Coenzyme Q10 Improves the Post-Thaw Sperm Quality in Dwarf Surfclam Mulinia lateralis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gamete Collection and Cryoprotective Solution Preparation
2.2. Experimental Design
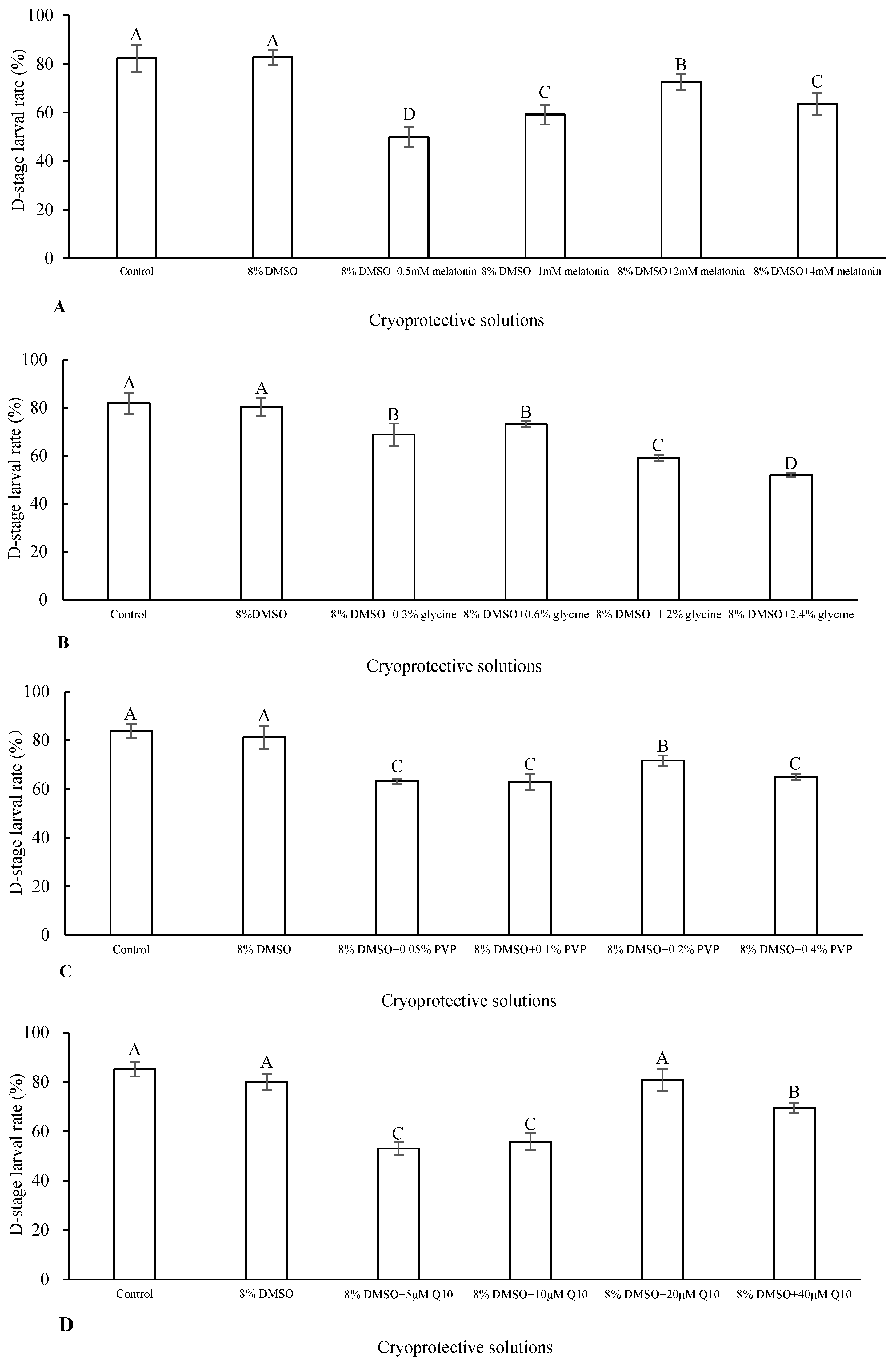
2.2.1. Effects of Types and Concentrations of Antioxidant on Post-Thaw D-Stage Larval Rate
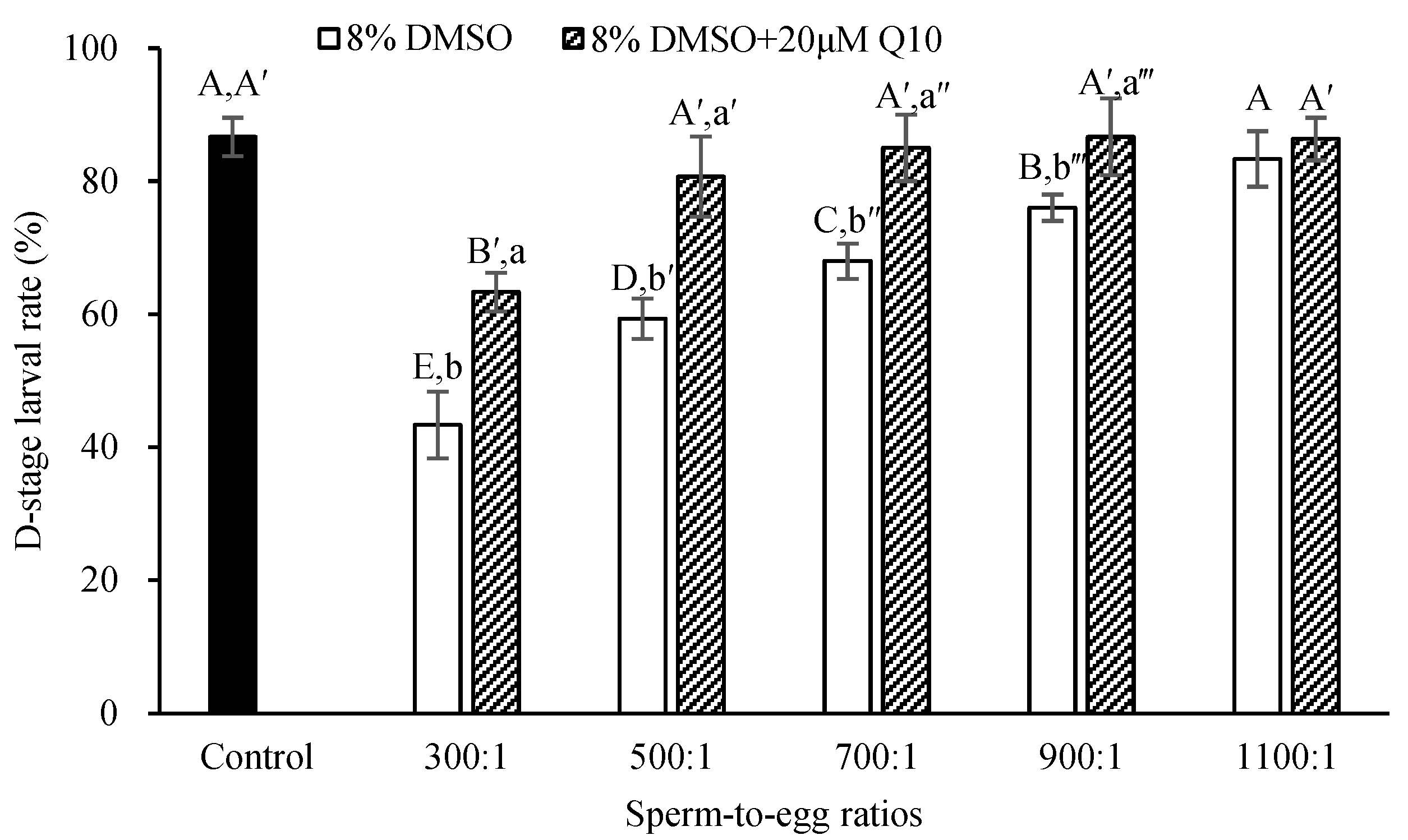
2.2.2. Comparison of Sperm-to-Egg Ratios on D-Stage Larval Rate between the Sperm Cryopreserved with 8% DMSO and 8% DMSO + 20 µM Q10
2.2.3. Comparison of Sperm Quality between Fresh and Cryopreserved Sperm
2.3. Sperm Quality Evaluation Methods
2.4. Statistical Analysis
3. Results
3.1. Effects of Types and Concentrations of Antioxidant on Post-Thaw D-Stage Larval Rate
3.2. Comparison of Sperm-to-Egg Ratio on D-Stage Larval Rate between the Sperm Cryopreserved with 8% DMSO and 8% DMSO + 20 µM Q10
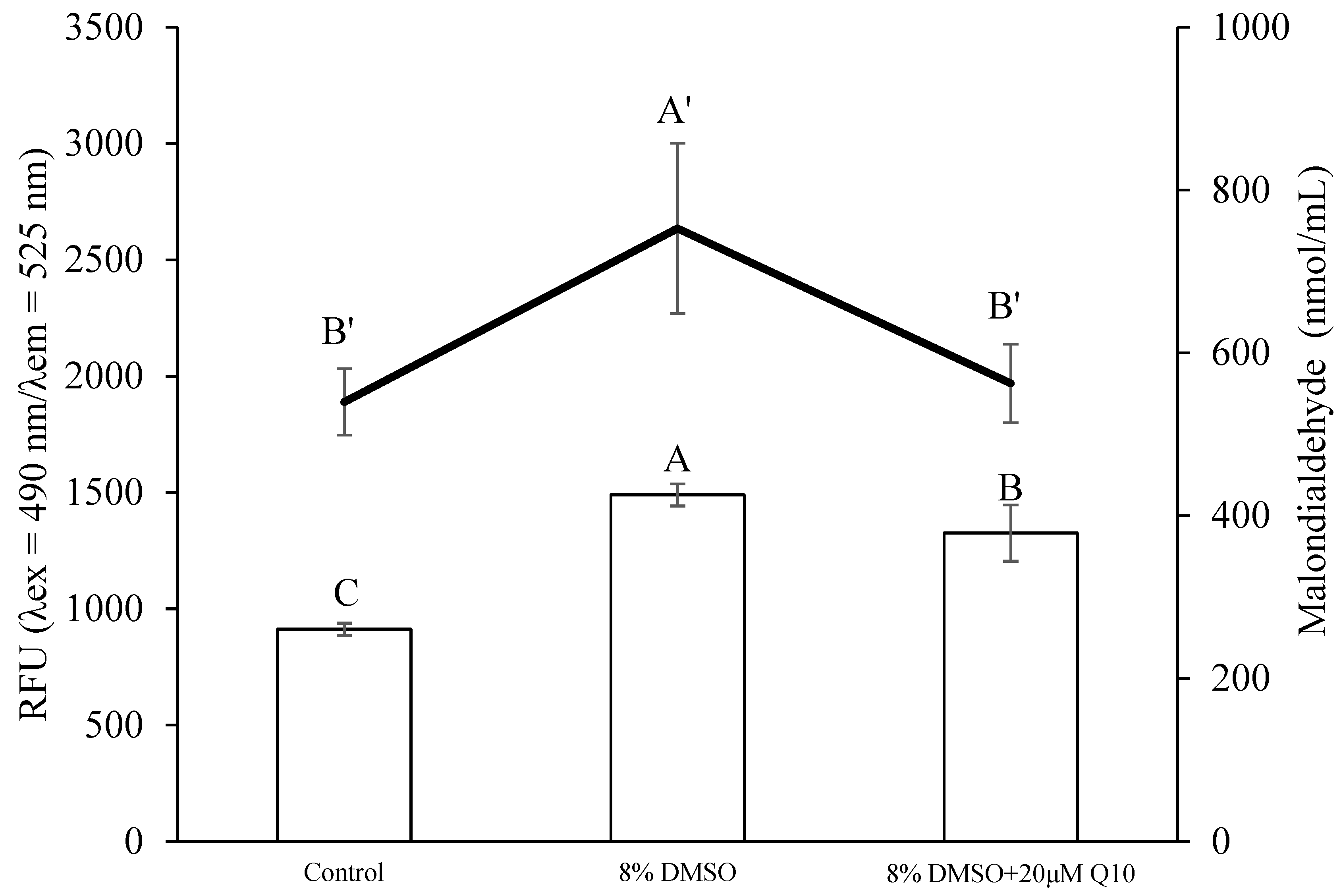
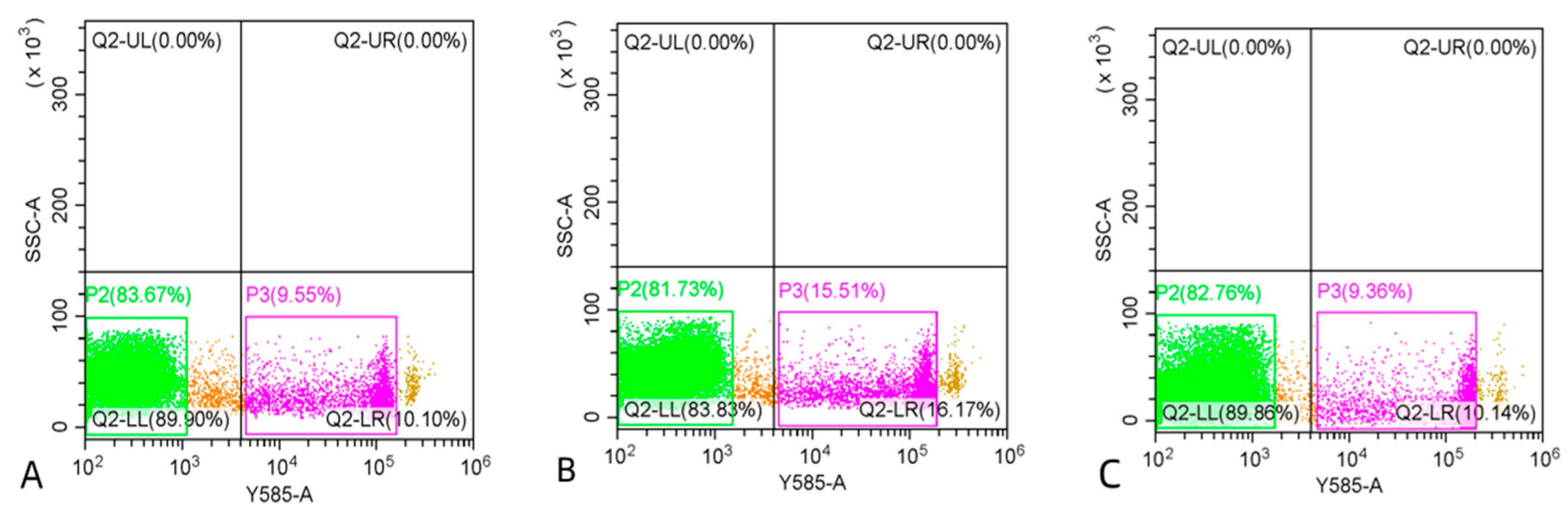
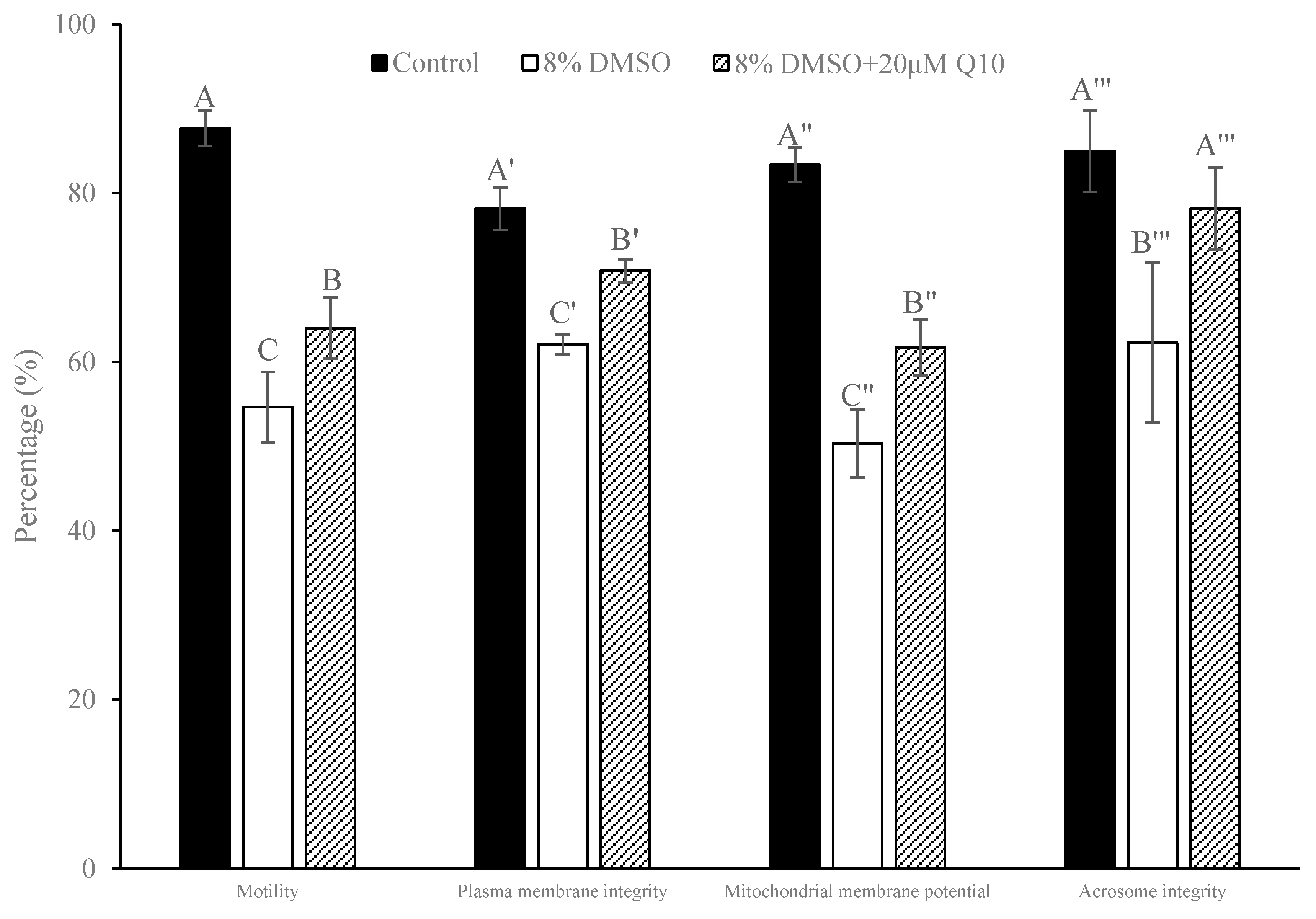
3.3. Comparison of Sperm Quality between Fresh and Cryopreserved Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willer, D.F.; Aldridge, D.C. Microencapsulated diets to improve bivalve shellfish aquaculture for global food security. Glob. Food Secur. 2019, 23, 64–73. [Google Scholar] [CrossRef]
- Fiorella, K.J.; Okronipa, H.; Baker, K.; Heilpern, S. Contemporary aquaculture: Implications for human nutrition. Curr. Opin. Biotechnol. 2021, 70, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Zhao, X.; Feng, K.S.; Hu, Y.C.; Tillotson, M.R.; Yang, L. Do mariculture products offer better environment and nutritional choices compared to land-based protein products in China? J. Clean. Prod. 2022, 372, 133697. [Google Scholar] [CrossRef]
- Bjørndal, T.; Dey, M.; Tusvik, A. Economic analysis of the contributions of aquaculture to future food security. Aquaculture 2024, 578, 740071. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture towards Blue Transformation; The Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- Willer, D.F.; Aldridge, D.C. Sustainable bivalve farming can deliver food security in the tropics. Nat. Food 2020, 1, 384–388. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Zheng, H. Selective breeding of edible bivalves and its implication of global climate change. Rev. Aquac. 2020, 12, 2559–2572. [Google Scholar] [CrossRef]
- Nascimento-Schulze, J.C.; Bean, T.P.; Houston, R.D.; Santos, E.M.; Sanders, M.B.; Lewis, C.; Ellis, R.P. Optimizing hatchery practices for genetic improvement of marine bivalves. Rev. Aquac. 2021, 13, 2289–2304. [Google Scholar] [CrossRef]
- Potts, R.W.A.; Gutierrez, A.P.; Penaloza, C.S.; Regan, T.; Bean, T.P.; Houston, R.D. Potential of genomic technologies to improve disease resistance in molluscan aquaculture. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200168. [Google Scholar] [CrossRef]
- Hassan, M.; Qin, J.G.; Li, X. Sperm cryopreservation in oysters: A review of its current status and potentials for future application in aquaculture. Aquaculture 2015, 438, 24–32. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Robinson, N.; Qin, J. Sperm cryopreservation in marine mollusk: A review. Aquac. Int. 2015, 23, 1505–1524. [Google Scholar] [CrossRef]
- Müller, T.; Matsubara, H.; Kubara, Y.; Horváth, Á.; Kolics, B.; Taller, J.; Stéger, V.; Kovács, B.; Horváth, L.; Asturiano, J.F.; et al. Testing cryopreserved European eel sperm for hybridization (A. japonica × A. anguilla). Theriogenology 2018, 113, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Nusbaumer, D.; da Cunha, L.M.; Wedekind, C. Sperm cryopreservation reduces offspring growth. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191644. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F.; Adams, S.L.; Gale, S.L.; McGowan, L.T.; Tervit, H.R.; Roberts, R.D. Cryopreservation of greenshellTM mussel (Perna canaliculus) sperm. I. Establishment of freezing protocol. Aquaculture 2012, 334–337, 199–204. [Google Scholar] [CrossRef]
- Guo, X.L.; Li, X.X.; Zhao, F.; Liu, D.W.; Yang, Z.J.; Li, M.L.; Li, Y.J.; Wei, H.L.; Wang, H.; Qin, Z.K.; et al. Full-length transcriptome analysis provides insights into larval shell formation in Mulinia lateralis. Front. Mar. Sci. 2023, 9, 1111241. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Z.; Bao, L.; Li, X.; Huang, X.; Liu, Y. Development of a non-programmable sperm cryopreservation technique in dwarf surfclams Mulinia lateralis–A potential model species for bivalve research. Front. Mar. Sci. 2024, 11, 1357454. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, L.J.; Zhang, L.J.; Wei, H.L.; Wu, S.X.; Liu, T.; Shu, Y.; Yang, Y.X.; Yang, Z.J.; Wang, S.; et al. Dynamic transcriptome analysis reveals the gene network of gonadal development from the early history life stages in dwarf surfclam Mulinia lateralis. Biol. Sex Differ. 2022, 13, 69. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Mirό, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
- Xin, M.; Niksirat, H.; Shaliutina-Kolešová, A.; Siddique, M.A.M.; Sterba, J.; Boryshpolets, S.; Linhart, O. Molecular and subcellular cryoinjury of fish spermatozoa and approaches to improve cryopreservation. Rev. Aquac. 2020, 12, 909–924. [Google Scholar] [CrossRef]
- Sandoval-Vargas, L.; Jiménez, M.S.; González, J.R.; Villalobos, E.F.; Cabrita, E.; Isler, I.V. Oxidative stress and use of antioxidants in fish semen cryopreservation. Rev. Aquac. 2021, 13, 365–387. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Nashtaei, M.S.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.G.; Pereira, R.R.; Bitencourt, J.L.; Milan, B.; Reis, W.V.; Junior, M.V.; Acácio, B.R.; Sampaio, B.F.; Souza, M.I. Coenzyme Q10 and melatonin protect cryopreserved equine sperm against lipoperoxidation. Anim. Reprod. Sci. 2022, 243, 107027. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z.; Kohram, H.; Nejati-Amiri, E.; Karimi, H.; Khodaei-Motlagh, M.; Shahverdi, A. Supplementation of extender with coenzyme Q10 improves the function and fertility potential of rooster spermatozoa after cryopreservation. Anim. Reprod. Sci. 2018, 198, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Lv, Y.; Zeng, W. Melatonin protects rabbit spermatozoa from cryo-damage via decreasing oxidative stress. Cryobiology 2019, 88, 1–8. [Google Scholar] [CrossRef]
- Jian, X.; Qian, F.; Wang, J.; Li, X.; Zhang, S.; Zhang, Z.; Yu, Y.; Gao, K.; Diao, Y.; Zhang, S.; et al. Effect of polyvinylpyrrolidone on cryopreservation of boar semen. China Anim. Husbandary Vet. Med. 2020, 47, 2553–2560, (In Chinese with English abstract). [Google Scholar]
- Nazif, M.S.; Rehman, Z.U.; Khan, H.; Khan, F.A.; Hussain, T.; Ahmad, A.; Farmanullah, A.; Husnain, A.; Muhammad, S.; Murtaza, G.; et al. Glycine Improved Cryopreserved Spermatozoa Quality in Achai Bull. BioMed Res. Int. 2022, 2022, 8282387. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Wang, H.; Pan, H.; Wang, X.; Teng, M.; Ren, Q.; Bao, Z. Effects of microalgae diets and stocking density on larval growth, survival and metamorphosis of dwarf surfclam, Mulinia lateralis. Aquaculture 2021, 536, 736440. [Google Scholar] [CrossRef]
- Gwo, J.; Chen, C.; Cheng, H. Semen cryopreservation of small abalone (Haliotis diversicolor supertexa). Theriogenology 2002, 58, 1563–1578. [Google Scholar] [CrossRef]
- Dong, Q.; Eudeline, B.; Huang, C.; Allen, S.K., Jr.; Tiersch, T.R. Commercial-scale sperm cryopreservation of diploid and tetraploid Pacific oysters, Crassostrea gigas. Cryobiology 2005, 50, 1–16. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, J.; Ye, T.; Wang, C.; Zhu, Y.; Dahms, H.; Jin, F.; Yang, W. Improvement of single-cell gel electrophoresis (SCGE) alkaline comet assay. Aquat. Biol. 2013, 18, 293–295. [Google Scholar] [CrossRef]
- Martins, C.; Costa, P.M. Technical Updates to the Comet Assay In Vivo for Assessing DNA Damage in Zebrafish Embryos from Fresh and Frozen Cell Suspensions. Zebrafish 2020, 17, 220–228. [Google Scholar] [CrossRef]
- Shaliutina-Loginova, A.; Loginov, D.S. Oxidative stress and DNA fragmentation in frozen/thawed common Carp Cyprinus carpio sperm with and without supplemental proteins. Anim. Reprod. Sci. 2023, 251, 107213. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Am-In, N.; Kirkwood; Techakumphu, M.; Tantasuparuk, W. Lipid profiles of sperm and seminal plasma from boars having normal or low sperm motility. Theriogenology 2011, 75, 897–903. [Google Scholar] [CrossRef]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef]
- Hai, E.; Li, B.; Zhang, J. Sperm freezing damage: The role of regulated cell death. Cell Death Discov. 2024, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Xu, T.; Robinson, N.; Qin, J. Improvement in non-programmable sperm cryopreservation technique in farmed greenlip abalone Haliotis laevigata. Aquaculture 2014, 434, 362–366. [Google Scholar] [CrossRef]
- Li, Y.; Si, W.; Zhang, X.; Dinnyes, A.; Ji, W. Effect of amino acids on cryopreservation of cynomolgus monkey (Macaca fascicularis) sperm. Am. J. Primatol. 2003, 59, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, J.; Qazi, I.H.; Fang, Y.; Zhou, G. Use of melatonin in sperm cryopreservation of farm animals: A brief review. Anim. Reprod. Sci. 2021, 233, 106850. [Google Scholar] [CrossRef]
- Yang, S.; Fan, B.; Chen, X.; Meng, Z. Supplementation of the freezing medium with Coenzyme Q10 attenuates oxidative stress and improves function of frozen-thawed giant grouper (Epinephelus lanceolatus) spermatozoa. Theriogenology 2021, 175, 77–82. [Google Scholar] [CrossRef]
- Khan, B.I.; Akhter, S.; Aslam, S.; Ejaz, R. Effect of Supplementation of Polyvinylpyrrolidone in Extender on Buffalo Semen Parameters. Pak. J. Zool. 2020, 53, 9–15. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Zafar, Z.; Hussain, I.; Santiago-Moreno, J.; Blesbois, E. Cryopreservation of Indian red jungle fowl (Gallus gallus murghi) semen with polyvinylpyrrolidone. Cryobiology 2017, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, R.; Sharafi, M.; Shahneh, A.Z. Effects of CoQ10 on the quality of ram sperm during cryopreservation in plant and animal based extenders. Anim. Reprod. Sci. 2019, 208, 106103. [Google Scholar] [CrossRef]
- Ahmed, H.; Ijaz, M.U.; Jahan, S.; Riaz, M.; Samir, H.; Swelum, A.A. Coenzyme Q10 improves the quality and in vitro fertility of post-thawed buffalo (Bubalus bubalis) semen via its antioxidative effect. Reprod. Domest. Anim. 2023, 59, e14515. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; di Emidio, G.; Vento, M.; Ciriminna, R.; Artini, P.G. Cryopreservation and oxidative stress in reproductive cells. Gynecol. Endocrinol. 2010, 26, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Yeste, M.; Zambrano, F.; Uribe, P.; Risopatrón, J.; Sánchez, R. Antioxidants and their effect on the oxidative/nitrosative stress of frozen-thawed boar sperm. Cryobiology 2021, 98, 5–11. [Google Scholar] [CrossRef]
- Yousefian, I.; Emamverdi, M.; Karamzadeh-Dehaghani, A.; Sabzian-Melei, R.; Zhandi, M.; Zare-Shahneh, A. Attenuation of cryopreservation-induced oxidative stress by antioxidant: Impact of Coenzyme Q10 on the quality of post-thawed buck spermatozoa. Cryobiology 2018, 81, 88–93. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Boulais, M.; Demoy-Schneider, M.; Alavi, S.M.H.; Cosson, J. Spermatozoa motility in bivalves: Signaling, flagellar beating behavior, and energetics. Theriogenology 2019, 136, 15–27. [Google Scholar] [CrossRef]
- Díaz, R.; Lee-Estevez, M.; Quiñones, J.; Dumorné, K.; Short, S.; Ulloa-Rodríguez, P.; Valdebenito, I.; Sepúlveda, N.; Farías, J.G. Changes in Atlantic salmon (Salmo salar) sperm morphology and membrane lipid composition related to cold storage and cryopreservation. Anim. Reprod. Sci. 2019, 204, 50–59. [Google Scholar] [CrossRef]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.C.C.R.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Kogan, T.; Dahan, D.G.; Laor, R.; Argov-Argaman, N.; Zeron, Y.; Komsky-Elbaz, A.; Kalo, D.; Roth, Z. Association between fatty acid composition, cryotolerance and fertility competence of progressively motile bovine spermatozoa. Animals 2021, 11, 2948. [Google Scholar] [CrossRef]
- Oddi, S.; Carluccio, A.; Ciaramellano, F.; Mascini, M.; Bucci, R.; Maccarrone, M.; Robbe, D.; Dainese, E. Cryotolerance of equine spermatozoa correlates with specific fatty acid pattern: A pilot study. Theriogenology 2021, 172, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.C.; Dinh, T.T.N.; Ugur, M.R.; Hitit, M.; Sajeev, D.; Kaya, A.; Topper, E.; Nicodemus, M.C.; Smith, G.D.; Memili, E. Lipidomic markers of sperm cryotolerance in cattle. Sci. Rep. 2020, 10, 20192. [Google Scholar] [CrossRef]
- García, B.M.; Fernández, L.G.; Ferrusola, C.O.; Salazar-Sandoval, C.; Rodríguez, A.M.; Martinez, H.R.; Tapia, J.; Morcuende, D.; Peña, F. Membrane Lipids of the Stallion Spermatozoon in Relation to Sperm Quality and Susceptibility to Lipid Peroxidation. Reprod. Domest. Anim. 2011, 46, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Beirão, J.; Zilli, L.; Vilella, S.; Cabrita, E.; Schiavone, R.; Herráez, M.P. Improving sperm cryopreservation with antifreeze proteins: Effect on gilthead seabream (Sparus aurata) plasma membrane lipids. Biol. Reprod. 2012, 86, 1–9. [Google Scholar] [CrossRef]
- Lewis, S.E.M.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell. Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef]
- Prochowska, S.; Bonarska-Kujawa, D.; Bobak, Ł.; Eberhardt, M.; Niżański, W. Fatty acid composition and biophysical characteristics of the cell membrane of feline spermatozoa. Sci. Rep. 2024, 14, 10214. [Google Scholar] [CrossRef]
- Melville, D.F.; Johnston, S.D.; Miller, R.R., Jr. Flying-fox (Pteropus spp.) sperm membrane fatty acid composition, its relationship to cold shock injury and implications for cryopreservation success. Cryobiology 2012, 65, 224–229. [Google Scholar] [CrossRef]
- Horokhovatskyi, Y.; Sampels, S.; Cosson, J.; Linhart, O.; Rodina, M.; Fedorov, P.; Blecha, M.; Dzyuba, B. Lipid composition in common carp (Cyprinus carpio) sperm possessing different cryoresistance. Cryobiology 2016, 73, 282–285. [Google Scholar] [CrossRef]
- Kaka, A.; Wahid, H.; Rosnina, Y.; Yimer, N.; Khumran, A.; Sarsaifi, K.; Behan, A.A.; Kaka, U.; Ebrahimi, M. α-Linolenic acid supplementation in BioXcell® extender can improve the quality of post-cooling and frozen-thawed bovine sperm. Anim. Reprod. Sci. 2015, 153, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R., Jr.; Sheffer, C.J.; Cornett, C.L.; McClean, R.; MacCallum, C.; Johnston, S.D. Sperm membrane fatty acid composition in the Eastern grey kangaroo (Macropus giganteus), koala (Phascolarctos cinereus), and common wombat (Vombatus ursinus) and its relationship to cold shock injury and cryopreservation success. Cryobiology 2004, 49, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Maldjian, A.; Pizzi, F.; Gliozzi, T.; Cerolini, S.; Penny, P.; Noble, R. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Theriogenology 2005, 63, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, N.; Boroda, A.; Velansky, P.; Kostetsky, E. The fatty acid profile changes in marine invertebrate larval cells during cryopreservation. Cryobiology 2009, 59, 335–343. [Google Scholar] [CrossRef]
- Surai, P.F.; Fujihara, N.; Speake, B.K.; Brillard, J.P.; Wishart, G.J.; Sparks, N.H.C. Polyunsaturated fatty acid, lipid peroxidation and antioxidant protection in avian semen-review. Asian-Aust. J. Anim. Sci. 2001, 14, 1024–1050. [Google Scholar] [CrossRef]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Appiah, M.O.; Asante-Badu, B.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Possible protective mechanisms of coenzyme Q10 action on spermatozoa during cryopreservation or cooled-stored condition. Cryoletters 2020, 41, 246–256. [Google Scholar]
| Enzyme Activity | Group | p Value | ||
|---|---|---|---|---|
| Control | 8% DMSO | 8% DMSO + 20 μM Q10 | ||
| SOD (U/mL) | 18.1 ± 1.0 A | 8.8 ± 1.5 B | 10.0 ± 0.5 B | <0.001 |
| CAT (U/mL) | 8.5 ± 0.8 A′ | 4.8 ± 0.7 B′ | 7.7 ± 1.6 A′ | 0.015 |
| GSH (μM) | 3.5 ± 0.4 A″ | 1.7 ± 0.2 B″ | 2.7 ± 0.7 A″ | 0.012 |
| Group | p Value | |||
|---|---|---|---|---|
| Control | 8% DMSO | 8% DMSO + 20 μM Q10 | ||
| Grade 0 (%) | 67.7 ± 2.1 A | 29.7 ± 5.5 C | 44.3 ± 3.2 B | <0.001 |
| Grade I (%) | 29.7 ± 1.5 B | 38.0 ± 1.00 A | 33.0 ± 2.6 B | 0.005 |
| Grade II (%) | 2.7 ± 1.2 C | 23.0 ± 2.6 A | 18.0 ± 1.0 B | <0.001 |
| Grade III (%) | 0 C | 9.3 ± 1.2 A | 4.7 ± 1.2 B | <0.001 |
| Grade IV (%) | 0 | 0 | 0 | |
| Comet rate (%) | 32.3 ± 2.1 C | 70.3 ± 5.5 A | 55.7 ± 3.2 B | <0.001 |
| Group | p Value | |||
|---|---|---|---|---|
| Control | 8% DMSO | 8% DMSO + 20 μM Q10 | ||
| Damage coefficient | 35.0 ± 3.0 C | 112.0 ± 15.1 A | 83.0 ± 4.4 B | <0.001 |
| Tail DNA (%) | 5.0 ± 0.4 C | 16.2 ± 2.8 A | 11.7 ± 0.6 B | <0.001 |
| Tail length (μm) | 5.5 ± 0.4 B | 10.5 ± 2.4 A | 9.1 ± 0.7 A | 0.014 |
| Comet length (μm) | 36.5 ± 1.6 C | 48.4 ± 3.2 A | 40.4 ± 0.2 B | 0.001 |
| Tail moment | 0.4 ± 0.1 C | 2.7 ± 0.3 A | 1.9 ± 0.2 B | <0.001 |
| Olive tail moment | 0.7 ± 0.1 C | 3.0 ± 0.2 A | 2.0 ± 0.2 B | <0.001 |
| Fatty Acid (%) | Control | 8% DMSO | 8% DMSO + 20 μM Q10 | p Value |
|---|---|---|---|---|
| Saturated | 80.728 ± 1.106 A | 76.077 ± 1.314 B | 77.042 ± 0.770 B | 0.004 |
| Butyric acid, C4:0 | 5.211 ± 0.585 A | 2.339 ± 0.417 B | 1.988 ± 0.377 B | <0.001 |
| Hexanoic acid, C6:0 | 4.350 ± 0.942 | 4.705 ± 0.388 | 3.789 ± 0.381 | 0.261 |
| Octanoic acid, C8:0 | 0.563 ± 0.110 C | 3.604 ± 0.512 B | 4.625 ± 0.478 A | <0.001 |
| Decanoic acid, C10:0 | 2.819 ± 0.659 | 3.203 ± 0.155 | 3.192 ± 0.376 | 0.493 |
| Undecanoic acid, C11:0 | 7.222 ± 0.779 | 7.241 ± 0.116 | 7.437 ± 0.439 | 0.854 |
| Dodecanoic acid, C12:0 | 7.107 ± 0.813 | 6.806 ± 0.097 | 7.153 ± 0.279 | 0.676 |
| Tridecylic acid, C13:0 | 26.570 ± 2.189 | 24.380 ± 1.949 | 24.030 ± 1.003 | 0.252 |
| Tetradecanoic acid, C14:0 | 4.447 ± 0.649 A | 2.792 ± 0.072 B | 3.397 ± 0.169 B | 0.004 |
| Pentadecanoic acid, C15:0 | 2.038 ± 0.488 | 1.606 ± 0.089 | 1.725 ± 0.111 | 0.255 |
| Hexadecanoic acid, C16:0 | 2.231 ± 0.110 A | 2.227 ± 0.125 A | 1.767 ± 0.065 B | 0.002 |
| Heptadecanoic acid, C17:0 | 1.521 ± 0.182 | 1.740 ± 0.138 | 1.508 ± 0.103 | 0.176 |
| Octadecanoic acid, C18:0 | 15.739 ± 0.503 | 14.760 ± 1.079 | 15.479 ± 1.535 | 0.574 |
| Eicosanic acid, C20:0 | 0.808 ± 0.137 A | 0.381 ± 0.016 B | 0.775 ± 0.045 A | 0.001 |
| Heneicosanoic acid, C21:0 | 0.050 ± 0.006 A | 0.013 ± 0.004 C | 0.025 ± 0.003 B | <0.001 |
| Docosanoic acid, C22:0 | 0.025 ± 0.002 B | 0.128 ± 0.003 A | 0.015 ± 0.002 C | <0.001 |
| Tricosylic acid, C23:0 | 0.018 ± 0.004 B | 0.036 ± 0.001 A | 0.029 ± 0.004 A | 0.002 |
| Tetracosanoic acid, C24:0 | 0.010 ± 0.003 B | 0.117 ± 0.008 A | 0.106 ± 0.004 A | <0.001 |
| Monounsaturated | 12.457 ± 1.026 B | 17.201 ± 1.111 A | 15.863 ± 0.522 A | 0.002 |
| Tetradecenoic acid, C14:1 | 0.027 ± 0.005 B | 0.039 ± 0.001 A | 0.045 ± 0.003 A | 0.004 |
| Pentadecenoic acid, C15:1 | 8.230 ± 0.781 B | 11.390 ± 0.850 A | 9.809 ± 0.891 AB | 0.011 |
| Palmitoleic acid, C16:1 | 0.106 ± 0.010 B | 0.148 ± 0.003 A | 0.152 ± 0.009 A | 0.001 |
| Heptadecenoic acid, C17:1 | 1.052 ± 0.032 A | 0.939 ± 0.048 B | 1.028 ± 0.032 A | 0.026 |
| Octadecenoic acid, C18:1T | 1.735 ± 0.218 B | 2.382 ± 0.187 A | 2.057 ± 0.285 AB | 0.041 |
| Oleic acid, C18:1 | 0.203 ± 0.035 | 0.240 ± 0.040 | 0.216 ± 0.027 | 0.459 |
| Eicosenoicacid, C20:1 | 1.063 ± 0.158 B | 1.380 ± 0.125 AB | 1.700 ± 0.227 A | 0.012 |
| Erucic acid, C22:1 | 0.028 ± 0.007 C | 0.638 ± 0.056 B | 0.821 ± 0.030 A | <0.001 |
| Nervonic acid, C24:1 | 0.014 ± 0.001 C | 0.044 ± 0.004 A | 0.035 ± 0.004 B | <0.001 |
| Polyunsaturated | 6.815 ± 0.202 | 6.722 ± 0.250 | 7.095 ± 0.305 | 0.257 |
| Linoleic acid, C18:2 | 1.826 ± 0.095 B | 1.652 ± 0.072 B | 2.575 ± 0.117 A | <0.001 |
| Linolenic acid, C18:3 | 0.016 ± 0.004 B | 0.021 ± 0.003 AB | 0.026 ± 0.002 A | 0.030 |
| Eicosadienoic acid, C20:2 | 0.356 ± 0.040 B | 0.448 ± 0.057 A | 0.386 ± 0.010 AB | 0.079 |
| Eicosatrienoic acid, C20:3 | 0.091 ± 0.010 A | 0.077 ± 0.006 A | 0.053 ± 0.005 B | 0.002 |
| Arachidonic acid, C20:4 | 0.465 ± 0.043 | 0.446 ± 0.021 | 0.421 ± 0.012 | 0.232 |
| Eicosapentaenoic acid, C20:5 | 3.725 ± 0.095 A | 3.405 ± 0.098 B | 3.059 ± 0.202 C | 0.004 |
| Docosadienoic acid, C22:2 | 0.086 ± 0.010 C | 0.182 ± 0.004 A | 0.144 ± 0.005 B | <0.001 |
| Docosatetraenoic acid, C22:4 | 0.075 ± 0.006 B | 0.097 ± 0.007 A | 0.079 ± 0.004 B | 0.007 |
| Docosapentaenoic acid, C22:5 | 0.034 ± 0.011 | 0.034 ± 0.006 | 0.028 ± 0.004 | 0.592 |
| Osbond acid, C22:5 | 0.011 ± 0.001 A | 0.009 ± 0.001 B | 0.009 ± 0.000 B | 0.014 |
| Docosahexaenoic acid, C22:6 | 0.129 ± 0.018 B | 0.350 ± 0.020 A | 0.314 ± 0.065 A | 0.001 |
| Unsaturated/saturated ratio | 0.239 ± 0.017 B | 0.315 ± 0.023 A | 0.298 ± 0.013 A | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Yang, Z.; Bao, L.; Lu, B.; Li, X.; Zhan, X.; Huang, X.; Liu, Y. Coenzyme Q10 Improves the Post-Thaw Sperm Quality in Dwarf Surfclam Mulinia lateralis. Antioxidants 2024, 13, 1085. https://doi.org/10.3390/antiox13091085
Xu Z, Yang Z, Bao L, Lu B, Li X, Zhan X, Huang X, Liu Y. Coenzyme Q10 Improves the Post-Thaw Sperm Quality in Dwarf Surfclam Mulinia lateralis. Antioxidants. 2024; 13(9):1085. https://doi.org/10.3390/antiox13091085
Chicago/Turabian StyleXu, Zhen, Zujing Yang, Lisui Bao, Bei Lu, Xiaoxu Li, Xin Zhan, Xiaoting Huang, and Yibing Liu. 2024. "Coenzyme Q10 Improves the Post-Thaw Sperm Quality in Dwarf Surfclam Mulinia lateralis" Antioxidants 13, no. 9: 1085. https://doi.org/10.3390/antiox13091085
APA StyleXu, Z., Yang, Z., Bao, L., Lu, B., Li, X., Zhan, X., Huang, X., & Liu, Y. (2024). Coenzyme Q10 Improves the Post-Thaw Sperm Quality in Dwarf Surfclam Mulinia lateralis. Antioxidants, 13(9), 1085. https://doi.org/10.3390/antiox13091085






