Abstract
The placenta plays a crucial role in nutrient transport and waste exchange between the dam and fetus, sustaining fetal growth. While the positive effects of 25-hydroxyvitamin D3 (25-OH-D3) on animal performance have been reported, its impact on placental function remains largely unknown. Therefore, this study aimed to investigate the effects of supplementing 25-OH-D3 in the diet of primiparous sows on reproductive performance, antioxidant capacity, placental oxidative stress, nutrient transport, and inflammatory response during mid-to-late gestation. A total of 45 healthy Landrace × Yorkshire primiparous sows on day 60 of gestation were selected and randomly allocated to three treatment groups based on body weight and backfat thickness: the control group (corn-soybean meal basal diet), the VD3 group (basal diet + 2000 IU VD3), and the 25-OH-D3 group (basal diet + 50 μg/kg 25-OH-D3). The results demonstrated that supplementation with 25-OH-D3 in the diet enhanced sows’ average litter weight and birth weight during mid-to-late gestation. Additionally, plasma malondialdehyde (MDA) concentrations in sows significantly decreased in the VD3 and 25-OH-D3 groups (p < 0.05). Furthermore, lower gene expressions of placental HO-1, GPX2, IL-8, and IL-6 were found in the VD3 or 25-OH-D3 groups (p < 0.05 or p < 0.10), while higher gene expressions of GLUT1 and SNAT2 in the placenta of sows were observed in the VD3 and 25-OH-D3 groups, respectively (p < 0.05). These findings indicate that the supplementation of VD3 and 25-OH-D3 in the diet of sows can improve their plasma oxidative stress status, enhance placental antioxidant capacity and nutrient transport, and reduce placental inflammatory responses, with more pronounced improvements in sow performance observed in sows fed diets supplemented with 25-OH-D3.
1. Introduction
In commercial swine production, the reproductive performance of sows, which includes the number of piglets born and alive, birth weight, and litter weight, is the most crucial economic indicator closely associated with pig production efficiency [1]. It is wellknown that sow pregnancy is a dynamic and precise process involving embryonic development, physiological changes, fetal growth, placental development, and maternal body reconstruction, leading to increased metabolic demands on sows, especially during mid-to-late gestation [2,3]. Numerous studies have demonstrated that maternal metabolism significantly increases during late gestation to meet the rapid fetal growth’s energy and nutrient needs. However, this process can also lead to excessive reactive oxygen species (ROS) production, resulting in maternal oxidative stress, particularly for hyper-prolific sows, which can impact energy balance, reproductive performance of sows, and subsequent piglet livability [4,5,6]. Furthermore, there is evidence suggesting that maternal oxidative stress also impacts placental oxidative stress status and function, such as trophoblast proliferation and differentiation and angiogenesis, which can limit the nutrient delivery from the dam to the fetus, ultimately affecting fetal metabolism and development [7,8,9].
Vitamin D3 (VD3) is a critical fat-soluble vitamin with a unique absorption mechanism that involves two hydroxylation processes. It is converted to the active form, 25-hydroxyvitamin D3 (25-OH-D3), via 25-hydroxylase (CYP24A1) in the liver and then converted to 1,25-dihydroxy vitamin D3 (1,25-(OH)2-D3) by 1 α-hydroxylase (CYP27B1) in the kidney [10]. VD3 participates in multiple biological processes, including intestinal calcium and phosphorus absorption, bone and muscle development, cell proliferation and differentiation, and the regulation of oxidative stress and inflammation [11,12,13]. In recent years, some reports revealed that exposure to oxidative stress affects the absorption of VD3 and inhibits its conversion to 25-OH-D3 in the liver. In contrast, dietary supplementation of 25-OH-D3 could enhance maternal antioxidant capacity and improve the VD3 status of the mother and fetus during the late gestation and lactation periods of sows [14,15,16]. However, there is a lack of knowledge regarding the dietary supplementation of 25-OH-D3 in alleviating placental oxidative stress and its effects on nutrient transfer and fetal development in sows. Therefore, the present study aims to investigate the effects of the active and original forms of VD3 on the reproductive performance, placenta antioxidant capacity, and function of primiparous sows via supplementing equivalent doses of VD3 and 25-OH-D3 to the diet during mid-to-late gestation.
2. Materials and Methods
2.1. Ethic Statement
The animal experiment was conducted at the Shandong Liaocheng Experimental Pig Farm, and all the procedures in this study were performed following the Institutional Animal Care and Use Committee of the Institute of Feed Research of the Chinese Academy of Agricultural Sciences (IFR-CAAS-20230908).
2.2. Animals and Experimental Design
On day 60 of gestation, a total of 45 healthy Landrace × Yorkshire primiparous sows were selected and randomly allocated into 3 groups based on body weight (198.3 ± 10.2 kg) and backfat thickness (18.85 ± 0.44 mm). Each group had 15 replicates, with 1 sow per replicate. The control group (CT) was fed the commercial gestation diet, the VD3 group was fed the CT diet supplemented with 2000 IU/kg VD3, and the 25-OH-D3 group was fed the CT diet supplemented with 50 µg/kg 25-OH-D3, whose active substance equaled to 2000 IU/kg VD3. The products of cholecalciferol (>99% VD3) and calcifediol monohydrate (>98% 25-OH-D3) were provided by Shandong Tonghui Biotechnology Co., Ltd. (Binzhou, China). The commercial gestation diet for sows was formulated according to the National Research Council’s (NRC, 2012) nutritional requirements (shown in Table 1). The entire experimental period spanned 54 d, from day 60 of gestation to the end of farrowing.

Table 1.
Ingredient composition and nutritional levels of the basal diets for pregnant sows (%, as-fed basis).
On day 60 of gestation, the sows were housed in the same room and fed 2.5 kg of experimental diet per day (from day 60 to day 85 of gestation) and 3.0 kg of experimental diet per day (from day 85 of gestation to farrowing), with equivalent feedings at 8:00 am and 4:00 pm. On day 107 of gestation, each sow was transferred to a separate farrowing crate (2.20 m × 1.80 m) following the pre-farrowing feeding procedure. The gestation and farrowing rooms were maintained at 22 °C and 25 °C, respectively. All the sows were managed in accordance with routine immunization and deworming procedures.
2.3. Reproductive Performance Analysis
The body weight and backfat thickness of sows were measured on days 60 and 107 of gestation. Backfat thickness was measured using ultrasound equipment (Renco Lean-Meatier; Renco Corporation, Manchester, MA, USA) at a point 65 mm to the right of the dorsal midline of the last rib. At farrowing, the number of total piglets born, born alive, stillborn, mummies, and piglets weighing less than 1.0 kg were recorded for each sow. The piglets were weighed within 24 h after farrowing, and the average birth weight and average litter weight were calculated.
2.4. Sample Collection
On days 60 and 107 of gestation, 10 mL of blood was collected from the ear vein of each sow using a heparin tube. At farrowing, 10 mL of umbilical cord blood was extracted by pressing the umbilical cord blood from maternal side to fetus at a point 10–15 cm from the root. From each sow, 3 umbilical cord blood samples were collected from 3 different fetuses. All blood samples were centrifuged at 3000 rpm at 4 °C for 15 min to obtain plasma and stored at −20 °C for antioxidant indicator analysis.
In addition, a total of 3 placental tissues from each sow were immediately separated and placed in liquid nitrogen after farrowing. All samples were ground and crushed under liquid nitrogen conditions, transferred to the 2 mL cryopreservation tubes, and stored at −80 °C for further use.
2.5. Assay of Antioxidant Indicators
The antioxidant indicators in plasma and umbilical cord blood were determined, which included measuring the concentrations of malondialdehyde (MDA) and the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT). The determination of the antioxidant indicators strictly followed the protocols of the individual ELISA kit specifically designed for pigs (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.6. Gene Expression Analysis
The total RNA was extracted from placental tissues using TRIzol reagent with an A260/280 value ranging between 1.8 and 2.0 and subsequently frozen at −80 °C until further use. The cDNA was obtained using the TakaRa reverse transcription kit (PrimeScriptTM RT reagent Kit with gDNA Eraser; Takara; Beijing, China). A quantitative real-time PCR (qRT-PCR) protocol was developed to measure the mRNA expression levels of antioxidant genes (HO-1, Nrf2, SOD1, SOD2, CAT, GPX1, and GPX2), nutrient transporter genes (GLUT1, SNAT1, SNAT2, and VEGFA), and inflammation genes (IL-8, IL-6, IL-1β, and TNF-α) according to the manufacturer’s instructions (TB Green® Premix Ex Taq ™; Takara; Beijing, China). The cycling process of qRT-PCR was as follows: 95 °C for 3 min, 40 cycles of denaturing at 95 °C for 10 s and annealing at 60 °C for 30 s, and the final elongation at 60 °C for 5 s and ending at 95 °C for 10 s. The obtained data were analyzed to calculate the expression of target genes relative to GAPDH as a housekeeping gene using the 2-∆∆Ct method described by Livak et al. [17]. All primer sequences for the genes and GADPH (as a housekeeping gene) were designed by Primer 3.0, and the primers’ quality was tested using agarose gel electrophoresis. All primer sequences used in this study are listed in Table 2.

Table 2.
Primer sequences used for quantitative real-time PCR.
2.7. Statistical Analysis
All experimental data were analyzed using the one-way ANOVA in SPSS 22.0. The Tukey method was performed for multiple comparisons. All the data were considered a significant difference among treatments when p < 0.05 and a trend difference among treatments when 0.05 ≤ p < 0.10.
3. Results
3.1. Reproductive Performance
The effect of dietary supplementation with VD3 and 25-OH-D3 on the reproductive performance of primiparous sows is presented in Table 3. The results showed that there were no differences in sows’ body weight and average backfat thickness on days 60 and 107 among the three treatments (p > 0.05). Additionally, no differences were obtained in the number of total born, born alive, stillborn, and mummified among the treatments. Furthermore, the average litter weight and average piglet weight were higher in the 25-OH-D3 group, showing a significant increase compared to the VD3 group (p < 0.05). Moreover, it was found that the proportions of piglets with a birth weight less than 1.0 kg were lower in sows fed diets supplemented with VD3 and 25-OH-D3 during the mid-to-late gestation period compared to the CT group.

Table 3.
The effect of dietary supplementation with VD3 and 25-OH-D3 on the reproductive performance of primiparous sows during mid-to-late gestation.
3.2. Plasma Antioxidant Capacities of Sows
The effect of dietary supplementation with VD3 and 25-OH-D3 on the plasma antioxidant capacity of primiparous sows is presented in Table 4. The results demonstrated that there were no significant differences in the concentrations of plasma MDA and the activities of SOD, GSH-Px, and CAT among the treatment groups on day 60 of gestation (p > 0.05). However, on day 107 of gestation, sows fed diets supplemented with VD3 and 25-OH-D3 reduced the concentration of plasma MDA (p < 0.05), whereas no significant impact was found on the activities of plasma SOD, GSH-Px, and CAT (p > 0.05).

Table 4.
The effect of dietary supplementation with VD3 and 25-OH-D3 on the plasma antioxidant capacity of primiparous sows during mid-to-late gestation.
3.3. Placenta Antioxidant Capacity
To further investigate the effects of dietary supplementation with VD3 and 25-OH-D3 on placental antioxidant capacity, we determined the antioxidant indicators in umbilical cord blood and the mRNA expression of the key antioxidant-related genes in placental tissue. The results revealed that there were no significant differences in the concentrations of MDA and the activities of SOD, GSH-Px, and CAT in the umbilical cord blood among the treatment groups (p > 0.05) (shown in Table 5).

Table 5.
The effect of maternal dietary supplementation with VD3 and 25-OH-D3 during mid-to-late gestation on the antioxidant capacity in the umbilical cord blood of newborn piglets.
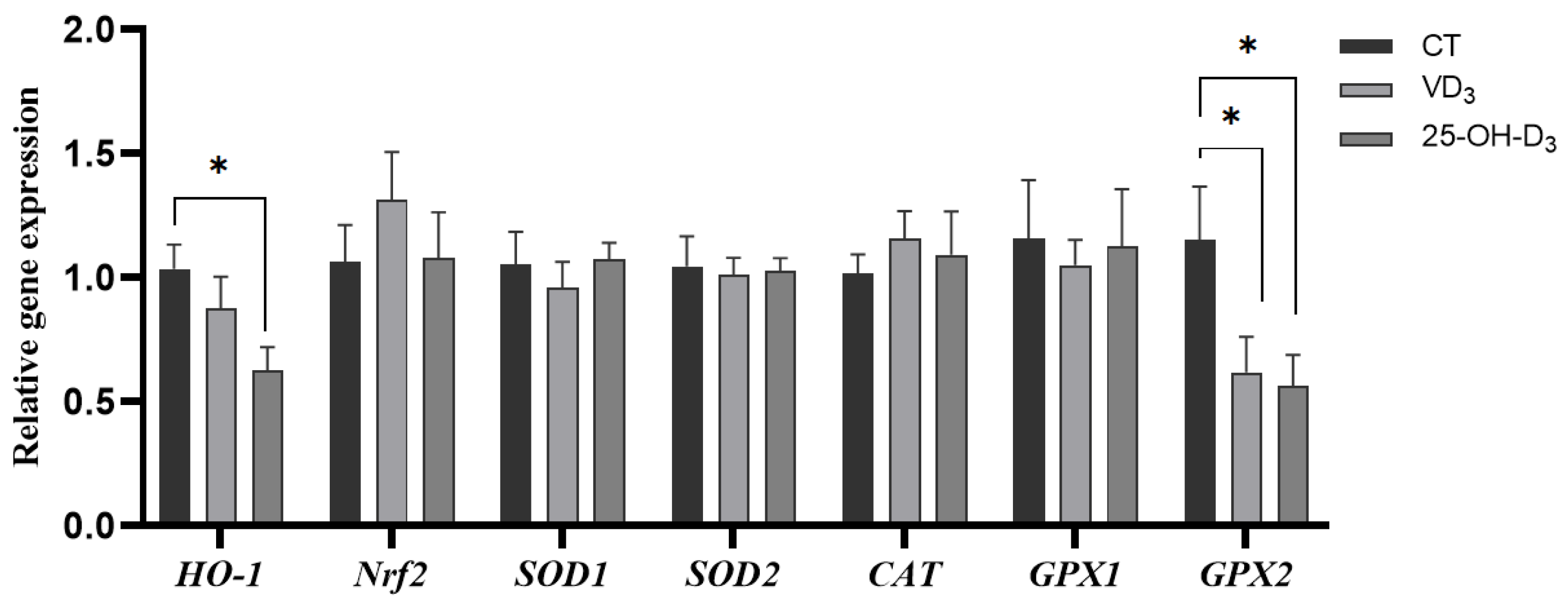
Furthermore, the gene expressions of placental HO-1 and GPX2 as the main antioxidant enzyme genes were significantly reduced in sows fed diet supplemented with 25-OH-D3 during the mid-to-late gestation, and the gene expression of GPX2 also decreased in the VD3 group compared to the CT group (p < 0.05). This indicated a lower oxidative damage response during nutrient transportation between sows and fetuses in the 25-OH-D3 and VD3 groups (shown in Figure 1).

Figure 1.
The effect of supplementing VD3 and 25-OH-D3 to the sow diet on the mRNA expression of placental antioxidant-related genes during mid-to-late gestation. The results are presented as mean ± SEM, n = 8. * represents a significant difference (p < 0.05). CT = control group fed with the basal diet; VD3 = VD3 group fed with the supplementation of 2000 IU/kg Vitamin D3 in the basal diet; 25-OH-D3 = 25-OH-D3 group fed with the supplementation of 50 µg/kg 25-OH-D3 in the basal diet. HO-1 = heme oxygenase-1; Nrf2 = nuclear factor-erythroid 2-related factor 2; SOD1 = superoxide dismutase 1; SOD2 = superoxide dismutase 2; CAT = catalase; GPX1 = glutathione peroxidase 1; GPX2 = glutathione peroxidase 2.
3.4. Placental Function
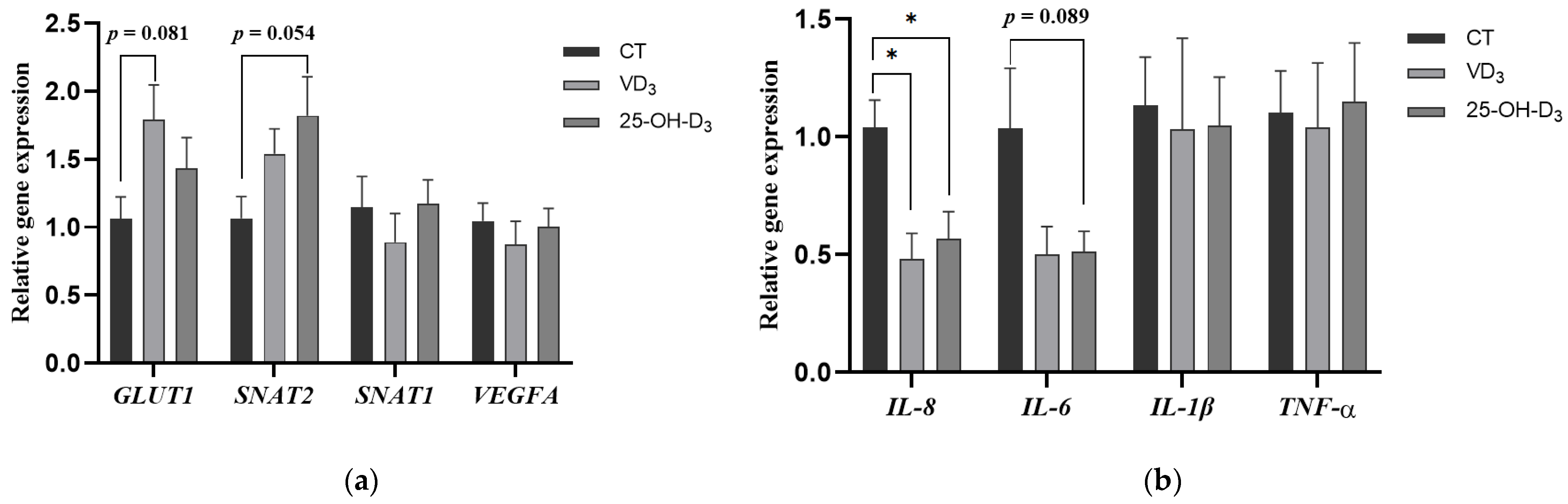
To further evaluate the effect of dietary supplementation with VD3 and 25-OH-D3 on placental function, we determined the gene expression of nutrient transport and immune-related genes in placental tissues. It was shown that the gene expression of glucose transporter GLUT1 in the placenta of sows fed the diet supplemented with VD3 tended to increase compared to the CT group (p = 0.081). Similarly, the gene expression of the neutral amino acid transporter SNAT2 in placental tissue from the 25-OH-D3 group showed a tendency toward upregulation compared to the CT group (p = 0.054). However, no significant differences were observed in the gene expression levels of SNAT1 and VEGFA in the placenta among the treatment groups (p > 0.05) (shown in Figure 2a).
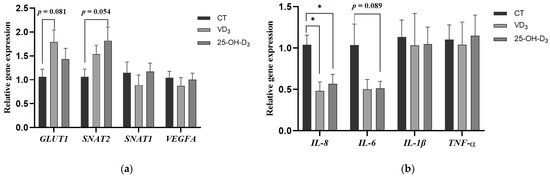
Figure 2.
The effect of supplementing VD3 and 25-OH-D3 into the sow diet on the mRNA expression of placental nutrient transport (a) and immune-related genes (b) during mid-to-late gestation. The results are presented as mean ± SEM, n = 8. * represents a significant difference (p < 0.05). CT = control group fed with the basal diet; VD3 = VD3 group fed with the supplementation of 2000 IU/kg Vitamin D3 in the basal diet; 25-OH-D3 = 25-OH-D3 group fed with the supplementation of 50 µg/kg 25-OH-D3 in the basal diet. GLUT1 = glucose transporter type 1; SNAT2 = sodium coupled neutral amino acid transporter 2; SNAT1 = sodium coupled neutral amino acid transporter 1; VEGFA = vascular endothelial growth factor A; IL-8 = interleukin-8; IL-6 = interleukin-6; IL-1β = interleukin-1β; TNF-α = tumor necrosis factor-α.
In addition, the gene expression of pro-inflammatory factor IL-8 in the placenta of sows fed diets supplemented with VD3 and 25-OH-D3 was significantly decreased compared to the CT group (p < 0.05), and the gene expression of placental IL-6 in the 25-OH-D3 group tended to be reduced compared to the CT group (p = 0.089) (shown in Figure 2b).
4. Discussion
Sow reproductive performance plays a crucial role in pig production. Generally, sow reproduction relies on key performance indicators, primarily including litter size, litter weight, the number of born alive and stillborn piglets, etc [18]. Our study indicated that dietary supplementation with 25-OH-D3 enhanced the average litter weight and average piglet weight of primiparous sows during mid-to-late gestation, which was consistent with previous findings in multiparous sows [19,20]. Some researchers have reported that the supplementation of 25-OH-D3 to the diet of primiparous sows also increased the number of live-born piglets and the weaned litter size [15,21], but no significant improvement in the number of live-born piglets was observed in our findings. However, another study demonstrated that there was no significant effect on the reproductive performance of multiparous sows when fed diets supplemented with different doses of VD3 and 25-OH-D3 during early gestation, although high doses of VD3 and 25-OH-D3 could significantly reduce the number of stillbirths [22]. Furthermore, our study revealed no difference in sows’ performance between the CT group and the VD3 group, indicating that supplementing the sow diet with the same dose of 25-OH-D3 may be more efficient in improving the reproductive performance of sows during mid-to-late gestation.
Previous studies have demonstrated that the supplementation of 25-OH-D3 in the diet could alleviate oxidative stress and inflammatory responses through increasing plasma SOD and GSH-Px activity and reducing tumor necrosis factor (TNF)-α levels when animals were challenged with lipopolysaccharide (LPS) or sulfate colistin [23,24]. It is well known that sows suffer oxidative stress due to multiple factors, including increased metabolism, rapid fetal development, as well as the processes of parturition and milk production during gestation and lactation. Previous research has indicated that 25-OH-D3 could increase the antioxidant capacity of pregnant sows and their offspring [25], and high doses of VD3 have also been shown to improve the activities of antioxidant enzymes (GSH-Px, T-AOC, and T-SOD) and reduce MDA concentrations [26]. Consistent with these findings, the present study suggests that the same dose of 25-OH-D3 and VD3 can significantly reduce plasma MDA concentrations in primiparous sows and enhance maternal antioxidant capacity during late gestation.
In mammals, the placenta serves as a unique connecting structure between the mother and fetus, delivering the nutrients and waste to support fetal development via the umbilical cord. However, placental function can be impacted by maternal oxidative stress, ultimately reflecting the health status of both maternal and fetal growth [27]. A previous study has shown that an increase in maternal antioxidant capacity could enhance the antioxidant function of umbilical cord blood [28]. Although it did not show a significant improvement in the antioxidant indicators of the umbilical cord in the 25-OH-D3 group, the level of GSH-Px was numerically higher than that in other groups. In addition, some researchers reported that the gene expressions of placental SOD, GSX, and CAT were upregulated, and the livability of piglets and reproductive performance of sows were reduced when the maternal oxidative stress increased, indicating that the placenta initiated a compensatory mechanism by activating the Nrf2 signaling pathway to alleviate placenta damage induced by oxidative stress and maintain fetal growth [29,30,31,32]. In the present study, our findings illustrated that the supplementation with 25-OH-D3 in the sow diet downregulated the gene expressions of HO-1 and GPX2. Additionally, the gene expression of GPX2 decreased in the VD3 group compared to that in the CT group, suggesting both 25-OH-D3 and VD3 can alleviate the placental compensatory effects caused by oxidative stress and improve placental antioxidant capacity.
Placental nutrient transporters, including those for glucose, amino acids, and fatty acids, play a critical role in modulating nutrients transfer to the fetus across the placenta. This process is influenced by multiple factors, including placental metabolism, oxidative stress, and blood flow [33,34]. In this study, the gene expression of GULT1 (one of the key glucose transporters) was increased in the placenta of sows fed the diet with the supplementation of VD3, suggesting that VD3 might enhance placental glucose transport from the sow to the fetus. Studies have shown that intrauterine growth retardation (IUGR) is strongly associated with placental amino acid transport capacity for protein deposition and fetal tissue growth [35,36,37]. Here, we found that the gene expression of SNAT2 (one of the main neutral amino acid transporters) in the placenta was accelerated in the 25-OH-D3 group, which might explain the higher litter weight of piglets at farrowing in the reproductive performance of sows fed the diet with supplementation of 25-OH-D3. Furthermore, it was also found that placental cytokines (IL-8 and IL-6) were decreased in the 25-OH-D3 group, indicating that this might repress placental inflammation and inhibit the inflammation response in the maternal–fetal interface at farrowing.
5. Conclusions
Our findings indicate that feeding primiparous sows a diet supplemented with 25-OH-D3 during mid-to-late gestation resulted in better sow performance. This improvement was likely associated with a reduction in maternal MDA concentrations and the gene expressions of placental HO-1, GPX2, IL-6, and IL-8 and an increase in the gene expression of amino acid transporter SNAT2. These changes helped alleviate oxidative stress and inflammation in both the mother and fetus and enhanced nutrient transport for fetal growth. Additionally, VD3 also facilitated maternal antioxidant capacity and placental function. However, no improvement in sow reproductive performance was observed in this study, indicating that 25-OH-D3 might be more effective for sow performance than VD3 at the same inclusion level.
Author Contributions
Conceptualization, J.L.; data curation, Q.B.; formal analysis, Y.P. and X.J.; methodology, X.J.; resources, Y.L. and Y.P.; writing—original draft preparation, J.L. and Q.B.; investigation, Y.P. and X.J.; writing—review and editing X.L.; supervision, X.L.; funding acquisition, Y.L. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2023-IFR-12).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the Animal Care and Use Committee of the Institute of Feed Research of the Chinese Academy of Agricultural Sciences (IFR-CAAS-20230908).
Informed Consent Statement
Not application.
Data Availability Statement
All data are included in the article.
Conflicts of Interest
The author declares that there are no conflicts of interest with other people or organizations, and there are no professional or other personal interests of any nature or kind in any product, service, and/or company that could be construed as influencing the content of this paper.
References
- Ampode, K.M.B.; Mun, H.S.; Lagua, E.B.; Chem, V.; Park, H.R.; Kim, Y.H.; Yang, C.J. Bump Feeding Improves Sow Reproductive Performance, Milk Yield, Piglet Birth Weight, and Farrowing Behavior. Animals 2023, 13, 3148. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Rasmussen, M.H.; Piening, B.; Shen, X.; Chen, S.; Röst, H.; Snyder, J.K.; Tibshirani, R.; Skotte, L.; Lee, N.C. Metabolic Dynamics and Prediction of Gestational Age and Time to Delivery in Pregnant Women. Cell 2020, 181, 1680–1692.e1615. [Google Scholar] [CrossRef]
- Yang, X.; Hu, R.; Shi, M.; Wang, L.; Yan, J.; Gong, J.; Zhang, Q.; He, J.; Wu, S. Placental Malfunction, Fetal Survival and Development Caused by Sow Metabolic Disorder: The Impact of Maternal Oxidative Stress. Antioxidants 2023, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, S.W. Oxidative stress status and reproductive performance of sows during gestation and lactation under different thermal environments. Asian-Australas. J. Anim. 2020, 33, 722–731. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L.A. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Xie, C.; Wu, X.; Long, C.; Wang, Q.; Fan, Z.; Li, S.; Yin, Y. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. BMC Vet. Res. 2016, 12, 243. [Google Scholar] [CrossRef]
- Serdar, Z.; Gür, E.; Colakoethullarý, M.; Develioethlu, O.; Sarandöl, E. Lipid and protein oxidation and antioxidant function in women with mild and severe preeclampsia. Arch. Gynecol. Obstet. 2003, 268, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Fatemi, S.A.; Alqhtani, A.; Elliott, K.E.C.; Bello, A.; Zhang, H.; Peebles, E.D. Effects of the in ovo injection of vitamin D(3) and 25-hydroxyvitamin D(3) in Ross 708 broilers subsequently fed commercial or calcium and phosphorus-restricted diets. I. Performance, carcass characteristics, and incidence of woody breast myopathy(1,2,3). Poultry Sci. 2021, 100, 101220. [Google Scholar] [CrossRef]
- Zhan, D.; Zhao, J.; Shi, Q.; Lou, J.; Wang, W. 25-hydroxyvitamin D3 inhibits oxidative stress and ferroptosis in retinal microvascular endothelial cells induced by high glucose through down-regulation of miR-93. BMC Ophthalmol. 2023, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Pittas, A.G. Vitamin D and diabetes. Endo. Crin. Metab. Clin. 2014, 43, 205–232. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcified Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Anandabaskar, N.; Selvarajan, S.; Dkhar, S.A.; Kamalanathan, S.K.; Tamilarasu, K.; Bobby, Z. Effect of vitamin D supplementation on vascular functions and oxidative stress in type 2 diabetic patients with vitamin D deficiency. Indian J. Endocrinol. Metab. 2017, 21, 555–563. [Google Scholar]
- Zhou, H.; Chen, Y.; Zhuo, Y.; Lv, G.; Lin, Y.; Feng, B.; Fang, Z.; Che, L.; Li, J.; Xu, S.; et al. Effects of 25-hydroxycholecalciferol supplementation in maternal diets on milk quality and serum bone status markers of sows and bone quality of piglets. Anim. Sci. 2017, 88, 476–483. [Google Scholar] [CrossRef]
- Lütke-Dörhoff, M.; Schulz, J.; Westendarp, H.; Visscher, C.; Wilkens, M.R. Dietary supplementation of 25-hydroxycholecalciferol as an alternative to cholecalciferol in swine diets: A review. J. Anim. Physiol. An. Nutri 2022, 106, 1288–1305. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Koketsu, Y.; Tani, S.; Iida, R. Factors for improving reproductive performance of sows and herd productivity in commercial breeding herds. Porcine Health Manag. 2017, 3, 1. [Google Scholar] [CrossRef]
- Weber, G.M.; Witschi, A.K.; Wenk, C.; Martens, H. Triennial Growth Symposium--Effects of dietary 25-hydroxycholecalciferol and cholecalciferol on blood vitamin D and mineral status, bone turnover, milk composition, and reproductive performance of sows. J. Anim. Sci. 2014, 92, 899–909. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Zhang, D.; Wang, R.; Zhao, Z.; Feng, M.; Wei, H.; Li, L.; Zhang, S. Effects of 25-hydroxycholecalciferol supplementation in maternal diets on reproductive performance and the expression of genes that regulate lactation in sows. Anim. Sci. J. 2020, 91, e13391. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Jung, Y.J.; Kim, Y.M.; Chung, T.K.; Kim, I.H. Effects of dietary supplementation with 25-OH-D3 during gestation and lactation on reproduction, sow characteristics and piglet performance to weaning: 25-hydroxyvitamin D3 in sows. Anim. Feed Sci. Tech. 2021, 271, 114732. [Google Scholar] [CrossRef]
- Lauridsen, C.; Halekoh, U.; Larsen, T.; Jensen, S.K. Reproductive performance and bone status markers of gilts and lactating sows supplemented with two different forms of vitamin D1. J. Anim. Sci. 2010, 88, 202–213. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Piao, X. Potential Effects of 25-Hydroxycholecalciferol on the Growth Performance, Blood Antioxidant Capacity, Intestinal Barrier Function and Microbiota in Broilers under Lipopolysaccharide Challenge. Antioxidants 2022, 11, 2094. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Xu, Y.; Zhang, Z.; Wu, Y.; Cao, J.; Qiu, B.; Qin, X.; Han, D.; Piao, X. Dietary supplementation of 25-hydroxyvitamin D3 improves growth performance, antioxidant capacity and immune function in weaned piglets. Antioxidants 2022, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Li, M.; Piao, X. Effects of maternal 25-hydroxycholecalciferol during the last week of gestation and lactation on serum parameters, intestinal morphology and microbiota in suckling piglets. Arch. Anim. Nutr. 2020, 74, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Mao, Z.; Mou, D.; Huang, L.; Yang, M.; Ding, D.; Yan, H.; Fang, Z.; Che, L.; Zhuo, Y.; et al. Maternal cholecalciferol supplementation during gestation improves antioxidant capacities in gilts and piglets. Italian J. Anim. Sci. 2021, 20, 1201–1210. [Google Scholar] [CrossRef]
- Xu, K.; Liu, G.; Fu, C. The Tryptophan Pathway Targeting Antioxidant Capacity in the Placenta. Oxid. Med. Cell Longev. 2018, 2018, 1054797. [Google Scholar] [CrossRef]
- Suhail, M.; Suhail, S.; Gupta, B.K.; Bharat, V. Malondialdehyde and Antioxidant Enzymes in Maternal and Cord Blood, and their Correlation in Normotensive and Preeclamptic Women. J. Clin. Med. Res. 2009, 1, 150–157. [Google Scholar] [CrossRef][Green Version]
- El-Hashash, A.H.; Warburton, D.; Kimber, S.J. Genes and signals regulating murine trophoblast cell development. Mech. Develop. 2010, 127, 1–20. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free. Radic. Bio. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Zhao, Y.; Lin, J.; Jiang, S.; Li, W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp. Mol. Med. 2022, 54, 1067–1075. [Google Scholar] [CrossRef]
- Jones, H.N.; Powell, T.L.; Jansson, T. Regulation of Placental Nutrient Transport—A Review. Placenta 2007, 28, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Lager, S.; Powell, T.L. Regulation of Nutrient Transport across the Placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef]
- Cetin, I.; Ronzoni, S.; Marconi, A.M.; Perugino, G.; Corbetta, C.; Battaglia, F.C.; Pardi, G. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am. J. Obstet. Gynecol. 1996, 174, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Paolini, C.L.; Marconi, A.M.; Ronzoni, S.; Di Noio, M.; Fennessey, P.V.; Pardi, G.; Battaglia, F.C. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J. Clin. Endocr. Metab. 2001, 86, 5427–5432. [Google Scholar] [CrossRef]
- Vaughan Owen, R.; Maksym, K.; Silva, E.; Barentsen, K.; Anthony Russel, V.; Brown Thomas, L.; Hillman Sara, L.; Spencer, R.; David Anna, L.; Rosario Fredrick, J.R.; et al. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin. Sci. 2021, 135, 2049–2066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).