Caffeine: The Story beyond Oxygen-Induced Lung and Brain Injury in Neonatal Animal Models—A Narrative Review
Abstract
1. Introduction
1.1. Caffeine in Preterm Infants
1.2. Pharmacology of Caffeine
1.3. Postnatal Oxidative Stress Injury Models for the Immature Lung and Developing Brain
1.3.1. Oxidative Stress in Premature
1.3.2. Postnatal Oxidative Stress Injury Models
1.3.3. Rodent Model for Preterm Brain Development
1.3.4. Rodent Model for Postnatal Pulmonal Maturation
1.4. Objective of This Study
2. Materials and Methods
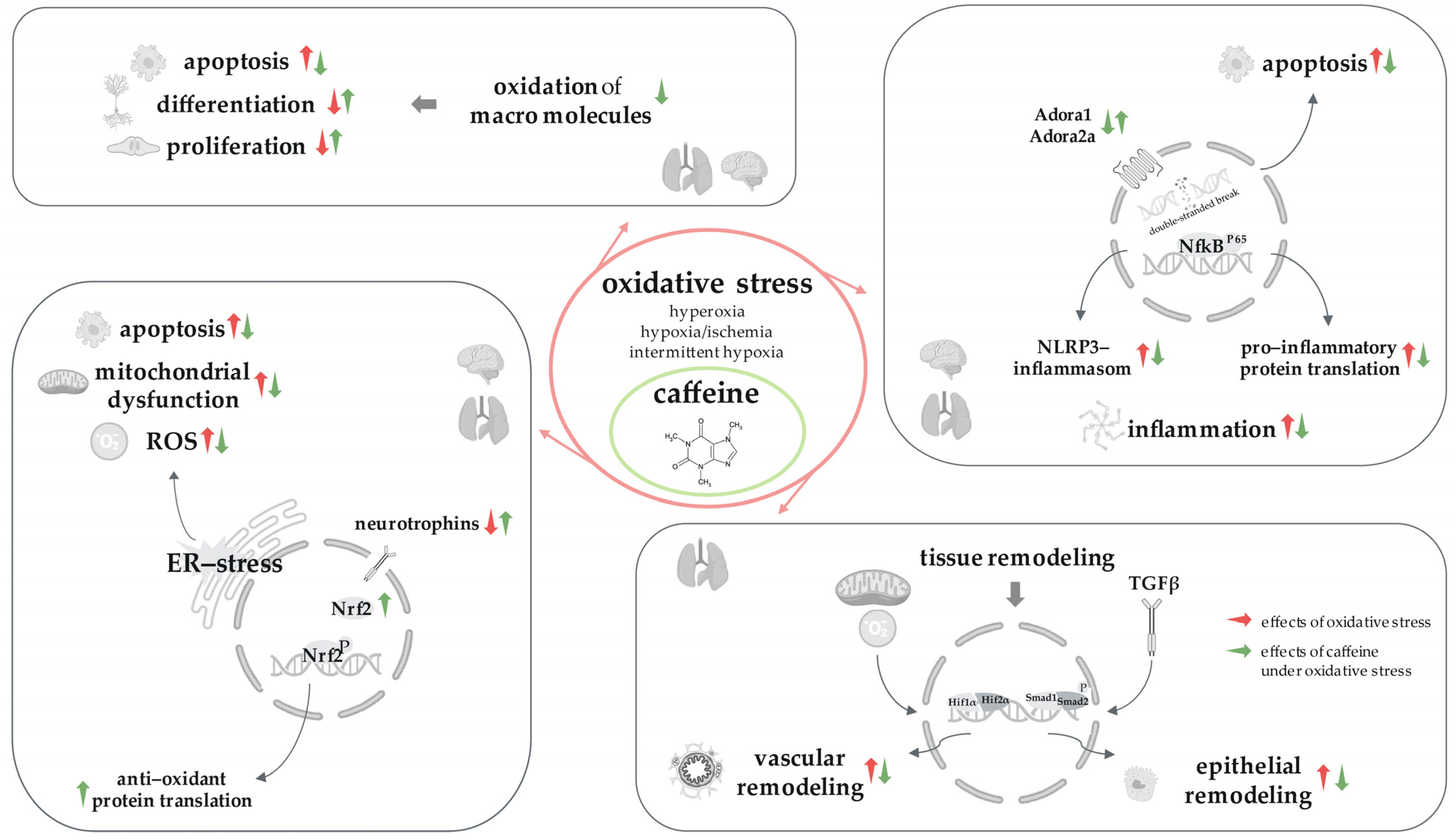
3. Results
3.1. Caffeine and the Hyperoxic-Injury Model
3.1.1. Caffeine Protects the Immature Lungs Affected by Hyperoxia
3.1.2. Caffeine Protects the Developing Brain Affected by Hyperoxia
3.1.3. Caffeine Protects the Lung–Brain Axis Affected by Hyperoxia
3.2. Caffeine and the Hypoxic-Injury Model
Caffeine Protects the Developing Brain Affected by Hypoxia
3.3. Caffeine and the Hypoxic–Ischemic-Injury Model
Caffeine Protects the Developing Brain Affected by Hypoxia–Ischemia
3.4. Caffeine and the Intermittent-Hypoxic Injury Model
3.4.1. Caffeine Protects the Immature Lung Affected by Intermittent Hypoxia
3.4.2. Caffeine Protects the Developing Brain Affected by Intermittent Hypoxia
3.5. Caffeine and the Immature Lung and the Developing Brain
3.5.1. Caffeine in the Immature Lung without Oxidative Stress
3.5.2. Caffeine in the Developing Brain without Oxidative Stress
3.5.3. Caffeine in the Lung–Brain Axis without Oxidative Stress
4. Discussion
4.1. Caffeine, the ‘Gamechanger’ for Oxidative Stress Injury in the Brain–Lung Axis
4.2. Caffeine, Forever and Ever?
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aranda, J.V.; Gorman, W.; Bergsteinsson, H.; Gunn, T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J. Pediatr. 1977, 90, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shaweesh, J.M.; Martin, R.J. Caffeine use in the neonatal intensive care unit. Semin. Fetal Neonatal Med. 2017, 22, 342–347. [Google Scholar] [CrossRef]
- Aranda, J.V.; Beharry, K.D. Pharmacokinetics, pharmacodynamics and metabolism of caffeine in newborns. Semin. Fetal Neonatal Med. 2020, 25, 101183. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Y.; Guo, H.L.; He, X.; Hu, Y.H.; Xia, Y.; Cheng, R.; Ding, X.S.; Chen, F.; Xu, J. Caffeine for the Pharmacological Treatment of Apnea of Prematurity in the NICU: Dose Selection Conundrum, Therapeutic Drug Monitoring and Genetic Factors. Front. Pharmacol. 2021, 12, 681842. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Smart, D.J.; De Paoli, A.G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. 2010, 12, Cd000140. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Davis, P.G. Prophylactic methylxanthines for endotracheal extubation in preterm infants. Cochrane Database Syst. Rev. 2010, 8, CD000139. [Google Scholar] [CrossRef]
- Marques, K.A.; Bruschettini, M.; Roehr, C.C.; Davis, P.G.; Fiander, M.; Soll, R. Methylxanthine for the prevention and treatment of apnea in preterm infants. Cochrane Database Syst. Rev. 2023, 10, CD013830. [Google Scholar] [CrossRef]
- Patel, R.M.; Leong, T.; Carlton, D.P.; Vyas-Read, S. Early caffeine therapy and clinical outcomes in extremely preterm infants. J. Perinatol. 2013, 33, 134–140. [Google Scholar] [CrossRef]
- Doyle, L.W.; Schmidt, B.; Anderson, P.J.; Davis, P.G.; Moddemann, D.; Grunau, R.E.; O’Brien, K.; Sankaran, K.; Herlenius, E.; Roberts, R. Reduction in developmental coordination disorder with neonatal caffeine therapy. J. Pediatr. 2014, 165, 356–359.e2. [Google Scholar] [CrossRef]
- Murner-Lavanchy, I.M.; Doyle, L.W.; Schmidt, B.; Roberts, R.S.; Asztalos, E.V.; Costantini, L.; Davis, P.G.; Dewey, D.; D’Ilario, J.; Grunau, R.E.; et al. Neurobehavioral Outcomes 11 Years After Neonatal Caffeine Therapy for Apnea of Prematurity. Pediatrics 2018, 141, e20174047. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Anderson, P.J.; Doyle, L.W.; Dewey, D.; Grunau, R.E.; Asztalos, E.V.; Davis, P.G.; Tin, W.; Moddemann, D.; Solimano, A.; et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012, 307, 275–282. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Anderson, P.J.; Asztalos, E.V.; Costantini, L.; Davis, P.G.; Dewey, D.; D’Ilario, J.; Doyle, L.W.; Grunau, R.E.; et al. Academic Performance, Motor Function, and Behavior 11 Years After Neonatal Caffeine Citrate Therapy for Apnea of Prematurity: An 11-Year Follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 564–572. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Steer, P. Prophylactic caffeine to prevent postoperative apnea following general anesthesia in preterm infants. Cochrane Database Syst. Rev. 2001, 4, Cd000048. [Google Scholar] [CrossRef]
- Karlinski Vizentin, V.; Madeira de Sá Pacheco, I.; Fahel Vilas Bôas Azevêdo, T.; Florêncio de Mesquita, C.; Alvim Pereira, R. Early versus Late Caffeine Therapy Administration in Preterm Neonates: An Updated Systematic Review and Meta-Analysis. Neonatology 2023, 121, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Entz, R.; Synnes, A.; Creighton, D.; Yusuf, K.; Lapointe, A.; Yang, J.; Shah, P.S. Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics 2019, 143, e20181348. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Davis, P.G.; Roberts, R.S. Timing of Caffeine Therapy in Very Low Birth Weight Infants. J. Pediatr. 2014, 164, 957–958. [Google Scholar] [CrossRef]
- Hoecker, C.; Nelle, M.; Poeschl, J.; Beedgen, B.; Linderkamp, O. Caffeine impairs cerebral and intestinal blood flow velocity in preterm infants. Pediatrics 2002, 109, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.B.; Klimek, J.; Hinder, M.; Ponnampalam, G.; Tracy, S.K. Does caffeine impair cerebral oxygenation and blood flow velocity in preterm infants? Acta Paediatr. 2010, 99, 1319–1323. [Google Scholar] [CrossRef]
- Dix, L.M.L.; van Bel, F.; Baerts, W.; Lemmers, P.M.A. Effects of caffeine on the preterm brain: An observational study. Early Hum. Dev. 2018, 120, 17–20. [Google Scholar] [CrossRef]
- Chavez Valdez, R.; Ahlawat, R.; Wills-Karp, M.; Nathan, A.; Ezell, T.; Gauda, E.B. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. J. Pediatr. 2011, 158, 57–64. [Google Scholar] [CrossRef]
- Vesoulis, Z.A.; McPherson, C.; Neil, J.J.; Mathur, A.M.; Inder, T.E. Early High-Dose Caffeine Increases Seizure Burden in Extremely Preterm Neonates: A Preliminary Study. J. Caffeine Res. 2016, 6, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Parladori, R.; Austin, T.; Smielewski, P.; Czosnyka, M.; Paoletti, V.; Vitali, F.; Corvaglia, L.; Martini, S. Cardiovascular and cerebrovascular effects of caffeine maintenance in preterm infants during the transitional period. Pediatr. Res. 2024; ahead of print. [Google Scholar] [CrossRef]
- Ősz, B.E.; Jîtcă, G.; Ștefănescu, R.E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.E. Caffeine and Its Antioxidant Properties-It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef]
- al-Alaiyan, S.; al-Rawithi, S.; Raines, D.; Yusuf, A.; Legayada, E.; Shoukri, M.M.; el-Yazigi, A. Caffeine metabolism in premature infants. J. Clin. Pharmacol. 2001, 41, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Aklillu, E.; McDonagh, E.M.; Klein, T.E.; Altman, R.B. PharmGKB summary: Caffeine pathway. Pharmacogenet Genom. 2012, 22, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Sun, X.; Hines, R.N.; McCarver, D.G.; Lake, B.G.; Osimitz, T.G.; Creek, M.R.; Clewell, H.J.; Yoon, M. Determination of Human Hepatic CYP2C8 and CYP1A2 Age-Dependent Expression to Support Human Health Risk Assessment for Early Ages. Drug Metab. Dispos. 2017, 45, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.C.; Fernández de Gatta, M.M.; Delgado Iribarnegaray, M.F.; Santos Buelga, D.; García, M.J.; Dominguez-Gil, A.; Lanao, J.M. Population pharmacokinetics of caffeine in premature neonates. Eur. J. Clin. Pharmacol. 1997, 52, 211–217. [Google Scholar] [CrossRef]
- Engbers, A.G.J.; Völler, S.; Poets, C.F.; Knibbe, C.A.J.; Reiss, I.K.M.; Koch, B.C.P.; Flint, R.B.; Simons, S.H.P. The Pharmacokinetics of Caffeine in Preterm Newborns: No Influence of Doxapram but Important Maturation with Age. Neonatology 2021, 118, 106–113. [Google Scholar] [CrossRef]
- Sohn, J.A.; Kim, H.S.; Oh, J.; Cho, J.Y.; Yu, K.S.; Lee, J.; Shin, S.H.; Lee, J.A.; Choi, C.W.; Kim, E.K.; et al. Prediction of serum theophylline concentrations and cytochrome P450 1A2 activity by analyzing urinary metabolites in preterm infants. Br. J. Clin. Pharmacol. 2017, 83, 1279–1286. [Google Scholar] [CrossRef]
- Natarajan, G.; Lulic-Botica, M.; Aranda, J. Pharmacology review: Clinical pharmacology of caffeine in the newborn. NeoReviews 2007, 8, e214–e221. [Google Scholar] [CrossRef]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine therapy in preterm infants. World J. Clin. Pediatr. 2015, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Chen, J.F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Yang, J.; Wang, Y. Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Aspects Med. 2017, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Faudone, G.; Arifi, S.; Merk, D. The Medicinal Chemistry of Caffeine. J. Med. Chem. 2021, 64, 7156–7178. [Google Scholar] [CrossRef]
- Kumral, A.; Tuzun, F.; Yesilirmak, D.C.; Duman, N.; Ozkan, H. Genetic basis of apnoea of prematurity and caffeine treatment response: Role of adenosine receptor polymorphisms. Acta Paediatr. 2012, 101, e299–e303. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S.; Jain, A.C. Antioxidant behaviour of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem. Toxicol. 1991, 29, 1–6. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Strauss, E.; Schlor, A.; Sifringer, M.; Scheuer, T.; Buhrer, C.; Schmitz, T. Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef]
- Endesfelder, S.; Strauß, E.; Scheuer, T.; Schmitz, T.; Bührer, C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir. Res. 2019, 20, 88. [Google Scholar] [CrossRef]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef]
- Rogers, S.; Witz, G.; Anwar, M.; Hiatt, M.; Hegyi, T. Antioxidant capacity and oxygen radical diseases in the preterm newborn. Arch. Pediatr. Adolesc. Med. 2000, 154, 544–548. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bührer, C.; Heller, G.; Thome, U.H. Population-Based Outcome Data of Extremely Preterm Infants in Germany during 2010–2017. Neonatology 2022, 119, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Thome, U.H.; Dreyhaupt, J.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.G.; Zimmermann, A.; et al. Influence of PCO2 Control on Clinical and Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants. Neonatology 2018, 113, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Chou, Y.H. Antioxidant profiles in full term and preterm neonates. Chang. Gung Med. J. 2005, 28, 846–851. [Google Scholar] [PubMed]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sanchez-Illana, A.; Nunez-Ramiro, A.; Kuligowski, J.; Chafer-Pericas, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef]
- Tarnow-Mordi, W.; Stenson, B.; Kirby, A.; Juszczak, E.; Donoghoe, M.; Deshpande, S.; Morley, C.; King, A.; Doyle, L.W.; Fleck, B.W.; et al. Outcomes of Two Trials of Oxygen-Saturation Targets in Preterm Infants. N. Engl. J. Med. 2016, 374, 749–760. [Google Scholar] [CrossRef]
- Obst, S.; Herz, J.; Alejandre Alcazar, M.A.; Endesfelder, S.; Möbius, M.A.; Rüdiger, M.; Felderhoff-Müser, U.; Bendix, I. Perinatal Hyperoxia and Developmental Consequences on the Lung-Brain Axis. Oxid. Med. Cell Longev. 2022, 2022, 5784146. [Google Scholar] [CrossRef]
- Wu, T.-J.; Jing, X.; Teng, M.; Pritchard, K.A.; Day, B.W.; Naylor, S.; Teng, R.-J. Role of Myeloperoxidase, Oxidative Stress, and Inflammation in Bronchopulmonary Dysplasia. Antioxidants 2024, 13, 889. [Google Scholar] [CrossRef]
- Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 2020, 150, 105187. [Google Scholar] [CrossRef]
- Crump, C.; Sundquist, J.; Sundquist, K. Preterm or early term birth and long-term risk of asthma into midadulthood: A national cohort and cosibling study. Thorax 2023, 78, 653–660. [Google Scholar] [CrossRef]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Brumbaugh, J.E.; Bell, E.F.; Grey, S.F.; DeMauro, S.B.; Vohr, B.R.; Harmon, H.M.; Bann, C.M.; Rysavy, M.A.; Logan, J.W.; Colaizy, T.T.; et al. Behavior Profiles at 2 Years for Children Born Extremely Preterm with Bronchopulmonary Dysplasia. J. Pediatr. 2020, 219, 152–159.e5. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.; Hadchouel, A.; Torchin, H.; Rozé, J.C.; Arnaud, C.; Bellino, A.; Couderc, L.; Marret, S.; Mittaine, M.; Pinquier, D.; et al. Educational and health outcomes associated with bronchopulmonary dysplasia in 15-year-olds born preterm. PLoS ONE 2019, 14, e0222286. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Raj, J.U. Regulation of the pulmonary circulation in the fetus and newborn. Physiol. Rev. 2010, 90, 1291–1335. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Vento, M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir. Physiol. Neurobiol. 2019, 266, 121–129. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; MacFarlane, P.M.; Martin, R.J. Intermittent Hypoxemia in Preterm Infants. Clin. Perinatol. 2019, 46, 553–565. [Google Scholar] [CrossRef]
- Di Fiore, J.M.; Raffay, T.M. The relationship between intermittent hypoxemia events and neural outcomes in neonates. Exp. Neurol. 2021, 342, 113753. [Google Scholar] [CrossRef]
- Vlassaks, E.; Gavilanes, A.W.; Vles, J.S.; Deville, S.; Kramer, B.W.; Strackx, E.; Martinez-Martinez, P. The effects of fetal and perinatal asphyxia on neuronal cytokine levels and ceramide metabolism in adulthood. J. Neuroimmunol. 2013, 255, 97–101. [Google Scholar] [CrossRef]
- Piešová, M.; Mach, M. Impact of perinatal hypoxia on the developing brain. Physiol. Res. 2020, 69, 199–213. [Google Scholar] [CrossRef]
- Ten, V.S.; Starkov, A. Hypoxic-ischemic injury in the developing brain: The role of reactive oxygen species originating in mitochondria. Neurol. Res. Int. 2012, 2012, 542976. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.; Ritter, J.; Mueller, S.; Felderhoff-Mueser, U.; Chew, L.J.; Gallo, V. Cellular changes underlying hyperoxia-induced delay of white matter development. J. Neurosci. 2011, 31, 4327–4344. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.; Endesfelder, S.; Reinert, M.C.; Klinker, F.; Muller, S.; Buhrer, C.; Liebetanz, D. Adolescent hyperactivity and impaired coordination after neonatal hyperoxia. Exp. Neurol. 2012, 235, 374–379. [Google Scholar] [CrossRef]
- Scheuer, T.; Brockmoller, V.; Blanco Knowlton, M.; Weitkamp, J.H.; Ruhwedel, T.; Mueller, S.; Endesfelder, S.; Buhrer, C.; Schmitz, T. Oligodendroglial maldevelopment in the cerebellum after postnatal hyperoxia and its prevention by minocycline. Glia 2015, 63, 1825–1839. [Google Scholar] [CrossRef] [PubMed]
- Nardiello, C.; Mižíková, I.; Morty, R.E. Looking ahead: Where to next for animal models of bronchopulmonary dysplasia? Cell Tissue Res. 2017, 367, 457–468. [Google Scholar] [CrossRef]
- Endesfelder, S.; Makki, H.; von Haefen, C.; Spies, C.D.; Buhrer, C.; Sifringer, M. Neuroprotective effects of dexmedetomidine against hyperoxia-induced injury in the developing rat brain. PLoS ONE 2017, 12, e0171498. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Strauß, E.; Bendix, I.; Schmitz, T.; Bührer, C. Prevention of Oxygen-Induced Inflammatory Lung Injury by Caffeine in Neonatal Rats. Oxid. Med. Cell Longev. 2020, 2020, 3840124. [Google Scholar] [CrossRef]
- Endesfelder, S.; Zaak, I.; Weichelt, U.; Bührer, C.; Schmitz, T. Caffeine protects neuronal cells against injury caused by hyperoxia in the immature brain. Free Radic. Biol. Med. 2014, 67, 221–234. [Google Scholar] [CrossRef]
- Giszas, V.; Strauß, E.; Bührer, C.; Endesfelder, S. The Conflicting Role of Caffeine Supplementation on Hyperoxia-Induced Injury on the Cerebellar Granular Cell Neurogenesis of Newborn Rats. Oxid. Med. Cell Longev. 2022, 2022, 5769784. [Google Scholar] [CrossRef]
- Heise, J.; Schmitz, T.; Bührer, C.; Endesfelder, S. Protective Effects of Early Caffeine Administration in Hyperoxia-Induced Neurotoxicity in the Juvenile Rat. Antioxidants 2023, 12, 295. [Google Scholar] [CrossRef]
- Puls, R.; von Haefen, C.; Bührer, C.; Endesfelder, S. Protective Effect of Dexmedetomidine against Hyperoxia-Damaged Cerebral Neurodevelopment in the Juvenile Rat. Antioxidants 2023, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Puls, R.; von Haefen, C.; Bührer, C.; Endesfelder, S. Dexmedetomidine Protects Cerebellar Neurons against Hyperoxia-Induced Oxidative Stress and Apoptosis in the Juvenile Rat. Int. J. Mol. Sci. 2023, 24, 7804. [Google Scholar] [CrossRef]
- Scheuer, T.; Klein, L.S.; Buhrer, C.; Endesfelder, S.; Schmitz, T. Transient Improvement of Cerebellar Oligodendroglial Development in a Neonatal Hyperoxia Model by PDGFA Treatment. Dev. Neurobiol. 2019, 79, 222–235. [Google Scholar] [CrossRef]
- Weichelt, U.; Cay, R.; Schmitz, T.; Strauss, E.; Sifringer, M.; Buhrer, C.; Endesfelder, S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 2013, 41, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.L.; Vaccarino, F.; Chacon, M.; Yan, W.L.; Ment, L.R.; Stewart, W.B. Chronic neonatal hypoxia leads to long term decreases in the volume and cell number of the rat cerebral cortex. Semin. Perinatol. 2004, 28, 379–388. [Google Scholar] [CrossRef]
- Curristin, S.M.; Cao, A.; Stewart, W.B.; Zhang, H.; Madri, J.A.; Morrow, J.S.; Ment, L.R. Disrupted synaptic development in the hypoxic newborn brain. Proc. Natl. Acad. Sci. USA 2002, 99, 15729–15734. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Tang, J.R.; Maxey, A.; Plopper, C.G.; Abman, S.H. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L964–L971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raman, L.; Georgieff, M.K.; Rao, R. The role of chronic hypoxia in the development of neurocognitive abnormalities in preterm infants with bronchopulmonary dysplasia. Dev. Sci. 2006, 9, 359–367. [Google Scholar] [CrossRef]
- Cai, J.; Tuong, C.M.; Gozal, D. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir. Physiol. Neurobiol. 2011, 178, 210–217. [Google Scholar] [CrossRef]
- Elberson, V.D.; Nielsen, L.C.; Wang, H.; Kumar, H.S.V. Effects of intermittent hypoxia and hyperoxia on angiogenesis and lung development in newborn mice. J. Neonatal-Perinat. Med. 2015, 8, 313–322. [Google Scholar] [CrossRef]
- Leroux, S.; Rodriguez-Duboc, A.; Arabo, A.; Basille-Dugay, M.; Vaudry, D.; Burel, D. Intermittent hypoxia in a mouse model of apnea of prematurity leads to a retardation of cerebellar development and long-term functional deficits. Cell Biosci. 2022, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Back, S.A. The Vannucci Model of Hypoxic-Ischemic Injury in the Neonatal Rodent: 40 years Later. Dev. Neurosci. 2022, 44, 186–193. [Google Scholar] [CrossRef]
- Martin, R.J.; Wang, K.; Köroğlu, O.; Di Fiore, J.; Kc, P. Intermittent hypoxic episodes in preterm infants: Do they matter? Neonatology 2011, 100, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Serdar, M.; Kempe, K.; Rizazad, M.; Herz, J.; Bendix, I.; Felderhoff-Müser, U.; Sabir, H. Early Pro-inflammatory Microglia Activation After Inflammation-Sensitized Hypoxic-Ischemic Brain Injury in Neonatal Rats. Front. Cell Neurosci. 2019, 13, 237. [Google Scholar] [CrossRef]
- Xue-Jiao, H.; Jian-Hua, F. A review of the effects of early postnatal hyperoxia exposure on the immature brain. Exp. Neurol. 2023, 370, 114550. [Google Scholar] [CrossRef]
- Bendix, I.; Weichelt, U.; Strasser, K.; Serdar, M.; Endesfelder, S.; von Haefen, C.; Heumann, R.; Ehrkamp, A.; Felderhoff-Mueser, U.; Sifringer, M. Hyperoxia changes the balance of the thioredoxin/peroxiredoxin system in the neonatal rat brain. Brain Res. 2012, 1484, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kaindl, A.M.; Sifringer, M.; Zabel, C.; Nebrich, G.; Wacker, M.A.; Felderhoff-Mueser, U.; Endesfelder, S.; von der Hagen, M.; Stefovska, V.; Klose, J.; et al. Acute and long-term proteome changes induced by oxidative stress in the developing brain. Cell Death Differ. 2006, 13, 1097–1109. [Google Scholar] [CrossRef]
- Morty, R.E. Using Experimental Models to Identify Pathogenic Pathways and Putative Disease Management Targets in Bronchopulmonary Dysplasia. Neonatology 2020, 117, 233–239. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Chizhikov, V.V. Cerebellar development after preterm birth. Front. Cell Dev. Biol. 2022, 10, 1068288. [Google Scholar] [CrossRef]
- Auvin, S.; Pressler, R. Comparison of Brain Maturation among Species: An Example in Translational Research Suggesting the Possible Use of Bumetanide in Newborn. Front. Neurol. 2013, 4, 36. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dobbing, J.; Sands, J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979, 3, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 511–533. [Google Scholar] [CrossRef]
- Morty, R.E. Recent advances in the pathogenesis of BPD. Semin. Perinatol. 2018, 42, 404–412. [Google Scholar] [CrossRef]
- Burri, P.H. Structural aspects of postnatal lung development-alveolar formation and growth. Biol. Neonate. 2006, 89, 313–322. [Google Scholar] [CrossRef]
- Kassim, Z.; Greenough, A.; Rafferty, G.F. Effect of caffeine on respiratory muscle strength and lung function in prematurely born, ventilated infants. Eur. J. Pediatr. 2009, 168, 1491–1495. [Google Scholar] [CrossRef]
- Silva, D.M.; Nardiello, C.; Pozarska, A.; Morty, R.E. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1239–L1272. [Google Scholar] [CrossRef]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Zhong, D.; Li, C.; Du, L. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway. Respir. Res. 2020, 21, 140. [Google Scholar] [CrossRef]
- Dumpa, V.; Nielsen, L.; Wang, H.; Kumar, V.H.S. Caffeine is associated with improved alveolarization and angiogenesis in male mice following hyperoxia induced lung injury. BMC Pulm. Med. 2019, 19, 138. [Google Scholar] [CrossRef]
- Teng, R.J.; Jing, X.; Michalkiewicz, T.; Afolayan, A.J.; Wu, T.J.; Konduri, G.G. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L586–L598. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.; Nardiello, C.; Surate Solaligue, D.E.; Agius, R.; Mizikova, I.; Huhn, S.; Mayer, K.; Vadasz, I.; Herold, S.; Runkel, F.; et al. Caffeine administration modulates TGF-beta signaling but does not attenuate blunted alveolarization in a hyperoxia-based mouse model of bronchopulmonary dysplasia. Pediatr. Res. 2017, 81, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Huang, Y.W.; Jarzembowski, J.; Shi, Y.; Konduri, G.G.; Teng, R.J. Caffeine ameliorates hyperoxia-induced lung injury by protecting GCH1 function in neonatal rat pups. Pediatr. Res. 2017, 82, 483–489. [Google Scholar] [CrossRef]
- Sadek, A.; Khattab, R.; Amer, A.; Youssef, A. Protective role of caffeine versus N-acetylcysteine in hyperoxic acute lung injury in neonatal rats. J. Morphol. Sci. 2017, 34, 058–067. [Google Scholar] [CrossRef]
- Nagatomo, T.; Jimenez, J.; Richter, J.; De Baere, S.; Vanoirbeek, J.; Naulaers, G.; Allegaert, K.; Croubels, S.; Deprest, J.A.; Toelen, J. Caffeine Prevents Hyperoxia-Induced Functional and Structural Lung Damage in Preterm Rabbits. Neonatology 2016, 109, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Dayanim, S.; Lopez, B.; Maisonet, T.M.; Grewal, S.; Londhe, V.A. Caffeine induces alveolar apoptosis in the hyperoxia-exposed developing mouse lung. Pediatr. Res. 2014, 75, 395–402. [Google Scholar] [CrossRef][Green Version]
- Batool, M.; Cai, C.L.; Aranda, J.V.; Hand, I.; Beharry, K.D. Early versus late caffeine and/or non-steroidal anti-inflammatory drugs (NSAIDS) for prevention of intermittent hypoxia-induced neuroinflammation in the neonatal rat. Int. J. Dev. Neurosci. 2024, 84, 227–250. [Google Scholar] [CrossRef]
- Soontarapornchai, K.; Cai, C.L.; Ahmad, T.; Aranda, J.V.; Hand, I.; Beharry, K.D. Pharmacodynamic Effects of Standard versus High Caffeine Doses in the Developing Brain of Neonatal Rats Exposed to Intermittent Hypoxia. Int. J. Mol. Sci. 2021, 22, 3473. [Google Scholar] [CrossRef]
- Li, H.-L.; Zaghloul, N.; Ahmed, I.; Omelchenko, A.; Firestein, B.L.; Huang, H.; Collins, L. Caffeine inhibits hypoxia-induced nuclear accumulation in HIF-1α and promotes neonatal neuronal survival. Exp. Neurol. 2019, 317, 66–77. [Google Scholar] [CrossRef]
- Back, S.A.; Craig, A.; Luo, N.L.; Ren, J.; Akundi, R.S.; Ribeiro, I.; Rivkees, S.A. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann. Neurol. 2006, 60, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Dou, H.; Wang, S.; Qu, D.; Peng, X.; Zou, N.; Yang, L. Caffeine improves mitochondrial dysfunction in the white matter of neonatal rats with hypoxia-ischemia through deacetylation: A proteomic analysis of lysine acetylation. Front. Mol. Neurosci. 2024, 17, 1394886. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Li, D.; Xue, X.; Fu, J. Proteomic analysis of the effects of caffeine in a neonatal rat model of hypoxic-ischemic white matter damage. CNS Neurosci. Ther. 2022, 28, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, X.; Zhang, Y.; Liu, N.; Xue, X.; Fu, J. Caffeine treatment started before injury reduces hypoxic–ischemic white-matter damage in neonatal rats by regulating phenotypic microglia polarization. Pediatr. Res. 2022, 92, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gonzalez, F.; McQuillen, P.S. Caffeine Restores Background EEG Activity Independent of Infarct Reduction after Neonatal Hypoxic Ischemic Brain Injury. Dev. Neurosci. 2020, 42, 72–82. [Google Scholar] [CrossRef]
- Di Martino, E.; Bocchetta, E.; Tsuji, S.; Mukai, T.; Harris, R.A.; Blomgren, K.; Ådén, U. Defining a Time Window for Neuroprotection and Glia Modulation by Caffeine After Neonatal Hypoxia-Ischaemia. Mol. Neurobiol. 2020, 57, 2194–2205. [Google Scholar] [CrossRef]
- Potter, M.; Rosenkrantz, T.; Fitch, R.H. Behavioral and neuroanatomical outcomes in a rat model of preterm hypoxic-ischemic brain Injury: Effects of caffeine and hypothermia. Int. J. Dev. Neurosci. 2018, 70, 46–55. [Google Scholar] [CrossRef]
- Winerdal, M.; Urmaliya, V.; Winerdal, M.E.; Fredholm, B.B.; Winqvist, O.; Ådén, U. Single Dose Caffeine Protects the Neonatal Mouse Brain against Hypoxia Ischemia. PLoS ONE 2017, 12, e0170545. [Google Scholar] [CrossRef]
- Kilicdag, H.; Daglioglu, Y.K.; Erdogan, S.; Zorludemir, S. Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J. Matern. Fetal Neonatal Med. 2014, 27, 1470–1475. [Google Scholar] [CrossRef]
- Alexander, M.; Smith, A.L.; Rosenkrantz, T.S.; Fitch, R.H. Therapeutic effect of caffeine treatment immediately following neonatal hypoxic-ischemic injury on spatial memory in male rats. Brain Sci. 2013, 3, 177–190. [Google Scholar] [CrossRef]
- Julien, C.A.; Joseph, V.; Bairam, A. Alteration of carotid body chemoreflexes after neonatal intermittent hypoxia and caffeine treatment in rat pups. Respir. Physiol. Neurobiol. 2011, 177, 301–312. [Google Scholar] [CrossRef]
- Julien, C.A.; Joseph, V.; Bairam, A. Caffeine reduces apnea frequency and enhances ventilatory long-term facilitation in rat pups raised in chronic intermittent hypoxia. Pediatr. Res. 2010, 68, 105–111. [Google Scholar] [CrossRef]
- Köroğlu, Ö.A.; MacFarlane, P.M.; Balan, K.V.; Zenebe, W.J.; Jafri, A.; Martin, R.J.; Kc, P. Anti-Inflammatory Effect of Caffeine Is Associated with Improved Lung Function after Lipopolysaccharide-Induced Amnionitis. Neonatology 2014, 106, 235–240. [Google Scholar] [CrossRef]
- Gubas, A.; Dikic, I. ER remodeling via ER-phagy. Mol. Cell. 2022, 82, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Sagara, H.; Okada, T.; Okumura, K.; Ogawa, H.; Ra, C.; Fukuda, T.; Nakao, A. Activation of TGF-β/Smad2 signaling is associated with airway remodeling in asthma. J. Allergy Clin. Immunol. 2002, 110, 249–254. [Google Scholar] [CrossRef]
- Ramos, A.C.; de Mattos Hungria, F.; Camerini, B.A.; Suiama, M.A.; Calzavara, M.B. Potential beneficial effects of caffeine administration in the neonatal period of an animal model of schizophrenia. Behav. Brain Res. 2020, 391, 112674. [Google Scholar] [CrossRef] [PubMed]
- Kasala, S.; Briyal, S.; Prazad, P.; Ranjan, A.K.; Stefanov, G.; Donovan, R.; Gulati, A. Exposure to Morphine and Caffeine Induces Apoptosis and Mitochondrial Dysfunction in a Neonatal Rat Brain. Front. Pediatr. 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Winter, K.; von Haefen, C.; Buhrer, C. Caffeine Protects Against Anticonvulsant-Induced Impaired Neurogenesis in the Developing Rat Brain. Neurotox. Res. 2018, 34, 173–187. [Google Scholar] [CrossRef]
- Abu-Sa’da, O.S.; Armstrong, E.A.; Scott, O.; Shaw, O.; Nguyen, A.I.; Shen, K.; Cheung, P.; Baker, G.; Yager, J.Y. The Effect of Caffeine on the Neuropathological and Neurobehavioral Outcome in the Newborn Rat. J. Caffeine Adenosine Res. 2018, 8, 143–152. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Sifringer, M.; Bendix, I.; Buhrer, C. Caffeine Protects Against Anticonvulsant-Induced Neurotoxicity in the Developing Rat Brain. Neurotox. Res. 2017, 32, 460–472. [Google Scholar] [CrossRef]
- Gaytan, S.P.; Pasaro, R. Neonatal caffeine treatment up-regulates adenosine receptors in brainstem and hypothalamic cardio-respiratory related nuclei of rat pups. Exp. Neurol. 2012, 237, 247–259. [Google Scholar] [CrossRef]
- Desfrere, L.; Olivier, P.; Schwendimann, L.; Verney, C.; Gressens, P. Transient inhibition of astrocytogenesis in developing mouse brain following postnatal caffeine exposure. Pediatr. Res. 2007, 62, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Tchekalarova, J.; Kubova, H.; Mares, P. Postnatal caffeine exposure: Effects on motor skills and locomotor activity during ontogenesis. Behav. Brain Res. 2005, 160, 99–106. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Falsaperla, R.; Lombardo, F.; Filosco, F.; Romano, C.; Saporito, M.A.N.; Puglisi, F.; Piro, E.; Ruggieri, M.; Pavone, P. Oxidative Stress in Preterm Infants: Overview of Current Evidence and Future Prospects. Pharmaceuticals 2020, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Cannavò, L.; Rulli, I.; Falsaperla, R.; Corsello, G.; Gitto, E. Ventilation, oxidative stress and risk of brain injury in preterm newborn. Ital. J. Pediatr. 2020, 46, 100. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Granese, R.; Falsaperla, R.; Reiter, R.J.; Corsello, G.; Gitto, E. Role of oxidative stress in neonatal respiratory distress syndrome. Free Radic. Biol. Med. 2019, 142, 132–137. [Google Scholar] [CrossRef]
- Cannavò, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef]
- Kumar, V.H.S.; Lipshultz, S.E. Caffeine and Clinical Outcomes in Premature Neonates. Children 2019, 6, 118. [Google Scholar] [CrossRef]
- Panfoli, I.; Candiano, G.; Malova, M.; De Angelis, L.; Cardiello, V.; Buonocore, G.; Ramenghi, L.A. Oxidative Stress as a Primary Risk Factor for Brain Damage in Preterm Newborns. Front. Pediatr. 2018, 6, 369. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Grassi, F.; Caporilli, C.; Boscarino, G.; Carbone, G.; Petrolini, C.; Gambini, L.M.; Di Peri, A.; Moretti, S.; Buonocore, G.; et al. Brain Damage in Preterm and Full-Term Neonates: Serum Biomarkers for the Early Diagnosis and Intervention. Antioxidants 2023, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T. Development and application of oxidative stress biomarkers. Biosci. Biotechnol. Biochem. 2018, 82, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Yamamoto, K.-I. DNA damage responses to oxidative stress. DNA Repair. 2004, 3, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.; Desai, A.; Chandel, D. Role of Oxidative Stress and DNA Damage on Preterm Birth Outcome. Biol. Res. Nurs. 2024, 26, 410–417. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; Lavail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Arnold, S.M.; Miller, C.N.; Wu, J.; Li, J.; Gunnison, K.M.; Mori, K.; Sadighi Akha, A.A.; Raden, D.; Kaufman, R.J. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006, 4, e374. [Google Scholar] [CrossRef]
- Ratner, V.; Starkov, A.; Matsiukevich, D.; Polin, R.A.; Ten, V.S. Mitochondrial dysfunction contributes to alveolar developmental arrest in hyperoxia-exposed mice. Am. J. Respir. Cell Mol. Biol. 2009, 40, 511–518. [Google Scholar] [CrossRef]
- Tong, X.; Li, M.; Liu, N.; Huang, W.; Xue, X.; Fu, J. Hyperoxia induces endoplasmic reticulum stress-associated apoptosis via the IRE1α pathway in rats with bronchopulmonary dysplasia. Mol. Med. Rep. 2021, 23, 11671. [Google Scholar] [CrossRef]
- Lu, H.Y.; Zhang, J.; Wang, Q.X.; Tang, W.; Zhang, L.J. Activation of the endoplasmic reticulum stress pathway involving CHOP in the lungs of rats with hyperoxia-induced bronchopulmonary dysplasia. Mol. Med. Rep. 2015, 12, 4494–4500. [Google Scholar] [CrossRef][Green Version]
- Thornton, C.; Baburamani, A.A.; Kichev, A.; Hagberg, H. Oxidative stress and endoplasmic reticulum (ER) stress in the development of neonatal hypoxic–ischaemic brain injury. Biochem. Soc. Trans. 2017, 45, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Abate, A.; George, A.G.; Weng, Y.H.; Dennery, P.A. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J. Clin. Investig. 2004, 114, 669–678. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, M.; Husted, C.; Chen, C.; Thiagarajan, K.; Johns, J.L.; Rao, S.P.; Alvira, C.M. Activation of the nuclear factor-κB pathway during postnatal lung inflammation preserves alveolarization by suppressing macrophage inflammatory protein-2. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L593–L604. [Google Scholar] [CrossRef] [PubMed]
- Sussan, T.E.; Sudini, K.; Talbot, C.C.; Wang, X.; Wills-Karp, M.; Burd, I.; Biswal, S. Nrf2 regulates gene-environment interactions in an animal model of intrauterine inflammation: Implications for preterm birth and prematurity. Sci. Rep. 2017, 7, 40194. [Google Scholar] [CrossRef]
- Tamatam, C.M.; Reddy, N.M.; Potteti, H.R.; Ankireddy, A.; Noone, P.M.; Yamamoto, M.; Kensler, T.W.; Reddy, S.P. Preconditioning the immature lung with enhanced Nrf2 activity protects against oxidant-induced hypoalveolarization in mice. Sci. Rep. 2020, 10, 19034. [Google Scholar] [CrossRef] [PubMed]
- Krey, F.C.; Stocchero, B.A.; Creutzberg, K.C.; Heberle, B.A.; Tractenberg, S.G.; Xiang, L.; Wei, W.; Kluwe-Schiavon, B.; Viola, T.W. Neurotrophic Factor Levels in Preterm Infants: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 643576. [Google Scholar] [CrossRef]
- Ghassabian, A.; Sundaram, R.; Chahal, N.; McLain, A.C.; Bell, E.; Lawrence, D.A.; Yeung, E.H. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev. Psychopathol. 2017, 29, 1499–1511. [Google Scholar] [CrossRef]
- Dingsdale, H.; Garay, S.M.; Tyson, H.R.; Savory, K.A.; Sumption, L.A.; Kelleher, J.S.; Langley, K.; Van Goozen, S.; John, R.M. Cord serum brain-derived neurotrophic factor levels at birth associate with temperament outcomes at one year. J. Psychiatr. Res. 2022, 150, 47–53. [Google Scholar] [CrossRef]
- Simpson, S.L.; Grayson, S.; Peterson, J.H.; Moore, J.J.; Mhanna, M.J.; Perez, M.K.; Rezaee, F.; Piedimonte, G. Serum neurotrophins at birth correlate with respiratory and neurodevelopmental outcomes of premature infants. Pediatr. Pulmonol. 2019, 54, 303–312. [Google Scholar] [CrossRef]
- Bokobza, C.; Van Steenwinckel, J.; Mani, S.; Mezger, V.; Fleiss, B.; Gressens, P. Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2019, 85, 155–165. [Google Scholar] [CrossRef]
- Yao, Q.; Zaidi, S.I.; Haxhiu, M.A.; Martin, R.J. Neonatal lung and airway injury: A role for neurotrophins. Semin. Perinatol. 2006, 30, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, M.; Liu, A.; Di, W.; Zhao, F.; Tian, Y.; Jia, J. Changes of inflammatory cytokines and neurotrophins emphasized their roles in hypoxic-ischemic brain damage. Int. J. Neurosci. 2013, 123, 191–195. [Google Scholar] [CrossRef]
- Higano, N.S.; Spielberg, D.R.; Fleck, R.J.; Schapiro, A.H.; Walkup, L.L.; Hahn, A.D.; Tkach, J.A.; Kingma, P.S.; Merhar, S.L.; Fain, S.B.; et al. Neonatal Pulmonary Magnetic Resonance Imaging of Bronchopulmonary Dysplasia Predicts Short-Term Clinical Outcomes. Am. J. Respir. Crit. Care Med. 2018, 198, 1302–1311. [Google Scholar] [CrossRef]
- Thebaud, B.; Abman, S.H. Bronchopulmonary dysplasia: Where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 2007, 175, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Alejandre-Alcázar, M.A.; Kwapiszewska, G.; Reiss, I.; Amarie, O.V.; Marsh, L.M.; Sevilla-Pérez, J.; Wygrecka, M.; Eul, B.; Köbrich, S.; Hesse, M.; et al. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L537–L549. [Google Scholar] [CrossRef] [PubMed]
- Ambalavanan, N.; Nicola, T.; Hagood, J.; Bulger, A.; Serra, R.; Murphy-Ullrich, J.; Oparil, S.; Chen, Y.F. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L86–L95. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Manti, S.; Buttarelli, L.; Petrolini, C.; Boscarino, G.; Filonzi, L.; Gitto, E.; Esposito, S.M.R.; Nonnis Marzano, F. Vascular Endothelial Growth Factor as Molecular Target for Bronchopulmonary Dysplasia Prevention in Very Low Birth Weight Infants. Int. J. Mol. Sci. 2023, 24, 2729. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Zhang, J.G.; Hepburn, L.; Cruz, G.; Borman, R.A.; Clark, K.L. The role of adenosine A2A and A2B receptors in the regulation of TNF-alpha production by human monocytes. Biochem. Pharmacol. 2005, 69, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Aceti, A.; Della Gatta, A.N.; Beghetti, I.; Marsico, C.; Pilu, G.; Corvaglia, L. Antenatal and Postnatal Sequelae of Oxidative Stress in Preterm Infants: A Narrative Review Targeting Pathophysiological Mechanisms. Antioxidants 2023, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B. Caffeine for Apnea of Prematurity: Too Much or Too Little of a Good Thing. J. Pediatr. 2023, 259, 113488. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Smart, D.J.; De Paoli, A.G. Prophylactic methylxanthine for prevention of apnoea in preterm infants. Cochrane Database Syst. Rev. 2010, 12, CD000432. [Google Scholar] [CrossRef]
- Erenberg, A.; Leff, R.D.; Haack, D.G.; Mosdell, K.W.; Hicks, G.M.; Wynne, B.A. Caffeine citrate for the treatment of apnea of prematurity: A double-blind, placebo-controlled study. Pharmacotherapy 2000, 20, 644–652. [Google Scholar] [CrossRef]
- Oliphant, E.A.; Hanning, S.M.; McKinlay, C.J.D.; Alsweiler, J.M. Caffeine for apnea and prevention of neurodevelopmental impairment in preterm infants: Systematic review and meta-analysis. J. Perinatol. 2024, 44, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.; Dobson, N.R.; Hunt, C.E. Immature control of breathing and apnea of prematurity: The known and unknown. J. Perinatol. 2021, 41, 2111–2123. [Google Scholar] [CrossRef]
- Ji, D.; Smith, P.B.; Clark, R.H.; Zimmerman, K.O.; Laughon, M.; Ku, L.; Greenberg, R.G. Wide variation in caffeine discontinuation timing in premature infants. J. Perinatol. 2020, 40, 288–293. [Google Scholar] [CrossRef]
- Bakewell-Sachs, S.; Medoff-Cooper, B.; Escobar, G.J.; Silber, J.H.; Lorch, S.A. Infant functional status: The timing of physiologic maturation of premature infants. Pediatrics 2009, 123, e878–e886. [Google Scholar] [CrossRef]
- Haddad, W.; Sajous, C.; Hummel, P.; Guo, R. Discontinuing caffeine in preterm infants at 33-35 weeks corrected gestational age: Failure rate and predictive factors. J. Neonatal Perinatal Med. 2015, 8, 41–45. [Google Scholar] [CrossRef]
- Rhein, L.M.; Dobson, N.R.; Darnall, R.A.; Corwin, M.J.; Heeren, T.C.; Poets, C.F.; McEntire, B.L.; Hunt, C.E. Effects of caffeine on intermittent hypoxia in infants born prematurely: A randomized clinical trial. JAMA Pediatr. 2014, 168, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Dobson, N.R.; Rhein, L.M.; Darnall, R.A.; Corwin, M.J.; Heeren, T.C.; Eichenwald, E.; James, L.P.; McEntire, B.L.; Hunt, C.E.; Consenstein, L.; et al. Caffeine decreases intermittent hypoxia in preterm infants nearing term-equivalent age. J. Perinatol. 2017, 37, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Urru, S.A.M.; Geist, M.; Carlinger, R.; Bodrero, E.; Bruschettini, M. Strategies for cessation of caffeine administration in preterm infants. Cochrane Database Syst. Rev. 2024, 7, CD015802. [Google Scholar] [CrossRef]
- Prakash, R.; Pournami, F.; Prabhakar, J.; Nandakumar, A.; Nair, P.M.C.; Jain, N. Duration of Caffeine for Apnea of Prematurity-A Randomized Controlled Trial. Indian. J. Pediatr. 2021, 88, 1174–1179. [Google Scholar] [CrossRef]
- Pradhap, K.; Solaiappan, M.; Ramya, S.; Muthukumaran, N. Effect of Short Course Caffeine on Recurrence of Apnea of Prematurity in Preterm Infants Less than 32 Weeks: A Randomized Controlled Trial. J. Neonatol. 2023, 38, 375–381. [Google Scholar] [CrossRef]
- Leon-Carmona, J.R.; Galano, A. Is caffeine a good scavenger of oxygenated free radicals? J. Phys. Chem. B. 2011, 115, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Rivkees, S.A.; Wendler, C.C. Adverse and protective influences of adenosine on the newborn and embryo: Implications for preterm white matter injury and embryo protection. Pediatr. Res. 2011, 69, 271–278. [Google Scholar] [CrossRef]
- Leviton, A.; Allred, E.N.; Fichorova, R.N.; Kuban, K.C.; Michael O’Shea, T.; Dammann, O. Systemic inflammation on postnatal days 21 and 28 and indicators of brain dysfunction 2years later among children born before the 28th week of gestation. Early Hum. Dev. 2016, 93, 25–32. [Google Scholar] [CrossRef]
- Leviton, A.; Joseph, R.M.; Allred, E.N.; Fichorova, R.N.; O’Shea, T.M.; Kuban, K.K.C.; Dammann, O. The risk of neurodevelopmental disorders at age 10years associated with blood concentrations of interleukins 4 and 10 during the first postnatal month of children born extremely preterm. Cytokine 2018, 110, 181–188. [Google Scholar] [CrossRef]
- Yanni, D.; Korzeniewski, S.J.; Allred, E.N.; Fichorova, R.N.; O’Shea, T.M.; Kuban, K.; Dammann, O.; Leviton, A. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatr. Res. 2017, 82, 691–696. [Google Scholar] [CrossRef]
- Chavez-Valdez, R.; Wills-Karp, M.; Ahlawat, R.; Cristofalo, E.A.; Nathan, A.; Gauda, E.B. Caffeine modulates TNF-alpha production by cord blood monocytes: The role of adenosine receptors. Pediatr. Res. 2009, 65, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Kawasaki, Y.; Katsuma, A.; Mito, A.; Tamura, K.; Makimoto, M.; Yoshida, T. Pharmacokinetic Variability of Caffeine in Routinely Treated Preterm Infants: Preliminary Considerations on Developmental Changes of Systemic Clearance. Biol. Pharm. Bull. 2021, 44, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.G.; Matricon, P.; Eddy, M.T.; Carlsson, J. Adenosine A(2A) receptor antagonists: From caffeine to selective non-xanthines. Br. J. Pharmacol. 2022, 179, 3496–3511. [Google Scholar] [CrossRef] [PubMed]
| Species | Oxygen Exposure | Caffeine | Caffeine and Pulmonary Hyperoxia | Ref. | |
|---|---|---|---|---|---|
 | |||||
 C57Bl/6 mice |  | 1 g/L lactate dam P0–P14 | Pulmonary protection antioxidative anti-apoptotic anti-inflammatory reduced lung architecture simplification increased surfactant protein | Pulmonary side effects modulated NfκB signaling pathway modulated AdoRA2a expression Side effects weight gain | [101] |
 C57Bl/6 mice |  | 20 mg/kg i.p. P0–P4 | Pulmonary protection improved microvascularization (male) reduced lung architecture simplification | Pulmonary side effects modulated HIF signaling pathway Side effects weight gain | [102] |
 SD rat |  | 20 mg/kg i.p. P2–P10/P21 | Pulmonary protection antioxidative anti-apoptotic improved microvascularization reduced lung architecture simplification reduced endoplasmic (ER) stress reduced mitochondrial dysfunction | [103] | |
 C57Bl/6 mice |  | 25 mg/kg i.p. P0–P13 | Pulmonary protection none | Pulmonary side effects increased phosphorylation Smad2 Side effects weight gain | [104] |
 SD rat | 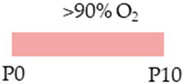 | 20 mg/kg i.p. P2–P21 | Pulmonary protection improved microvascularization reduced lung architecture simplification | Pulmonary side effects reduced cAMP level Side effects reduced mortality weight gain | [105] |
 SD rat |  | 10 mg/kg i.p. P7 | Pulmonary protection antioxidant anti-apoptotic anti-inflammatory reduced lung architecture simplification | [106] | |
 New Zealand white rabbit |  | 10 mg/kg i.p. P0 5 mg/kg i.p. P1–P5 | Pulmonary protection anti-inflammatory improved lung function reduced lung architecture simplification | [107] | |
 FVB/n mice | 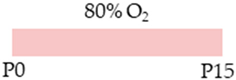 | 20 mg/kg i.p. P1 10 mg/kg i.p. P2–P15 | Pulmonary protection none | Pulmonary damage pro-apoptotic pro-inflammatory exacerbated lung architecture reduced AECII counts Pulmonary side effects reduced AdoRA2a expression | [108] |
| Species | Oxygen Exposure | Caffeine | Caffeine and Neuronal Hyperoxia | Ref. | |
|---|---|---|---|---|---|
 | |||||
 SD rat |  | 20 mg/kg i.p. P0 5 mg/kg i.p. P1–P14 | Neuroprotection anti-apoptotic anti-inflammatory protected against demyelination | Neurodegeneration/side effects n/a | [109] |
 SD rat |  | LoC: 20 mg/kg i.p. P0 5 mg/kg i.p. P1–P14 HiC: 80 mg/kg i.p. P0 20 mg/kg i.p. P1–P14 | Neuroprotection LoC: antioxidant anti-apoptotic reduced neuronal pyknosis increased neurons and dendritogenesis HiC: antioxidant anti-apoptotic increased neurons and dendritogenesis protected from demyelination | Neurodegeneration/side effects LoC: reduced AdoRA1a/2a expression weight gain HiC: intraventricular haemorrhages histopathological abnormalities reduced AdoRA1a/2a expression weight loss | [110] |
| Species | Oxygen Exposure | Caffeine | Caffeine and Pulmonary-Neuronal Hyperoxia | Ref. | |
|---|---|---|---|---|---|
 |  | ||||
 Wistar rat | 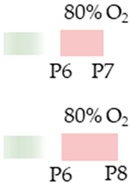 | 10 mg/kg i.p. P6 10 mg/kg i.p. P6 | Pulmonary protection anti-inflammatory reduced lung architecture simplification | Neuroprotection antioxidant anti-apoptotic anti-inflammatory protected from tissue remodeling protected from impaired hippocampal neurogenesis Neuronal side effects modulated NfκB/Nrf2 signaling pathway | [38,69,75] The studies use the identical cohorts. |
 Wistar rat | 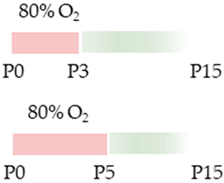 | 10 mg/kg i.p. P0–P2 10 mg/kg i.p. P0–P4 | Pulmonale Protection antioxidant anti-apoptotic anti-inflammatory Pulmonary side effects modulated NfκB/Nrf2 signaling pathway modulated AdoRA1 expression | Neuroprotection protected from impaired hippocampal neurogenesis protected from impaired cerebellar neurogenesis and migration rescued neurotrophins | [39,68,70,71] The studies use the identical cohorts. |
| Species | Oxygen Exposure | Caffeine | Caffeine and Neuronal Hypoxia | Ref. | |
|---|---|---|---|---|---|
 | |||||
 mice | 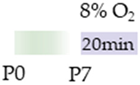 | 20 mg/kg p.o. P4 15 mg/kg p.o. P5–P7 | Neuroprotection reduced HIF1α cortical accumulation | Neurodegeneration/side effects n/a | [111] |
 C57Bl/6 mice | 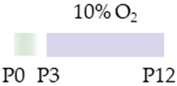 | 0.3 g/L lactate dam P2–P12 | Neuroprotection reduced ventriculomegaly protected against demyelination | Neurodegeneration/side effects n/a | [112] |
| Species | Oxygen Exposure | Caffeine | Caffeine and Neuronal Hypoxia-Ischemia | Ref. | |
|---|---|---|---|---|---|
 | |||||
 SD rat |  | 10 mg/kg i.p. P2–P6 before HI and post HI | Neuroprotection anti-apoptotic | Neuronal side effects improved mitochondrial function promoted effects of deacetylation | [113] |
 SD rat |  | 10 mg/kg i.p. P2–P6 before HI and post HI | Neuroprotection anti-inflammatory restored reduced synapses proteins alleviated the suppressed cognitive impairment reduced ventriculomegaly protected against demyelination promote microglial polarization to the M2 phenotype | Neurodegeneration/side effects n/a | [114,115] The studies use the identical cohorts. |
 SD rat |  | 0.3 g/L lactate dam E8–P16 before HI and P3–P16 post HI | Neuroprotection before HI: reduced number of brain infarcts reduced infarct volume restored suppressed EEG brain activity post HI: restored suppressed EEG brain activity | Neurodegeneration/side effects n/a | [116] |
 C57Bl/6 mice | 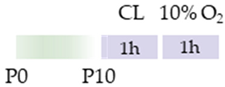 | 5 mg/kg i.p. 0 h, 6 h, 12 h, and 24 h post HI | Neuroprotection anti-apoptotic anti-inflammatory reduced infarct volume protected against demyelination reduced microglia-activation | Neurodegeneration/side effects n/a | [117] |
 Wistar rat | 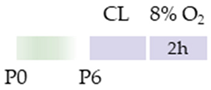 | 10 mg/kg i.p. 0 h, 24 h post HI | Neuroprotection improved cognitive and motoric learning (male) | Neurodegeneration/side effects n/a | [118] |
 C57Bl/6 mice |  | 5 mg/kg i.p. 0 h post HI | Neuroprotection improved motoric learning | Neurodegeneration/side effects n/a | [119] |
 Wistar rat | 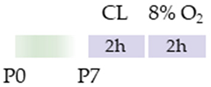 | 10 mg/kg i.p. before HI and 0 h, 24 h, 48 h, and 72 h post HI | Neuroprotection anti-apoptotic (HC, cortex) | Neurodegeneration/side effects n/a | [120] |
 Wistar rat |  | 10 mg/kg i.p. 0 h post HI | Neuroprotection improved spatial learning (male) | Neurodegeneration/side effects n/a | [121] |
| Species | Oxygen Exposure | Caffeine | Caffeine and Pulmonal Intermittent Hypoxia | Ref. | |
|---|---|---|---|---|---|
 | |||||
 SD rat |  | 15 mg/kg p.o. P3–P15 * 10 mg/kg i.p. P3–P15 ** | Pulmonary protection reduced frequency of apnea | Pulmonary side effects n/a | [122] * [123] ** |
| Species | Oxygen Exposure | Caffeine | Caffeine and Neuronal Intermittent Hypoxia | Ref. | |
|---|---|---|---|---|---|
 | |||||
 SD rat | 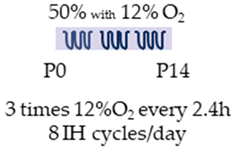 | 20 mg/kg i.p. P0 5 mg/kg i.p. P1–P14 | Neuroprotection anti-inflammatory reduced histopathological abnormalities | Neuronal side effects hypermyelination Side effects reduced enhanced brain/body weight ratio | [109] |
 SD rat |  | LoC: 20 mg/kg i.p. P0 5 mg/kg i.p. P1–P14 HiC: 80 mg/kg i.p. P0 20 mg/kg i.p. P1–P14 | Neuroprotection/side effects LoC: anti-apoptotic increased neurons and dendritogenesis HiC: antioxidant anti-apoptotic increased neurons and dendritogenesis | Neurodegeneration/side effects LoC: pro-oxidant reinforced demyelination histopathological abnormalities Neuronal side effects LoC: reduced AdoRA1a expression induced AdoRA2a expression HiC: reduced AdoRA1a expression Side effects LoC: induced body length HiC: reduced brain weight weight loss | [110] |
| Species | Oxygen Exposure | Caffeine | Caffeine and the Immature Lung | Ref. | |
|---|---|---|---|---|---|
 | |||||
 C57Bl/6 mice | 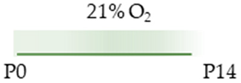 | 1 g/L lactate dam P0–P14 | Pulmonary damage none | Pulmonary side effects reduced AdoRA2a expression | [101] |
 C57Bl/6 mice | 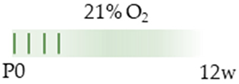 | 20 mg/kg i.p. P0–P4 | Pulmonary damage none | Pulmonary side effects induced HIF mapped transcripts induced vascular-associated transcripts weight gain (male) | [102] |
 SD rat | 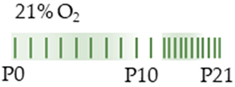 | 20 mg/kg i.p. P2–P10/P21 | Pulmonary damage increased ER-phagy associated protein activated UPR (linked to fibrosis and inflammation) decreased blood vessel formation | Pulmonary side effects n/a | [103] |
 C57Bl/6 mice |  | 25 mg/kg i.p. P0–P13 | Pulmonary damage decreased phosphorylation Smad2 (linked to epithelial remodeling) | Pulmonary side effects n/a | [104] |
 SD rat | 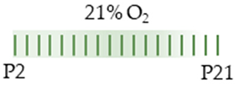 | 20 mg/kg i.p. P2–P21 | Pulmonary damage n/a | Side effects weight loss | [105] |
 SD rat |  | 10 mg/kg p.o. P1–P14 | Pulmonary damage n/a | Pulmonary side effects n/a | [124] |
 FVB/n mice | 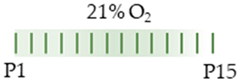 | 20 mg/kg i.p. P1 10 mg/kg i.p. P2–P15 | Pulmonary damage pro-apoptotic pro-inflammatory with MΦ infiltration and chemokine-induction reduced surfactant protein C transcript reduced AEC II | Pulmonary side effects n/a | [108] |
 SD rat | 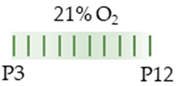 | 15 mg/kg p.o. P3–P12 | Pulmonary damage n/a | Pulmonary side effects increased minute ventilation increased breathing frequency | [122] |
| Species | Oxygen Exposure | Caffeine | Caffeine and the Developing Bain | Ref. | |
|---|---|---|---|---|---|
 | |||||
 SD rat | 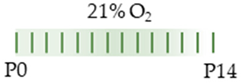 | 20 mg/kg i.p. P0 5 mg/kg i.p. P1–P14 | Neurodegeneration pro-inflammatory | Neuronal side effects hypermyelination | [109] |
 SD rat | 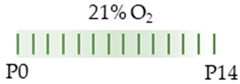 | LoC: 20 mg/kg i.p. P0 5 mg/kg i.p. P1–P14 HiC: 80 mg/kg i.p. P0 20 mg/kg i.p. P1–P14 | Neurodegeneration LoC: pro-oxidant neuronal disorganization HiC: pro-oxidant pro-apoptotic pro-necrotic neuronal degeneration and disorganization | Neuronal side effects LoC: hypermyelination increased neurons per area and dendrites per neuron reduced AdoRA1 and AdoRA2a expression HiC: hypermyelination increased neurons per area and dendrites per neuron reduced AdoRA1 and AdoRA2a expression | [110] |
 Wistar rat |  | 10 mg/kg s.c. P7 | Neurodevelopment hyperlocomotion reduced social interaction reduced contextual fear conditioning | Neuronal side effects n/a | [128] |
 SD rat |  | 50 mg/kg s.c. P3–P6 | Neurodegeneration pro-apoptotic impaired mitochondrial dysfunction | Neuronal side effects n/a | [129] |
 Wistar rat |  | 10 mg/kg i.p. P6 | Neurodegeneration reduced hippocampal proliferation capacity reduced differentiation potential in hippocampal neural linage | Neuronal side effects increased neurotrophin expression | [130] |
 Long Evans rat | 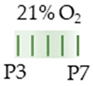 | 20 mg/kg s.c. P3 15 mg/kg s.c. P4–P7 | Neurodegeneration reduced neurons (Hth and CA1) pro-apoptotic (CA1, Hth, Th, WM) | Neuronal side effects n/a | [131] |
 Wistar rat | 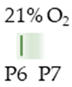 | 10 mg/kg i.p. P6 | Neurodegeneration pro-apoptotic (DG, Hth) pro-inflammatory | Neuronal side effects increased AdoRA1 and AdoRA2a expression | [132] |
 SD rat | 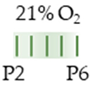 | 20 mg/kg i.p. P2 15 mg/kg i.p. P3–P6 | Neurodegeneration none | Neuronal side effects induced early maturation adenosinergic system (Hth, brain stem) induced AdoRA1 and AdoRA2a expression | [133] |
 mice | 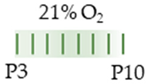 | 10 mg/kg i.p. P3 2.5 mg/kg i.p. P4–P10 | Neurodegeneration reduced proliferation SVZ and WM reduced astrocytogenesis cortex and WM | Neuronal side effects n/a | [134] |
 Wistar rat Wistar rat | 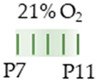 | 10 mg/kg s.c. P7–P11 20 mg/kg s.c. P7–P11 | Neurodevelopment reduced motoric performance hyperactivity | Neuronal side effects n/a | [135] |
| Species | Oxygen Exposure | Caffeine | Caffeine and the Immature Lung and the Developing Brain | Ref. | |
|---|---|---|---|---|---|
 |  | ||||
 Wistar rat |  | 10 mg/kg i.p. P6 10 mg/kg i.p. P6 | Pulmonary damage pro-inflammatory | Neurodegeneration pro-inflammatory modulated NfκB signaling pathway reduced hippocampal proliferation reduced hippocampal neurogenesis inhibited physiological apoptosis | [38,69,75] The studies use the identical cohorts. |
 Wistar rat |  | 10 mg/kg i.p. P0–P2 10 mg/kg i.p. P0–P4 | Pulmonary damage pro-inflammatory Pulmonary side effects modulated NfκB/Nrf2 signaling pathway and AdoRa1 expression Side effects gain loss | Neurodegeneration reduced hippocampal neurogenesis reduced hippocampal proliferation reduced neurotrophin expression Neuronal side effects increased AdoRA1, AdoRA2a, and AdoRA2b expression modulated cerebellar neurogenesis and migration induced cerebellar proliferation | [39,68,70,71] The studies use the identical cohorts. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endesfelder, S. Caffeine: The Story beyond Oxygen-Induced Lung and Brain Injury in Neonatal Animal Models—A Narrative Review. Antioxidants 2024, 13, 1076. https://doi.org/10.3390/antiox13091076
Endesfelder S. Caffeine: The Story beyond Oxygen-Induced Lung and Brain Injury in Neonatal Animal Models—A Narrative Review. Antioxidants. 2024; 13(9):1076. https://doi.org/10.3390/antiox13091076
Chicago/Turabian StyleEndesfelder, Stefanie. 2024. "Caffeine: The Story beyond Oxygen-Induced Lung and Brain Injury in Neonatal Animal Models—A Narrative Review" Antioxidants 13, no. 9: 1076. https://doi.org/10.3390/antiox13091076
APA StyleEndesfelder, S. (2024). Caffeine: The Story beyond Oxygen-Induced Lung and Brain Injury in Neonatal Animal Models—A Narrative Review. Antioxidants, 13(9), 1076. https://doi.org/10.3390/antiox13091076








