.; Cifone, M.G.; Cimini, A. The emerging role of probiotics in neurodegenerative diseases: New hope for Parkinson’s disease? Neural Regen. Res. 2021, 16, 628–634. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Huang, Y.Y.; Tsai, S.Y.; Kuo, Y.W.; Lin, J.H.; Ho, H.H.; Chen, J.F.; Hsia, K.C.; Sun, Y. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer’s Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Sanzone, J.; Life, M.; Reiss, D.; May, D.; Hartley, B.; Spiddle, P.; Al-Kirwi, J.; Grigoryan, T.; Costin, J. Uses of Fecal Microbiota Transplantation in Neurodegenerative Disease: A Scoping Review. Cureus 2024, 16, e62265. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation With Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid. Based Integr. Med. 2020, 25, 2515690X20957225. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Xing, D. The Current and Future Perspectives of Postbiotics. Probiotics Antimicrob. Proteins 2023, 15, 1626–1643. [Google Scholar] [CrossRef]
- Jang, J.; Kim, S.R.; Lee, J.E.; Lee, S.; Son, H.J.; Choe, W.; Yoon, K.S.; Kim, S.S.; Yeo, E.J.; Kang, I. Molecular Mechanisms of Neuroprotection by Ketone Bodies and Ketogenic Diet in Cerebral Ischemia and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 25, 124. [Google Scholar] [CrossRef]
- Armeli, F.; Bonucci, A.; Maggi, E.; Pinto, A.; Businaro, R. Mediterranean Diet and Neurodegenerative Diseases: The Neglected Role of Nutrition in the Modulation of the Endocannabinoid System. Biomolecules 2021, 11, 790. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox® Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Milano, L.; Gautam, A.; Caldecott, K.W. DNA damage and transcription stress. Mol. Cell 2024, 84, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.P.; Collins, A.E.; Nelson, M.L.; Chen, H.; Kalisch, B.E. Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease. Curr. Issues Mol. Biol. 2024, 46, 4379–4402. [Google Scholar] [CrossRef]
- Mir, F.A.; Amanullah, A.; Jain, B.P.; Hyderi, Z.; Gautam, A. Neuroepigenetics of ageing and neurodegeneration-associated dementia: An updated review. Ageing Res. Rev. 2023, 91, 102067. [Google Scholar] [CrossRef]
- Dogaru, B.G.; Munteanu, C. The Role of Hydrogen Sulfide (H2S) in Epigenetic Regulation of Neurodegenerative Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12555. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Zhao, A.; Xu, W.; Han, R.; Wei, J.; Yu, Q.; Wang, M.; Li, H.; Li, M.; Chi, G. Role of histone modifications in neurogenesis and neurodegenerative disease development. Ageing Res. Rev. 2024, 98, 102324. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Qu, J.; Liu, G.H. Emerging epigenetic insights into aging mechanisms and interventions. Trends Pharmacol. Sci. 2024, 45, 157–172. [Google Scholar] [CrossRef]
- Parikh, D.; Shah, M. A comprehensive study on epigenetic biomarkers in early detection and prognosis of Alzheimer’s disease. Biomed. Anal. 2024, 1, 138–153. [Google Scholar] [CrossRef]
- Piergiorge, R.M.; Vasconcelos, A.T.R.; Santos-Rebouças, C.B. Understanding the (epi)genetic dysregulation in Parkinson’s disease through an integrative brain competitive endogenous RNA network. Mech. Ageing Dev. 2024, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.G.; Fandy, T.E. DNA methylation inhibitors: Retrospective and perspective view. Adv. Cancer Res. 2021, 152, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Rathod, S.S.S.; Ghoneim, M.M.; Alshehri, S.; Ahmad, J.; Mishra, A.; Alhakamy, N.A. DNA Methylation: A Promising Approach in Management of Alzheimer’s Disease and Other Neurodegenerative Disorders. Biology 2022, 11, 90. [Google Scholar] [CrossRef]
- Fantacuzzi, M.; Amoroso, R.; Carradori, S.; De Filippis, B. Resveratrol-based compounds and neurodegeneration: Recent insight in multitarget therapy. Eur. J. Med. Chem. 2022, 233, 114242. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef]
- Ali, J.; Choe, K.; Park, J.S.; Park, H.Y.; Kang, H.; Park, T.J.; Kim, M.O. The Interplay of Protein Aggregation, Genetics, and Oxidative Stress in Alzheimer’s Disease: Role for Natural Antioxidants and Immunotherapeutics. Antioxidants 2024, 13, 862. [Google Scholar] [CrossRef]
- Lu, L.; Deng, Y.; Xu, R. Current potential therapeutics of amyotrophic lateral sclerosis. Front. Neurol. 2024, 15, 1402962. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.J.; Jiang, X.Y.; Du, S.Y.; Zhang, K.; Chen, Z. miR-107-5p ameliorates neurological damage, oxidative stress, and immune responses in mice with Alzheimer’s disease by suppressing the Toll-like receptor 4 (TLR4)/nuclear factor-kappaB(NF-κB) pathway. Kaohsiung J. Med. Sci. 2024, 40, 119–130. [Google Scholar] [CrossRef]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Ren, D.; Jeon, B.; Liu, H.W. S-Adenosylmethionine: More than just a methyl donor. Natural Product Reports. R. Soc. Chem. 2023, 40, 1521–1549. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2021, 38, 223–244. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- García-Cabrerizo, R.; Carbia, C.; ORiordan, K.J.; Schellekens, H.; Cryan, J.F. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2021, 157, 1495–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Tian, S.; Chen, M. Global research progress of gut microbiota and epigenetics: Bibliometrics and visualized analysis. Front. Immunol. 2024, 15, 1412640. [Google Scholar] [CrossRef]
- Al Theyab, A.; Almutairi, T.; Al-Suwaidi, A.M.; Bendriss, G.; McVeigh, C.; Chaari, A. Epigenetic Effects of Gut Metabolites: Exploring the Path of Dietary Prevention of Type 1 Diabetes. Front. Nutr. 2020, 7, 563605. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: The regulation and intervention. Signal Transduct. Target. Ther. 2021, 6, 245. [Google Scholar] [CrossRef]
- Nagu, P.; Parashar, A.; Behl, T.; Mehta, V. Gut Microbiota Composition and Epigenetic Molecular Changes Connected to the Pathogenesis of Alzheimer’s Disease. J. Mol. Neurosci. 2021, 71, 1436–1455. [Google Scholar] [CrossRef]
- Munteanu, C.; Dogaru, G.; Rotariu, M.; Onose, G. Therapeutic gases used in balneotherapy and rehabilitation medicine-scientific relevance in the last ten years (2011–2020)-Synthetic literature review. Balneo PRM Res. J. 2021, 12, 111–122. [Google Scholar] [CrossRef]
- Munteanu, C.; Călin, M.A.; Manea, D.; Popescu, C.; Iliescu, M.; Ionescu, E.V.; Stanciu, L.; Minea, M.; Oprea, C.; Oprea, D.; et al. Current data regarding homeostasis of tissues oxygenation in pathophysiological and therapeutic circumstances. Balneo PRM Res. J. 2023, 14, 1–30. [Google Scholar] [CrossRef]
- Munteanu, C.; Hoteteu, M.; Munteanu, D.; Onose, G. The effects of Mineral Waters from Slănic Moldova’s Spring 1 and Spring 1 bis on Fibroblast activity: An In Vitro Study. Balneo PRM Res. J. 2023, 14, 591. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Zhang, S.; Qi, C.; Zhang, Z.; Ma, R.; Xiang, L.; Chen, L.; Zhu, Y.; Tang, C.; et al. Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: A multi-omics Mendelian randomization study. BMC Med. 2023, 21, 179. [Google Scholar] [CrossRef] [PubMed]

| Keywords | PubMed | Scopus | Web of Science | Google Scholar | Total |
|---|---|---|---|---|---|
| “Neurodegenerative Disease” + “Oxidative Stress” | 7 | 1 | 0 | 0 | 8 |
| “Alzheimer’s Disease” + “Oxidative Stress” | 13 | 3 | 14 | 22 | 53 |
| “Parkinson’s Disease” + “Oxidative Stress” | 8 | 8 | 9 | 14 | 39 |
| “Huntington’s Disease” + “Oxidative Stress” | 1 | 1 | 1 | 2 | 5 |
| “Amyotrophic Lateral Sclerosis” + “Oxidative Stress” | 4 | 0 | 0 | 3 | 7 |
| “Multiple Sclerosis” + “Oxidative Stress” | 6 | 1 | 5 | 9 | 21 |
| “Gut Microbiota” + “Neurodegenerative Disease” | 2 | 0 | 3 | 3 | 8 |
| “Microbiota” + “Neurodegenerative Disease” | 4 | 0 | 4 | 3 | 11 |
| “Epigenetic” + “Neurodegenerative Disease” | 1 | 1 | 0 | 0 | 2 |
| “DNA Methylation” + “Neurodegenerative Disease” | 0 | 0 | 0 | 0 | 0 |
| “Histone Modification” + “Neurodegenerative Disease” | 0 | 1 | 0 | 0 | 1 |
| “RNA” + “Neurodegenerative Disease” | 2 | 1 | 1 | 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, C.; Galaction, A.I.; Turnea, M.; Blendea, C.D.; Rotariu, M.; Poștaru, M. Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases: A Systematic Review. Antioxidants 2024, 13, 1062. https://doi.org/10.3390/antiox13091062
Munteanu C, Galaction AI, Turnea M, Blendea CD, Rotariu M, Poștaru M. Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases: A Systematic Review. Antioxidants. 2024; 13(9):1062. https://doi.org/10.3390/antiox13091062
Chicago/Turabian StyleMunteanu, Constantin, Anca Irina Galaction, Marius Turnea, Corneliu Dan Blendea, Mariana Rotariu, and Mădălina Poștaru. 2024. "Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases: A Systematic Review" Antioxidants 13, no. 9: 1062. https://doi.org/10.3390/antiox13091062






